Effect of Air Pollution on the Basal DNA Damage of Mother–Newborn Couples of México City
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Pollution Data
2.3. Alkaline Comet Assay
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dondi, A.; Carbone, C.; Manieri, E.; Zama, D.; Del Bono, C.; Betti, L.; Biagi, C.; Lanari, M. Outdoor Air Pollution and Childhood Respiratory Disease: The Role of Oxidative Stress. Int. J. Mol. Sci. 2023, 24, 4345. [Google Scholar] [CrossRef]
- Molina, L.T.; Velasco, E.; Retama, A.; Zavala, M. Experience from Integrated Air Quality Management in the Mexico City Metropolitan Area and Singapore. Atmosphere 2019, 10, 512. [Google Scholar] [CrossRef]
- Mamkhezri, J.; Bohara, A.K.; Islas Camargo, A. Air pollution and daily mortality in the Mexico City Metropolitan Area. Atmósfera 2020, 33, 249–267. [Google Scholar] [CrossRef]
- Texcalac-Sangrador, J.L.; Hurtado-Díaz, M.; Félix-Arellano, E.E.; Guerrero-López, C.M.; Riojas-Rodríguez, H. Health and Economic Impacts Assessment of O3 Exposure in Mexico. Int. J. Environ. Res. Public Health 2021, 18, 11646. [Google Scholar] [CrossRef] [PubMed]
- Cerón, R.M.; Cerón, J.G.; Rangel, M.; Ruíz, A.; Aguilar, C.; Montalvo, C.; Canedo, Y.; García, R.; Uc, M.; Galván, A. Association between Short-Term Exposure to Criteria Air Pollutants and Daily Mortality in Mexico City: A Time Series Study. Atmosphere 2023, 14, 955. [Google Scholar] [CrossRef]
- Gutiérrez-Avila, I.; Riojas-Rodríguez, H.; Colicino, E.; Rush, J.; Tamayo-Ortiz, M.; Borja-Aburto, V.H.; Just, A.C. Daily exposure to PM2.5 and 1.5 million deaths: A time-stratified case-crossover analysis in the Mexico City Metropolitan Area. medRxiv 2023. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; Ayala, A. Fine particle air pollution and lung cancer risk: Extending the long list of health risks. Cell 2023, 186, 2285–2287. [Google Scholar] [CrossRef]
- Romieu, I.; Gouveia, N.; Cifuentes, L.A.; de Leon, A.P.; Junger, W.; Vera, J.; Strappa, V.; Hurtado-Díaz, M.; Miranda-Soberanis, V.; Rojas-Bracho, L.; et al. Multicity study of air pollution and mortality in Latin America (the ESCALA study). Res. Rep. Health Eff. Inst. 2012, 171, 5–86. [Google Scholar]
- GBD 2019 Chronic Respiratory Diseases Collaborators. Global burden of chronic respiratory diseases and risk factors, 1990–2019: An update from the Global Burden of Disease Study 2019. EClinicalMedicine 2023, 59, 101936. [Google Scholar] [CrossRef]
- Tellez-Rojo, M.M.; Romieu, I.; Polo-Pena, M.; Ruiz-Velasco, S.; Meneses-Gonzalez, F.; Hernandez-Avila, M. Effect of environmental pollution on medical visits for respiratory infections in children in Mexico City. Salud Publica Mex. 1997, 39, 513–522. Available online: http://www.ncbi.nlm.nih.gov/pubmed/9477733 (accessed on 17 September 2017).
- Pereira, L.A.; Loomis, D.; Conceicao, G.M.; Braga, A.L.F.; Arcas, R.M.; Kishi, H.S.; Singer, J.M.; Bohm, G.M.; Saldiva, P.H. Association between air pollution and intrauterine mortality in Sao Paulo, Brazil. Environ. Health Perspect. 1998, 106, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Davis, D.L.; Saldiva, P.H.N. Urban Air Pollution Risks to Children: A Global Environmental Health Indicator. 1999. Available online: http://www.wri.org/pubs/pubs_description.cfm?pid=3004 (accessed on 17 September 2017).
