Urinary N-acetylglucosaminidase in People Environmentally Exposed to Cadmium Is Minimally Related to Cadmium-Induced Nephron Destruction
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Quantitation of Exposure and Its Effects
2.3. Normalization of Excretion Rate of Cd, β2M, alb and NAG
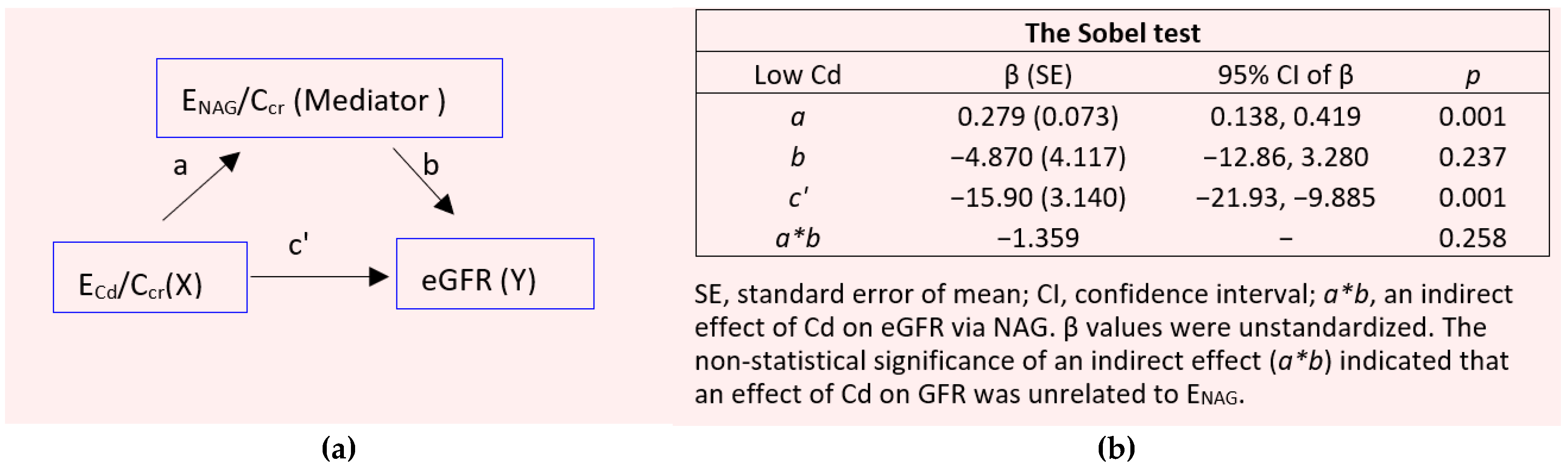
2.4. Mediation Analysis
2.5. Statistical Analysis
3. Results
3.1. Characteristics of Study Subjects
3.2. The Risks of a Low eGFR and Albuminuria Increase with the Severity of Tubular Proteinuria
3.3. Associations of Eβ2M with ECd, eGFR, and ENAG
3.4. Moderate-to-Strong Association of ENAG/Ccr with ECd/Ccr
3.5. Quantification of Effects of Cadmium and Tubular Injury on eGFR
3.6. A Cause-Effect Inference Analysis of Cd Exposure, Tubular Injury (ENAG), and GFR Decline
4. Discussion
4.1. Use of β2-Microglobulinuria in Toxicological Risk Assessment of Cadmium Exposure
4.2. Cadmium Body Burden at Which Adverse Kidney Outcomes Were Observed
4.3. The Tubulo-Glomerular Effects of Cadmium
4.4. Cadmium-Induced Microalbuminuria
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Foreman, K.J.; Marquez, N.; Dolgert, A.; Fukutaki, K.; Fullman, N.; McGaughey, M.; Pletcher, M.A.; Smith, A.E.; Tang, K.; Yuan, C.W.; et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: Reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet 2018, 392, 2052–2090. [Google Scholar] [CrossRef] [PubMed]
- GBD 2021 Forecasting Collaborators. Burden of disease scenarios for 204 countries and territories, 2022–2050: A forecasting analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 2204–2256. [Google Scholar] [CrossRef] [PubMed]
- Murton, M.; Goff-Leggett, D.; Bobrowska, A.; Garcia Sanchez, J.J.; James, G.; Wittbrodt, E.; Nolan, S.; Sörstadius, E.; Pecoits-Filho, R.; Tuttle, K. Burden of Chronic Kidney Disease by KDIGO Categories of Glomerular Filtration Rate and Albuminuria: A Systematic Review. Adv. Ther. 2021, 38, 180–200. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Jafar, T.H.; Nitsch, D.; Neuen, B.L.; Perkovic, V. Chronic kidney disease. Lancet 2021, 398, 786–802. [Google Scholar] [CrossRef]
- Farrell, D.R.; Vassalotti, J.A. Screening, identifying, and treating chronic kidney disease: Why, who, when, how, and what? BMC Nephrol. 2024, 25, 34. [Google Scholar] [CrossRef]
- Satarug, S.; Vesey, D.A.; Gobe, G.C.; Phelps, K.R. Estimation of health risks associated with dietary cadmium exposure. Arch. Toxicol. 2023, 97, 329–358. [Google Scholar] [CrossRef]
- Fechner, C.; Hackethal, C.; Höpfner, T.; Dietrich, J.; Bloch, D.; Lindtner, O.; Sarvan, I. Results of the BfR MEAL Study: In Germany, mercury is mostly contained in fish and seafood while cadmium, lead, and nickel are present in a broad spectrum of foods. Food Chem. X 2022, 14, 100326. [Google Scholar] [CrossRef]
- Watanabe, T.; Kataoka, Y.; Hayashi, K.; Matsuda, R.; Uneyama, C. Dietary exposure of the Japanese general population to elements: Total diet study 2013–2018. Food Saf. 2022, 10, 83–101. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.T.; Jandev, V.; Petroni, M.; Atallah-Yunes, N.; Bendinskas, K.; Brann, L.S.; Heffernan, K.; Larsen, D.A.; MacKenzie, J.A.; Palmer, C.D.; et al. Airborne levels of cadmium are correlated with urinary cadmium concentrations among young children living in the New York state city of Syracuse, USA. Environ. Res. 2023, 223, 115450. [Google Scholar] [CrossRef]
