Author Contributions
Conceptualization, S.S. and R.G.; Methodology, D.V., S.J. and S.B.; Software, D.V., S.J., S. and I.T.; Formal analysis, D.V., S.J., S.B., S.S. and R.G.; Investigation, D.V., S.J., S.B., S.S. and R.G.; Resources, S.S.; Data curation, S.J. and S.S.; Writing—original draft, D.V., S.J., S.B., S.S., R.G., S. and I.T.; Writing—review & editing, D.V., S.J., S.B., S.S., R.G., S. and I.T.; Visualization, S.B., S. and I.T.; Supervision, S.S. and R.G.; Project administration, R.G.; Funding acquisition, R.G. All authors have read and agreed to the published version of the manuscript.
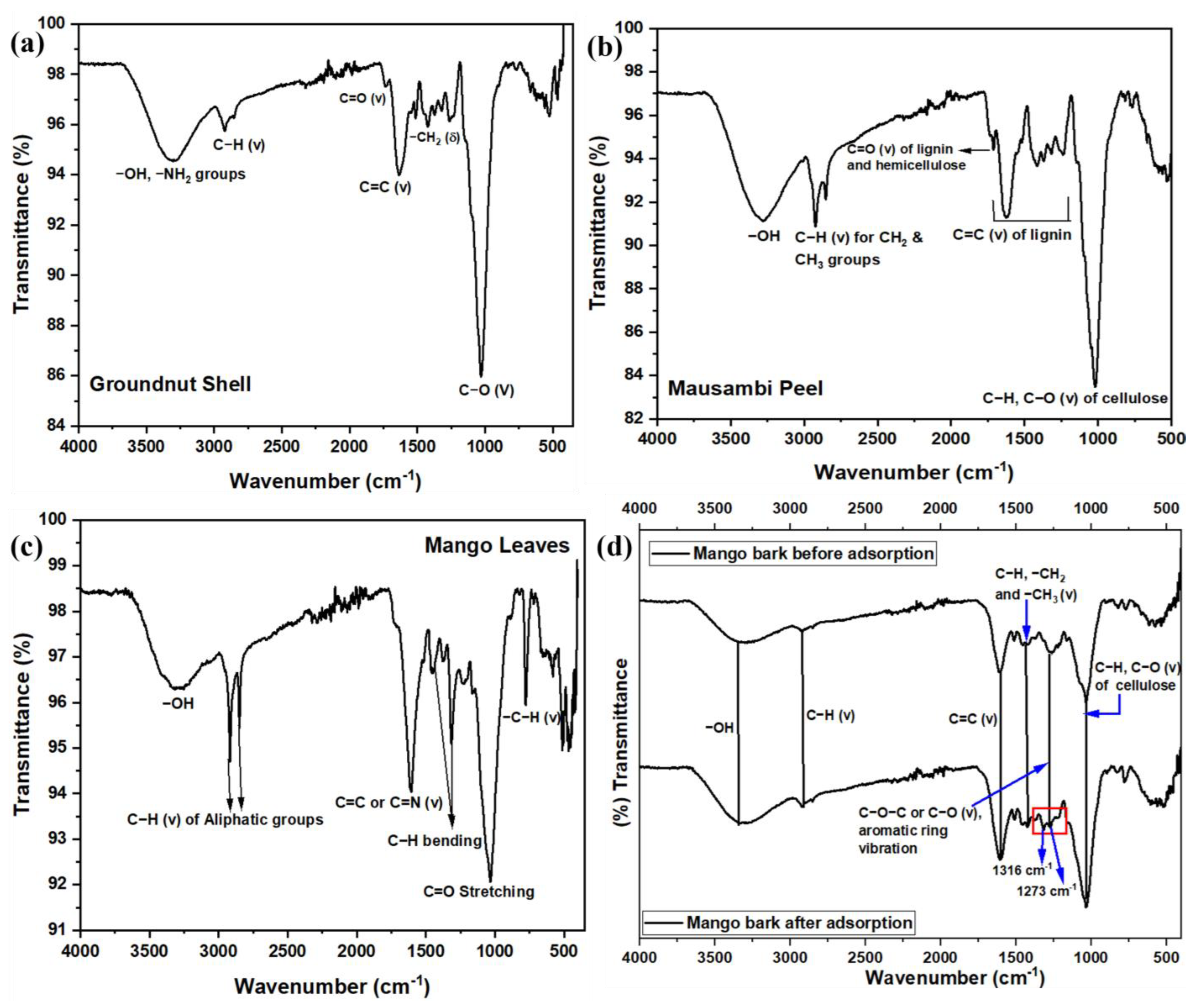
Figure 1.
FT-IR spectra for all the adsorbents (a) GS, (b) MP, (c) ML, and (d) MBARK before and after adsorption.
Figure 1.
FT-IR spectra for all the adsorbents (a) GS, (b) MP, (c) ML, and (d) MBARK before and after adsorption.