- Loomis, D.; Castillejos, M.; Gold, D.R.; McDonnell, W.; Borja-Aburto, V.H. Air pollution and infant mortality in Mexico City. Epidemiology 1999, 10, 118–123. [Google Scholar] [CrossRef]
- Yang, Y.; Runkui, L.; Wenjing, L.; Wang, M.; Cao, Y.; Wu, Z.; Xu, Q. The Association between Ambient Air Pollution and Daily Mortality in Beijing after the 2008 Olympics: A Time Series Study. PLoS ONE 2013, 8, e76759. [Google Scholar] [CrossRef] [PubMed]
- Torrico-Lavayen, R.; Vargas-Alarcón, G.; Riojas-Rodriguez, H.; Sánchez Guerra, M.A.; Texcalac-Sangrador, J.L.; Ortiz-Panozo, E.; Gutiérrez-Avila, I.; De Vizcaya-Ruiz, A.; Cardenas, A.; Posadas-Sánchez, R.; et al. Long-term exposure to ambient fine particulate matter and carotid intima media thickness at bilateral, left and right in adults from Mexico City: Results from GEA study. Chemosphere 2023, 335, 139009. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Martinez, R.; Perez-Padilla, R.; Olaiz-Fernandez, G.; Mendoza-Alvarado, L.; Moreno-Macias, H.; Fortoul, T.; Mc Donnell, W.; Loomis, D.; Romieu, I. Lung function growth in children with long-term exposure to air pollutants in Mexico City. Am. J. Respir. Crit. Care Med. 2007, 176, 377–384. [Google Scholar] [CrossRef]
- Escamilla-Nunez, M.C.; Barraza-Villareal, A.; Hernandez-Cadena, L.; Moreno-Macias, H.; Ramirez-Aguilar, M.; Sienra-Monje, J.J.; Cortez-Luga, M.; Texcalac, J.L.; del Rio-Navarro, B.; Romieu, I. Traffic-related air pollution and respiratory symptoms among asthmatic children, resident in Mexico City: The EVA cohort study. Respir. Res. 2008, 9, 74. [Google Scholar] [CrossRef]
- Barraza-Villarreal, A.; Sunyer, J.; Hernandez-Cadena, L.; Escamilla Nunez, M.C.; Sienra-Monje, J.J.; Ramirez-Aguilar, M.; Cortez-Lugo, M.; Holguin, F.; Diaz-Sanchez, D.; Olin, A.C.; et al. Air pollution, airway inflammation, and lung function in a cohort Study of Mexico City Schoolchildren. Environ. Health Perspect. 2008, 116, 832–838. [Google Scholar] [CrossRef]
- Barraza-Villarreal, A.; Escamilla-Nunez, M.C.; Hernandez-Cadena, L.; Texcalac-Sangrador, J.L.; Sienra-Monje, J.J.; del Rio-Navarro, B.E.; Cortez-Lugo, M.; Sly, P.D.; Romieu, I. Elemental carbon exposure and lung function in schoolchildren from Mexico City. Eur. Respir. J. 2011, 38, 548–552. [Google Scholar] [CrossRef]
- Linares, B.; Guizar, J.M.; Amador, N.; Garcia, A.; Miranda, V.; Perez, J.R.; Chapela, R. Impact of air pollution on pulmonary function and respiratory symptoms in children. Longitudinal repeated-measures study. BMC Pulm. Med. 2010, 10, 62. [Google Scholar] [CrossRef]