- Almerud, P.; Zamaratskaia, G.; Lindroos, A.K.; Bjermo, H.; Andersson, E.M.; Lundh, T.; Ankarberg, E.H.; Lignell, S. Cadmium, total mercury, and lead in blood and associations with diet, sociodemographic factors, and smoking in Swedish adolescents. Environ. Res. 2021, 197, 110991. [Google Scholar] [CrossRef]
- Pappas, R.S.; Fresquez, M.R.; Watson, C.H. Cigarette smoke cadmium breakthrough from traditional filters: Implications for exposure. J. Anal. Toxicol. 2015, 39, 45–51. [Google Scholar] [CrossRef] [PubMed]
- JECFA Summary and Conclusions. In Proceedings of the Joint FAO/WHO Expert Committee on Food Additives and Contaminants, Seventy-Third Meeting, Geneva, Switzerland, 8–17 June 2010; JECFA/73/SC; Food and Agriculture Organization of the United Nations/World Health Organization: Geneva, Switzerland, 2011. Available online: https://apps.who.int/iris/handle/10665/44521 (accessed on 12 August 2024).
- Satarug, S.; Vesey, D.A.; Ruangyuttikarn, W.; Nishijo, M.; Gobe, G.C.; Phelps, K.R. The Source and Pathophysiologic Significance of Excreted Cadmium. Toxics 2019, 7, 55. [Google Scholar] [CrossRef] [PubMed]
- Doccioli, C.; Sera, F.; Francavilla, A.; Cupisti, A.; Biggeri, A. Association of cadmium environmental exposure with chronic kidney disease: A systematic review and meta-analysis. Sci. Total Environ. 2024, 906, 167165. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, Y.; Nomiyama, T.; Kumagai, N.; Dekio, F.; Uemura, T.; Takebayashi, T.; Nishiwaki, Y.; Matsumoto, Y.; Sano, Y.; Hosoda, K.; et al. Uptake of cadmium in meals from the digestive tract of young non-smoking Japanese female volunteers. J. Occup. Health 2003, 45, 43–52. [Google Scholar] [CrossRef]
- Horiguchi, H.; Oguma, E.; Sasaki, S.; Miyamoto, K.; Ikeda, Y.; Machida, M.; Kayama, F. Comprehensive study of the effects of age, iron deficiency, diabetes mellitus, and cadmium burden on dietary cadmium absorption in cadmium-exposed female Japanese farmers. Toxicol. Appl. Pharmacol. 2004, 196, 114–123. [Google Scholar] [CrossRef]
- Peng, X.; Li, C.; Zhao, D.; Huang, L. Associations of micronutrients exposure with cadmium body burden among population: A systematic review. Ecotoxicol. Environ. Saf. 2023, 256, 114878. [Google Scholar] [CrossRef]
- Satarug, S.; Baker, J.R.; Reilly, P.E.; Moore, M.R.; Williams, D.J. Cadmium levels in the lung, liver, kidney cortex, and urine samples from Australians without occupational exposure to metals. Arch. Environ. Health 2002, 57, 69–77. [Google Scholar] [CrossRef]
- Akerstrom, M.; Barregard, L.; Lundh, T.; Sallsten, G. The relationship between cadmium in kidney and cadmium in urine and blood in an environmentally exposed population. Toxicol. Appl. Pharmacol. 2013, 268, 286–293. [Google Scholar] [CrossRef]
- Barregard, L.; Sallsten, G.; Lundh, T.; Mölne, J. Low-level exposure to lead, cadmium and mercury, and histopathological findings in kidney biopsies. Environ. Res. 2022, 211, 113119. [Google Scholar] [CrossRef]
- Thévenod, F.; Lee, W.K.; Garrick, M.D. Iron and cadmium entry into renal mitochondria: Physiological and toxicological implications. Front. Cell Dev. Biol. 2020, 8, 848. [Google Scholar] [CrossRef]
