A Theoretical Study on the Degradation Mechanism, Kinetics, and Ecotoxicity of Metronidazole (MNZ) in •OH- and SO4•−-Assisted Advanced Oxidation Processes
Abstract
:1. Introduction
2. Computational Methods
2.1. Electronic Structure and Kinetic Calculations
2.2. Ecotoxicity Assessment
3. Results and Discussion
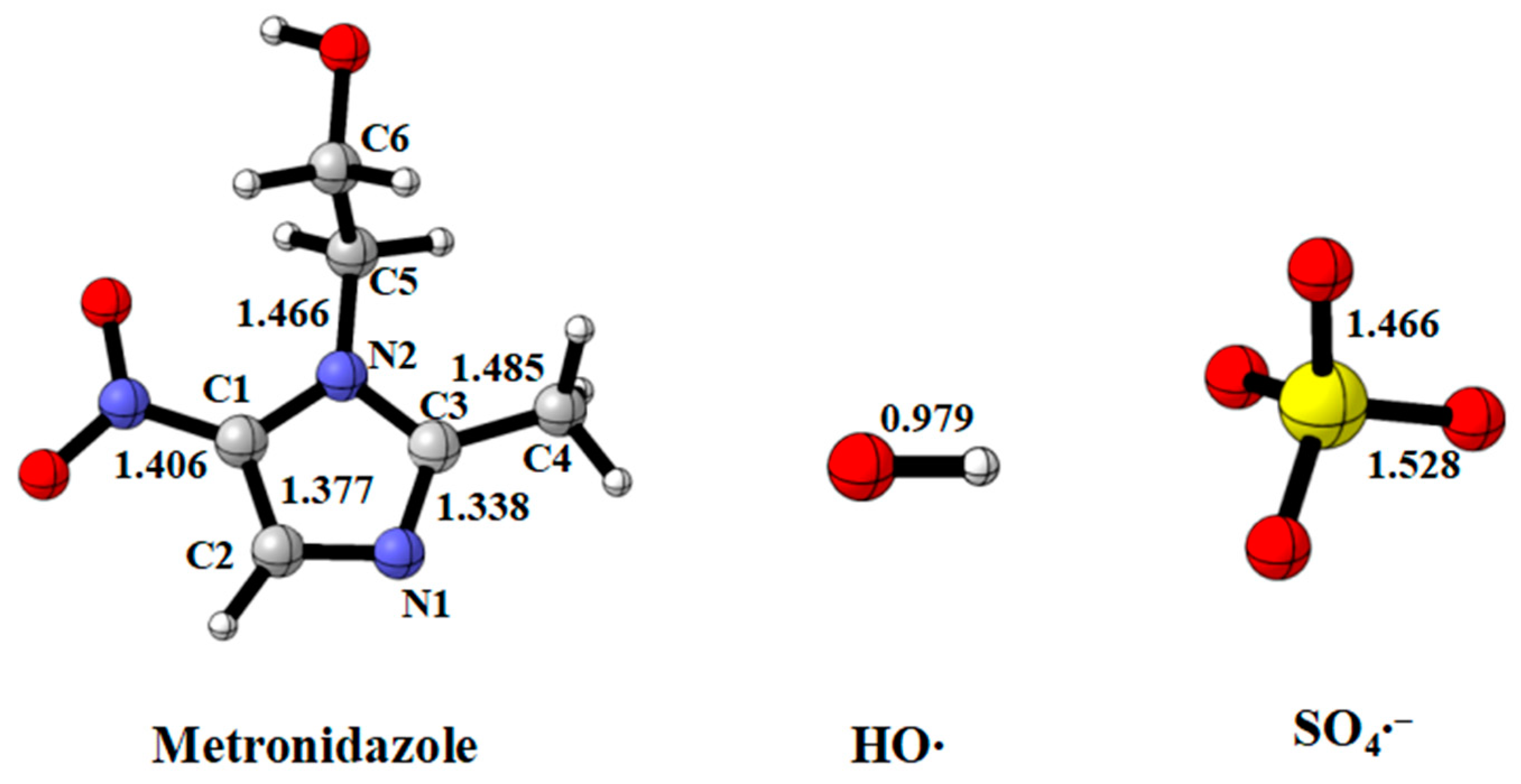