- Li, P.; Xin, J.; Wang, Y.; Li, G.; Pan, X.; Wang, S.; Cheng, M.; Wen, T.; Wang, G.; Liu, Z. Association between particulate matter and its chemical constituents of urban air pollution and daily mortality or morbidity in Beijing City. Environ. Sci. Pollut. Res. 2014, 22, 358–368. [Google Scholar] [CrossRef]
- Pedersen, M.; Mendez, M.A.; Schoket, B.; Godschalk, R.W.; Espinosa, A.; Landström, A.; Villanueva, C.M.; Merlo, D.F.; Fthenou, E.; Gracia-Lavedan, E.; et al. Environmental, dietary, maternal, and fetal predictors of bulky DNA adducts in cord blood: A European mother–child study (NewGeneris). Environ. Health Perspect. 2015, 123, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Valverde, M.; Rojas, E. Chapter 11: Comet Assay in Human Biomonitoring. In Issues in Toxicology; Dhawan, A., Anderson, D., Eds.; RCS Publishing: Cambridge, UK, 2017; pp. 264–313. [Google Scholar] [CrossRef]
- Singh, N.P.; McCoy, M.T.; Tice, R.R.; Schneider, E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 1988, 175, 184–191. [Google Scholar] [CrossRef]
- Collins, A.; Møller, P.; Gajski, G.; Vodenková, S.; Abdulwahed, A.; Anderson, D.; Bankoglu, E.E.; Bonassi, S.; Boutet-Robinet, E.; Brunborg, G.; et al. Measuring DNA modifications with the comet assay: A compendium of protocols. Nat. Protoc. 2023, 18, 929–989. [Google Scholar] [CrossRef]
- Calderon-Garciduenas, L.; Osnaya-Brizuela, N.; Ramirez-Martinez, L.; Villarreal-Calderon, A. DNA Strand Breaks in Human Nasal Respiratory Epithelium Are Induced upon Exposure to Urban Pollution. Environ. Health Perspect. 1996, 104, 160. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Calderón-Garcidueñas, L.; Osnaya, N.; Rodríguez-Alcaraz, A.; Villarreal-Calderón, A. DNA damage in nasal respiratory epithelium from children exposed to urban pollution. Environ. Mol. Mutagen. 1997, 30, 11–20. [Google Scholar] [CrossRef]
- Tovalin, H.; Valverde, M.; Morandi, M.T.; Blanco, S.; Whitehead, L.; Rojas, E. DNA damage in outdoor workers occupationally exposed to environmental air pollutants. Occup. Environ. Med. 2006, 63, 230–236. [Google Scholar] [CrossRef]
- Valverde, M.; López, M.C.; López, I.; Sánchez, I.; Fortoul, T.I.; Ostrosky-Wegman, P.; Rojas, E. DNA damage in leukocytes and buccal and nasal epithelial cells of individuals exposed to air pollution in Mexico City. Environ. Mol. Mutagen. 1997, 30, 147–152. [Google Scholar] [CrossRef]