- Ning, B.; Guo, C.; Kong, A.; Li, K.; Xie, Y.; Shi, H.; Gu, J. Calcium signaling mediates cell death and crosstalk with autophagy in kidney disease. Cells 2021, 10, 3204. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Guo, C.; Ruan, J.; Ning, B.; Wong, C.K.-C.; Shi, H.; Gu, J. Cadmium disrupted ER Ca2+ homeostasis by inhibiting SERCA2 expression and activity to induce apoptosis in renal proximal tubular cells. Int. J. Mol. Sci. 2023, 24, 5979. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.T.; Liu, T.B.; Li, Y.; Wang, Z.Y.; Lian, C.Y.; Wang, L. HO-1 activation contributes to cadmium-induced ferroptosis in renal tubular epithelial cells via increasing the labile iron pool and promoting mitochondrial ROS generation. Chem. Biol. Interact. 2024, 399, 111152. [Google Scholar] [CrossRef]
- Satarug, S. Is Chronic Kidney Disease Due to Cadmium Exposure Inevitable and Can It Be Reversed? Biomedicines 2024, 12, 718. [Google Scholar] [CrossRef] [PubMed]
- Price, R.G. Measurement of N-acetyl-beta-glucosaminidase and its isoenzymes in urine: Methods and clinical applications. Eur. J. Clin. Chem. Clin. Biochem. 1992, 30, 693–705. [Google Scholar]
- Pócsi, I.; Dockrell, M.E.; Price, R.G. Nephrotoxic biomarkers with specific indications for metallic pollutants: Implications for environmental health. Biomark. Insights 2022, 17, 11772719221111882. [Google Scholar] [CrossRef]
- Argyropoulos, C.P.; Chen, S.S.; Ng, Y.H.; Roumelioti, M.E.; Shaffi, K.; Singh, P.P.; Tzamaloukas, A.H. Rediscovering beta-2 microglobulin as a biomarker across the spectrum of kidney diseases. Front. Med. 2017, 4, 73. [Google Scholar] [CrossRef]
- Portman, R.J.; Kissane, J.M.; Robson, A.M. Use of B2-microglobulin to diagnose tubulo-interstitial renal lesions in children. Kidney Int. 1986, 30, 91–98. [Google Scholar] [CrossRef]
- Gauthier, C.; Nguyen-Simonnet, H.; Vincent, C.; Revillard, J.-P.; Pellet, M.V. Renal tubular absorption of beta 2 micro-globulin. Kidney Int. 1984, 26, 170–175. [Google Scholar] [CrossRef]
- Nielsen, R.; Christensen, E.I.; Birn, H. Megalin and cubilin in proximal tubule protein reabsorption: From experimental models to human disease. Kidney Int. 2016, 89, 58–67. [Google Scholar] [CrossRef]
- Molitoris, B.A.; Sandoval, R.M.; Yadav, S.P.S.; Wagner, M.C. Albumin uptake and processing by the proximal tubule: Physiological, pathological, and therapeutic implications. Physiol. Rev. 2022, 102, 1625–1667. [Google Scholar] [CrossRef] [PubMed]
- Comper, W.D.; Vuchkova, J.; McCarthy, K.J. New insights into proteinuria/albuminuria. Front. Physiol. 2022, 13, 991756. [Google Scholar] [CrossRef] [PubMed]
- Benzing, T.; Salant, D. Insights into glomerular filtration and albuminuria. N. Engl. J. Med. 2021, 384, 1437–1446. [Google Scholar] [CrossRef] [PubMed]
- Suwatvitayakorn, P.; Ko, M.S.; Kim, K.W.; Chanpiwat, P. Human health risk assessment of cadmium exposure through rice consumption in cadmium-contaminated areas of the Mae Tao sub-district, Tak, Thailand. Environ. Geochem. Health 2020, 42, 2331–2344. [Google Scholar] [CrossRef]
- Swaddiwudhipong, W.; Nguntra, P.; Kaewnate, Y.; Mahasakpan, P.; Limpatanachote, P.; Aunjai, T.; Jeekeeree, W.; Punta, B.; Funkhiew, T.; Phopueng, I. Human health effects from cadmium exposure: Comparison between persons living in cadmium-contaminated and non-contaminated areas in northwestern Thailand. Southeast Asian J. Trop. Med. Publ. Health 2015, 46, 133–142. [Google Scholar]