3.1. Initial Reaction of MNZ with HO• and SO4•−
3.1.1. Radical Adduct Formation Reactions
3.1.2. Hydrogen Abstraction Reactions
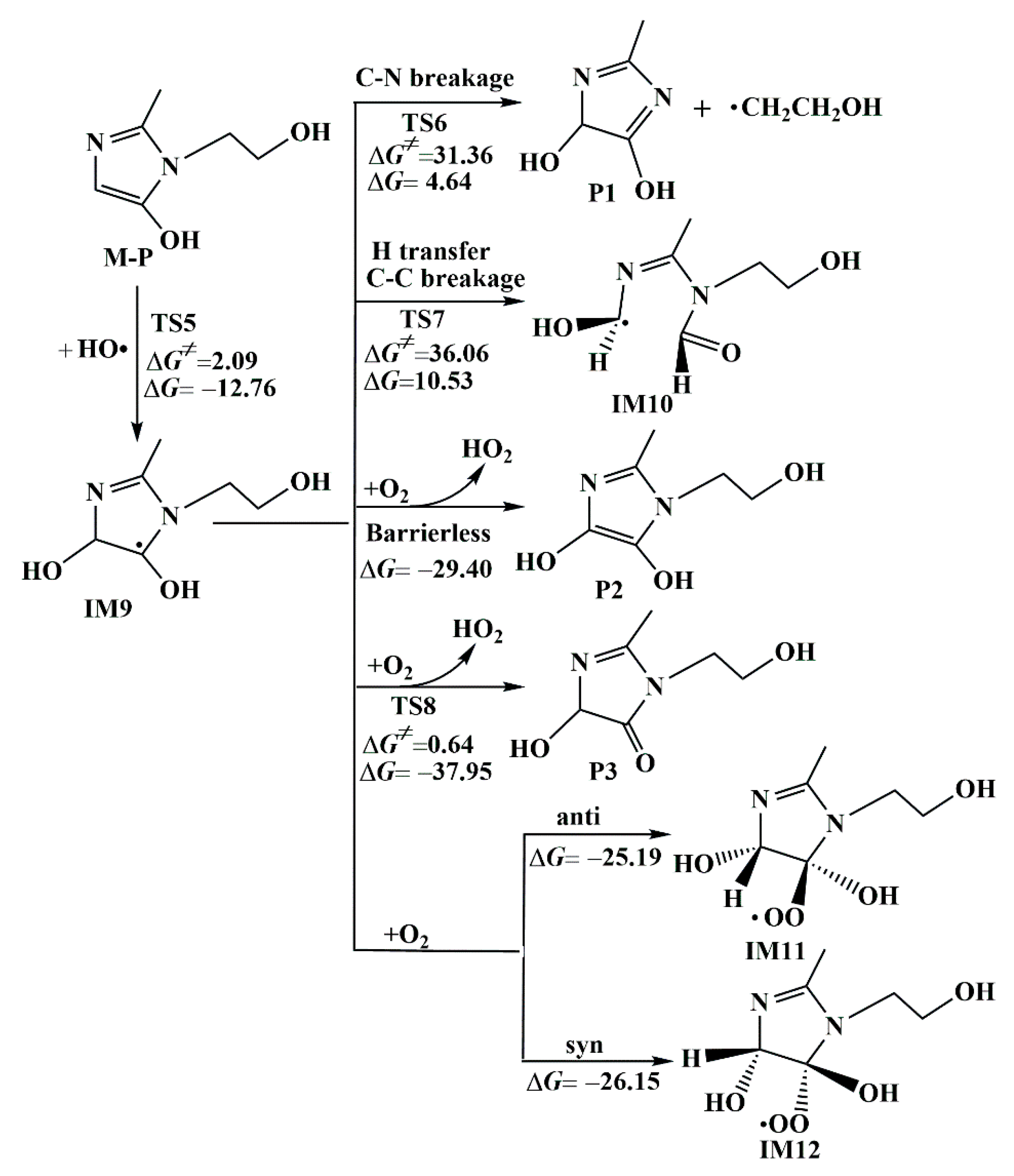
3.1.3. Further Conversion Reactions of M-P
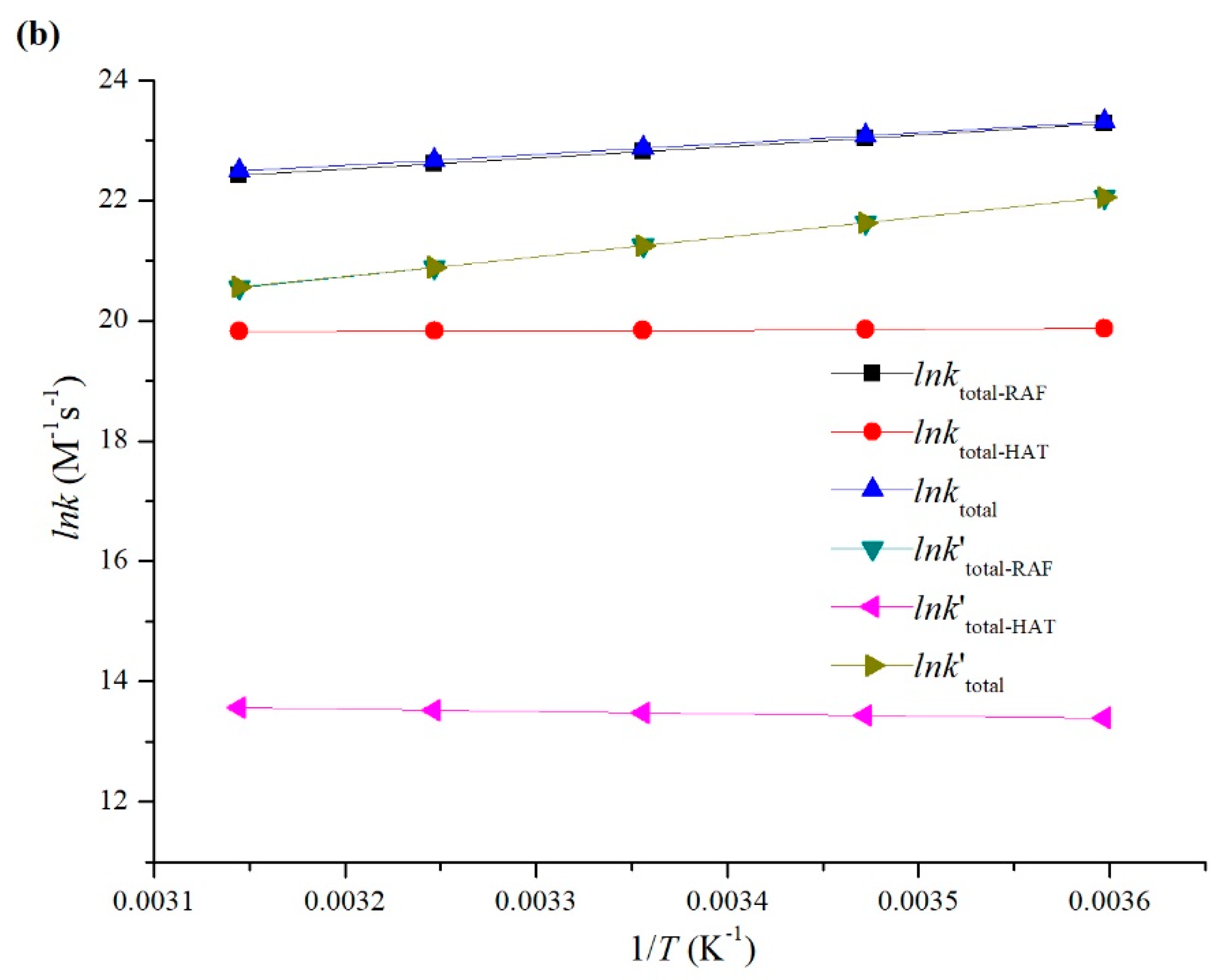
3.2. Kinetic Study
3.3. Risk Predictions
4. Conclusions
- (1)
- The main reaction channel for both HO• and SO4•−-initiated MNZ was the RAF reaction. The main reaction site was the C1 atom on the imidazole ring of MNZ. The main by-products obtained in the subsequent hydroxylation reaction were P2 and P3.
- (2)
- The total rate constants of MNZ with HO• and SO4•− were determined to be 8.52 × 109 and 1.69 × 109 M−1 s−1 at 298 K in an aqueous environment, which is in agreement with the experimental values.