- Rojas, E.; Valverde, M.; López, M.C.; Naufal, I.; Bizarro, P.; López, I.; Fortoul, T.I.; Ostrosky-Wegman, P. Evaluation of DNA damage in exfoliated tear duct epithelial cells from individuals exposed to air pollution assessed by single cell gel electrophoresis assay. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2000, 468, 11–17. [Google Scholar] [CrossRef]
- Tice, R.R.; Agurell, E.; Burlinson, B.; Hartmann, A.; Kobayashi, H.; Miyame, Y.; Rojas, E.; Ryu, J.C.; Sasaki, Y.F. Single cell gel/comet assay: Guidelines for in vitro and in vivo genetic toxicology testing. Environ. Mol. Mutagen. 2000, 35, 206–221. [Google Scholar] [CrossRef]
- Scalera, F.; Fischer, T.; Schlembach, D.; Beinder, E. Serum from healthy pregnant women reduces oxidative stress in human umbilical vein endothelial cells. Clin. Sci. 2002, 103, 53–57. [Google Scholar] [CrossRef]
- Baydas, G.; Karatas, F.; Gursu, M.F.; Bozkurt, H.A.; Ilhan, N.; Yasar, A.; Canatan, H. Antioxidant vitamin levels in term and preterm infants and their relation to maternal vitamin status. Arch. Med. Res. 2002, 33, 276–280. [Google Scholar] [CrossRef]
- Neri, M.; Ugolini, D.; Bonassi, S.; Ficic, A.; Holland, N.; Knudsen, L.E.; Sram, R.J.; Ceppi, M.; Bocchini, V.; Merlo, D.F. Children’s exposure to environmental pollutants and biomarkers of genetic damage: II. Results of a comprehensive literature search and meta-analysis. Mutat. Res. Rev. Mutat. Res. 2006, 612, 14–39. [Google Scholar] [CrossRef]
- Šrám, R.J.; Podrazilová, K.; Dejmek, J.; Mračková, G.; Pilčík, T. Single cell gel electrophoresis assay: Sensitivity of peripheral white blood cells in human population studies. Mutagenesis 1998, 13, 99–103. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Whyatt, R.M.; Perera, F.P. Application of biologic markers to studies of environmental risks in children and the developing fetus. Environ. Health Perspect. 1995, 103 (Suppl. S6), 105–110. [Google Scholar] [CrossRef]
- Dussias, V.; Stefos, T.; Stefanidis, K.; Paraskevaidis, E.; Karabini, F.; Lolis, D. Lead concentrations in maternal and umbilical cord blood in areas with high and low air pollution. Clin. Exp. Obstet. Gynecol. 1997, 24, 187–189. Available online: http://www.ncbi.nlm.nih.gov/pubmed/9478314 (accessed on 17 January 2020). [PubMed]
- Whyatt, R.M.; Santella, R.M.; Jadrychowsky, W.; Garte, S.J.; Bell, D.A.; Ottman, R.; Gladek-Yarborough, A.; Cosma, G.; Young, T.L.; Cooper, T.B.; et al. Relationship between ambient air pollution and DNA damage in Polish mothers and newborns. Environ. Health Perspect. 1998, 106 (Suppl. S3), 821–826. [Google Scholar] [CrossRef]