- Satarug, S.; Swaddiwudhipong, W.; Ruangyuttikarn, W.; Nishijo, M.; Ruiz, P. Modeling cadmium exposures in low- and high-exposure areas in Thailand. Environ. Health Perspect. 2013, 121, 531–536. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Scmid, C.H.; Zhang, Y.; Castro, A.F., III; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- White, C.A.; Allen, C.M.; Akbari, A.; Collier, C.P.; Holland, D.C.; Day, A.G.; Knoll, G.A. Comparison of the new and traditional CKD-EPI GFR estimation equations with urinary inulin clearance: A study of equation performance. Clin. Chim. Acta 2019, 488, 189–195. [Google Scholar] [CrossRef]
- Hornung, R.W.; Reed, L.D. Estimation of average concentration in the presence of nondetectable values. Appl. Occup. Environ. Hyg. 1990, 5, 46–51. [Google Scholar] [CrossRef]
- Spencer, K. Analytical reviews in clinical biochemistry: The estimation of creatinine. Ann. Clin. Biochem. 1985, 23, 1–25. [Google Scholar] [CrossRef]
- Bargnoux, A.S.; Barrot, A.; Fesler, P.; Kuster, N.; Badiou, S.; Dupuy, A.M.; Ribstein, J.; Cristol, J.P. Evaluation of five immunoturbidimetric assays for urinary albumin quantification and their impact on albuminuria categorization. Clin. Biochem. 2014, 47, 250–253. [Google Scholar] [CrossRef] [PubMed]
- Kok, M.B.; Tegelaers, F.P.; van Dam, B.; van Rijn, J.L.; van Pelt, J. Carbamylation of albumin is a cause for discrepancies between albumin assays. Clin. Chim. Acta 2014, 434, 6–10. [Google Scholar] [CrossRef]
- Phelps, K.R.; Gosmanova, E.O. A generic method for analysis of plasma concentrations. Clin. Nephrol. 2020, 94, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S.; Vesey, D.A.; Gobe, G.C.; Đorđević, A.B. The Validity of Benchmark Dose Limit Analysis for Estimating Permissible Accumulation of Cadmium. Int. J. Environ. Res. Public Health 2022, 19, 15697. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S.; Đorđević, A.B.; Yimthiang, S.; Vesey, D.A.; Gobe, G.C. The NOAEL Equivalent of Environmental Cadmium Exposure Associated with GFR Reduction and Chronic Kidney Disease. Toxics 2022, 10, 614. [Google Scholar] [CrossRef]
- Preacher, K.J. Advances in mediation analysis: A survey and synthesis of new developments. Annu. Rev. Psychol. 2015, 66, 825–852. [Google Scholar] [CrossRef]
- MacKinnon, D.P.; Warsi, G.; Dwyer, J.H. A simulation study of mediated effect measures. Multiv. Behav. Res. 1995, 30, 41–62. [Google Scholar] [CrossRef]
- Preacher, K.J.; Hayes, A.F. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav. Res. Meth. Instrum. Comput. 2004, 36, 717–731. [Google Scholar] [CrossRef]
- Mashima, Y.; Konta, T.; Kudo, K.; Takasaki, S.; Ichikawa, K.; Suzuki, K.; Shibata, Y.; Watanabe, T.; Kato, T.; Kawata, S.; et al. Increases in urinary albumin and beta2-microglobulin are independently associated with blood pressure in the Japanese general population: The Takahata Study. Hypertens. Res. 2011, 34, 831–835. [Google Scholar] [CrossRef]
- Kudo, K.; Konta, T.; Mashima, Y.; Ichikawa, K.; Takasaki, S.; Ikeda, A.; Hoshikawa, M.; Suzuki, K.; Shibata, Y.; Watanabe, T.; et al. The association between renal tubular damage and rapid renal deterioration in the Japanese population: The Takahata study. Clin. Exp. Nephrol. 2011, 15, 235–241. [Google Scholar] [CrossRef]