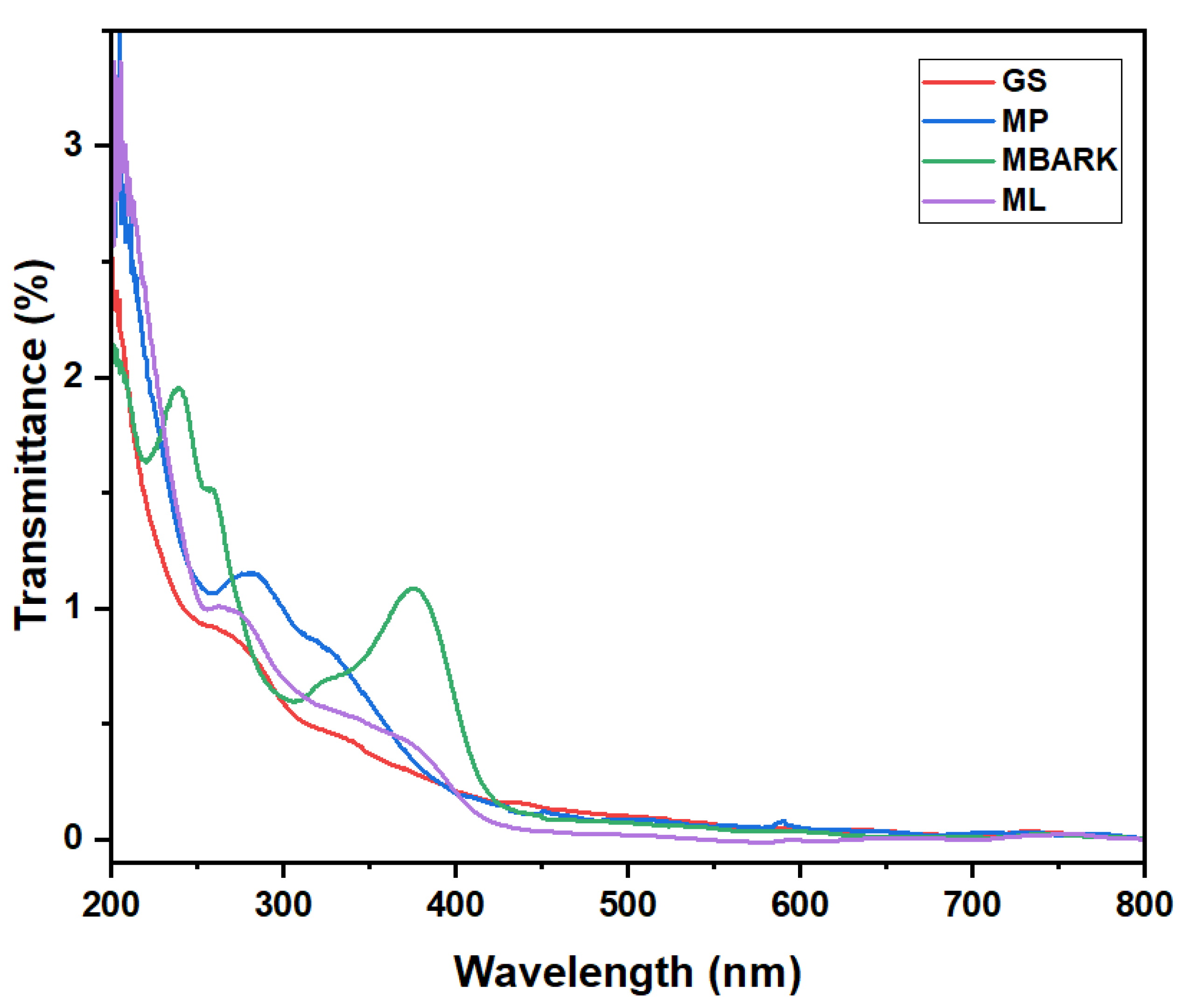
Figure 2.
UV–Vis spectra of aqueous solution of Groundnut Shell (GS), Mosambi Peel (MP), Mango Bark (MBARK), and Mango Leaves (ML).
Figure 2.
UV–Vis spectra of aqueous solution of Groundnut Shell (GS), Mosambi Peel (MP), Mango Bark (MBARK), and Mango Leaves (ML).
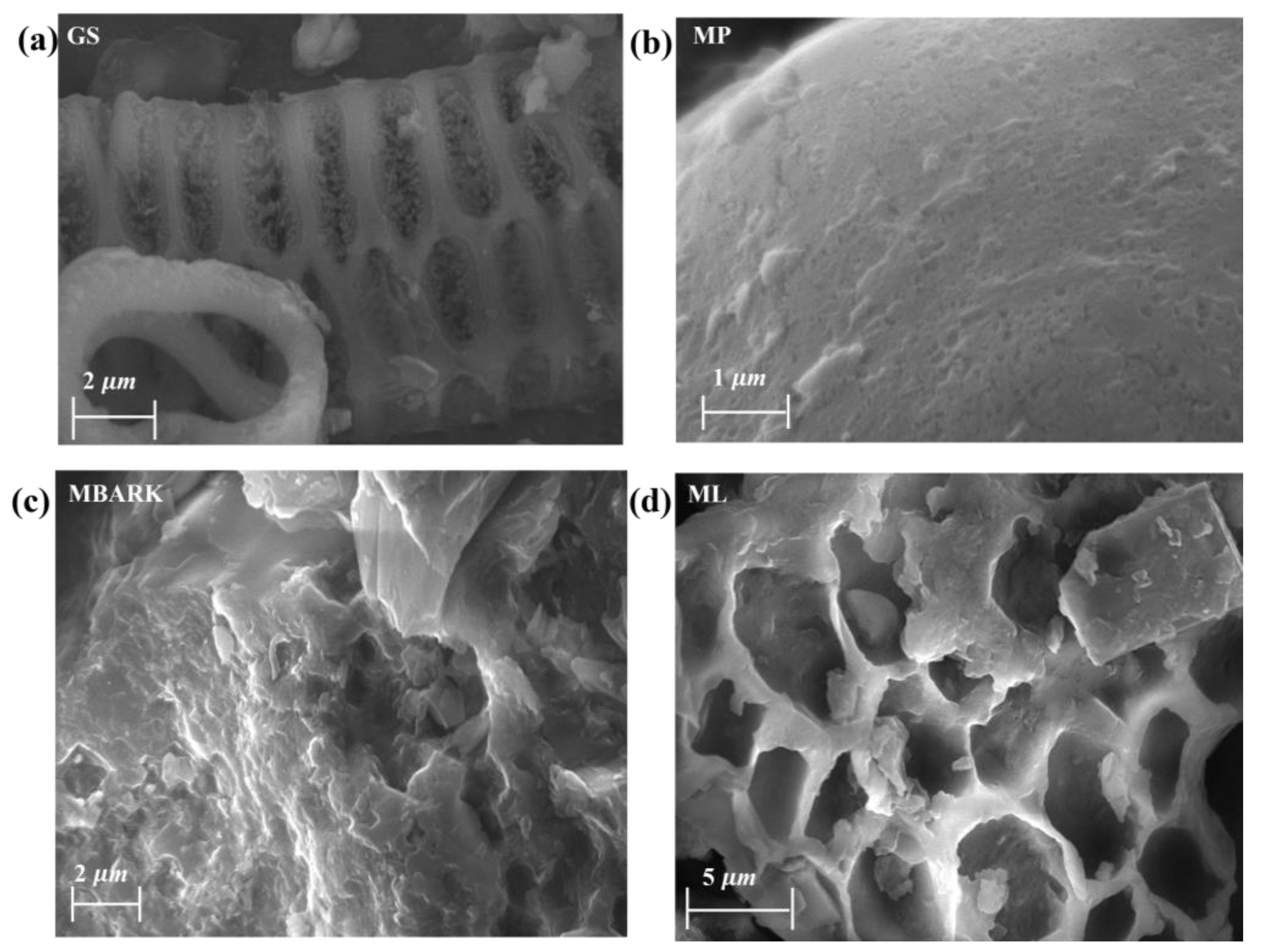
Figure 3.
SEM images of the adsorbents (a) GS, (b) MP, (c) MBARK, and (d) ML.
Figure 3.
SEM images of the adsorbents (a) GS, (b) MP, (c) MBARK, and (d) ML.
