Exposure to Benzo[a]pyrene and 1-Nitropyrene in Particulate Matter Increases Oxidative Stress in the Human Body
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. BaP and 1-NP Atmospheric Air Concentration Measurement
2.3. 8-Hydroxydeoxyguanosine (8-OHdG) Urinary Concentration Measurement
2.4. Thiobarbituric Acid-Reactive Substance (TBARS) Urinary Concentration Measurement
2.5. 1-OHP Urinary Concentration Measurement
2.6. 6-OHNP and 1-NAAP Urinary Concentration Measurement
2.7. Statistical Analysis
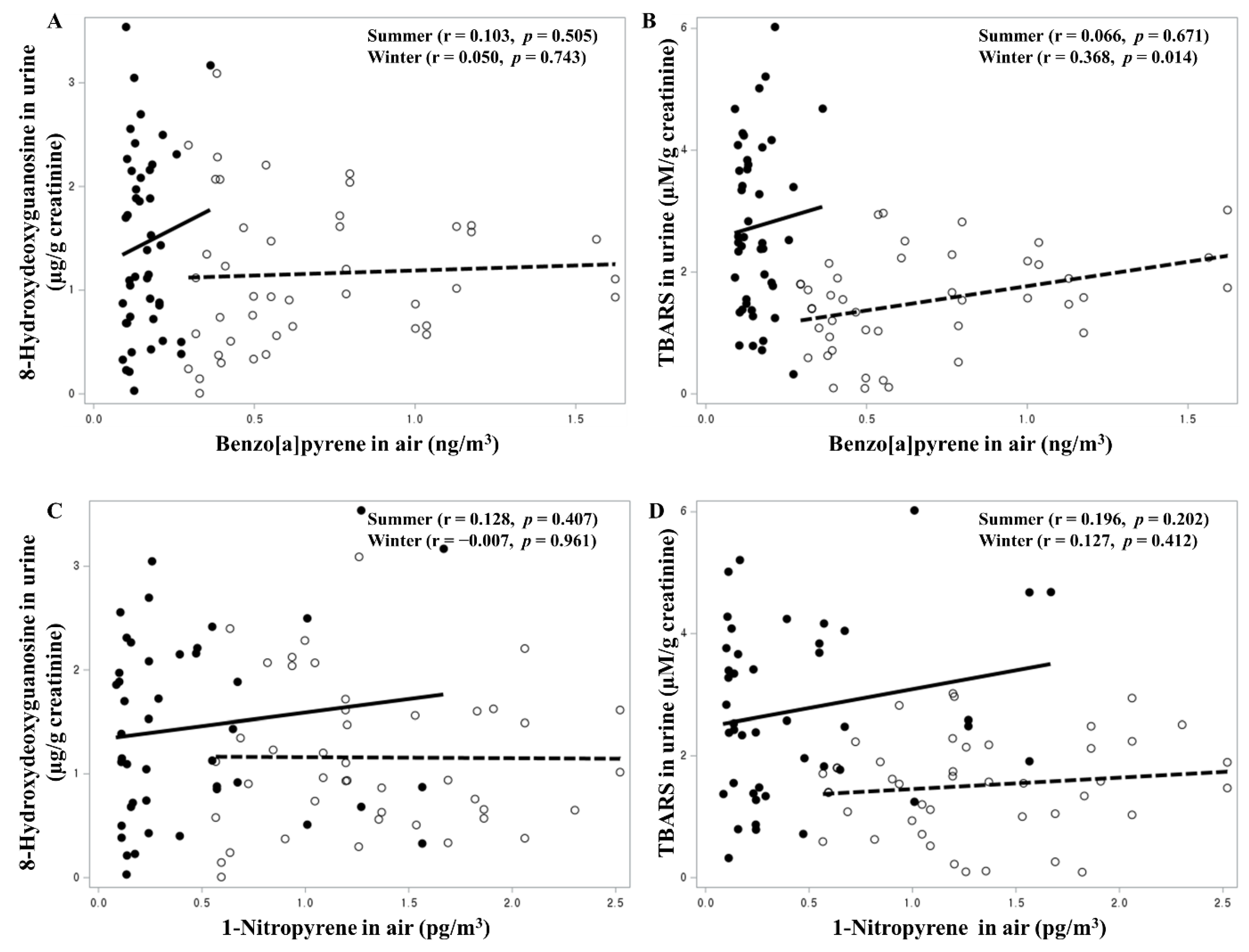
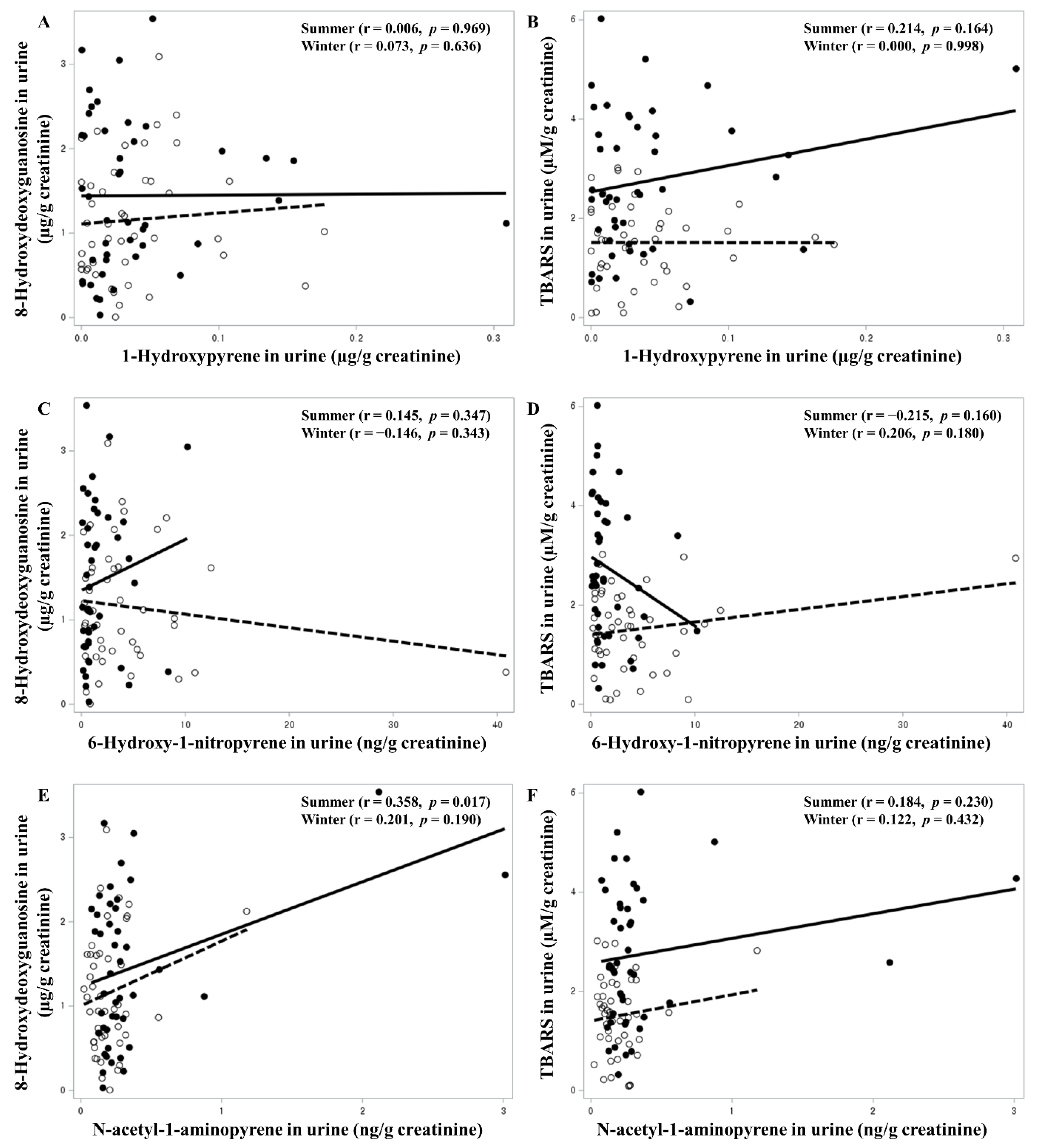
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Niu, Q.; Zhang, H.; Li, X.; Li, M. Benzo[a]pyrene-induced neurobehavioral function and neurotransmitter alterations in coke oven workers. Occup. Environ. Med. 2010, 67, 444–448. [Google Scholar] [CrossRef]
- Perera, F.P.; Rauh, V.; Whyatt, R.M.; Tsai, W.Y.; Tang, D.; Diaz, D.; Hoepner, L.; Barr, D.; Tu, Y.H.; Camann, D.; et al. Effect of prenatal exposure to airborne polycyclic aromatic hydrocarbons on neurodevelopment in the first 3 years of life among inner-city children. Environ. Health Perspect. 2006, 114, 1287–1292. [Google Scholar] [CrossRef] [PubMed]
- IARC Working Group Benzo[a]Pyrene. Iarc Monographs on the Identification of Carcinogenic Hazards to Humans. 2010. Available online: https://monographs.iarc.who.int/wp-content/uploads/2018/06/mono100F-14.pdf (accessed on 22 August 2023).