- Satarug, S.; Vesey, D.A.; Nishijo, M.; Ruangyuttikarn, W.; Gobe, G.C. The inverse association of glomerular function and urinary β2-MG excretion and its implications for cadmium health risk assessment. Environ. Res. 2019, 173, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Moffett, D.B.; Mumtaz, M.M.; Sullivan, D.W., Jr.; Whittaker, M.H. Chapter 13, General Considerations of Dose-Effect and Dose-Response Relationships. In Handbook on the Toxicology of Metals, 5th ed.; Volume I: General Considerations; Nordberg, G., Costa, M., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 299–317. [Google Scholar]
- Thomas, D.K.; Hodgson, S.; Nieuwenhuijsen, M.; Jarup, L. Early kidney damage in a population exposed to cadmium and other heavy metals. Environ. Health Perspect. 2009, 117, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, Y.; Zhu, C.; Dong, Z.; Zhang, K.; Zhao, Y.; Xu, Y. Benchmark dose for cadmium exposure and elevated N-acetyl-β-D-glucosaminidase: A meta-analysis. Environ. Sci. Pollut. Res. Int. 2016, 23, 20528–20538. [Google Scholar] [CrossRef] [PubMed]
- Suwazono, Y.; Sand, S.; Vahter, M.; Filipsson, A.F.; Skerfving, S.; Lidfeldt, J.; Akesson, A. Benchmark dose for cadmium-induced renal effects in humans. Environ. Health Perspect. 2006, 114, 1072–1076. [Google Scholar] [CrossRef]
- Shi, Z.; Taylor, A.W.; Riley, M.; Byles, J.; Liu, J.; Noakes, M. Association between dietary patterns, cadmium intake and chronic kidney disease among adults. Clin. Nutr. 2018, 37, 276–284. [Google Scholar] [CrossRef]
- Lang, S.M.; Schiffl, H. Smoking status, cadmium, and chronic kidney disease. Ren. Replace. Ther. 2024, 10, 17. [Google Scholar] [CrossRef]
- Nath, K.A. Tubulointerstitial changes as a major determinant in the progression of renal damage. Am. J. Kidney Dis. 1992, 20, 1–17. [Google Scholar] [CrossRef]
- Sharma, S.; Smyth, B. From proteinuria to fibrosis: An update on pathophysiology and treatment options. Kidney Blood Press. Res. 2021, 46, 411–420. [Google Scholar] [CrossRef]
- Risdon, R.A.; Sloper, J.C.; De Wardener, H.E. Relationship between renal function and histological changes found in renal-biopsy specimens from patients with persistent glomerular nephritis. Lancet 1968, 292, 363–366. [Google Scholar] [CrossRef]
- Schainuck, L.I.; Striker, G.E.; Cutler, R.E.; Benditt, E.P. Structural-functional correlations in renal disease: Part II: The correlations. Hum. Pathol. 1970, 1, 631–641. [Google Scholar] [CrossRef]
- Bohle, A.; von Gise, H.; Mackensen-Haen, S.; Stark-Jakob, B. The obliteration of the postglomerular capillaries and its influence upon the function of both glomeruli and tubuli. Functional interpretation of morphologic findings. Klin. Wochenschr. 1981, 59, 1043–1051. [Google Scholar] [CrossRef] [PubMed]
- Kazeminia, S.; Eirin, A. Role of mitochondria in endogenous renal repair. Clin. Sci. 2024, 138, 963–973. [Google Scholar] [CrossRef] [PubMed]
- Kramann, R.; Kusaba, T.; Humphreys, B.D. Who regenerates the kidney tubule? Nephrol. Dial. Transpl. 2015, 30, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S.; Vesey, D.A.; Yimthiang, S.; Khamphaya, T.; Pouyfung, P.; Đorđević, A.B. Environmental Cadmium Exposure Induces an Increase in Systolic Blood Pressure by Its Effect on GFR. Stresses 2024, 4, 436–451. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, M.; Xie, R. Associations between cadmium exposure and whole-body aging: Mediation analysis in the NHANES. BMC Public Health 2023, 23, 1675. [Google Scholar] [CrossRef]
- Dong, W.; Zhang, K.; Gong, Z.; Luo, T.; Li, J.; Wang, X.; Zou, H.; Song, R.; Zhu, J.; Ma, Y.; et al. N-acetylcysteine delayed cadmium-induced chronic kidney injury by activating the sirtuin 1-P53 signaling pathway. Chem. Biol. Interact. 2023, 369, 110299. [Google Scholar] [CrossRef]