- (3)
- The toxicity analysis showed that the end products were not harmful to aquatic organisms. The toxicity level of the initial degradation product of M-P was higher than the parent of MNZ.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Philip, J.M.; Aravind, U.K.; Aravindakumar, C.T. Emerging contaminants in Indian environmental matrices–A review. Chemosphere 2018, 190, 307–326. [Google Scholar] [CrossRef]
- Wilkinson, J.; Hooda, P.S.; Barker, J.; Barton, S.; Swinden, J. Occurrence, fate and transformation of emerging contaminants in water: An overarching review of the field. Environ. Pollut. 2017, 231, 954–970. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Kanekar, S.; Sabat, S.; Thamburaj, K. Metronidazole-induced cerebellar toxicity. Neurol. Int. 2016, 8, 6365. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.Q.; Chen, J.H.; Qiu, X.H.; Qiu, X.Q.; Cheng, W.; Zhu, L.C. Effective removal of antibiotic metronidazole from water by nanoscale zero-valent iron particles. Desalination 2011, 268, 60–67. [Google Scholar] [CrossRef]
- Asquith, J.C.; Foster, J.L.; Willson, R.L.; Ings, R.; McFadzean, J.A. Metronidazole (“Flagyl”). A radiosensitizer of hypoxic cells. Brit. J. Radiol. 1974, 47, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Willson, R.L.; Cramp, W.A.; Ings, R.M.J. Metronidazole (“Flagyl”): Mechanisms of radiosensitization. Int. J. Radiat. Biol. 1974, 26, 557–569. [Google Scholar] [CrossRef]
- Wardman, P. Some reactions and properties of nitro radical-anions important in biology and medicine. Environ. Health Persp. 1985, 64, 309–320. [Google Scholar] [CrossRef]
- Wagil, M.; Maszkowska, J.; Białk-Bielińska, A.; Caban, M.; Stepnowski, P.; Kumirska, J. Determination of metronidazole residues in water, sediment and fish tissue samples. Chemosphere 2015, 119, S28–S34. [Google Scholar] [CrossRef]
- Ramavandi, B.; Akbarzadeh, S. Removal of metronidazole antibiotic from contaminated water using a coagulant extracted from Plantago ovata. Desalin. Water. Treat. 2015, 55, 2221–2228. [Google Scholar] [CrossRef]
- Rivera-Utrilla, J.; Prados-Joya, G.; Sánchez-Polo, M.; Ferro-García, M.A.; Bautista-Toledo, I. Removal of nitroimidazole antibiotics from aqueous solution by adsorption/bioadsorption on activated carbon. J. Hazard. Mater. 2009, 170, 298–305. [Google Scholar] [CrossRef]
- Forouzesh, M.; Ebadi, A.; Aghaeinejad-Meybodi, A. Degradation of metronidazole antibiotic in aqueous medium using activated carbon as a persulfate activator. Sep. Purif. Technol. 2019, 210, 145–151. [Google Scholar] [CrossRef]
- Sánchez-Polo, M.; López-Peñalver, J.; Prados-Joya, G.; Ferro-García, M.A.; Rivera-Utrilla, J. Gamma irradiation of pharmaceutical compounds, nitroimidazoles, as a new alternative for water treatment. Water. Res. 2009, 43, 4028–4036. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.H.; Wu, Z.H.; Yan, S.W.; Yao, B.; Song, W.H.; Hua, Z.C.; Zhang, X.W.; Kong, X.J.; Li, X.C.; Fang, J.Y. Comparison of the UV/chlorine and UV/H2O2 processes in the degradation of PPCPs in simulated drinking water and wastewater: Kinetics, radical mechanism and energy requirements. Water. Res. 2018, 147, 184–194. [Google Scholar] [CrossRef]
- Yang, Y.; Ok, Y.S.; Kim, K.-H.; Kwon, E.E.; Tsang, Y.F. Occurrences and removal of pharmaceuticals and personal care products (PPCPs) in drinking water and water/ sewage treatment plants: A review. Sci. Total Environ. 2017, 596–597, 303–320. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.C.; Meng, T.; Huang, C.H.; Ben, W.W.; Yao, H.; Liu, R.N.; Sun, P.Z. PPCP degradation by chlorine−UV processes in ammoniacal water: New reaction insights, kinetic modeling, and DBP formation. Environ. Sci. Technol. 2018, 52, 7833–7841. [Google Scholar] [CrossRef]