- Binkova, B.; Veselyý, D.; Veselá, D.; Jelínek, R.; Sram, R.J. Genotoxicity and embryotoxicity of urban air particulate matter collected during winter and summer period in two different districts of the Czech Republic. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 1999, 440, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Dejmek, J.; Selevan, S.G.; Benes, I.; Solansky, I.; Sram, R.J. Fetal growth and maternal exposure to particulate matter during pregnancy. Environ. Health Perspect. 1999, 107, 475–480. [Google Scholar] [CrossRef]
- Mattison, D.R. Environmental exposures and development. Curr. Opin. Pediatr. 2010, 22, 208–218. [Google Scholar] [CrossRef]
- Furnees, D.L.; Dekker, G.A.; Roberts, C.T. DNA damage and health in pregnancy. J. Reprod. Immunol. 2011, 89, 153–162. [Google Scholar] [CrossRef]
- Sardas, S.; Karahatil, B.; Akyol, D.; Kukner, S.; Karakaya, A.E. Assessment of smoking-induced DNA damage in lymphocytes of smoking mothers of newborn infants using the alkaline single-cell gel electrophoresis technique. Mutat. Res. Mutagen. Relat. Subj. 1995, 335, 213–217. [Google Scholar] [CrossRef]
- Sardas, S.; Walker, D.; Akyol, D.; Karakaya, A.E. The effect of smoking on sister chromatid exchange rate of newborn infants born to smoking mothers. Mutat. Res. Toxicol. 1995, 341, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Betancourt, M.; Ortiz, R.; Gonzalez, C.; Perez, P.; Cortes, L.; Rodriguez, L.; Villasenor, L. Assessment of DNA damage in leukocytes from infected and malnourished children by single cell gel electrophoresis/comet assay. Mutat. Res. Fundam. Mol. Mech. Mutagen. 1995, 331, 65–77. [Google Scholar] [CrossRef]
- Perera, F.P.; Whyat, R.M.; Rauh, V.; Whyatt, R.M. 1 Recent developments in molecular, epidemiology: A study of the effects of environmental polycyclic aromatic hydrocarbons on birth outcomes in Poland. Am. J. Epidemiol. 1998, 147, 309–314. [Google Scholar] [CrossRef]
- Perera, F.P.; Whyatt, R.M.; Jedrychowski, W.; Rauh, V.; Manchester, D.; Santella, R.M.; Ottman, R. Molecular epidemiologic research on the effects of environmental pollutants on the fetus. Environ. Health Perspect. 1999, 107 (Suppl. S3), 451–460. [Google Scholar] [CrossRef]
- de Assis, K.R.C.; Ladeira, M.S.P.; Bueno, R.C.A.; dos Santos, B.F.; Dalben, I.; Salvadori, D.M.F. Genotoxicity of cigarette smoking in maternal and newborn lymphocytes. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2009, 679, 72–78. [Google Scholar] [CrossRef]