Figure 4.
PZC plot for (a) GS, (b) MP, (c) MBARK, and (d) ML.
Figure 4.
PZC plot for (a) GS, (b) MP, (c) MBARK, and (d) ML.
Figure 5.
UV–Vis spectra showing adsorption of (a) CV, (b) MB, (c) RhB, and (d) MG as a single component system (adsorbent dosage = 1 mg/mL, Dye concentration = 10 mg/L, total contact time = 60 min).
Figure 5.
UV–Vis spectra showing adsorption of (a) CV, (b) MB, (c) RhB, and (d) MG as a single component system (adsorbent dosage = 1 mg/mL, Dye concentration = 10 mg/L, total contact time = 60 min).
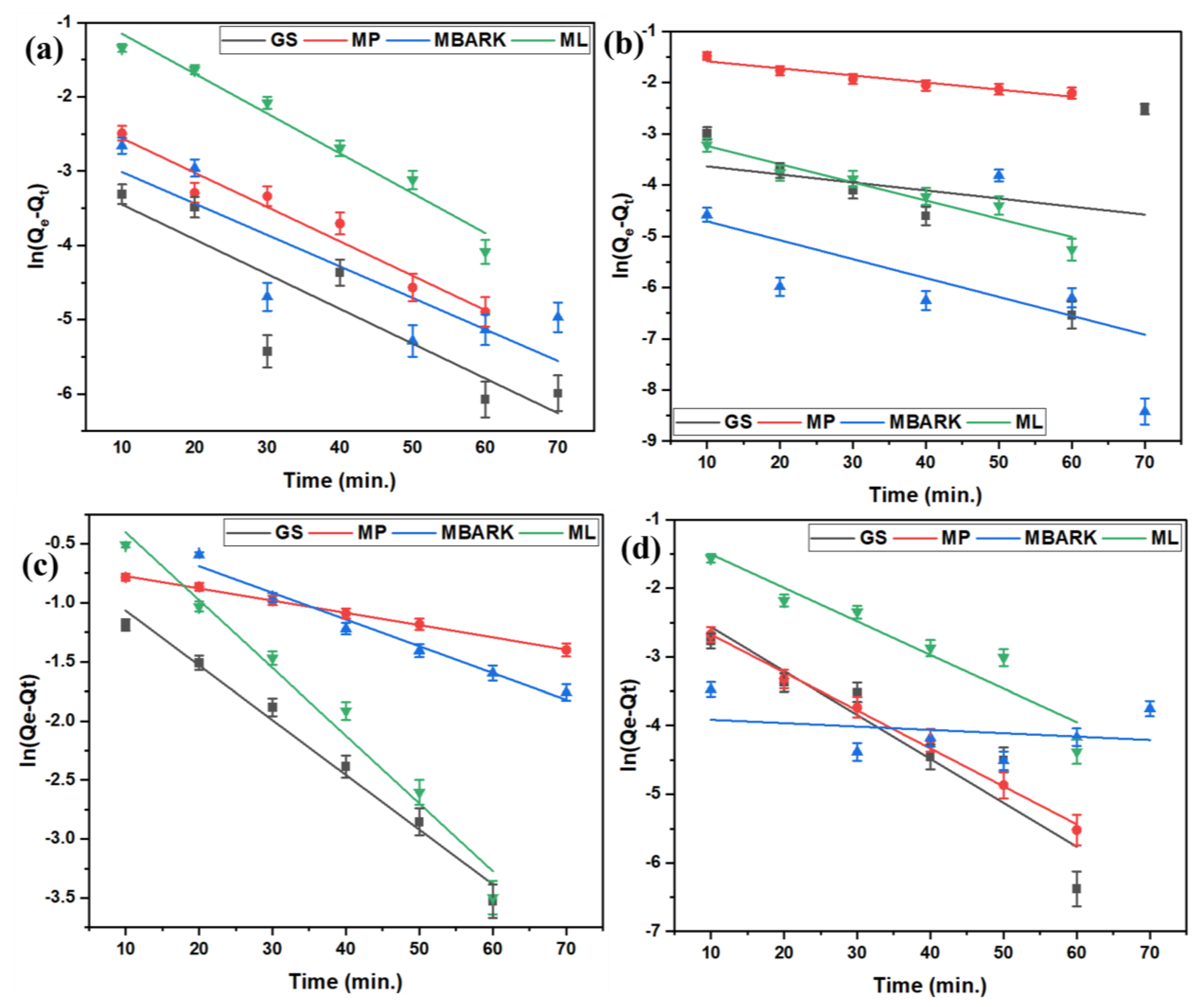
Figure 6.
First −order kinetic model for removal of dyes using all four adsorbents: (a) CV, (b) MB, (c) RhB, and (d) MG (adsorbent dosage = 1 mg/mL, dye concentration = 10 mg/L, total contact time = 70 min).
Figure 6.
First −order kinetic model for removal of dyes using all four adsorbents: (a) CV, (b) MB, (c) RhB, and (d) MG (adsorbent dosage = 1 mg/mL, dye concentration = 10 mg/L, total contact time = 70 min).