- Trojanowicz, M.; Bobrowski, K.; Szreder, T.; Bojanowska-Czajka, A. Gamma-ray, X-ray and electron beam based processes. Advanced oxidation processes for waste water treatment. In Advanced Oxidation Processes for Waste Water Treatment; Academic Press: Cambridge, MA, USA, 2018; pp. 257–331. [Google Scholar] [CrossRef]
- Kanakaraju, D.; Glass, B.D.; Oelgemöller, M. Advanced oxidation processmediated removal of pharmaceuticals from water: A review. J. Environ. Manag. 2018, 219, 189–207. [Google Scholar] [CrossRef]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hübner, U. Evaluation of advanced oxidation processes for water and wastewater treatment–a critical review. Water. Res. 2018, 139, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Li, A.L.; Wu, Z.H.; Wang, T.T.; Hou, S.D.; Huang, B.J.; Kong, X.J.; Li, X.C.; Guan, Y.H.; Qiu, R.L.; Fang, J.Y. Kinetics and mechanisms of the degradation of PPCPs by zero-valent iron (Fe°) activated peroxydisulfate (PDS) system in groundwater. J. Hazard Mater. 2018, 357, 207–216. [Google Scholar] [CrossRef]
- Zhang, R.C.; Sun, P.Z.; Boyer, T.H.; Zhao, L.; Huang, C.-H. Degradation of pharmaceuticals and metabolite in synthetic human urine by UV, UV/H2O2, and UV/PDS. Environ. Sci. Technol. 2015, 49, 3056–3066. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, R.Z. Advanced Oxidation Processes (AOPs) in Wastewater Treatment. Curr. Pollut. Rep. 2015, 1, 33–40. [Google Scholar] [CrossRef]
- Xiao, R.Y.; He, L.; Luo, Z.H.; Spinney, R.; Wei, Z.S.; Dionysiou, D.D.; Zhao, F.P. An experimental and theoretical study on the degradation of clonidine by hydroxyl and sulfate radicals. Sci. Total. Environ. 2020, 710, 136333. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.T.; Qi, Y.M.; Qu, R.J.; Al-Basher, G.; Pan, X.X.; Wang, Z.Y.; Huo, Z.L.; Zhu, F. Effective degradation of 2,4-dihydroxybenzophenone by zero−valent iron powder (Fe°)-activated persulfate in aqueous solution: Kinetic study, product identification and theoretical calculations. Sci. Total. Environ. 2021, 771, 144743. [Google Scholar] [CrossRef]
- Pan, X.X.; Chen, J.; Wu, N.N.; Qi, Y.M.; Xu, X.X.; Ge, J.L.; Wang, X.H.; Li, C.G.; Qu, R.J.; Sharma, V.K.; et al. Degradation of aqueous 2,4,4′-trihydroxybenzophenone by persulfate activated with nitrogen doped carbonaceous materials and the formation of dimer products. Water. Res. 2018, 240, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.M.; Wei, J.Y.; Qu, R.J.; Al-Basher, G.; Pan, X.X.; Ahmed Dar, A.; Shad, A.; Zhou, D.M.; Wang, Z.Y. Mixed oxidation of aqueous nonylphenol and triclosan by thermally activated persulfate: Reaction kinetics and formation of co-oligomerization products. Chem. Eng. J. 2021, 403, 126396. [Google Scholar] [CrossRef]
- Wacławek, S.; Lutze, H.V.; Grübel, K.; Padil, V.V.T.; Černík, M.; Dionysiou, D.D. Chemistry of persulfates in water and wastewater treatment: A review. Chem. Eng. J. 2017, 330, 44–62. [Google Scholar] [CrossRef]
- Wojnárovits, L.; Takács, E. Rate constants of sulfate radical anion reactions with organic molecules: A review. Chemosphere 2019, 220, 1014–1032. [Google Scholar] [CrossRef]
- Shemer, H.; Kunukcu, Y.K.; Linden, K.G. Degradation of the pharmaceutical Metronidazole via UV, Fenton and photo-Fenton processes. Chemosphere 2006, 63, 269–276. [Google Scholar] [CrossRef]
- Ammar, H.B. Sono-Fenton process for metronidazole degradation in aqueous solution: Effect of acoustic cavitation and peroxydisulfate anion. Ultrason. Sonochem. 2016, 33, 164–169. [Google Scholar] [CrossRef]
