Dielectric Barrier Discharge Plasma Coupled with Cobalt Oxyhydroxide for Methylene Blue Degradation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Catalyst Preparation
2.3. Experimental Setup
2.4. Methods and Analysis
3. Results
3.1. Characterization of CoOOH
3.2. MB Degradation Performance via DDBD with CoOOH Catalyst
3.2.1. Effect of CoOOH Dosage on MB Degradation
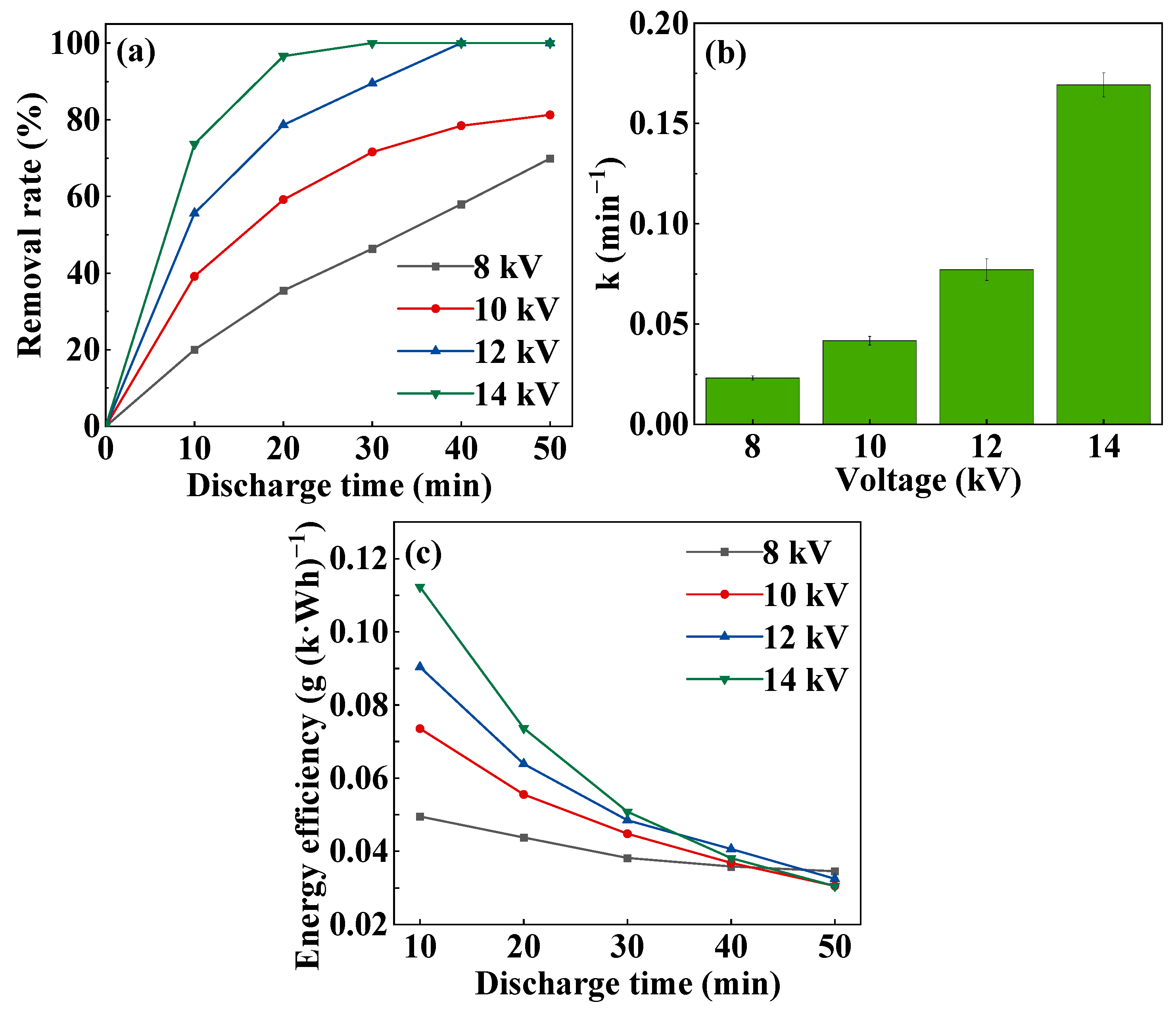
3.2.2. Effect of Discharge Voltage on MB Degradation
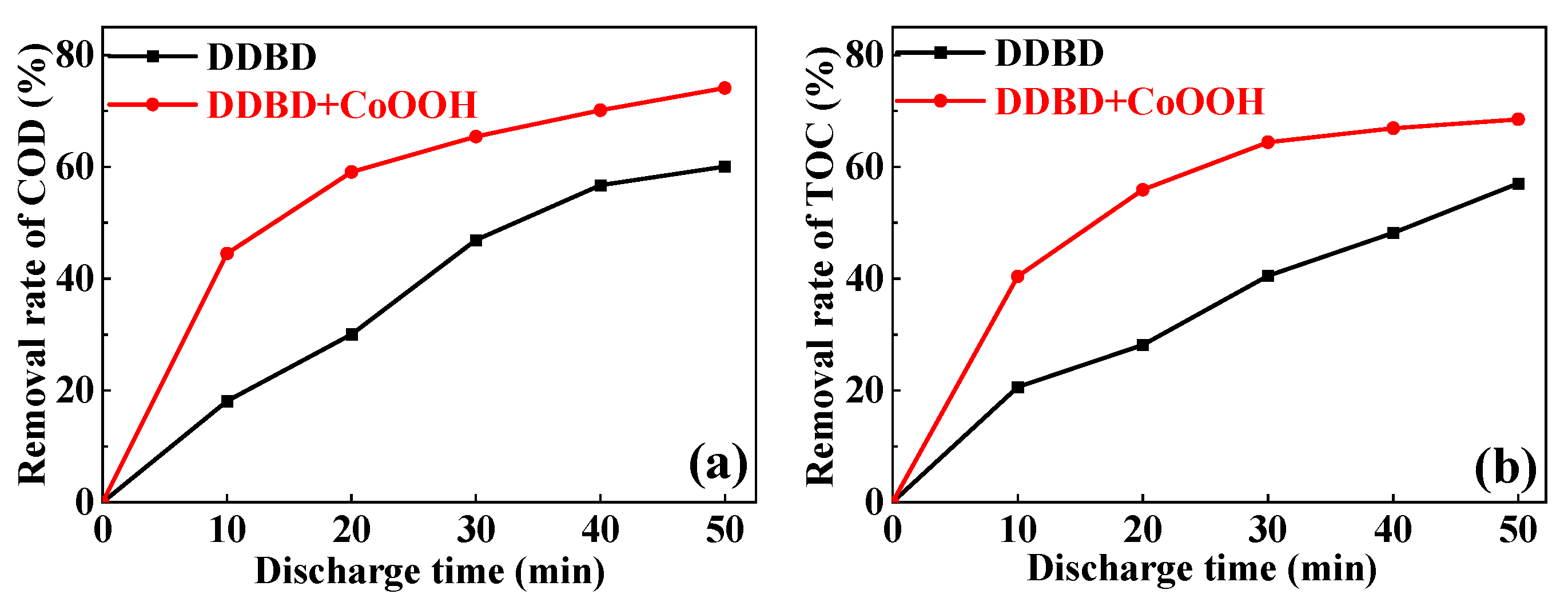
3.2.3. COD and TOC
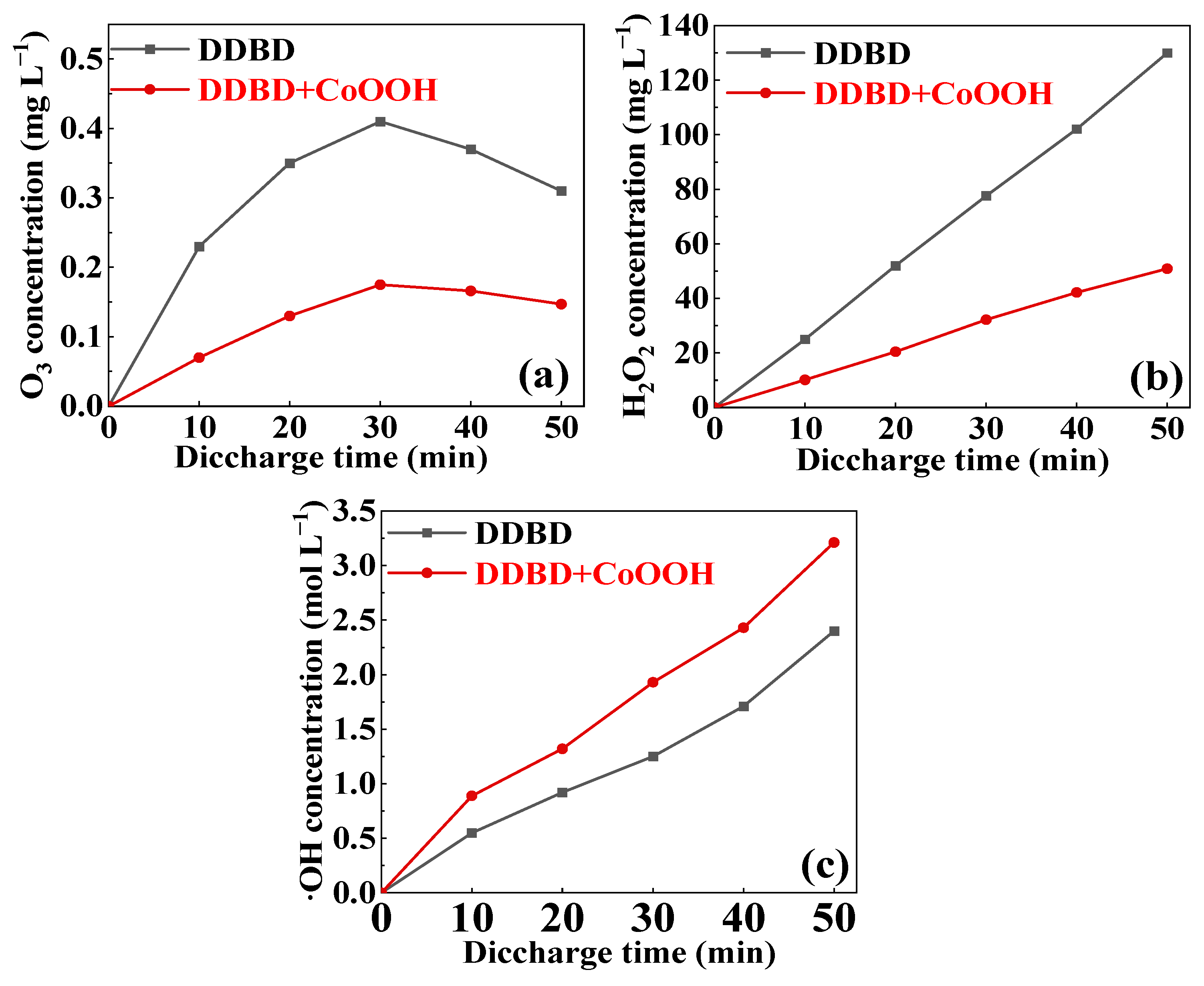
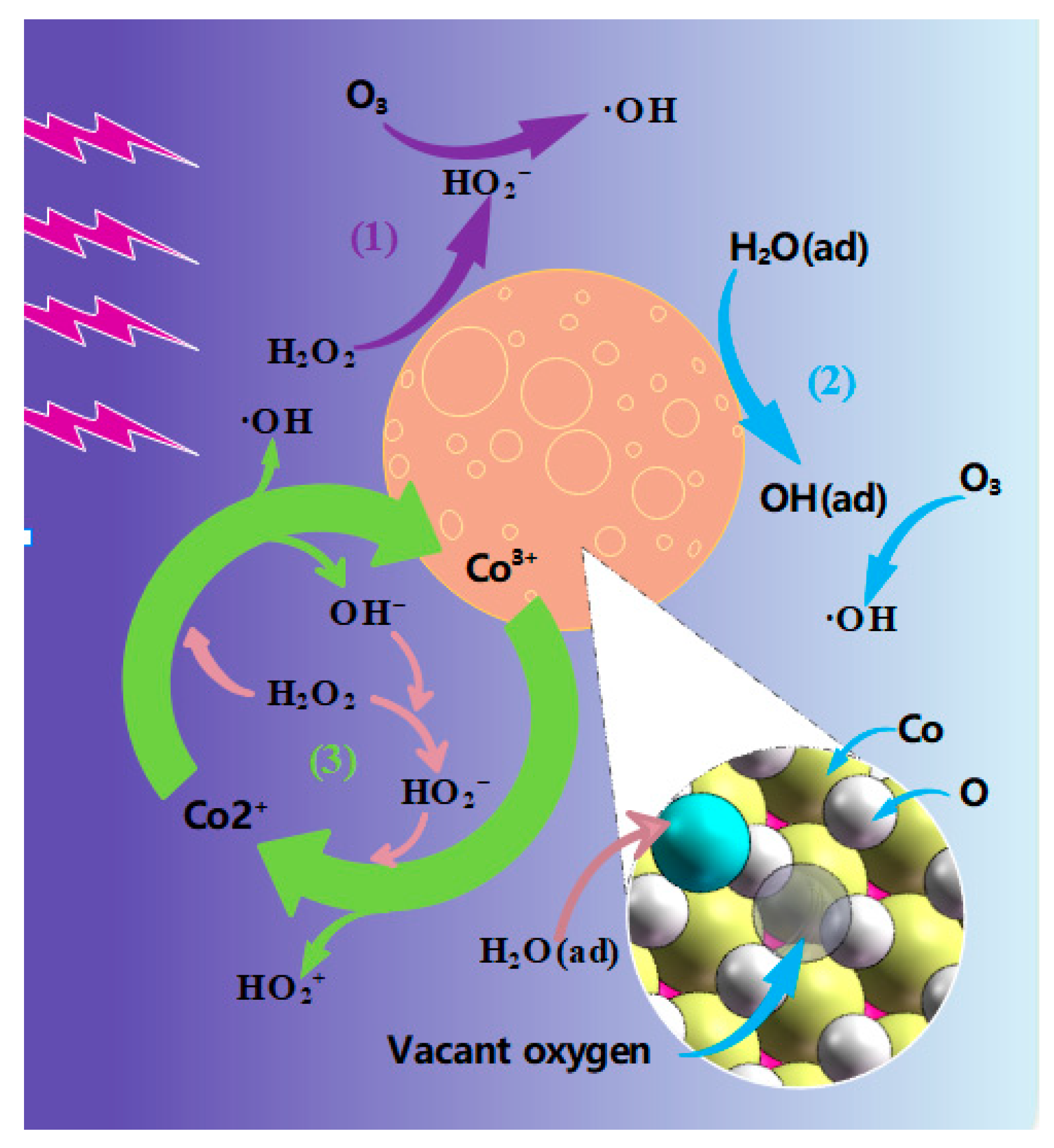
3.3. Mechanism of MB Degradation via DDBD Coupled with CoOOH
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kyere-Yeboah, K.; Bique, I.K.; Qiao, X.C. Advances of non-thermal plasma discharge technology in degrading recalcitrant wastewater pollutants. A comprehensive review. Chemosphere 2023, 320, 138061. [Google Scholar] [CrossRef]
- Topolovec, B.; Skoro, N.; Puac, N.; Petrovic, M. Pathways of organic micropollutants degradation in atmospheric pressure plasma processing—A review. Chemosphere 2022, 294, 133606. [Google Scholar] [CrossRef] [PubMed]
- Oturan, M.A.; Aaron, J.J. Advanced Oxidation Processes in Water/Wastewater Treatment: Principles and Applications. A Review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2577–2641. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, M.H.; Hao, X.L.; Lei, L.C. Degradation mechanisms of 4-chlorophenol in a novel gas-liquid hybrid discharge reactor by pulsed high voltage system with oxygen or nitrogen bubbling. Chemosphere 2007, 67, 702–711. [Google Scholar] [CrossRef] [PubMed]
