A 35-Year Record (1987–2022) of Hg Concentrations in Two of the Fish Species Most Consumed by People Living in the Upper Madeira River Basin, Brazilian Amazon Region
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Database
2.2. Sampling and Chemical Analysis
2.3. Total Hg Emissions from ASGM and Deforestation
2.4. Statistical Analyses
3. Results and Discussion
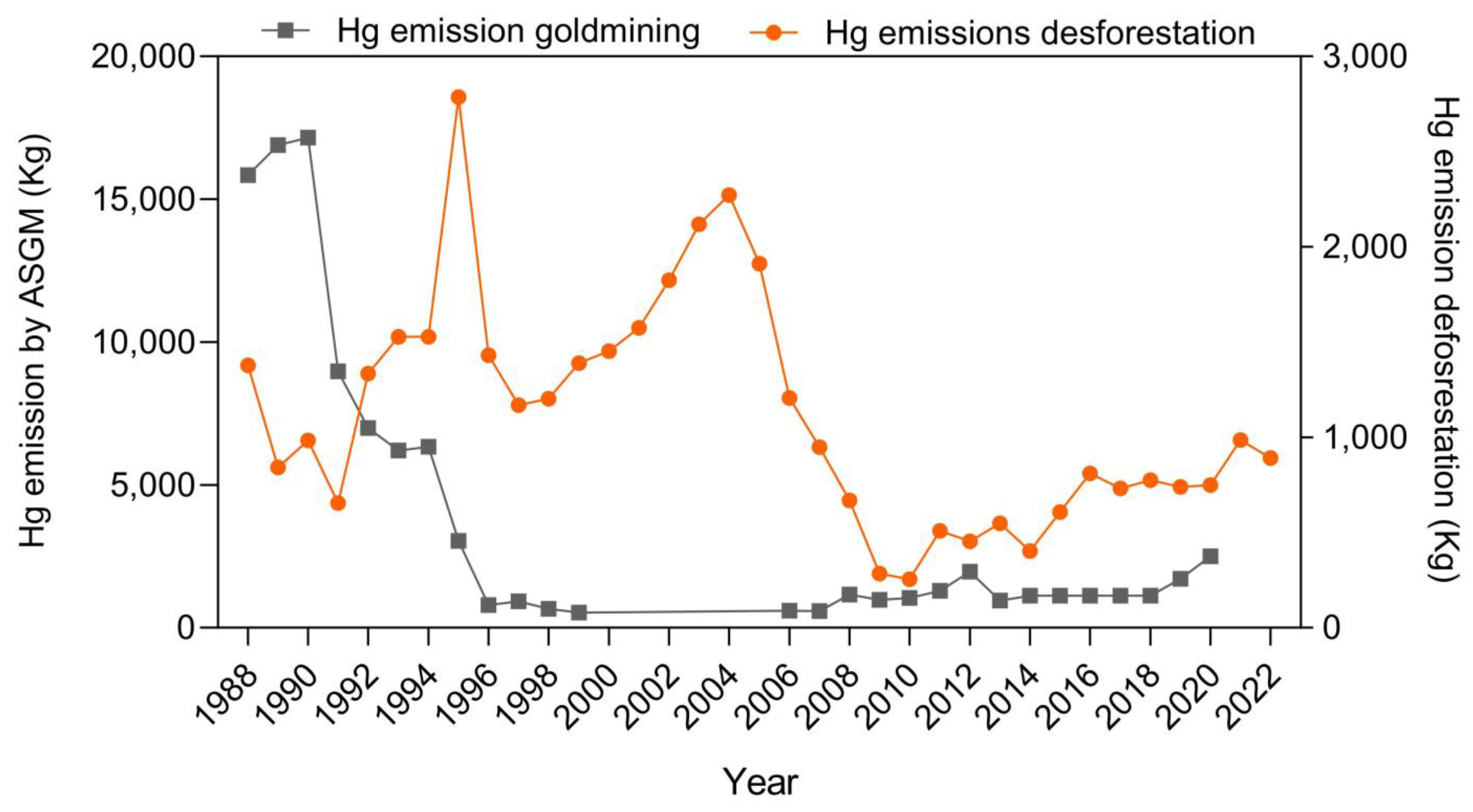
3.1. Mercury Emissions from ASGM and Deforestation
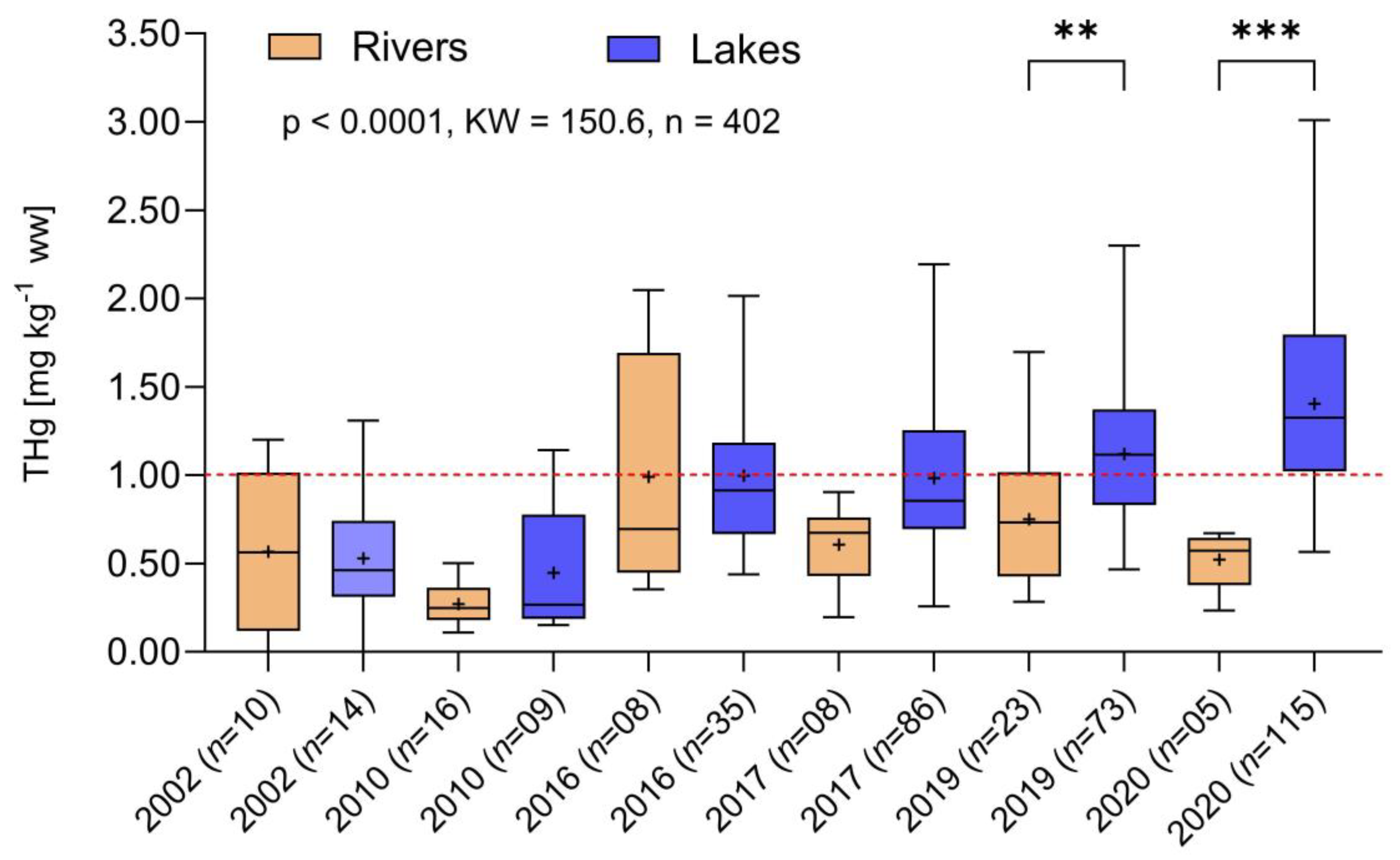
3.2. Long-Term Changes in Fish Mercury in the Madeira River Watershed
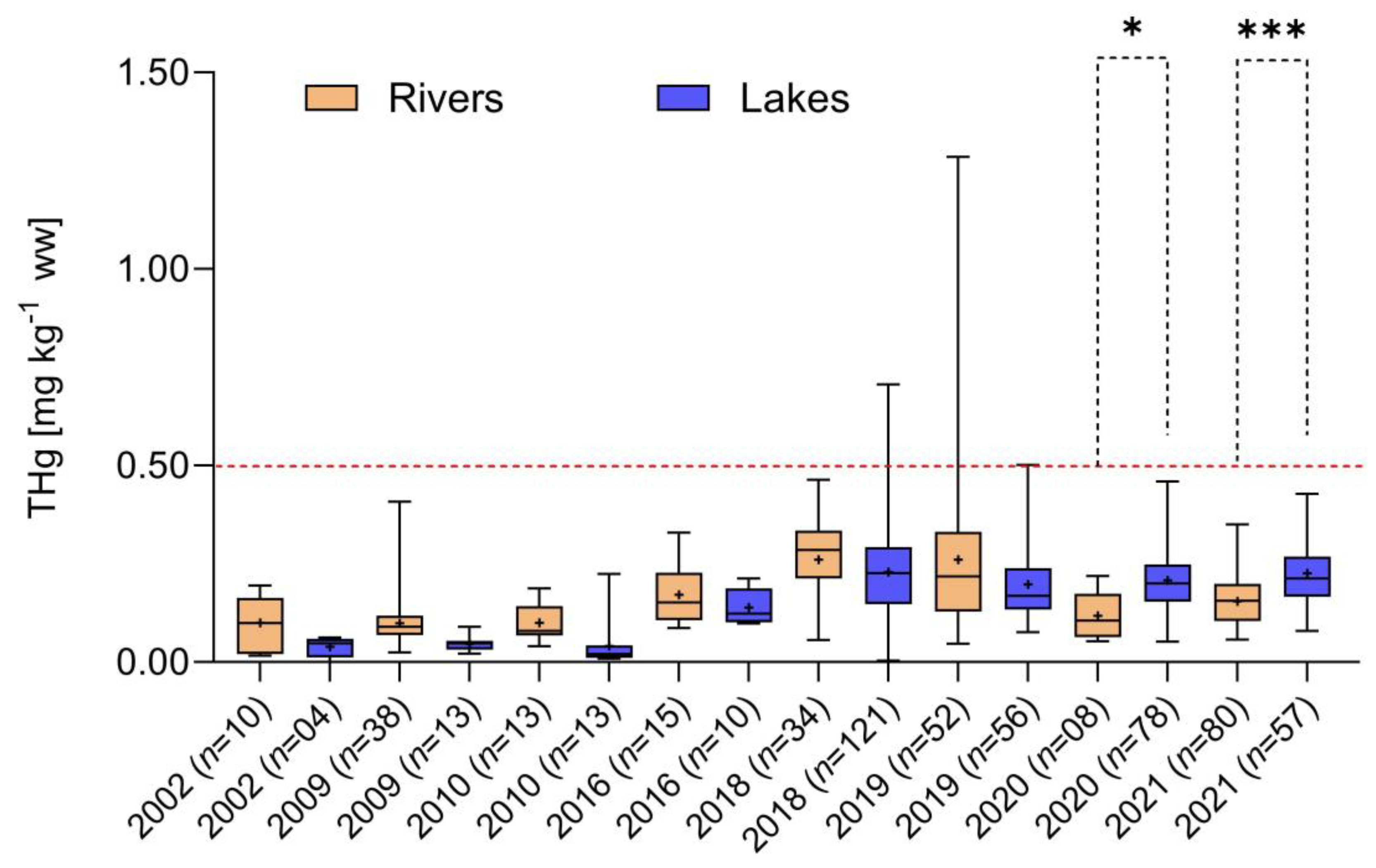
3.3. Temporal Trends in Hg Concentrations in Tributaries and Marginal Lakes versus Main River
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lacerda, L.D.; Salomons, W. Mercury from Gold and Silver Mining: A Chemical Time Bomb? Springer: Berlin/Heidelberg, Germany, 1998; 147p. [Google Scholar] [CrossRef]