- Ammar, H.B.; Brahim, M.B.; Abdelhédi, R.; Samet, Y. Enhanced degradation of metronidazole by sunlight via photo-Fenton process under gradual addition of hydrogen peroxide. J. Mol. Catal A Chem. 2016, 420, 222–227. [Google Scholar] [CrossRef]
- Cheng, W.; Yang, M.; Xie, Y.Y.; Liang, B.; Fang, Z.Q.; Tsang, E.P. Enhancement of mineralization of metronidazole by the electro-Fenton process with a Ce/SnO2-Sb coated titanium anode. Chem. Eng. J. 2013, 220, 214–220. [Google Scholar] [CrossRef]
- Lian, L.S.; Yao, B.; Hou, S.D.; Fang, J.Y.; Yan, S.W.; Song, W.H. Kinetic study of hydroxyl and sulfate radical-mediated oxidation of pharmaceuticals in wastewater effluents. Environ. Sci. Technol. 2017, 51, 2954–2962. [Google Scholar] [CrossRef] [PubMed]
- Whillans, D.W.; Adams, G.E.; Neta, P. Electron-affinic sensitization: VI. A pulse radiolysis and ESR comparison of some 2- and 5-nitroimidazoles. Radiat. Res. 1975, 62, 407–421. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.J.; Li, W.Y.; Désesquelles, P.; Van-Oanh, N.T.; Thomas, S.; Yang, J. A statistical model and DFT study of the fragmentation mechanisms of metronidazole by advanced oxidation processes. J. Phys. Chem. A 2019, 123, 933–942. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.F.; Tang, Y.Z.; Zhang, Y.J.; Ruan, M.; Wu, W.Z.; Sun, J.Y. New theoretical investigation of mechanism, kinetics, and toxicity in the degradation of dimetridazole and ornidazole by hydroxyl radicals in aqueous phase. J. Hazard. Mater. 2022, 422, 126930. [Google Scholar] [CrossRef]
- Qu, R.J.; Li, C.G.; Liu, J.Q.; Xiao, R.Y.; Pan, X.X.; Zeng, X.L.; Wang, Z.Y.; Wu, J.C. Hydroxyl radical based photocatalytic degradation of halogenated organic contaminants and paraffin on silica gel. Environ. Sci. Technol. 2018, 120, 7220–7229. [Google Scholar] [CrossRef]
- Xu, M.M.; Yan, S.D.; Liu, X.F.; Sun, S.M.; Khan, Z.U.H.; Wu, W.Z.; Sun, J.Y. Theoretical investigation on the degradation of sulfadiazine in water environments: Oxidation of •OH, SO4•− and CO3•− and reactivity of (TiO2)n clusters (n = 1−6). J. Environ. Chem. Eng. 2023, 11, 109994. [Google Scholar] [CrossRef]
- Xu, M.M.; Yao, J.F.; Sun, S.M.; Yan, S.D.; Sun, J.Y. Theoretical calculation on the reaction mechanisms, kinetics and toxicity of acetaminophen degradation initiated by hydroxyl and sulfate radicals in the aqueous phase. Toxics. 2021, 9, 234. [Google Scholar] [CrossRef]
- Xu, M.M.; Yan, S.D.; Sun, S.M.; Ni, Z.R.; Wu, W.Z.; Sun, J.Y. N, N-diethyl-m-toluamide (DEET) degradation by •OH and SO4•−-assisted AOPs in wastewater treatment: Theoretical studies into mechanisms, kinetics and toxicity. J. Environ. Chem. Eng. 2022, 10, 108435. [Google Scholar] [CrossRef]
- Yao, J.F.; Tang, Y.Z.; Zhang, Y.J.; Ruan, M.; Chen, F.; Wu, W.Z.; Sun, J.Y. Quantum chemical study of the mechanisms, kinetics, and ecotoxicity assessment of OH radical-initiated reactions of 2, 2′ 4, 4′, 5, 5′-hexabrominated diphenyl ether (BDE-153) in atmosphere and wastewater. Chem. Eng. J. 2021, 422, 129916. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A. Gaussian 09 (Revision D.01); Gaussian, Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Fukui, K. The path of chemical reactions-the IRC approach. Acc. Chem. Res. 1981, 14, 363–368. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B. 2009, 113, 6378–6396. [Google Scholar] [CrossRef] [PubMed]
- Li, M.X.; Sun, J.F.; Han, D.D.; Wei, B.; Mei, Q.; An, Z.X.; Wang, X.Y.; Cao, H.J.; Xie, J.; He, M.X. Theoretical investigation on the contribution of •OH, SO4•− and CO3•− radicals to the degradation of phenacetin in water: Mechanisms, kinetics, and toxicity evaluation. Ecotox. Environ. Safe. 2020, 204, 110977. [Google Scholar] [CrossRef]
- Truhlar, D.G.; Garrett, B.C.; Klippenstein, S.J. Current status of transition-state theory. J. Phys. Chem. 1996, 100, 12771–12800. [Google Scholar] [CrossRef]