- Duana, L.J.; Jiang, N.; Lu, N.; Shang, K.F.; Li, J.; Wu, Y. Synergetic effect of TiO2 and Fe3+ as co-catalysts for enhanced phenol degradation in pulsed discharge system. Appl. Catal. B-Environ. 2018, 221, 521–529. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, W.X.; Huang, J.W.; Wang, Y.W.; Guo, H. ZnO Promoted Persulfate Activation in Discharge Plasma System for Ofloxacin Degradation. Catalysts 2023, 13, 847. [Google Scholar] [CrossRef]
- Qian, X.F.; Ren, M.; Zhu, Y.; Yue, D.T.; Han, Y.; Jia, J.P.; Zhao, Y.X. Visible Light Assisted Heterogeneous Fenton-Like Degradation of Organic Pollutant via alpha-FeOOH/Mesoporous Carbon Composites. Environ. Sci. Technol. 2017, 51, 3993–4000. [Google Scholar] [CrossRef]
- Zhao, L.; Lin, Z.R.; Ma, X.H.; Dong, Y.H. Catalytic activity of different iron oxides: Insight from pollutant degradation and hydroxyl radical formation in heterogeneous Fenton-like systems. Chem. Eng. J. 2018, 352, 343–351. [Google Scholar] [CrossRef]
- Abdedayem, A.; Guiza, M.; Rivas Toledo, F.J.; Ouederni, A. Nitrobenzene degradation in aqueous solution using ozone/cobalt supported activated carbon coupling process: A kinetic approach. Sep. Purif. Technol. 2017, 184, 308–318. [Google Scholar] [CrossRef]
- Mahamallik, P.; Pal, A. Degradation of textile wastewater by modified photo-Fenton process: Application of Co(II) adsorbed surfactant-modified alumina as heterogeneous catalyst. J. Environ. Chem. Eng. 2017, 5, 2886–2893. [Google Scholar] [CrossRef]
- Xu, Z.Z.; Chen, Z.L.; Joll, C.; Ben, Y.; Shen, J.M.; Tao, H. Catalytic efficiency and stability of cobalt hydroxide for decomposition of ozone and p-chloronitrobenzene in water. Catal. Commun. 2009, 10, 1221–1225. [Google Scholar] [CrossRef]