- Chelchowska, M.; Ambroszkiewicz, J.; Gajewska, J.; Laskowska-Klita, T.; Leibschang, J. The effect of tobacco smoking during pregnancy on plasma oxidant and antioxidant status in mother and newborn. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 155, 132–136. [Google Scholar] [CrossRef]
- Xu, X.; Sharma, R.K.; Talbott, E.O.; Zborowski, J.V.; Rager, J.; Arena, V.C.; Volz, C.D. PM10 air pollution exposure during pregnancy and term low birth weight in Allegheny County, PA, 1994–2000. Int. Arch. Occup. Environ. Health 2011, 84, 251–257. [Google Scholar] [CrossRef]
- Anderson, T.; Alfredsson, L.; Kälberg, H.; Zdravkovic, S.; Ahlbom, A. Calculating measures of biological interaction. Eur. J. Epidemiol. 2005, 20, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Z.; Qian, Z.M.; Guo, Y.; Zhou, J.; Yang, Y.; Acharya, B.K.; Guo, S.; Zheng, Y.; Cummings-Vaughn, L.A.; Rigdon, S.E.; et al. Ambient fine particulate matter and ozone higher that certain thresholds associated with myopia in the elderly aged 50 years and above. Environ. Res. 2019, 177, 108581. [Google Scholar] [CrossRef]
- Borja-Aburto, V.H.; Castillejos, M.; Gold, D.R.; Bierzwinski, S.; Loomis, D. Mortality and ambient fine particles in southwest Mexico City, 1993–1995. Environ. Health Perspect. 1998, 106, 849–855. [Google Scholar] [CrossRef]
- Rojas, E.; López, M.C.; Valverde, M. Single cell gel electrophoresis assay: Methodology and applications. J. Chromatogr. B Biomed. Sci. Appl. 1999, 722, 225–254. [Google Scholar] [CrossRef]
- Hernandez-Haro, N.; Solis-Calero, C.; Casasnovas, R.; Morell, C.; Grand, A.; Frau, J.; Ortega-Castro, J. Formation mechanism of Inter-Crosslink in DNA by nitrogen oxides pollutants through a diazonium intermediate. Int. J. Mol. Sci. 2022, 23, 10621. [Google Scholar] [CrossRef]
- Vande Loock, K.; Decordier, I.; Plas, G.; Ciardelli, R.; Haumont, D.; Kirsch-Volders, M. Lower nucleotide excision repair capacity in newborns compared to their mothers: A pilot study. Reprod. Toxicol. 2014, 43, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Mesbah-Namin, S.A.; Shahidi, M.; Nakhshab, M. An increased genotoxic risk in lymphocytes from phototherapy-treated hyperbilirubinemic neonates. Iran. Biomed. J. 2017, 21, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Nazaroff, W.W.; Weschler, C.J. Indoor ozone: Concentrations and influencing factors. Indoor Air 2022, 32, e12942. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhao, B. Review of relationship between indoor and outdoor particles: I/O ratio, infiltration factor and penetration factor. Atmos. Environ. 2011, 45, 275–288. [Google Scholar] [CrossRef]