- Garrett, B.C.; Truhlar, D.G. Accuracy of tunneling corrections to transition state theory for thermal rate constants of atom transfer reactions. J. Phys. Chem. 1979, 83, 200–203. [Google Scholar] [CrossRef]
- Canneaux, S.; Bohr, F.; Henon, E. KiSThelP: A program to predict thermodynamic properties and rate constants from quantum chemistry results. J. Comput. Chem. 2014, 35, 82–93. [Google Scholar] [CrossRef]
- Wang, X.Y.; Sun, J.F.; Han, D.D.; Bao, L.; Mei, Q.; Wei, B.; An, Z.X.; He, M.X.; Yuan, S.L.; Xie, J.; et al. Gaseous and heterogeneous reactions of low-molecular-weight (LMW) unsaturated ketones with O3: Mechanisms, kinetics, and effects of mineral dust in tropospheric chemical processes. Chem. Eng. J. 2020, 395, 125083. [Google Scholar] [CrossRef]
- Yao, J.F.; Sun, Y.N.; Tang, Y.Z.; Zhang, Y.J.; Wu, W.Z.; Sun, J.Y. Atmospheric oxidation of 4-(2-methoxyethyl) phenol initiated by OH radical in the presence of O2 and NOx: A mechanistic and kinetic study. Int. J. Quantum. Chem. 2021, 121, e26650. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, S.J.; Sun, J.Y.; Zhang, Y.J.; Tang, Y.Z. OH-initiated degradation of 1, 2, 3-trimethylbenzene in the atmosphere and wastewater: Mechanisms, kinetics, and ecotoxicity. Sci. Total. Environ. 2023, 857, 159534. [Google Scholar] [CrossRef]
- ECOSAR V2.0. Available online: https://www.epa.gov/oppt/newchems/tools/21ecosar.htm (accessed on 1 September 2012).
- Qu, R.J.; Liu, H.X.; Feng, M.B.; Yang, X.; Wang, Z.Y. Investigation on intramolecular hydrogen bond and some thermodynamic properties of polyhydroxylated anthraquinones. J. Chem. Eng. Data. 2012, 57, 2442–2455. [Google Scholar] [CrossRef]
- Xiao, W.K.; Yan, S.D.; Liu, X.F.; Sun, S.M.; Khan, Z.U.H.; Sun, J.Y. Theoretical study on the degradation mechanism, kinetics and toxicity for aqueous ozonation reaction of furan derivatives. Chemosphere 2023, 332, 138782. [Google Scholar] [CrossRef]
- Xiao, W.K.; Sun, S.M.; Yan, S.D.; Wu, W.Z.; Sun, J.Y. Theoretical study on the formation of Criegee intermediates from ozonolysis of pentenal: An example of trans-2-pentenal. Chemosphere 2022, 303, 135142. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.N.; Yao, J.F.; Tang, Y.Z.; Zhang, Y.J.; Wu, W.Z.; Sun, J.Y. Theoretical study on the atmospheric degradation mechanism and subsequent products of E, E-2, 4-hexadienal with hydroxyl radical. Int. J. Quantum. Chem. 2021, 121, e26563. [Google Scholar] [CrossRef]
- Brezonik, P.L.; Fulkerson-Brekken, J. Nitrate-induced photolysis in natural Waters: Controls on concentrations of hydroxyl radical photo-intermediates by natural scavenging agents. Environ. Sci. Technol. 1998, 32, 3004–3010. [Google Scholar] [CrossRef]
- Burns, J.M.; Cooper, W.J.; Ferry, J.L.; King, D.W.; DiMento, B.P.; McNeill, K.; Miller, C.J.; Miller, W.L.; Peake, B.M.; Rusak, S.A.; et al. Methods for reactive oxygen species(ROS) detection in aqueous environments. Aquat. Sci. 2012, 74, 683–734. [Google Scholar] [CrossRef]
- Haag, W.R.; Hoigné, J. Photo-sensitized oxidation in natural water via OH radicals. Chemosphere 1985, 14, 1659–1671. [Google Scholar] [CrossRef]
- Bai, F.Y.; Ni, S.; Tang, Y.Z.; Pan, X.M.; Zhao, Z. Ciprofloxacin transformation in aqueous environments: Mechanism, kinetics, and toxicity assessment during •OH-mediated oxidation. Sci. Total. Environ. 2020, 699, 134190. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wang, S.J.; Zhang, Y.J.; Lu, C.G.; Tang, Y.Z. Degradation of mevinphos and monocrotophos by OH radicals in the environment: A computational investigation on mechanism, kinetic, and ecotoxicity. J. Hazard. Mater. 2023, 445, 130478. [Google Scholar] [CrossRef]
 .