- Ren, J.Y.; Jiang, N.; Shang, K.F.; Lu, N.; Li, J.; Wu, Y. Synergistic degradation of trans-ferulic acid by water falling film DBD plasma coupled with cobalt oxyhydroxide: Performance and mechanisms. Chem. Eng. J. 2019, 372, 321–331. [Google Scholar] [CrossRef]
- Mei, S.F.; Liu, Y.A. Degradation of Reactive Black 5 in Aqueous Solution by Double-Dielectric Barrier Discharge. In Proceedings of the 2nd International Conference on Energy, Environment and Sustainable Development (EESD 2012), Jilin, China, 12–14 October 2012; pp. 1616–1619. [Google Scholar]
- Zhang, H.B.; Li, K.; Shu, C.H.; Lou, Z.Y.; Sun, T.H.; Jia, J.P. Enhancement of styrene removal using a novel double-tube dielectric barrier discharge (DDBD) reactor. Chem. Eng. J. 2014, 256, 107–118. [Google Scholar] [CrossRef]
- Rong, S.P.; Sun, Y.B. Degradation of TAIC by water falling film dielectric barrier discharge-Influence of radical scavengers. J. Hazard. Mater. 2015, 287, 317–324. [Google Scholar] [CrossRef]
- Wu, J.L.; Xiong, Q.; Liang, J.L.; He, Q.; Yang, D.X.; Deng, R.Y.; Chen, Y. Degradation of benzotriazole by DBD plasma and peroxymonosulfate: Mechanism, degradation pathway and potential toxicity. Chem. Eng. J. 2020, 384, 123300. [Google Scholar] [CrossRef]
- Yao, X.; Fang, Y.; Guo, Y.; Xu, M. Degradation of methylene blue using a novel gas-liquid hybrid DDBD reactor: Performance and pathways. Chemosphere 2023, 336, 139172. [Google Scholar] [CrossRef]
- Jing, H.P.; Wang, C.C.; Zhang, Y.W.; Wang, P.; Li, R. Photocatalytic degradation of methylene blue in ZIF-8. Rsc Adv. 2014, 4, 54454–54462. [Google Scholar] [CrossRef]