- Michaelson, J.J.; Trump, S.; Rudzok, S.; Gräbsch, C.; Madureira, D.J.; Dautel, F.; Mai, J.; Attinger, S.; Schirmer, K.; von Bergen, M.; et al. Transcriptional signatures of regulatory and toxic responses to benzo-[a]-pyrene exposure. BMC Genom. 2011, 12, 502. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Yang, H.; Ramesh, A.; Goodwin, J.S.; Okoro, E.U.; Guo, Z. Overexpression of Catalase Enhances Benzo(a)pyrene Detoxification in Endothelial Microsomes. PLoS ONE 2016, 11, e0162561. [Google Scholar] [CrossRef]
- Jongeneelen, F.J.; Bos, R.P.; Anzion, R.B.; Theuws, J.L.; Henderson, P.T. Biological monitoring of polycyclic aromatic hydrocarbons. Metabolites in urine. Scand. J. Work. Environ. Health 1986, 12, 137–143. [Google Scholar] [CrossRef]
- Frey, J.W.; Corn, M. Physical and chemical characteristics of particulates in a diesel exhaust. Am. Ind. Hyg. Assoc. J. 1967, 28, 468–478. [Google Scholar] [CrossRef] [PubMed]
- Ris, C.U.S. EPA health assessment for diesel engine exhaust: A review. Inhal. Toxicol. 2007, 19 (Suppl. S1), 229–239. [Google Scholar] [CrossRef] [PubMed]
- Attfield, M.D.; Schleiff, P.L.; Lubin, J.H.; Blair, A.; Stewart, P.A.; Vermeulen, R.; Coble, J.B.; Silverman, D.T. The Diesel Exhaust in Miners study: A cohort mortality study with emphasis on lung cancer. J. Natl. Cancer Inst. 2012, 104, 869–883. [Google Scholar] [CrossRef]
- Silverman, D.T.; Samanic, C.M.; Lubin, J.H.; Blair, A.E.; Stewart, P.A.; Vermeulen, R.; Coble, J.B.; Rothman, N.; Schleiff, P.L.; Travis, W.D.; et al. The Diesel Exhaust in Miners study: A nested case-control study of lung cancer and diesel exhaust. J. Natl. Cancer Inst. 2012, 104, 855–868. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Diesel and gasoline engine exhausts and some nitroarenes. Iarc monographs on the evaluation of carcinogenic risks to humans. IARC Monogr. Eval. Carcinog. Risks Hum. 2014, 105, 9–699. [Google Scholar]
- Bamford, H.A.; Bezabeh, D.Z.; Schantz, S.; Wise, S.A.; Baker, J.E. Determination and comparison of nitrated-polycyclic aromatic hydrocarbons measured in air and diesel particulate reference materials. Chemosphere 2003, 50, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Toriba, A.; Kitaoka, H.; Dills, R.L.; Mizukami, S.; Tanabe, K.; Takeuchi, N.; Ueno, M.; Kameda, T.; Tang, N.; Hayakawa, K.; et al. Identification and quantification of 1-nitropyrene metabolites in human urine as a proposed biomarker for exposure to diesel exhaust. Chem. Res. Toxicol. 2007, 20, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.D.; Ko, Y.J.; Kawamoto, T.; Kim, H. The effects of 1-nitropyrene on oxidative DNA damage and expression of DNA repair enzymes. J. Occup. Health 2005, 47, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Andersson, H.; Piras, E.; Demma, J.; Hellman, B.; Brittebo, E. Low levels of the air pollutant 1-nitropyrene induce DNA damage, increased levels of reactive oxygen species and endoplasmic reticulum stress in human endothelial cells. Toxicology 2009, 262, 57–64. [Google Scholar] [CrossRef]