- Pfeiffer, W.C.; Malm, O.; Souza, C.M.M.; Lacerda, L.D.; Silveira, E.G.; Bastos, W.R. Mercury in the Madeira River ecosystem, Rondônia, Brazil. For. Ecol. Manag. J. 1991, 38, 239–245. [Google Scholar] [CrossRef]
- Malm, O.; Castro, M.B.; Bastos, W.R.; Branches, F.J.P.; Guimarães, J.R.D.; Zuffo, C.E.; Pfeiffer, W.C. An assessment of Hg pollution in different goldmining areas, Amazon Brazil. Sci. Total Environ. 1995, 175, 127–140. [Google Scholar] [CrossRef]
- Passos, C.J.; Mergler, D. Human mercury exposure and adverse health effects in the Amazon: A review. Cad. Saúde Pública 2008, 24 (Suppl. 4), 503–520. [Google Scholar] [CrossRef]
- Pfeiffer, W.C.; Lacerda, L.D. Mercury inputs into the Amazon Region, Brazil. Environ. Technol. Lett. 1988, 9, 325–330. [Google Scholar] [CrossRef]
- Diringer, S.E.; Berky, A.J.; Marani, M.; Ortiz, E.J.; Karatum, O.; Plata, D.L.; Pan, W.K.; Hsu-Kim, H. Deforestation Due to Artisanal and Small-Scale Gold Mining Exacerbates Soil and Mercury Mobilization in Madre de Dios, Peru. Environ. Sci. Technol. 2019, 54, 286–296. [Google Scholar] [CrossRef]