- Din, M.I.; Khalid, R.; Najeeb, J.; Hussain, Z. Fundamentals and photocatalysis of methylene blue dye using various nanocatalytic assemblies-a critical review. J. Clean. Prod. 2021, 298, 126567. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Zekker, I.; Zhang, B.L.; Hendi, A.H.; Ahmad, A.; Ahmad, S.; Zada, N.; Ahmad, H.; Shah, L.A.; et al. Review on Methylene Blue: Its Properties, Uses, Toxicity and Photodegradation. Water 2022, 14, 242. [Google Scholar] [CrossRef]
- Suarasan, I.; Ghizdavu, L.; Ghizdavu, I.; Budu, S.; Dascalescu, L. Experimental characterization of multi-point corona discharge devices for direct ozonization of liquids. J. Electrost. 2002, 54, 207–214. [Google Scholar] [CrossRef]
- Renault, O.; Gosset, L.G.; Rouchon, D.; Ermolieff, A. Angle-resolved x-ray photoelectron spectroscopy of ultrathin Al2O3 films grown by atomic layer deposition. J. Vac. Sci. Technol. A 2002, 20, 1867–1876. [Google Scholar] [CrossRef]
- Rodríguez, J.L.; Poznyak, T.; Valenzuela, M.A.; Tiznado, H.; Chairez, I. Surface interactions and mechanistic studies of 2,4-dichlorophenoxyacetic acid degradation by catalytic ozonation in presence of Ni/TiO2. Chem. Eng. J. 2013, 222, 426–434. [Google Scholar] [CrossRef]
- Zhao, X.L.; Wang, Y.; Wu, H.Y.; Fang, L.C.; Liang, J.J.; Fan, Q.H.; Li, P. Insights into the effect of humic acid on Ni(II) sorption mechanism on illite: Batch, XPS and EXAFS investigations. J. Mol. Liq. 2017, 248, 1030–1038. [Google Scholar] [CrossRef]
- Peng, J.L.; Lai, L.D.; Jiang, X.; Jiang, W.J.; Lai, B. Catalytic ozonation of succinic acid in aqueous solution using the catalyst of Ni/Al2O3 prepared by electroless plating-calcination method. Sep. Purif. Technol. 2018, 195, 138–148. [Google Scholar] [CrossRef]