- Scheepers, P.T.; Martens, M.H.; Velders, D.D.; Fijneman, P.; van Kerkhoven, M.; Noordhoek, J.; Bos, R.P. 1-Nitropyrene as a marker for the mutagenicity of diesel exhaust-derived particulate matter in workplace atmospheres. Environ. Mol. Mutagen. 1995, 25, 134–147. [Google Scholar] [CrossRef]
- Liu, C.S.; Tsai, C.S.; Kuo, C.L.; Chen, H.W.; Lii, C.K.; Ma, Y.S.; Wei, Y.H. Oxidative stress-related alteration of the copy number of mitochondrial DNA in human leukocytes. Free Radic. Res. 2003, 37, 1307–1317. [Google Scholar] [CrossRef]
- Yun, S.-G.; Yoo, C. The Effects of spring and winter blocking on PM10 concentration in Korea. Atmosphere 2019, 10, 410. [Google Scholar] [CrossRef]
- Allabakash, S.; Lim, S.; Chong, K.-S.; Yamada, T.J. Particulate Matter Concentrations over South Korea: Impact of Meteorology and Other Pollutants. Remote Sens. 2022, 14, 4849. [Google Scholar] [CrossRef]
- Boongla, Y.; Orakij, W.; Nagaoka, Y.; Tang, N.; Hayakawa, K.; Toriba, A. Simultaneous determination of polycyclic aromatic hydrocarbons and their nitro-derivatives in airborne particulates by using two-dimensional high-performance liquid chromatography with on-line reduction and fluorescence detection. Asian J. Atmos. Environ. 2017, 11, 83–299. [Google Scholar] [CrossRef]
- Kil, H.N.; Eom, S.Y.; Park, J.D.; Kawamoto, T.; Kim, Y.D.; Kim, H. A rapid method for estimating the levels of urinary thiobarbituric Acid reactive substances for environmental epidemiologic survey. Toxicol. Res. 2014, 30, 7–11. [Google Scholar] [CrossRef]
- Jongeneelen, F.J.; Anzion, R.B.M.; Scheepers, P.T.J.; Bos, R.P.; Henderson, P.T.; Nijenhuis, E.H.; Veenstra, S.J.; Brouns, R.M.E.; Winkes, A. 1-Hydroxypyrene in urine as a biological indicator of exposure to polycyclic aromatic hydrocarbons in several work environments. Ann. Occup. Hyg. 1988, 32, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, P.J.; Oosthuizen, M.K.; Mitchell, C.; Blount, J.D.; Bennett, N.C. Heat and dehydration induced oxidative damage and antioxidant defenses following incubator heat stress and a simulated heat wave in wild caught four-striped field mice Rhabdomys dilectus. PLoS ONE 2020, 15, e0242279. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.K.; Siegrist, K.J.; Wolff, M.; Nield, L.; Brüning, T.; Upham, B.L.; Käfferlein, H.U.; Plöttner, S. The Carcinogenic Properties of Overlooked yet Prevalent Polycyclic Aromatic Hydrocarbons in Human Lung Epithelial Cells. Toxics 2022, 10, 28. [Google Scholar] [CrossRef] [PubMed]
- Romo, D.; Velmurugan, K.; Upham, B.L.; Dwyer-Nield, L.D.; Bauer, A.K. Dysregulation of Gap Junction Function and Cytokine Production in Response to Non-Genotoxic Polycyclic Aromatic Hydrocarbons in an In Vitro Lung Cell Model. Cancers 2019, 11, 572. [Google Scholar] [CrossRef] [PubMed]
- Riley, E.A.; Carpenter, E.E.; Ramsay, J.; Zamzow, E.; Pyke, C.; Paulsen, M.H.; Sheppard, L.; Spear, T.M.; Seixas, N.S.; Stephenson, D.J.; et al. Evaluation of 1-Nitropyrene as a Surrogate Measure for Diesel Exhaust. Ann. Work Expo. Health 2018, 62, 339–350. [Google Scholar] [CrossRef]
- Scheepers, P.T.; Micka, V.; Muzyka, V.; Anzion, R.; Dahmann, D.; Poole, J.; Bos, R.P. Exposure to dust and particle-associated 1-nitropyrene of drivers of diesel-powered equipment in underground mining. Ann. Occup. Hyg. 2003, 47, 379–388. [Google Scholar] [CrossRef][Green Version]