- Pinedo-Hernandéz, J.; Marrugo-Negrete, J.; Díez, S. Speciation and bioavailability of mercury in sediments impacted by gold mining in Colombia. Chemosphere 2015, 119, 1289–1295. [Google Scholar] [CrossRef]
- Lacerda, L.D. Atmospheric mercury and fish contamination in the Amazon. Ciência Cult. 1997, 49, 54–57. [Google Scholar]
- Roulet, M.; Lucotte, M.; Farella, N.; Serique, G.; Coelho, H.; Passos, C.J.S.; Silva, E.J.S.; Andrade, S.; Mergler, D.; Guimarães, J.R.D.; et al. Effects of recent human colonization on the presence of mercury in Amazonian ecosystems. Water Air Soil Pollut. 1999, 112, 297–313. [Google Scholar] [CrossRef]
- Almeida, M.D.; Lacerda, L.D.; Bastos, W.R.; Herrmann, J.C. Mercury loss from soils following conversion from forest to pasture in Rondônia, Western Amazon, Brazil. Environ. Pollut. 2005, 137, 179–186. [Google Scholar] [CrossRef]
- Lacerda, L.D.; Bastos, W.R.; Almeida, M.D. The impacts of land use changes in the mercury flux in the Madeira River, Western Amazon. An. Acad. Bras. Ciências 2012, 84, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Bastos, W.R.; Dórea, J.G.; Bernardi, J.V.E.; Manzatto, A.G.; Lauthartte, L.C.; Mussy, M.H.; Lacerda, L.D.; Malm, O. Mercury in fish of the Madeira River (temporal and spatial assessment), Brazilian Amazon. Environ. Res. 2015, 140, 191–197. [Google Scholar] [CrossRef]
- Passos, C.J.S.; Silva, D.S.; Lemire, M.; Fillion, M.; Guimarães, J.R.D.; Lucotte, M.; Mergler, D. Daily mercury intake in fish-eating populations in the Brazilian Amazon. J. Expo. Sci. Environ. Epidemiol. 2008, 18, 76–87. [Google Scholar] [CrossRef] [PubMed]
- UNEP. United Nations Environment Programme. Minamata Convention Agreed by Nations. 2013. Available online: https://www.unep.org/news-and-stories/press-release/minamata-convention-agreed-nations (accessed on 20 December 2023).