.
 .
.
 .
.
 .
.




| Reactions | HO• | Reactions | SO4•− | ||
|---|---|---|---|---|---|
| kaq | Г (%) | kaq | Г (%) | ||
| RAF-1 | 8.10 × 109 | 95 | RAF-5 | 1.68 × 109 | 99.5 |
| RAF-2 | 4.49 × 106 | RAF-6 | 5.78 × 106 | 0.3 | |
| RAF-3 | 4.32 × 106 | RAF-7 | 3.35 × 10−13 | ||
| RAF-4 | 1.36 × 105 | RAF-8 | 2.72 × 106 | 0.2 | |
| ktotal-RAF | 8.10 × 109 | 95 | k′total-RAF | 1.69 × 109 | 100 |
| HAT-1 | 2.44 × 10−18 | HAT-6 | 6.08 × 10−17 | ||
| HAT-2 | 1.38 × 107 | HAT-7 | 2.80 × 104 | ||
| HAT-3 | 6.46 × 105 | HAT-8 | 1.20 × 103 | ||
| HAT-4 | 3.98 × 108 | 5 | HAT-9 | 6.85 × 105 | |
| HAT-5 | 1.22 × 10−13 | HAT-10 | 2.70 × 10−9 | ||
| ktotal-HAT | 4.13 × 108 | 5 | k′total-HAT | 7.15 × 105 | |
| ktotal | 8.52 × 109 | k′total | 1.69 × 109 | ||
| kexperiment | (3.54 ± 0.42) × 109; (5.5 ± 0.2) × 109 | k′experiment | (2.74 ± 0.13) × 109 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Chu, R.; Khan, Z.U.H. A Theoretical Study on the Degradation Mechanism, Kinetics, and Ecotoxicity of Metronidazole (MNZ) in •OH- and SO4•−-Assisted Advanced Oxidation Processes. Toxics 2023, 11, 796. https://doi.org/10.3390/toxics11090796
Sun J, Chu R, Khan ZUH. A Theoretical Study on the Degradation Mechanism, Kinetics, and Ecotoxicity of Metronidazole (MNZ) in •OH- and SO4•−-Assisted Advanced Oxidation Processes. Toxics. 2023; 11(9):796. https://doi.org/10.3390/toxics11090796
Chicago/Turabian StyleSun, Jingyu, Ruijun Chu, and Zia Ul Haq Khan. 2023. "A Theoretical Study on the Degradation Mechanism, Kinetics, and Ecotoxicity of Metronidazole (MNZ) in •OH- and SO4•−-Assisted Advanced Oxidation Processes" Toxics 11, no. 9: 796. https://doi.org/10.3390/toxics11090796
APA StyleSun, J., Chu, R., & Khan, Z. U. H. (2023). A Theoretical Study on the Degradation Mechanism, Kinetics, and Ecotoxicity of Metronidazole (MNZ) in •OH- and SO4•−-Assisted Advanced Oxidation Processes. Toxics, 11(9), 796. https://doi.org/10.3390/toxics11090796