- Chou, X.; Li, X.; Min, Z.; Ding, F.; Ma, K.; Shen, Y.; Sun, D.; Wu, Q. Sirtuin-1 attenuates cadmium-induced renal cell senescence through p53 deacetylation. Ecotoxicol. Environ. Saf. 2022, 245, 114098. [Google Scholar] [CrossRef]
- Satarug, S.; Vesey, D.A.; Gobe, G.C.; Phelps, K.R. The pathogenesis of albuminuria in cadmium nephropathy. Curr. Res. Toxicol. 2023, 6, 100140. [Google Scholar] [CrossRef]
- Santoyo-Sánchez, M.P.; Pedraza-Chaverri, J.; Molina-Jijón, E.; Arreola-Mendoza, L.; Rodríguez-Muñoz, R.; Barbier, O.C. Impaired endocytosis in proximal tubule from subchronic exposure to cadmium involves angiotensin II type 1 and cubilin receptors. BMC Nephrol. 2013, 14, 211. [Google Scholar] [CrossRef]
- Gena, P.; Calamita, G.; Guggino, W.B. Cadmium impairs albumin reabsorption by down-regulating megalin and ClC5 channels in renal proximal tubule cells. Environ. Health Perspect. 2010, 118, 1551–1556. [Google Scholar] [CrossRef]
- Li, L.; Dong, F.; Xu, D.; Du, L.; Yan, S.; Hu, H.; Lobe, C.G.; Yi, F.; Kapron, C.M.; Liu, J. Short-term, low-dose cadmium exposure induces hyperpermeability in human renal glomerular endothelial cells. J. Appl. Toxicol. 2016, 36, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jiang, L.; Tao, T.; Su, W.; Guo, Y.; Yu, H.; Qin, J. Assessment of cadmium-induced nephrotoxicity using a kidney-on-a-chip device. Toxicol. Res. 2017, 6, 372–380. [Google Scholar] [CrossRef] [PubMed]
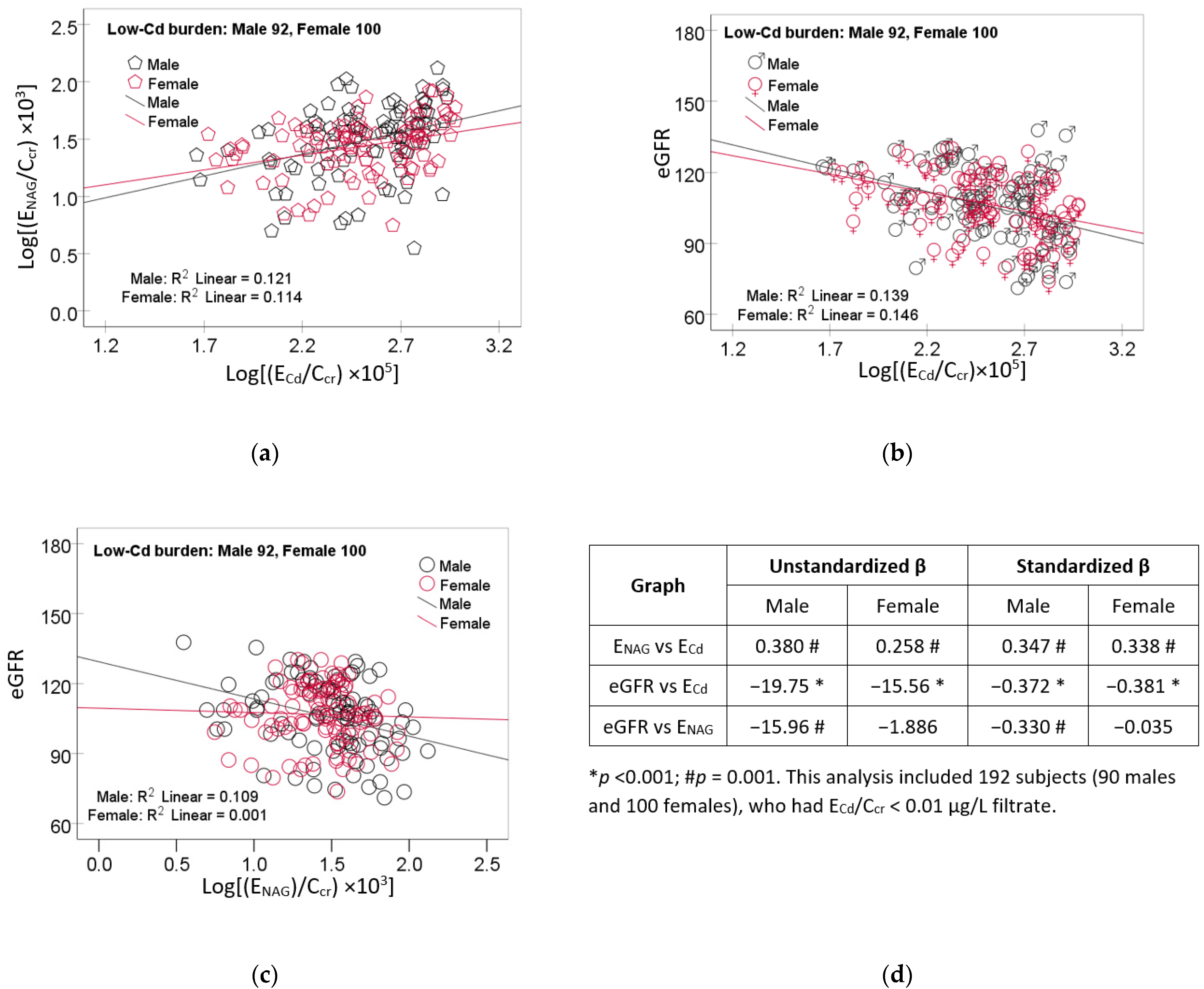


| Parameters | All, n = 737 | Low-Cd Burden a | High-Cd Burden | ||
|---|---|---|---|---|---|
| Males, n = 92 | Females, n = 100 | Males, n = 198 | Females, n = 347 | ||
| Age, years | 48.1 (11.0) | 35.8 (10.2) | 42.1 (9.1) *** | 52.8 (11.3) | 50.4 (8.2) |
| Age range, years | 16–87 | 16–87 | 23–60 | 30–87 | 33–84 |
| BMI, kg/m2 | 23.2 (3.8) | 23.3 (3.4) | 23.4 (3.8) | 22.0 (3.3) | 23.7 (4.1) *** |
| % Female | 60.7 | − | 52.0 | − | 63.7 |
| % Smoking | 42.7 | 43.5 | 1.0 *** | 81.8 | 32.3 *** |
| % Hypertension | 32.2 | 27.0 | 15.8 | 30.9 | 39.2 * |
| eGFR a, mL/min/1.73 m2 | 91 (22) | 106 (16) | 107 (13) | 83 (22) | 87 (21) * |
| % eGFR ≤ 60 mL/min/1.73 m2 | 9.1 | 0 | 0 | 15.2 | 10.7 |
| [cr]p, mg/dL | 0.88 (0.28) | 0.92 (0.12) | 0.67 (0.10) *** | 1.08 (0.34) | 0.82 (0.23) *** |
| [cr]u, mg/dL | 110.2 (73.8) | 89.8 (80.3) | 70.3 (58.3) | 135.6 (65.2) | 112.6 (74.5) *** |
| [Cd]u, µg/L | 6.53 (11.71) | 0.39 (0.48) | 0.44 (0.57) | 10.7 (18.7) | 7.52 (7.82) |
| Normalized to Ecr (Ex/Ecr) b | |||||
| ECd/Ecr, µg/g creatinine | 2.78 (0.60) | 0.25 (0.29) | 0.28 (0.31) | 4.72 (0.38) | 5.17 (0.33) * |
| ENAG/Ecr, units/g creatinine | 2.40 (1.41) | 3.12 (0.32) | 3.41 (0.32) | 2.37 (0.44) | 2.03 (0.40) |
| Ealb/Ecr, mg/g creatinine | 5.19 (0.71) | 4/34 (0.52) | 3.98 (0.27) | 4.89 (0.75) | 5.41 (0.70) |
| Eβ2M/Ecr, µg/g creatinine | 18.38 (1.27) | 6.7 (1.04) | 6.91 (1.42) | 47.19 (1.42) | 18.58 (1.20) ** |
| % Eβ2M/Ecr, µg/g creatinine | |||||
| <300 | 88.1 | 95.7 | 99 | 76.9 | 89.0 |
| 300–1000 | 6.3 | 3.3 | 1.0 | 11.3 | 6.0 |
| >1000 | 5.6 | 1.1 | 0 | 11.8 | 5.1 |
| Normalized to Ccr, (Ex/Ccr) c | |||||
| (ECd/Ccr) × 100, µg/L filtrate | 2.36 (0.63) | 0.32 (0.29) | 0.32 (0.32) | 5.27 (0.43) | 4.50 (0.36) |
| (ENAG/Ccr) × 100, µg/L filtrate | 5.43 (0.35) | 3.07 (0.32) | 2.73 (0.24) | 6.56 (0.31) | 6.92 (0.33) |