- Wang, Q.L.; Tang, M.H.; Peng, Y.Q.; Du, C.C.; Lu, S.Y. Ozone assisted oxidation of gaseous PCDD/Fs over CNTs-containing composite catalysts at low temperature. Chemosphere 2018, 199, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Miao, J.; Duan, X.; Guan, D.; Zhong, Y.; Wang, S.; Zhou, W.; Shao, Z. Postsynthesis Growth of CoOOH Nanostructure on SrCo0.6Ti0.4O3-δ Perovskite Surface for Enhanced Degradation of Aqueous Organic Contaminants. ACS Sustain. Chem. Eng. 2018, 6, 15737–15748. [Google Scholar] [CrossRef]
- Ren, J.Y.; Li, J.; Lv, L.; Wang, J. Degradation of caffeic acid by dielectric barrier discharge plasma combined with Ce doped CoOOH catalyst. J. Hazard. Mater. 2021, 402, 123772. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.H.; Chen, J.T.; Yao, T.; He, J.F.; Jiang, S.; Sun, Z.H.; Liu, Q.H.; Cheng, W.R.; Hu, F.C.; Jiang, Y.; et al. CoOOH Nanosheets with High Mass Activity for Water Oxidation. Angew. Chem. Int. Ed. 2015, 54, 8722–8727. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.H.; He, D.; Li, X.R.; Feng, W.; Lyu, C.; Zhang, Y.F. Mechanism and performance of singlet oxygen dominated peroxymonosulfate activation on CoOOH nanoparticles for 2,4-dichlorophenol degradation in water. J. Hazard. Mater. 2020, 384, 121350. [Google Scholar] [CrossRef]
- Wang, Z.M.; Ma, H.; Zhang, C.; Feng, J.; Pu, S.Y.; Ren, Y.M.; Wang, Y. Enhanced catalytic ozonation treatment of dibutyl phthalate enabled by porous magnetic Ag-doped ferrospinel MnFe2O4 materials: Performance and mechanism. Chem. Eng. J. 2018, 354, 42–52. [Google Scholar] [CrossRef]
- Hamada, K.; Qian, J.; Hirata, Y.; Satomura, K.; Mitsuishi, M. Effects of bolaform electrolytes on the interaction between a water-soluble polymer and sulphonated monoazo dyes. Part 3: Dyes containing two naphthalene rings. Dye. Pigment. 1996, 31, 19–30. [Google Scholar] [CrossRef]