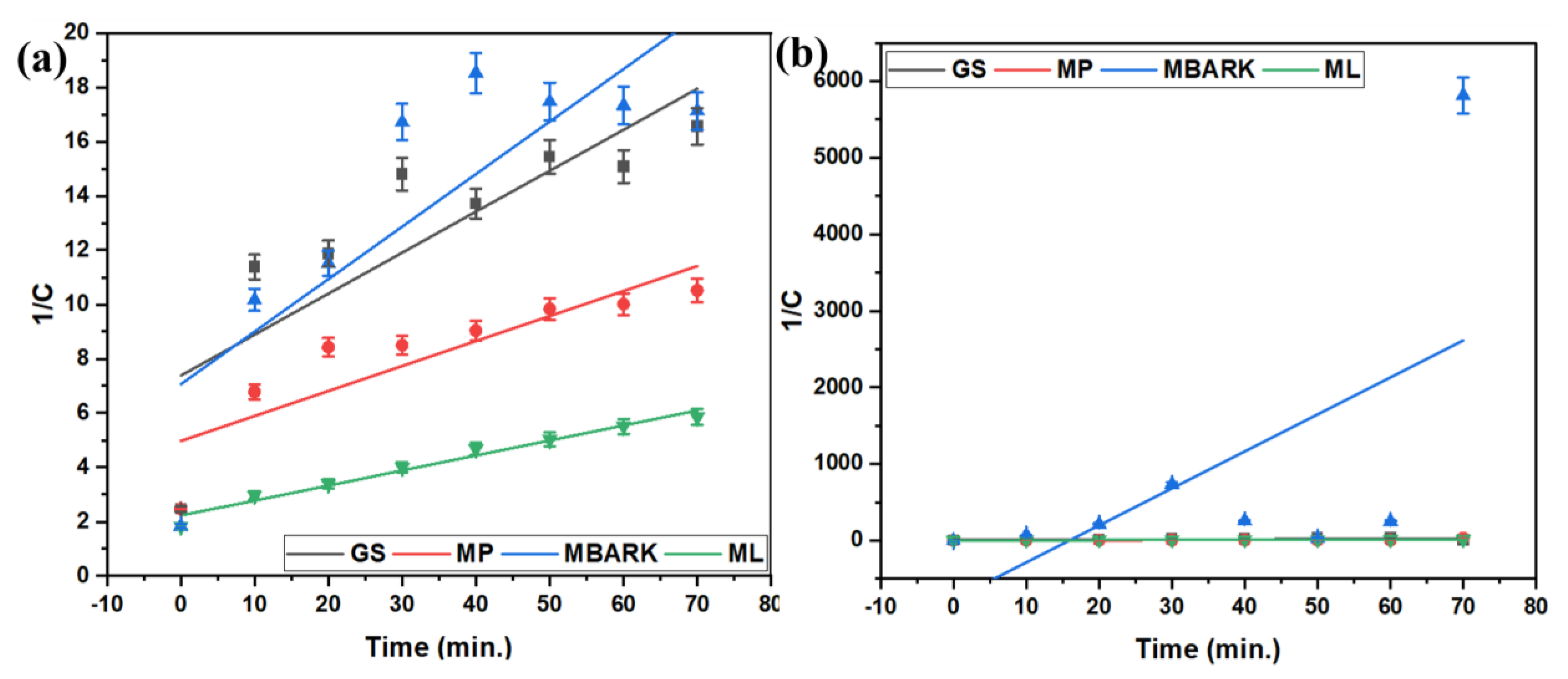
Figure 7.
Second−order kinetic model for removal of dyes using all four adsorbents (a) CV, (b) MB, (c) RhB, and (d) MG (adsorbent dosage = 1 mg/mL, dye concentration = 10 mg/L, total contact time = 70 min).
Figure 7.
Second−order kinetic model for removal of dyes using all four adsorbents (a) CV, (b) MB, (c) RhB, and (d) MG (adsorbent dosage = 1 mg/mL, dye concentration = 10 mg/L, total contact time = 70 min).
Figure 8.
Pseudo −first −order kinetic model for removal of dyes using all four adsorbents: (a) CV, (b) MB, (c) RhB, and (d) MG dyes (adsorbent dosage = 1 mg/mL, dye concentration = 10 mg/L, total contact time = 70 min).
Figure 8.
Pseudo −first −order kinetic model for removal of dyes using all four adsorbents: (a) CV, (b) MB, (c) RhB, and (d) MG dyes (adsorbent dosage = 1 mg/mL, dye concentration = 10 mg/L, total contact time = 70 min).
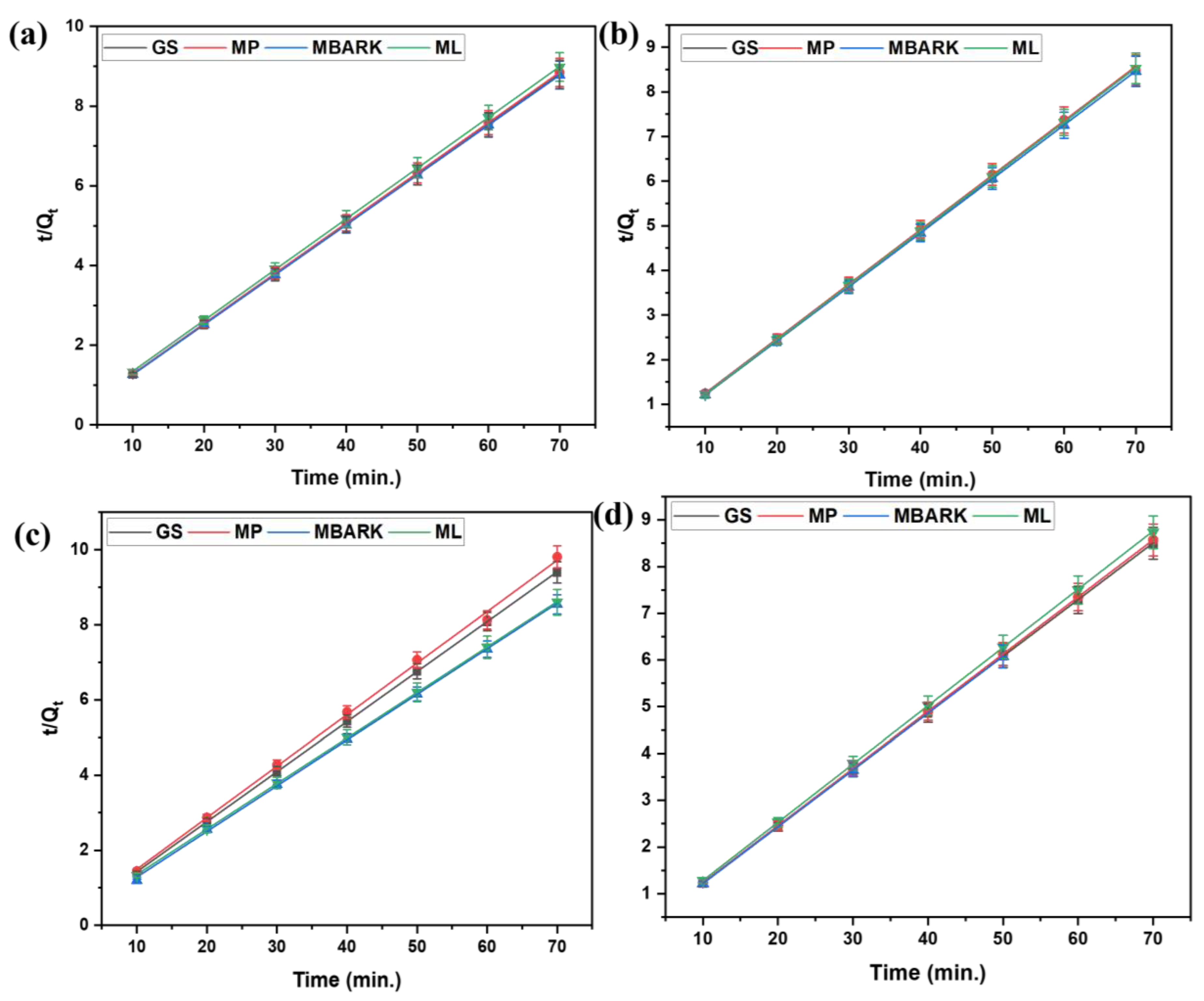
Figure 9.