- Wang, P.; Liu, J.; Wang, C.; Zhang, Z.; Li, J. A holistic performance assessment of duct-type electrostatic precipitators. J. Clean. Prod. 2022, 357, 131997. [Google Scholar] [CrossRef]
- Li, J.; Zuraimi, S.; Schiavon, S.; Wan, M.P.; Xiong, J.; Tham, K.W. Diurnal trends of indoor and outdoor fluorescent biological aerosol particles in a tropical urban area. Sci. Total Environ. 2022, 848, 157811. [Google Scholar] [CrossRef]
- Wang, P.; Liu, S.; Liu, J.; Wang, J.; Li, J. Size-resolved splashed cooking oil droplets from 1 to 1000 μm on surfaces: The impact of residential range hoods. Build. Environ. 2022, 210, 108705. [Google Scholar] [CrossRef]
- Bonassi, S.; Ceppi, M.; Møller, P.; Azqueta, A.; Milić, M.; Neri, M.; Brunborg, G.; Godschalk, R.; Koppen, G.; Langie, S.A.S.; et al. DNA damage in circulating leukocytes measured with the comet assay may predict the risk of death. Sci. Rep. 2021, 11, 16793. [Google Scholar] [CrossRef] [PubMed]
- Melody, S.M.; Ford, J.; Wills, K.; Venn, A.; Johnston, F.H. Maternal exposure to short to medium-term outdoor air pollution and obstetric and neonatal outcomes: A systematic review. Environ. Pollut. 2019, 244, 915–925. [Google Scholar] [CrossRef] [PubMed]
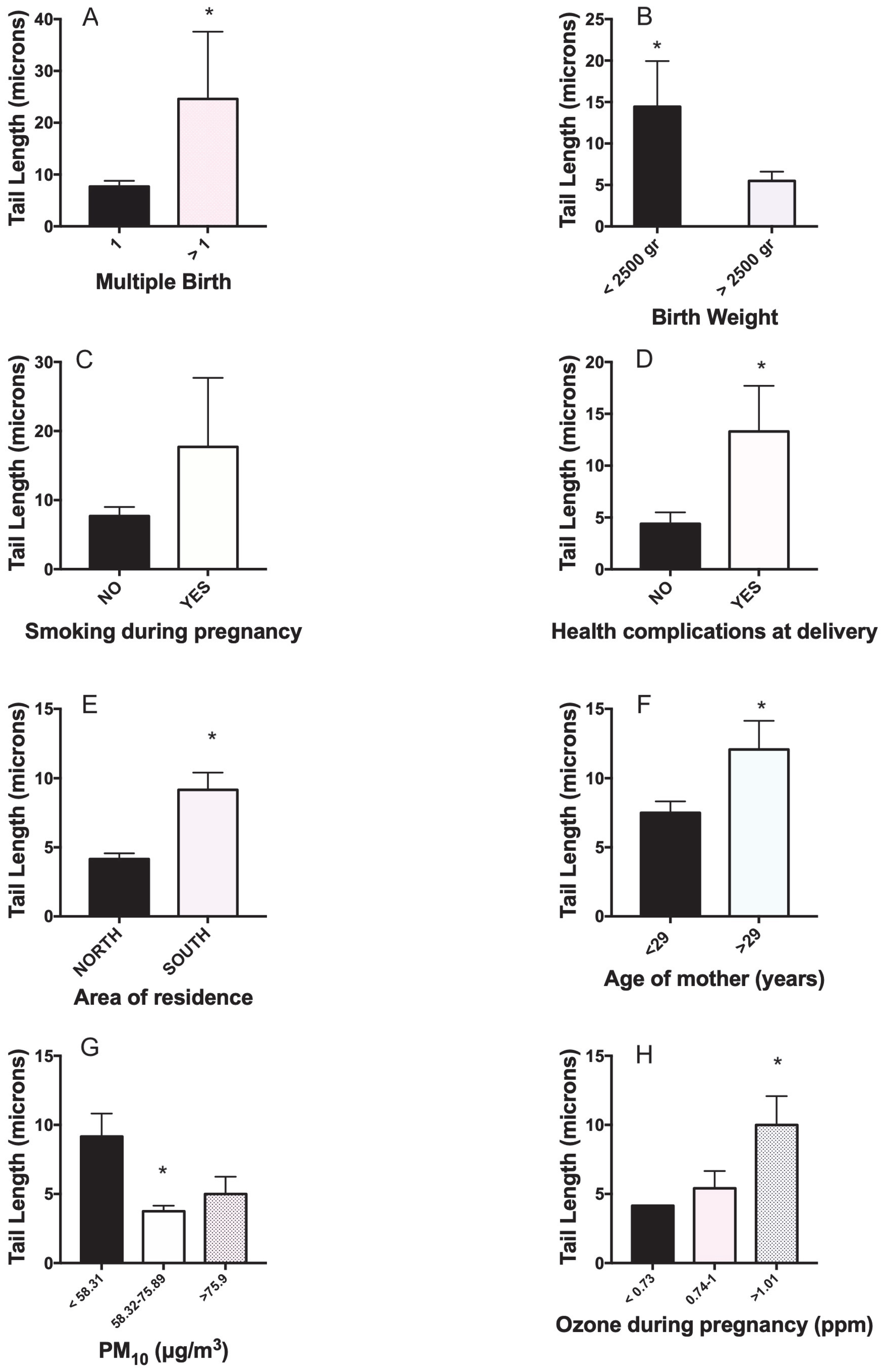
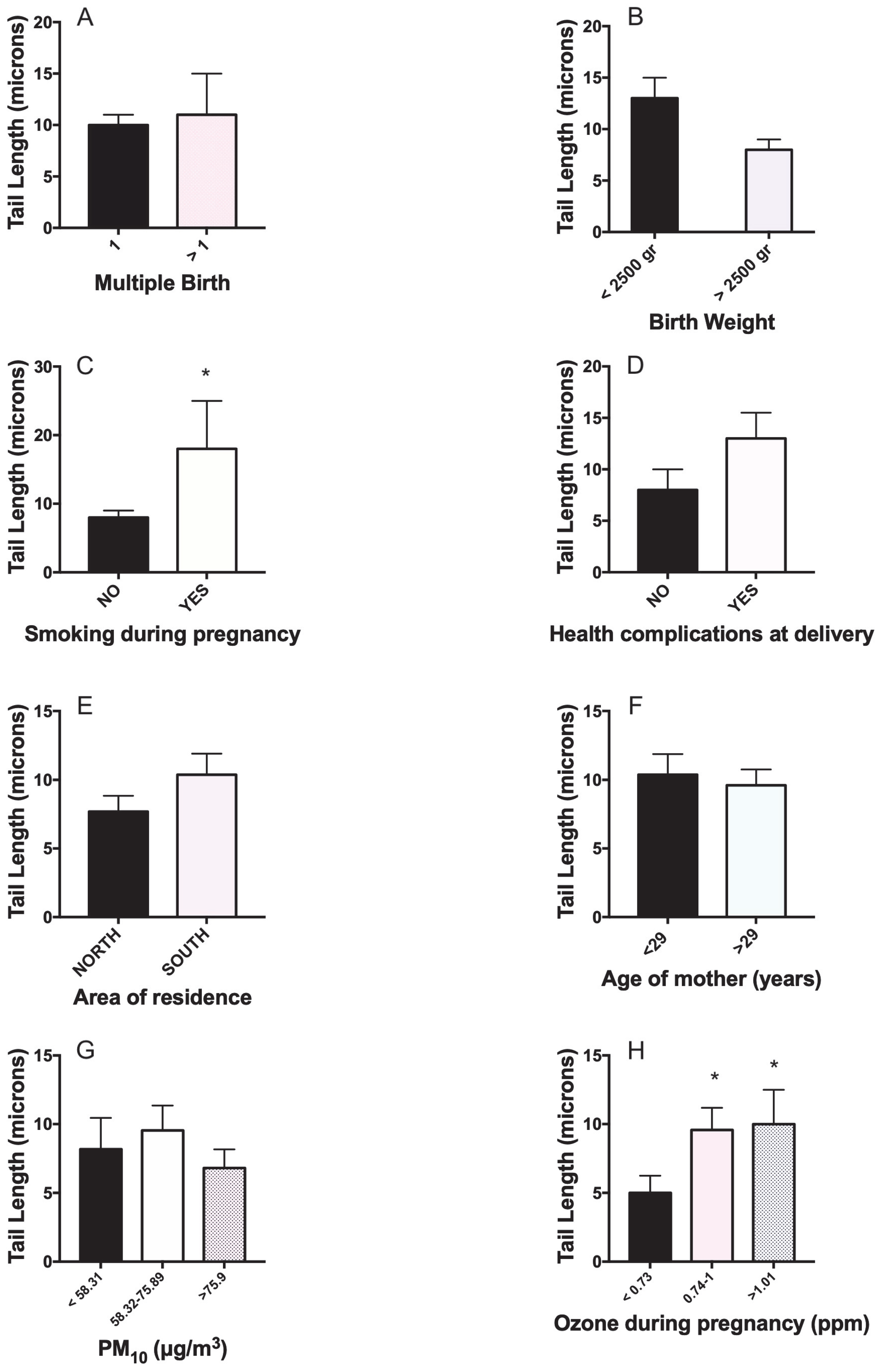

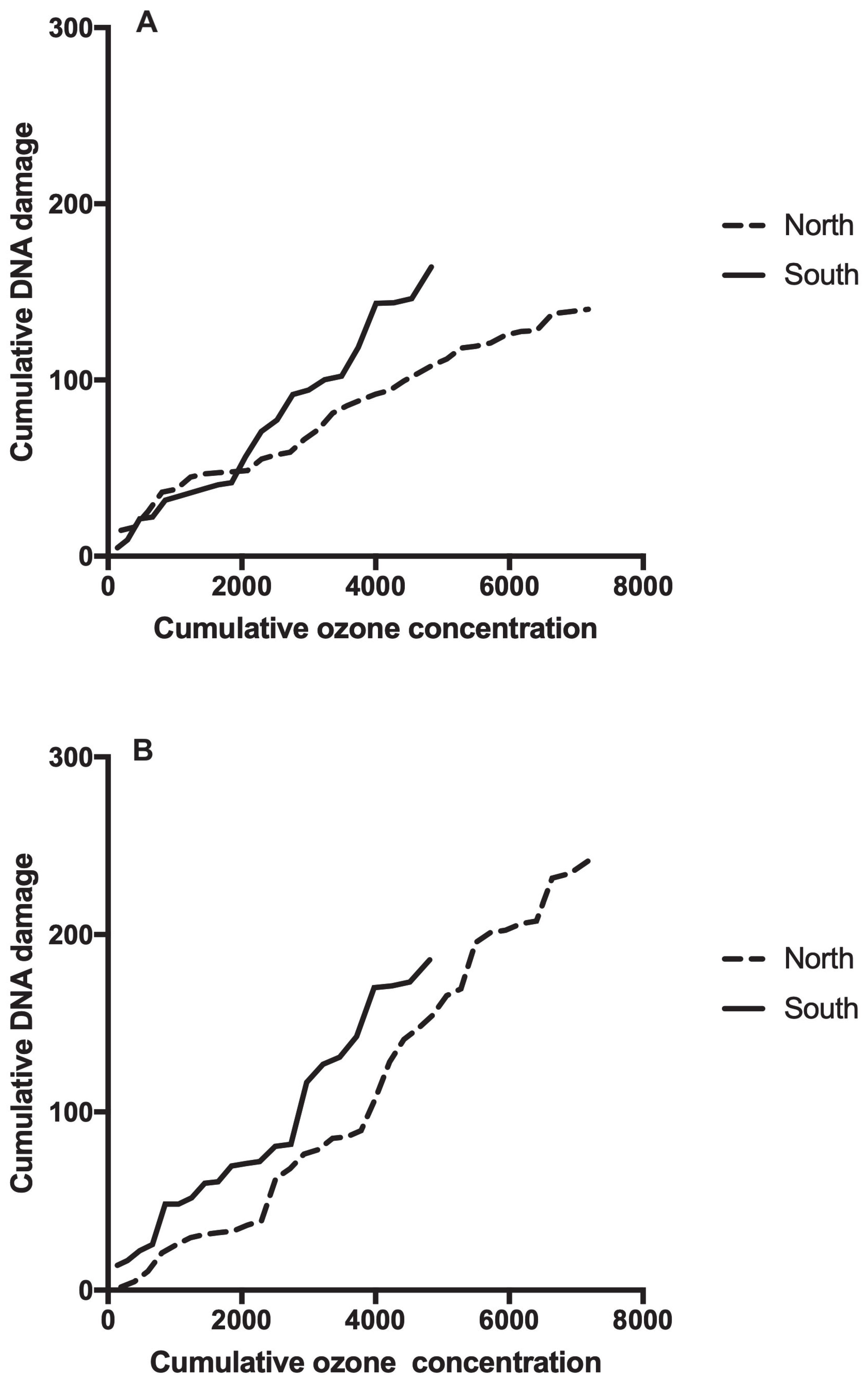
| Mother/Newborn/Delivery Characteristics | N | Mean ± SD or % | Min-Max | |
|---|---|---|---|---|
| Maternal age (years) | 79 | 28.5 ± 6.3 | 14–43 | |
| Multiple births | 1 | 94 | 90.4% | 1–4 |
| >1 | 10 | 9.6% | ||
| Number of pregnancies | 1 | 14 | 30.4% | 1–7 |
| >1 | 32 | 69.6% | ||
| Number of abortions | No | 33 | 68.8% | 0–4 |
| Yes | 15 | 31.3% | ||
| Infections during pregnancy | No | 71 | 73.2% | |
| Yes | 26 | 26.8% | ||
| Use of medications during pregnancy | No | 70 | 72.2% | |
| Yes | 27 | 27.8% | ||
| Smoking during pregnancy | No | 39 | 86.7% | 0–20 (Cigarettes/Day) |
| Yes | 6 | 13.3% | ||
| Weeks of gestational age | ≥37 | 47 | 54.0% | 27–42 (Weeks) |
| <37 | 40 | 46.0% | ||
| Birth weight (%) | ≥2500 g | 58 | 76.7% | 1114–4500 (g) |
| <2500 g | 24 | 29.3% | ||
| Apgar score at 1 min | 78 | 7.99 ± 1.15 | 1–9 | |
| Apgar score at 5 min | 80 | 8.81 ± 0.53 | 6–9 | |
| Area of residence (2 levels) | North | 42 | 58.9% | |
| South | 31 | 41.1% | ||
| Area of residence (4 levels) | North-West | 7 | 9.7% | |
| North-East | 35 | 47.9% | ||
| South-West | 19 | 26.0% | ||
| South-East | 12 | 16.4% |
| Exposure to Air Pollution | N | Mean ± SD | Min-Max |