- Yun, J.K.; Ochirpurev, B.; Eom, S.Y.; Toriba, A.; Kim, Y.D.; Kim, H. Effects of gene polymorphisms of CYP1A1, CYP1B1, EPHX1, NQO1, and NAT2 on urinary 1-nitropyrene metabolite concentrations. Heliyon 2022, 8, e10120. [Google Scholar] [CrossRef]
- Hayakawa, K.; Tang, N.; Nagato, E.; Toriba, A.; Lin, J.M.; Zhao, L.; Zhou, Z.; Qing, W.; Yang, X.; Mishukov, V.; et al. Long-term trends in urban atmospheric polycyclic aromatic hydrocarbons and nitropolycyclic aromatic hydrocarbons: China, Russia, and Korea from 1999 to 2014. Int. J. Environ. Res. Public Health 2020, 17, 431. [Google Scholar] [CrossRef]
- Murahashi, T.; Hayakawa, K. A sensitive method for the determination of 6-nitrochrysene, 2-nitrofluoranthene and 1-, 2- and 4-nitropyrenes in airborne particulates using high-performance liquid chromatography with chemiluminescence detection. Anal. Chim. Acta 1997, 343, 251–257. [Google Scholar] [CrossRef]
- Miller-Schulze, J.P.; Paulsen, M.; Toriba, A.; Tang, N.; Hayakawa, K.; Tamura, K.; Dong, L.; Zhang, X.; Simpson, C.D. Exposures to particulate air pollution and nitro-polycyclic aromatic hydrocarbons among taxi drivers in Shenyang, China. Environ. Sci. Technol. 2010, 44, 216–221. [Google Scholar] [CrossRef]
- Ball, L.M.; Kohan, M.J.; Inmon, J.P.; Claxton, L.D.; Lewtas, J. Metabolism of 1-nitro[14C]pyrene in vivo in the rat and mutagenicity of urinary metabolites. Carcinogenesis 1984, 5, 1557–1564. [Google Scholar] [CrossRef]
- Ochirpurev, B.; Eom, S.Y.; Toriba, A.; Kim, Y.D.; Kim, H. Urinary 1-aminopyrene level in Koreans as a biomarker for the amount of exposure to atmospheric 1-nitropyrene. Toxicol. Res. 2021, 38, 45–51. [Google Scholar] [CrossRef]
- Jelic, M.D.; Mandic, A.D.; Maricic, S.M.; Srdjenovic, B.U. Oxidative stress and its role in cancer. J. Cancer Res. Ther. 2021, 17, 22–28. [Google Scholar] [CrossRef]
- Jiang, H.; Gelhaus, S.L.; Mangal, D.; Harvey, R.G.; Blair, I.A.; Penning, T.M. Metabolism of benzo[a]pyrene in human bronchoalveolar H358 cells using liquid chromatography-mass spectrometry. Chem. Res. Toxicol. 2007, 20, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell Longev. 2014, 360438. [Google Scholar] [CrossRef] [PubMed]
- Cooke, M.S.; Evans, M.D.; Dizdaroglu, M.; Lunec, J. Oxidative DNA damage: Mechanisms, mutation, and disease. FASEB J. 2003, 17, 1195–1214. [Google Scholar] [CrossRef] [PubMed]
- Hahm, J.Y.; Park, J.; Jang, E.S.; Chi, S.W. 8-Oxoguanine: From oxidative damage to epigenetic and epitranscriptional modification. Exp. Mol. Med. 2022, 54, 1626–1642. [Google Scholar] [CrossRef]
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. Front. Pharmacol. 2021, 12, 643972. [Google Scholar] [CrossRef]
- Lelieveld, S.; Wilson, J.; Dovrou, E.; Mishra, A.; Lakey, P.S.J.; Shiraiwa, M.; Pöschl, U.; Berkemeier, T. Hydroxyl Radical Production by Air Pollutants in Epithelial Lining Fluid Governed by Interconversion and Scavenging of Reactive Oxygen Species. Environ. Sci. Technol. 2021, 55, 14069–14079. [Google Scholar] [CrossRef]
| Variables | N (%) | Mean ± S.D. | |