| (Ealb/Ccr) × 100, mg/L filtrate | 11.28 (1.02) | 4.05 (0.51) | 2.80 (0.28) | 17.89 (1.08) | 9.12 (0.97) ** |
| Eβ2M/Ccr × 100, µg/L filtrate | 41.53 (1.20) | 6.59 (1.17) | 5.5 (1.05) | 130.62 (1.20) | 63.01 (1.00) ** |
| % Eβ2M/Ccr × 100, µg/L filtrate | |||||
| <300 | 82.0 | 95.7 | 100 | 66.2 | 82.3 |
| 300–1000 | 8.0 | 3.3 | 0 | 14.6 | 7.8 |
| >1000 | 10.00 | 2.1 | 0 | 19.2 | 9.9 |
| Independent Variables/Factors | Low eGFR a | Albuminuria b | ||
|---|---|---|---|---|
| POR (95% CI) | p | POR (95% CI) | p | |
| Age, years | 1.133 (1.084, 1.183) | <0.001 | 1.100 (1.064, 1.137) | <0.001 |
| BMI, kg/m2 | 1.156 (1.039, 1.287) | 0.008 | 0.957 (0.896, 1.023) | 0.197 |
| Log2[(ECd/Ccr) × 105], µg/L filtrate | 2.093 (1.512, 2.898) | <0.001 | 1.977 (1.597, 2449) | <0.001 |
| Gender | 0.475 (0.202, 1.118) | 0.088 | 1.299 (0.771, 2.188) | 0.326 |
| Hypertension | 2.127 (0.939, 4.816) | 0.070 | 0.654 (0.408, 1.048) | 0.078 |
| Smoking | 1.371 (0.585, 3.212) | 0.467 | 1.059 (0.641, 1.749) | 0.823 |
| Eβ2M/Ccr, µg/L filtrate | ||||
| <3 | Referent | Referent | ||
| 3–10 | 3.227 (1.111, 9.373) | 0.031 | 2.334 (1.100, 4.955) | 0.027 |
| >10 | 16.20 (6.432, 20.78) | <0.001 | 3.904 (1.501, 10.16) | 0.005 |
| Independent Variables/ Factors | Log10[(Eβ2M/Ccr) × 103], µg/L Filtrate | |||||||
|---|---|---|---|---|---|---|---|---|
| Males, n = 277 | Females, n = 425 | Low-Cd Burden a, n = 166 | High-Cd Burden, n = 536 | |||||
| β | p | β | p | β | p | β | p | |
| Age, years | −0.101 | 0.171 | 0.037 | 0.472 | 0.082 | 0.512 | 0.051 | 0.241 |
| BMI, kg/m2 | −0.158 | 0.001 | −0.129 | 0.002 | −0.121 | 0.149 | −0.117 | 0.001 |
| Log2[(ECd/Ccr) × 105], µg/L filtrate | 0.316 | <0.001 | 0.331 | <0.001 | 0.007 | 0.942 | 0.310 | <0.001 |
| eGFR, mL/min/1.73 m2 | −0.472 | <0.001 | −0.287 | <0.001 | −0.035 | 0.752 | −0.367 | <0.001 |
| Hypertension | −0.030 | 0.509 | 0.000282 | 0.994 | 0.016 | 0.853 | 0.001 | 0.981 |
| Smoking | 0.052 | 0.259 | 0.069 | 0.099 | 0.137 | 0.132 | 0.035 | 0.358 |
| Gender | − | − | − | − | 0.045 | 0.648 | −0.037 | 0.311 |
| Adjusted R2 | 0.483 | <0.001 | 0.383 | <0.001 | −0.012 | 0.655 | 0.448 | <0.001 |
| Independent Variables/ Factors | Log10[(Ealb/Ccr) × 104], mg/L Filtrate | |||||||
|---|---|---|---|---|---|---|---|---|
| Males, n = 277 | Females, n = 425 | Low-Cd Burden a, n = 166 | High-Cd Burden, n = 536 | |||||
| β | p | β | p | β | p | β | p | |
| Age, years | 0.020 | 0.810 | 0.205 | <0.001 | 0.263 | 0.724 | 0.136 | 0.004 |
| BMI, kg/m2 | −0.034 | 0.593 | −0.071 | 0.159 | −0.349 | 0.512 | −0.059 | 0.145 |
| Log2[(ECd/Ccr) × 105], µg/L filtrate | 0.250 | <0.001 | 0.169 | 0.001 | 0.373 | 0.566 | 0.210 | <0.001 |
| eGFR, mL/min/1.73 m2 | −0.460 | <0.001 | −0.309 | <0.001 | 0.099 | 0.836 | −0.351 | <0.001 |
| Hypertension | −0.018 | 0.769 | −0.024 | 0.602 | 0.871 | 0.190 | −0.017 | 0.640 |
| Smoking | 0.018 | 0.759 | 0.029 | 0.546 | −0.420 | 0.416 | 0.042 | 0.309 |
| Gender | − | − | − | − | −0.614 | 0.321 | −0.024 | 0.546 |
| Adjusted R2 | 0.416 | <0.001 | 0.332 | <0.001 | −0.103 | 0.607 | 0.376 | <0.001 |
| Independent Variables/ Factors | Log10[(ENAG/Ccr) × 103], U/L Filtrate | |||||||
|---|---|---|---|---|---|---|---|---|
| Males, n = 277 | Females, n = 427 | Low-Cd Burden a, n = 186 | High-Cd Burden, n = 538 | |||||
| β | p | β | p | β | p | β | p | |
| Age, years | −0.012 | 0.892 | −0.170 | 0.003 | −0.094 | 0.419 | −0.124 | 0.026 |