|---|---|---|---|
| NOx (ppm) | 70 | 1.32 ± 0.35 | 0.425–2.72 |
| PM10 (μg/m3) | 52 | 69.25 ± 10.48 | 40.75–93.46 |
| Ozone (ppm) | 52 | 0.81 ± 0.10 | 0.51–1.90 |
| Ozone 1st trimester (ppm) | 70 | 0.89 ± 0.18 | 0.315–1.26 |
| Ozone at delivery (ppm) | 70 | 0.85 ±0.34 | 0.33–1.87 |
| Variables | β ± SD | MR [95% CI] | p-Value | |
|---|---|---|---|---|
| Area of Residence | NorthSouth | - | 1.00 | <0.05 |
| 0.66 ± 0.31 | 1.94; [1.03–3.63] | |||
| NOx (ppm) | ≤1.19 | - | 1.00 | |
| 1.20–1.96 | −0.77 ± 0.36 | 0.46; [0.24–0.94] | <0.05 | |
| ≥1.97 | 0.72 ± 0.41 | 0.38; [0.21–1.12] | n.s. | |
| PM10 (μg/m3) | ≤58.31 | - | 1.00 | |
| 58.32–75.89 | −0.75 ± 0.37 | 0.47; [0.23–0.99] | ≤0.05 | |
| ≥75.90 | −0.50 ± 0.37 | 0.61; [0.19–0.78] | ≤0.05 | |
| Ozone at delivery (ppm) | ≤0.73 | - | 1.00 | |
| 0.74–1.00 | 0.06 ± 0.32 | 1.06; [0.56–2.01] | n.s. | |
| ≥1.01 | 1.26 ± 0.41 | 3.51; [1.53–8.03] | <0.01 | |
| Maternal age | <29 | - | 1.00 | <0.05 |
| ≥29 | 0.88 ± 0.41 | 2.41; [1.04–5.60] | ||
| Difficult delivery | No | - | 1.00 | <0.05 |
| Yes | 0.80 ± 0.33 | 2.23; [1.16–4.31] |
| Variables | β ± SD | MR [95% CI] | p-Value | |
|---|---|---|---|---|
| Difficult delivery | No Yes | - | 1.00 | <0.05 |
| 0.58 ± 0.28 | 1.78; [1.02–3.11] | |||
| Birth weight | ≥2500 g | - | 1.00 | |
| <2500 g | 0.57 ± 0.28 | 1.76; [1.00–3.09] | <0.05 | |
| Smoking during pregnancy | No | - | 1.00 | <0.05 |
| Yes | 0.99 ± 0.46 | 2.72; [1.06–6.93] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valverde, M.; Granados, A.; Milić, M.; Ceppi, M.; Sollano, L.; Bonassi, S.; Rojas, E. Effect of Air Pollution on the Basal DNA Damage of Mother–Newborn Couples of México City. Toxics 2023, 11, 766. https://doi.org/10.3390/toxics11090766
Valverde M, Granados A, Milić M, Ceppi M, Sollano L, Bonassi S, Rojas E. Effect of Air Pollution on the Basal DNA Damage of Mother–Newborn Couples of México City. Toxics. 2023; 11(9):766. https://doi.org/10.3390/toxics11090766
Chicago/Turabian StyleValverde, Mahara, Adriana Granados, Mirta Milić, Marcello Ceppi, Leticia Sollano, Stefano Bonassi, and Emilio Rojas. 2023. "Effect of Air Pollution on the Basal DNA Damage of Mother–Newborn Couples of México City" Toxics 11, no. 9: 766. https://doi.org/10.3390/toxics11090766
APA StyleValverde, M., Granados, A., Milić, M., Ceppi, M., Sollano, L., Bonassi, S., & Rojas, E. (2023). Effect of Air Pollution on the Basal DNA Damage of Mother–Newborn Couples of México City. Toxics, 11(9), 766. https://doi.org/10.3390/toxics11090766