- Isaad, J.; El Achari, A. A novel glycoconjugated N-acetylamino aldehyde hydrazone azo dye as chromogenic probe for cyanide detection in water. Anal. Chim. Acta 2011, 694, 120–127. [Google Scholar] [CrossRef]
- Wang, H.J.; Shen, Z.; Yan, X.; Guo, H.; Mao, D.N.; Yi, C.W. Dielectric barrier discharge plasma coupled with WO3 for bisphenol A degradation. Chemosphere 2021, 274, 129722. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Jiang, J.; Lu, X.L.; Ma, J.; Liu, Y.Z. Production of Sulfate Radical and Hydroxyl Radical by Reaction of Ozone with Peroxymonosulfate: A Novel Advanced Oxidation Process. Environ. Sci. Technol. 2015, 49, 7330–7339. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.H.; Wang, J.P.; Hu, J.; Chen, Z.D.; Zheng, L.R.; Fu, Y.H.; Lei, Y.Q.; Ren, X.Z.; He, C.A.X.; Zhang, Q.L.; et al. Electrochemical Construction of Low-Crystalline CoOOH Nanosheets with Short-Range Ordered Grains to Improve Oxygen Evolution Activity. Acs Catal. 2021, 11, 6104–6112. [Google Scholar] [CrossRef]
- Zeng, H.X.; Zhu, H.; Deng, J.; Shi, Z.; Zhang, H.J.; Li, X.Y.; Deng, L. New insight into peroxymonosulfate activation by CoAl-LDH derived CoOOH: Oxygen vacancies rather than Co species redox pairs induced process. Chem. Eng. J. 2022, 442, 136251. [Google Scholar] [CrossRef]
- Staehelin, J.; Hoigne, J. Decomposition of ozone in water-rate of initiation by hydroxide ions and hydrogen-peroxide. Environ. Sci. Technol. 1982, 16, 676–681. [Google Scholar] [CrossRef]
- Jain, P.; Jha, S.; Ingole, P.P. Concurrently Engineered Lewis Acid Sites and Coordination Sphere Vacancies in CoFe Prussian Blue Analogues for Boosted Bifunctional Oxygen Electrocatalysis. ACS Appl. Energy Mater. 2023, 6, 3278–3290. [Google Scholar] [CrossRef]