Pseudo −second −order kinetic model for removal of dyes using all five adsorbents: (a) CV, (b) MB, (c) RhB, and (d) MG dyes (adsorbent dosage = 1 mg/mL, dye concentration = 10 mg/L, total contact time = 70 min).
Figure 9.
Pseudo −second −order kinetic model for removal of dyes using all five adsorbents: (a) CV, (b) MB, (c) RhB, and (d) MG dyes (adsorbent dosage = 1 mg/mL, dye concentration = 10 mg/L, total contact time = 70 min).
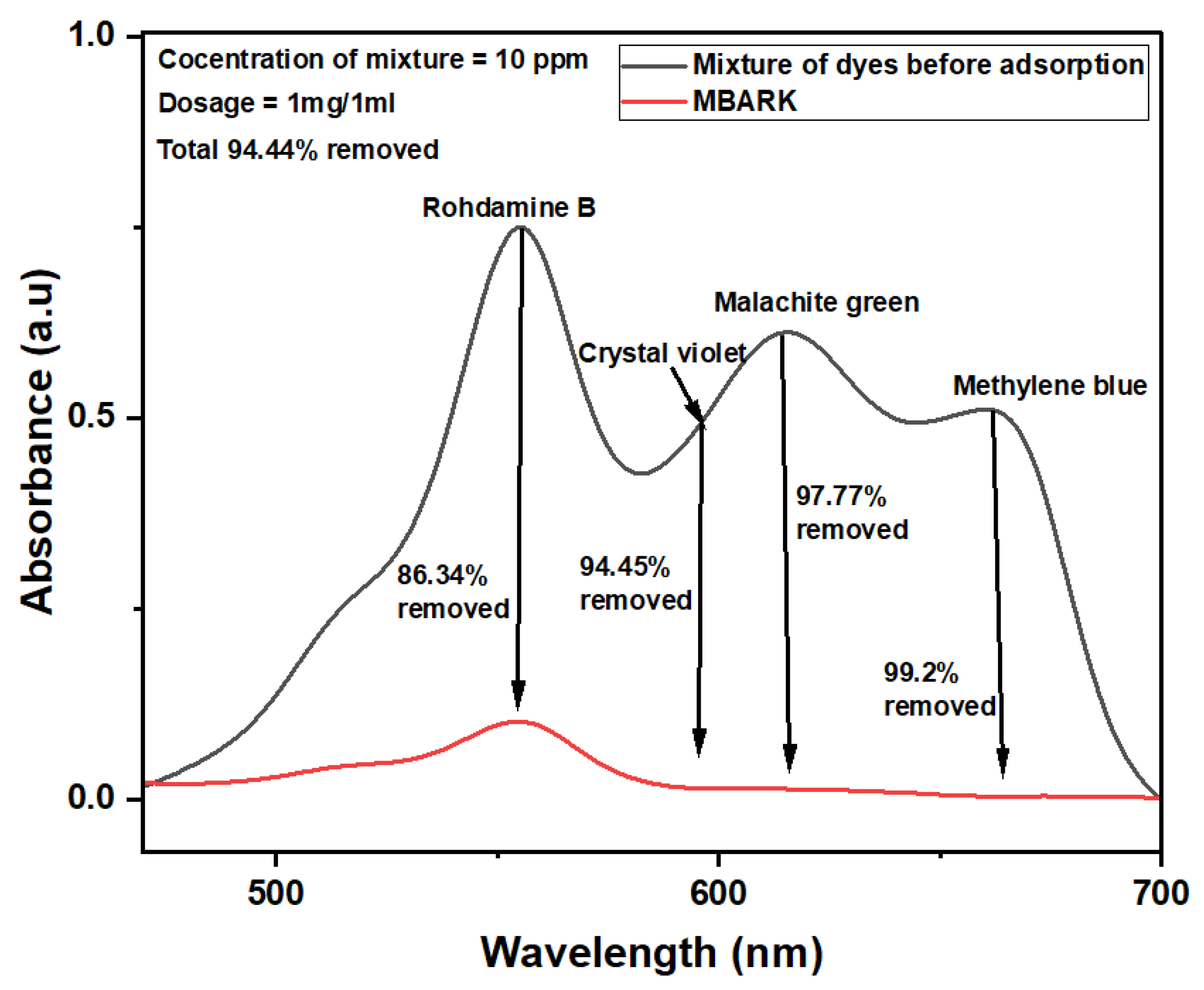
Figure 10.
UV–Vis spectra showing removal of CV, MB, RhB, and MG simultaneously from a mixture of dyes using MBARK (adsorbent dosage = 1 mg/mL, dye mixture concentration = 10 mg/L (each dye = 2.5 mg/L), pH of solution = 6, total contact time = 60 min).
Figure 10.
UV–Vis spectra showing removal of CV, MB, RhB, and MG simultaneously from a mixture of dyes using MBARK (adsorbent dosage = 1 mg/mL, dye mixture concentration = 10 mg/L (each dye = 2.5 mg/L), pH of solution = 6, total contact time = 60 min).