- Brasil. Decreto n° 9.470. Promulga a Convenção de Minamata sobre Mercúrio, firmada pela República Federativa do Brasil, em Kumamoto, em 10 de outubro de 2013. 2018. Available online: https://www.in.gov.br/materia/-/asset_publisher/Kujrw0TZC2Mb/content/id/36849570/do1-2018-08-15-decreto-n-9-470-de-14-de-agosto-de-2018-36849564 (accessed on 20 December 2023).
- Veiga, M.M.; Fadina, O. A review of the failed attempts to curb mercury use at artisanal gold mines and a proposed solution. Extr. Ind. Soc. 2020, 7, 1135–1146. [Google Scholar] [CrossRef]
- Pestana, I.A.; Rezende, C.E.; Almeida, R.; Lacerda, L.D.; Bastos, W.R. Let’s talk about mercury contamination in the Amazon (again): The case of the floating gold miners’ village on the Madeira River. Extr. Ind. Soc. 2022, 11, 101122. [Google Scholar] [CrossRef]
- Sirén, A.; Valbo-Jørgensen, J. Quantifying fish catches and fish consumption in the Amazon Basin. Aquat. Ecosyst. Health Manag. 2022, 25, 59–71. [Google Scholar] [CrossRef]
- Oliveira, R.C.; Dórea, J.G.; Bernardi, J.V.E.; Bastos, W.R.; Almeida, R.; Manzatto, A.G. Fish consumption by traditional subsistence villagers of the Rio Madeira (Amazon): Impact on hair mercury. Ann. Hum. Biol. 2010, 37, 629–642. [Google Scholar] [CrossRef]
- Wu, P.; Zhang, Y. Toward a global model of methylmercury biomagnification in marine food webs: Trophic dynamics and implications for human exposure. Environ. Sci. Technol. 2023, 57, 6563–6572. [Google Scholar] [CrossRef]
- Bastos, W.R.; Malm, O.; Pfeifer, W.C.; Cleary, D. Establishment and analytical quality control of laboratories for Hg determination in biological and geological samples in the Amazon Brazil. Ciência Cult. 1998, 50, 255–260. [Google Scholar]
- INPE. Instituto Nacional de Pesquisas Espaciais, Terra Brasilis, Taxas de Desmatamento. São José dos Campos. 2023. Available online: http://terrabrasilis.dpi.inpe.br/app/dashboard/deforestation/biomes/legal_amazon/rates (accessed on 16 October 2023).
- De Queiroz, L.J.; Ohara, W.M.; Vari, R.P. Prochilodontidae. In Peixes Do Rio Madeira; De Queiroz, L.J., Ohara, W.M., Pires, T.H.d.S., Torrente-Villara, G., Zuanon, J.A., Doria, C.R.D.C., Eds.; Dialeto Latin American Documentary: São Paulo, Brazil, 2013. [Google Scholar]
- Silva, E.A.; Stewart, D.J. Reproduction, feeding and migration patterns of Prochilodus nigricans (Characiformes: Prochilodontidae) in northeastern Ecuador. Neotrop. Ichthyol. 2017, 15, e160171. [Google Scholar] [CrossRef]
- dos Reis, R.M.; Lourenço, L.d.S.; da Silva, H.P.; Vendrusclo, J.; Fernandes, I.M. Length-weight relationships and condition factor of the predatory fish, Cichla pleiozona and Serrasalmus rhombeus, from two tropical reservoirs, Amazon basin, Brazil. Int. J. Fish. Aquat. Stud. 2020, 8, 355–360. [Google Scholar]
- Doria, C.R.C.; Duponchelle, F.; Lima, M.A.L.; Garcia, A.; Carvajal-Vallejos, F.M.; Méndez, C.C.; Catarino, M.F.; Freitas, C.E.d.C.; Vega, B.; Miranda-Chumacero, G.; et al. Review of Fisheries Resource Use and Status in the Madeira River Basin (Brazil, Bolivia, and Peru) Before Hydroelectric Dam Completion. Rev. Fish. Sci. Aquac. 2018, 26, 494–514. [Google Scholar] [CrossRef]