- Zhang, Q.R.; Qu, G.Z.; Wang, T.C.; Li, C.G.; Qiang, H.; Sun, Q.H.; Liang, D.L.; Hu, S.B. Humic acid removal from micro-polluted source water in the presence of inorganic salts in a gas-phase surface discharge plasma system. Sep. Purif. Technol. 2017, 187, 334–342. [Google Scholar] [CrossRef]
- Lin, Y.T.; Chen, C.H.; Lin, M.S. pH-Dependent Catalytic Behavior in Cathodic Application of Hydrogen Peroxide with Cobalt Oxide Modified Electrode and Its Application in Electrochemical Fenton Process in Alkaline Media. J. Electrochem. Soc. 2018, 165, H360–H364. [Google Scholar] [CrossRef]
- Ren, J.Y.; Jiang, N.; Li, J.; Shang, K.F.; Lu, N.; Wu, Y. Synergistic degradation of trans-ferulic acid in aqueous solution by dielectric barrier discharge plasma combined with ozone. Environ. Sci. Pollut. Res. 2018, 25, 35479–35491. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, X.; Fang, Y.; Cui, X.; Cheng, X.; Cheng, Z. Dielectric Barrier Discharge Plasma Coupled with Cobalt Oxyhydroxide for Methylene Blue Degradation. Toxics 2023, 11, 763. https://doi.org/10.3390/toxics11090763
Yao X, Fang Y, Cui X, Cheng X, Cheng Z. Dielectric Barrier Discharge Plasma Coupled with Cobalt Oxyhydroxide for Methylene Blue Degradation. Toxics. 2023; 11(9):763. https://doi.org/10.3390/toxics11090763
Chicago/Turabian StyleYao, Xiaomei, Yingbo Fang, Xiaochen Cui, Xian Cheng, and Zixia Cheng. 2023. "Dielectric Barrier Discharge Plasma Coupled with Cobalt Oxyhydroxide for Methylene Blue Degradation" Toxics 11, no. 9: 763. https://doi.org/10.3390/toxics11090763
APA StyleYao, X., Fang, Y., Cui, X., Cheng, X., & Cheng, Z. (2023). Dielectric Barrier Discharge Plasma Coupled with Cobalt Oxyhydroxide for Methylene Blue Degradation. Toxics, 11(9), 763. https://doi.org/10.3390/toxics11090763