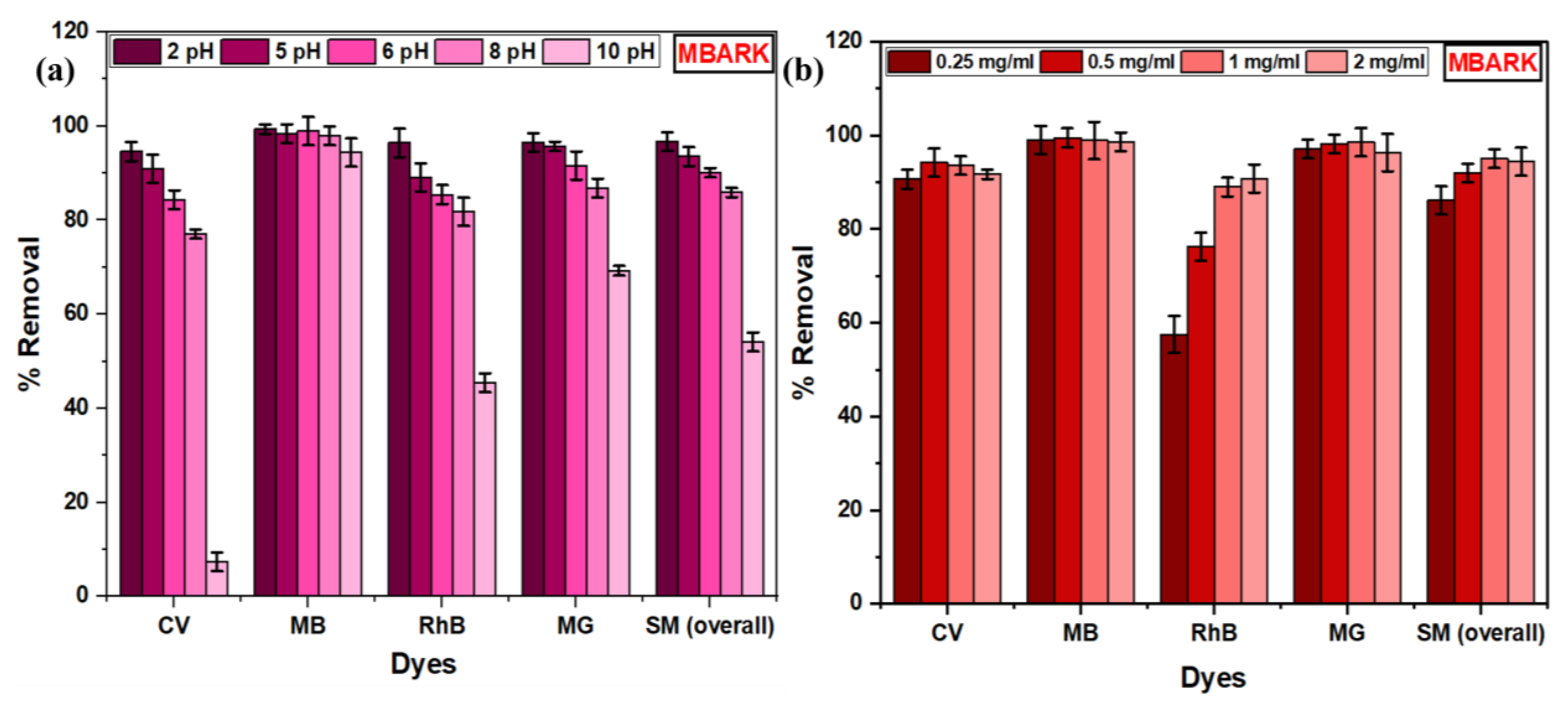
Figure 11.
Bar graphs of (a) Effect of pH, (b) Effect of dosage for MBARK on simultaneous removal of dyes (adsorbent dosage = 0.25 to 2 mg/mL, dye mixture concentration = 10 mg/L (each dye = 2.5 mg/mL), pH = 2 to 10, and total contact time = 60 min).
Figure 11.
Bar graphs of (a) Effect of pH, (b) Effect of dosage for MBARK on simultaneous removal of dyes (adsorbent dosage = 0.25 to 2 mg/mL, dye mixture concentration = 10 mg/L (each dye = 2.5 mg/mL), pH = 2 to 10, and total contact time = 60 min).
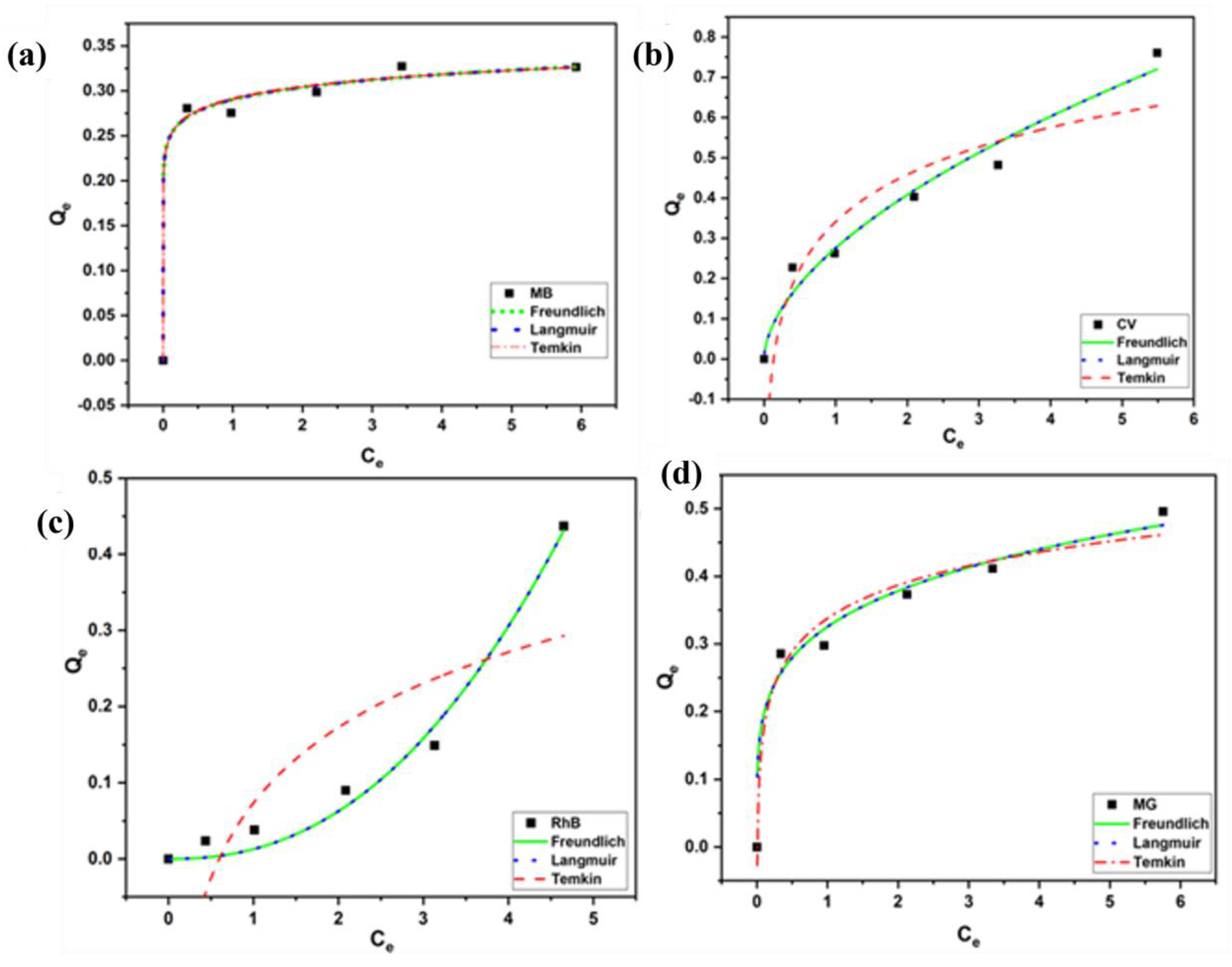
Figure 12.