- Bastos, W.R.; Dórea, J.G.; Bernardi, J.V.E.; Manzatto, A.G.; Mussy, M.H.; Lauthartte, L.C.; Lacerda, L.D.; Malm, O. Sex-related mercury bioaccumulation in fish from the Madeira River, Amazon. Environ. Res. 2016, 144, 73–80. [Google Scholar] [CrossRef]
- Michelazzo, P.A.M.; Fostier, A.H.; Magarelli, G.; Santos, J.C.; Carvalho, J.A. Mercury emissions from forest burning in southern Amazon. Geophys. Res. Lett. 2010, 37, L09809. [Google Scholar] [CrossRef]
- Melendez-Perez, J.J.; Fostier, A.H.; Carvalho, J.A.; Windmöller, C.C.; Santo, J.C.; Carpi, A. Soil and biomass mercury emissions during a prescribed fire in the Amazonian rain forest. Atmos. Environ. 2014, 96, 415–422. [Google Scholar] [CrossRef]
- Lacerda, L.D. Amazon Mercury Emissions. Nature 1995, 374, 20–21. [Google Scholar] [CrossRef]
- Almeida, M.D.; Marins, R.V.; Paraquetti, H.M.M.; Bastos, W.R.; Lacerda, L.D. Mercury degassing from forested and open field soils in Rondônia, Western Amazon, Brazil. Chemosphere 2009, 77, 60–66. [Google Scholar] [CrossRef]
- Almeida, M.D.; Lacerda, L.D.; Almeida, R.; Oliveira, R.C.; Bastos, W.R.; Marins, R.V. Degassing de mercúrio em solo de floresta e pastagem em candeia do Jamari—RO. Geochim. Bras. 2009, 23, 151–158. [Google Scholar]
- Manzolli, B.A. Diagnóstico da exploração de ouro no Brasil. 2021. Available online: https://mapbiomas-br-site.s3.amazonaws.com/Trabalhos%20vencedores%204ed/2oLugar_CategoriaJovem_BrunoAntonioManzolli.pdf (accessed on 16 October 2023).
- Manzolli, B.A.; Rajão, R.; Bragança, A.C.H.; Oliveira, P.T.M.; Alcântara, G.K.; Nunes, F.; Filho, B.S. Legalidade da Produção de ouro no Brasil; Editora IGC/UFMG: Belo Horizonte, Brazil, 2021; 43p, Available online: http://www.lagesa.org/wp-content/uploads/documents/Manzolli_Rajao_21_Ilegalidade%20cadeia%20do%20Ouro.pdf (accessed on 18 October 2023).
- MINACOOP; Cooperativa dos Garimpeiros, Mineração e Agroflorestal (Minacoop), em Porto Velho, Produção de Ouro em Rondônia, Porto Velho, Rondônia, Brazil. Personal communication, 2023.
- Helder, M. Dez anos da evolução do Ouro no Brasil. In the Mine, 15 April 2014. Available online: https://www.inthemine.com.br/site/evolucao-da-mineracao-de-ouro-no-brasil-2001-2012/ (accessed on 18 October 2023).
- MF. Ministério da Fazenda, Valores do IOF-Ouro distribuídos aos municípios. MF, Brasília. 2021. Available online: https://dados.gov.br/dataset/transferenciasobrigatorias-da-uniao-por-municipio/resource/4248cd95-6d79-4520-9e17-48322eab6259 (accessed on 16 October 2023).
- Lacerda, L.D.; Pfeiffer, W.C.; Ott, A.T.; Silveira, E.G. Mercury contamination in the Madeira River, Amazon: Hg inputs to the environment. Biotropica 1989, 21, 91–93. [Google Scholar] [CrossRef]
- UNEP. Global Mercury Assessment. UN Environment Programme Chemicals and Health Branch Geneva Switzerland. 2019. Available online: https://www.unenvironment.org/explore-topics/chemicals-waste (accessed on 16 October 2023).
- ANVISA. Agência Nacional de Vigilância Sanitária. Resolução—RDC Nº 42, de 29 de agosto de 2013. Regulamento Técnico MERCOSUL sobre Limites Máximos de Contaminantes Inorgânicos em Alimentos. 2013. Available online: https://bvsms.saude.gov.br/bvs/saudelegis/anvisa/2013/rdc0042_29_08_2013.html (accessed on 16 October 2023).