| BMI, kg/m2 | 0.002 | 0.974 | 0.132 | 0.003 | −0.008 | 0.924 | 0.087 | 0.063 |
| Log2[(ECd/Ccr) × 105], µg/L filtrate | 0.447 | <0.001 | 0.394 | <0.001 | 0.287 | 0.001 | 0.145 | 0.004 |
| eGFR, mL/min/1.73 m2 | −0.127 | 0.127 | −0.178 | 0.002 | −0.132 | 0.205 | −0.223 | <0.001 |
| Hypertension | 0.167 | 0.002 | 0.169 | <0.001 | 0.180 | 0.022 | 0.158 | <0.001 |
| Smoking | −0.037 | 0.496 | 0.111 | 0.016 | −0.055 | 0.517 | 0.045 | 0.356 |
| Gender | − | − | − | − | −0.061 | 0.511 | 0.045 | 0.343 |
| Adjusted R2 | 0.293 | <0.001 | 0.266 | <0.001 | 0.114 | <0.001 | 0.090 | <0.001 |
| Independent Variables/Factors | Low eGFR | ||||
|---|---|---|---|---|---|
| β Coefficients | POR | 95% CI | p | ||
| (SE) | Lower | Upper | |||
| Age, years | 0.156 (0.022) | 1.168 | 1.118 | 1.221 | <0.001 |
| BMI, kg/m2 | 0.104 (0.050) | 1.109 | 1.006 | 1.222 | 0.037 |
| Log2[(ECd/Ccr) × 105], µg/L filtrate | 0.998 (0.164) | 2.714 | 1.967 | 3.744 | <0.001 |
| Gender | −0.389 (0.429) | 0.678 | 0.292 | 1.572 | 0.365 |
| Hypertension | −0.707 (0.387) | 0.493 | 0.231 | 1.052 | 0.068 |
| Smoking | 0.268 (0.414) | 1.307 | 0.581 | 2.945 | 0.517 |
| Tubular injury a | |||||
| Minimal | Referent | ||||
| Mild | −0.529 (0.754) | 0.589 | 0.134 | 2.581 | 0.483 |
| Moderate | 0.218 (0.694) | 1.244 | 0.320 | 4.843 | 0.753 |
| Severe | 1.570 (0.648) | 4.804 | 1.350 | 17.09 | 0.015 |
| Independent Variables/ Factors | eGFR, mL/min/1.73 m2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Males, n = 277 | Females, n = 427 | Low-Cd Burden a, n = 186 | High-Cd Burden, n = 538 | |||||
| η2 | p | η2 | p | η2 | p | η2 | p | |
| Age, years | 0.340 | <0.001 | 0.249 | <0.001 | 0.380 | <0.001 | 0.300 | <0.001 |
| BMI, kg/m2 | 0.001 | 0.637 | 0.004 | 0.228 | 0.002 | 0.632 | 0.010 | 0.024 |
| Log2[(ECd/Ccr) × 105], µg/L filtrate | 0.081 | <0.001 | 0.114 | <0.001 | 0.000335 | 0.828 | 0.150 | <0.001 |
| Smoking | 0.002 | 0.434 | 0.000171 | 0.792 | 0.000407 | 0.811 | 0.000248 | 0.724 |
| Hypertension | 0.016 | 0.044 | 0.002 | 0.420 | 0.000012 | 0.968 | 0.004 | 0.167 |
| ENAG/Ccr quartiles | 0.015 | 0.275 | 0.034 | 0.003 | 0.018 | 0.460 | 0.013 | 0.085 |
| Gender | − | − | − | − | 0.051 | 0.007 | 0.003 | 0.256 |
| Smoking × Hypertension | − | − | 0.022 | 0.003 | 0.013 | 0.176 | 0.004 | 0.147 |
| Smoking × Hypertension × ENAG/Ccr quartile | − | − | − | − | 0.059 | 0.014 | − | − |
| Adjusted R2 | 0.633 | <0.001 | 0.440 | <0.001 | 0.494 | <0.001 | 0.493 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Satarug, S. Urinary N-acetylglucosaminidase in People Environmentally Exposed to Cadmium Is Minimally Related to Cadmium-Induced Nephron Destruction. Toxics 2024, 12, 775. https://doi.org/10.3390/toxics12110775
Satarug S. Urinary N-acetylglucosaminidase in People Environmentally Exposed to Cadmium Is Minimally Related to Cadmium-Induced Nephron Destruction. Toxics. 2024; 12(11):775. https://doi.org/10.3390/toxics12110775
Chicago/Turabian StyleSatarug, Soisungwan. 2024. "Urinary N-acetylglucosaminidase in People Environmentally Exposed to Cadmium Is Minimally Related to Cadmium-Induced Nephron Destruction" Toxics 12, no. 11: 775. https://doi.org/10.3390/toxics12110775
APA StyleSatarug, S. (2024). Urinary N-acetylglucosaminidase in People Environmentally Exposed to Cadmium Is Minimally Related to Cadmium-Induced Nephron Destruction. Toxics, 12(11), 775. https://doi.org/10.3390/toxics12110775