Freundlich, Langmuir, and Temkin isotherm models for (a) MB, (b) CV, (c) RhB, and (d) MG dyes using MBARK (adsorbent dosage = 1 mg/mL, dye concentration = 2.5 mg/L to 25 mg/L, contact time = 60 min).
Figure 12.
Freundlich, Langmuir, and Temkin isotherm models for (a) MB, (b) CV, (c) RhB, and (d) MG dyes using MBARK (adsorbent dosage = 1 mg/mL, dye concentration = 2.5 mg/L to 25 mg/L, contact time = 60 min).
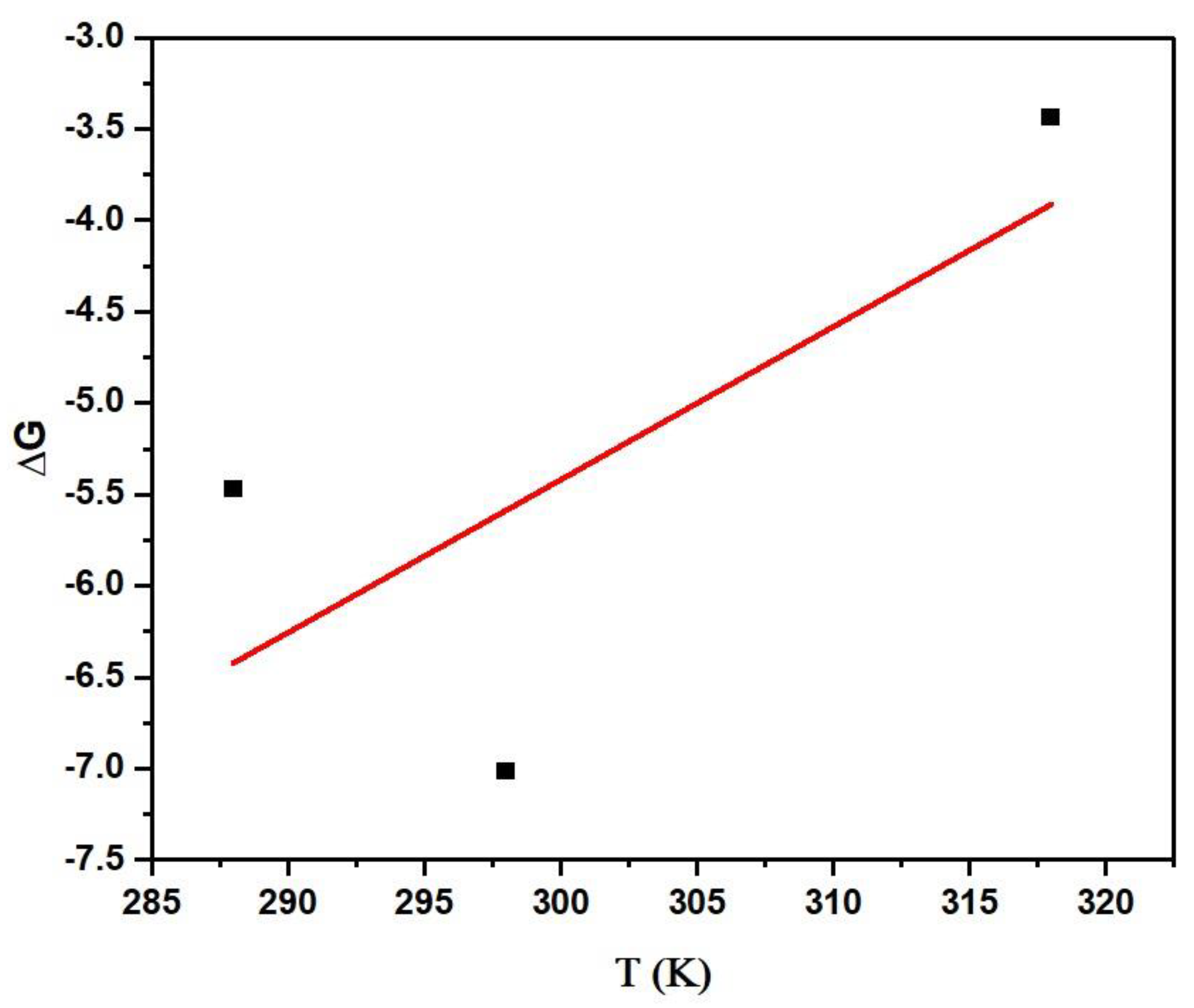
Figure 13.
Thermodynamic plot of the simultaneous reaction performed at varying temperatures (adsorbent dosage = 1 mg/mL, dye mixture concentration = 10 mg/L, contact time = 60 min).
Figure 13.
Thermodynamic plot of the simultaneous reaction performed at varying temperatures (adsorbent dosage = 1 mg/mL, dye mixture concentration = 10 mg/L, contact time = 60 min).
Figure 14.
Probable mechanism and interaction based on the functionalities of adsorbent (MBARK) and dye molecule.
Figure 14.
Probable mechanism and interaction based on the functionalities of adsorbent (MBARK) and dye molecule.
Table 1.
Advantages and disadvantages of various physical and chemical wastewater treatment methods.
Table 1.
Advantages and disadvantages of various physical and chemical wastewater treatment methods.
| Methods | Advantages | Disadvantages | Ref. |
|---|
| Ion exchange | Small amount of sludge | High operation cost | [4] |
| Coagulation/flocculation | low energy consumption | No complete removal of heavy metals | [5] |
| Chemical precipitation | Low capital cost | Large amount of sludge | [6] |
| Flotation | Economically efficient | Low elimination efficiency | [7] |
| Membrane filtration | High separation selectivity | High operational cost | [8] |
| Adsorption | Easy and simple operation | Weak selectivity | [9] |
Table 2.
Values of particle size (from Zeta sizer analysis), PZC, surface area, pore size, and pore volume for all adsorbents.
Table 2.
Values of particle size (from Zeta sizer analysis), PZC, surface area, pore size, and pore volume for all adsorbents.
| Adsorbents | Particle Size
(Z-Average (r.nm)) | PZC | BET Surface Area (m2/g) | Pore Size (nm) | Pore Volume (cm3/g) |
|---|
| GS | 748.6 | 6.9 | 1.857 | 12.279 | 0.009 |
| MP | 543.8 | 6.9 | 1.106 | 14.756 | 0.004 |
| MBARK | 533.2 | 8.0 | 2.463 | 11.065 | 0.007 |
| ML | 423.1 | 6.6 | 0.986 | 14.304 | 0.004 |
Table 3.