- Lacerda, L.D.; Souza, M.; Ribeiro, M.G. Effect of land use change on the mercury distribution is soils from Alta Floresta, southern Amazon. Environ. Pollut. 2004, 129, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Carpi, A.; Fostier, A.H.; Orta, O.; Santos, J.C.; Gittings, M. Gaseous mercury emissions from soil following forest loss and land use changes: Field experiments in the United States and Brazil. Atmos. Environ. 2014, 96, 426–429. [Google Scholar] [CrossRef]
- Almeida, R.; Bernardi, J.V.E.; Oliveira, R.C.; Carvalho, D.P.; Bastos, W.R.; Lacerda, L.D. Flood pulse and spatial dynamics of mercury in sediments in Puruzinho Lake, Brazilian Amazon, Brazil. Acta Amaz. 2014, 44, 99–106. [Google Scholar] [CrossRef]
- Bastos, W.R.; de Almeida, R.; Dórea, J.G.; Barbosa, A.G. Annual flooding and fish-mercury bioaccumulation in the environmentally impacted Rio Madeira (Amazon). Ecotoxicology 2007, 16, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Araujo, B.F.; Hintelmann, H.; Dimock, B.; Sobrinho, R.L.; Bernardes, M.C.; Almeida, M.G.; Krusche, A.V.; Rangel, T.P.; Thompson, F.; Rezende, C.E. Mercury speciation and Hg stable isotope ratios in sediments from Amazon floodplain lakes—Brazil. Limnol. Oceanogr. 2018, 63, 1334–1345. [Google Scholar] [CrossRef]
- Coquery, M.; Cossa, D.; Peretyazhko, T.; Azemard, S.; Charlet, L. Methylmercury formation in the anoxic waters of the Petit-Saut reservoir (French Guiana) and its spreading in the adjacent Sinnamary river. J. Phys. IV 2003, 107, 327–331. [Google Scholar] [CrossRef]
- Brendson, C.B.; Forsberg, B.R.; Kasper, D.; Amaral, J.H.F.; Vasconcelos, M.R.R.; Sousa, O.P.; Cunha, F.A.G.; Bastos, W.R. The influence of inundation and lake morphometry on the dynamics of mercury in the water and plankton in an Amazon floodplain lake. Hydrobiologia 2017, 790, 35–48. [Google Scholar]
- Pestana, I.A.; Almeida, M.G.; Bastos, W.R.; Souza, C.M.M. Total Hg and methylmercury dynamics in a river-floodplain system in the Western Amazon: Influence of seasonality, organic matter and physical and chemical parameters. Sci. Total Environ. 2019, 656, 88–399. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Lacerda, L.D.; de Almeida, R.; Bastos, W.R. A 35-Year Record (1987–2022) of Hg Concentrations in Two of the Fish Species Most Consumed by People Living in the Upper Madeira River Basin, Brazilian Amazon Region. Toxics 2024, 12, 144. https://doi.org/10.3390/toxics12020144
de Lacerda LD, de Almeida R, Bastos WR. A 35-Year Record (1987–2022) of Hg Concentrations in Two of the Fish Species Most Consumed by People Living in the Upper Madeira River Basin, Brazilian Amazon Region. Toxics. 2024; 12(2):144. https://doi.org/10.3390/toxics12020144
Chicago/Turabian Stylede Lacerda, Luiz Drude, Ronaldo de Almeida, and Wanderley Rodrigues Bastos. 2024. "A 35-Year Record (1987–2022) of Hg Concentrations in Two of the Fish Species Most Consumed by People Living in the Upper Madeira River Basin, Brazilian Amazon Region" Toxics 12, no. 2: 144. https://doi.org/10.3390/toxics12020144
APA Stylede Lacerda, L. D., de Almeida, R., & Bastos, W. R. (2024). A 35-Year Record (1987–2022) of Hg Concentrations in Two of the Fish Species Most Consumed by People Living in the Upper Madeira River Basin, Brazilian Amazon Region. Toxics, 12(2), 144. https://doi.org/10.3390/toxics12020144