|---|---|---|---|
| Age | 44 (100%) | 63.8 ± 12.1 yr | |
| Sex | Male | 18 (40.9%) | |
| Females | 26 (59.1%) | ||
| Smoking habit | Never smokers | 28 (63.6%) | |
| Ex-smokers | 9 (20.5%) | ||
| Current smokers | 7 (16.0%) | ||
| Residential area | Incinerator area | 16 (36.4%) | |
| Industrial complex area | 10 (22.7%) | ||
| Rural area | 18 (40.9%) |
| Variables | Mean ± SD | p-Value | |
|---|---|---|---|
| Winter (N = 44) | Summer (N = 44) | ||
| Benzo[a]pyrene in the atmospheric air (ng/m3) | 0.68 ± 0.37 | 0.15 ± 0.06 | <0.0001 |
| 1-Nitropyrene in the atmospheric air (pg/m3) | 1.31 ± 0.54 | 0.44 ± 0.44 | <0.0001 |
| TBARS in the urine (μM/g creatinine) | 1.51 ± 0.80 | 2.74 ± 1.39 | <0.0001 |
| 8-Hydroxydeoxyguanosine in the urine (μg/g creatinine) | 1.16 ± 0.70 | 1.44 ± 0.89 | 0.2142 |
| 1-OHP in the urine (μg/g creatinine) | 0.04 ± 0.04 | 0.04 ± 0.06 | 0.5424 |
| 6-OHNP in the urine (ng/g creatinine) | 4.30 ± 6.44 | 1.65 ± 2.15 | 0.0012 |
| 1-NAAP in the urine (ng/g creatinine) | 0.20 ± 0.18 | 0.35 ± 0.52 | 0.0027 |
| Variables | Season | TBARs in Urine (μM/g Creatinine) | 8-OHdG in Urine (μg/g Creatinine) | ||
|---|---|---|---|---|---|
| β | p-Value | β | p-Value | ||
| Current smoker (no = 0, yes = 1) | Winter | −0.106 | 0.7962 | −0.454 | 0.2485 |
| Summer | 14.41 | 0.0412 | 0.990 | 0.0466 | |
| Cumulative smoking amount (Pack year) | Winter | 0.011 | 0.2994 | 0.018 | 0.0748 |
| Summer | −0.052 | 0.0048 | 0.013 | 0.3089 | |
| Benzo[a]pyrene in air (ng/m3) | Winter | 0.760 | 0.0251 | 0.207 | 0.8833 |
| Summer | 5.566 | 0.1158 | 5.496 | 0.0313 | |
| 1-Nitropyrene in air (pg/m3) | Winter | −0.271 | 0.2531 | −0.156 | 0.5174 |
| Summer | 0.345 | 0.4593 | −0.309 | 0.3511 | |
| 1-OHP in urine (μg/g creatinine) | Winter | −432.7 | 0.1662 | 276.6 | 0.3503 |
| Summer | 407.0 | 0.4167 | −657.5 | 0.0651 | |
| 6-OHNP in urine (ng/g creatinine) | Winter | 0.762 | 0.7038 | −2.803 | 0.1482 |
| Summer | −11.22 | 0.2212 | 6.256 | 0.3324 | |
| 1-NAAP in urine (ng/g creatinine) | Winter | 49.08 | 0.4670 | 134.2 | 0.0373 |
| Summer | 39.72 | 0.2899 | 55.69 | 0.0300 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, S.-H.; Ochirpurev, B.; Toriba, A.; Won, J.-U.; Kim, H. Exposure to Benzo[a]pyrene and 1-Nitropyrene in Particulate Matter Increases Oxidative Stress in the Human Body. Toxics 2023, 11, 797. https://doi.org/10.3390/toxics11090797
Choi S-H, Ochirpurev B, Toriba A, Won J-U, Kim H. Exposure to Benzo[a]pyrene and 1-Nitropyrene in Particulate Matter Increases Oxidative Stress in the Human Body. Toxics. 2023; 11(9):797. https://doi.org/10.3390/toxics11090797
Chicago/Turabian StyleChoi, Sun-Haeng, Bolormaa Ochirpurev, Akira Toriba, Jong-Uk Won, and Heon Kim. 2023. "Exposure to Benzo[a]pyrene and 1-Nitropyrene in Particulate Matter Increases Oxidative Stress in the Human Body" Toxics 11, no. 9: 797. https://doi.org/10.3390/toxics11090797
APA StyleChoi, S.-H., Ochirpurev, B., Toriba, A., Won, J.-U., & Kim, H. (2023). Exposure to Benzo[a]pyrene and 1-Nitropyrene in Particulate Matter Increases Oxidative Stress in the Human Body. Toxics, 11(9), 797. https://doi.org/10.3390/toxics11090797