% Removal of dyes (MB, CV, RhB, and MG) individually for different adsorbents (adsorbent dosage = 1 mg/mL, Dye concentration = 10 mg/L, total contact time = 60 min).
Table 3.
% Removal of dyes (MB, CV, RhB, and MG) individually for different adsorbents (adsorbent dosage = 1 mg/mL, Dye concentration = 10 mg/L, total contact time = 60 min).
| Adsorbents | % Removal |
|---|
| MB | CV | RhB | MG |
|---|
| GS | 97.78 ± 2 | 90.39 ± 3 | 41.49 ± 2 | 97.3 ± 3 |
| MP | 78.01 ± 3 | 86.57 ± 2 | 24.34 ± 2 | 90.93 ± 1 |
| MBARK | 98.08 ± 1 | 88.42 ± 1 | 78.85 ± 2 | 98.18 ± 3 |
| ML | 78.6 ± 2 | 54.08 ± 1 | 71.55 ± 1 | 67.60 ± 1 |
Table 4.
Linear form of equations for different kinetic models.
Table 4.
Linear form of equations for different kinetic models.
| Kinetic Model | Linear Equation |
|---|
| First order | |
| Pseudo-first order | t |
| Second order | |
| Pseudo-second order | |
Table 5.
The values of k and R2 of different kinetic model fittings for the adsorption of different dyes using all the adsorbents (adsorbent dosage = 1 mg/mL, dye concentration = 10 mg/L, total contact time = 70 min).
Table 5.
The values of k and R2 of different kinetic model fittings for the adsorption of different dyes using all the adsorbents (adsorbent dosage = 1 mg/mL, dye concentration = 10 mg/L, total contact time = 70 min).
| Dye | Adsorbents | Qe(mg/g) | First Order | Second Order | Pseudo—First Order | Pseudo—Second Order |
|---|
| k | R2 | k | R2 | k | R2 | k | R2 |
|---|
| CV | GS | 1.62 | 0.01838 | 0.51562 | 0.15087 | 0.68036 | 0.04681 | 0.77812 | 1.94692 | 1 |
| MP | 1.53 | 0.01502 | 0.60066 | 0.09197 | 0.75561 | 0.04628 | 0.95367 | 0.84410 | 1 |
| MBARK | 1.57 | 0.02339 | 0.54096 | 0.19344 | 0.68527 | 0.04239 | 0.74484 | 1.34942 | 1 |
| ML | 0.83 | 0.01493 | 0.89678 | 0.5524 | 0.975 | 0.05367 | 0.96926 | 0.21479 | 0.99998 |
| MB | GS | 2.4 | 0.03007 | 0.32167 | 0.23314 | 0.28002 | 0.01569 | 0.06546 | 1.72565 | 0.99991 |
| MP | 1.66 | 0.03857 | 0.74471 | 0.29097 | 0.47652 | 0.01383 | 0.93041 | 0.25611 | 0.99983 |
| MBARK | 2.42 | 0.07657 | 0.49181 | 48.31715 | 0.35359 | 0.03688 | 0.28828 | 14.47806 | 1 |
| ML | 1.68 | 0.02755 | 0.47033 | 0.1145 | 0.70882 | 0.03557 | 0.93002 | 1.54918 | 1 |
| RhB | GS | 0.64 | 0.00736 | 0.74293 | 0.00519 | 0.82086 | 0.04637 | 0.98511 | 0.18305 | 0.99996 |
| MP | 0.03 | 0.0042 | 0.74749 | 0.00250 | 0.79498 | 0.01038 | 0.98511 | 0.14851 | 0.99859 |
| MBARK | 2.08 | 0.02602 | 0.89677 | 0.04258 | 0.99531 | 0.02263 | 0.97562 | 0.20824 | 0.99963 |
| ML | 1.81 | 0.02264 | 0.88044 | 0.03296 | 0.99111 | 0.05743 | 0.97893 | 0.10184 | 0.99996 |
| MG | GS | 2.68 | 0.03246 | 0.54845 | 0.23663 | 0.85798 | 0.06397 | 0.88389 | 1.03446 | 1 |
| MP | 2.46 | 0.02395 | 0.48837 | 0.09636 | 0.69503 | 0.0553 | 0.99402 | 1.03438 | 1 |
| MBARK | 2.71 | 0.02533 | 0.31694 | 0.12195 | 0.2404 | 0.00489 | 0.0725 | 4.30477 | 1 |
| ML | 1.67 | 0.0181 | 0.63893 | 0.04067 | 0.83123 | 0.04891 | 0.90187 | 0.30413 | 0.99998 |
Table 6.
Non-linear equations for Freundlich, Langmuir, and Temkin isotherm models.
Table 6.
Non-linear equations for Freundlich, Langmuir, and Temkin isotherm models.
| Isotherm Model | Non-Linear Form |
|---|
| Freundlich isotherm | |
| Langmuir isotherm | |
| Temkin isotherm | |
Table 7.
Values of different parameters derived from thermodynamic studies for simultaneous adsorption of dyes (adsorbent dosage = 1 mg/mL, dye mixture concentration = 10 mg/L, contact time = 60 min).
Table 7.
Values of different parameters derived from thermodynamic studies for simultaneous adsorption of dyes (adsorbent dosage = 1 mg/mL, dye mixture concentration = 10 mg/L, contact time = 60 min).
| ΔG (kJ/mole) at Varying Temperature | ΔH (kJ/mole) | ΔS (J/mole·K) |
|---|
| 288 K | 298 K | 318 K | −30.51 | 0.0836 |
| −5.47 | −7.01 | −3.43 |
Table 8.
Removal (%) of different dyes in the mixture up to 4 cycles using spent adsorbent (adsorbent dosage = 1 mg/mL, dye mixture concentration = 10 mg/L, contact time = 60 min).
Table 8.
Removal (%) of different dyes in the mixture up to 4 cycles using spent adsorbent (adsorbent dosage = 1 mg/mL, dye mixture concentration = 10 mg/L, contact time = 60 min).
| Dye | Cycle 1 | Cycle 2 | Cycle 3 | Cycle 4 |
|---|
| MB | 97.24 | 99.68 | 97.70 | 97.71 |
| CV | 94.90 | 99.54 | 96.39 | 93.39 |
| MG | 86.46 | 99.45 | 89.84 | 82.05 |
| RhB | 96.10 | 90.99 | 97.01 | 95.55 |
| Overall | 93.67 | 97.41 | 95.23 | 92.17 |