Preliminary Study on the Effect and Molecular Mechanism of Tetrandrine in Alleviating Pulmonary Inflammation and Fibrosis Induced by Silicon Dioxide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
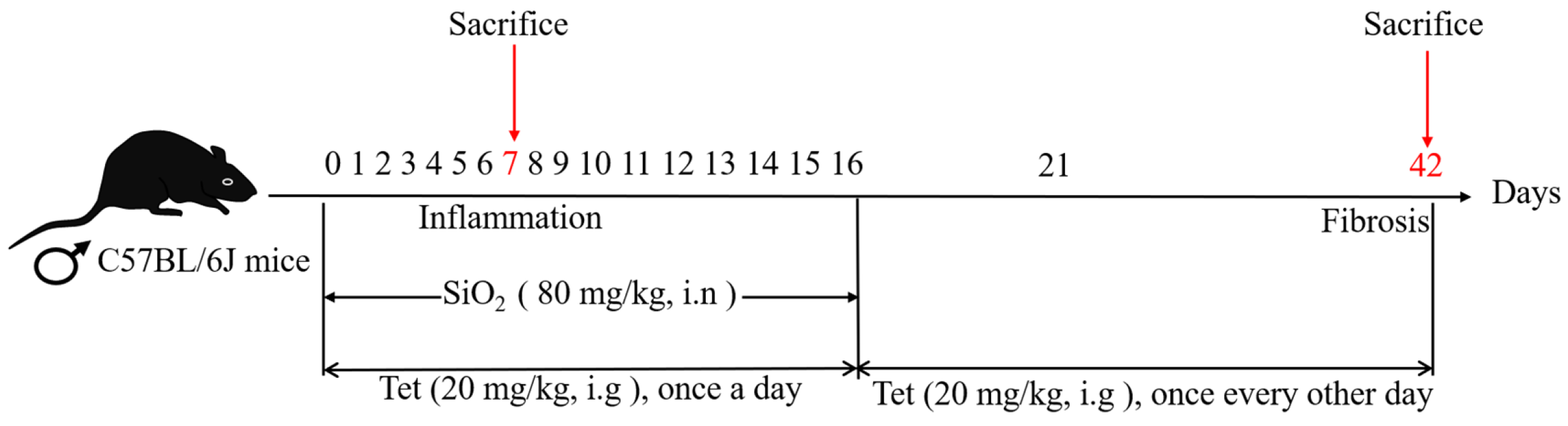
2.2. Experimental Animals and Groups
2.3. Organ Coefficient of Lung Tissues
2.4. Pathological Analysis of Lung Tissues
2.5. RT-qRCR
2.6. Determination of Hydroxyproline (HYP) Content
2.7. Immunohistochemistry
2.8. Western Blot (WB)
2.9. Immunofluorescence
2.10. Transmission Electron Microscopy (TEM)
2.11. Statistical Analysis
3. Results
3.1. Tet Could Counteract the Adverse Effects of SiO2 Exposure on Body Weight and Lung Organ Coefficient of Mice to a Certain Extent
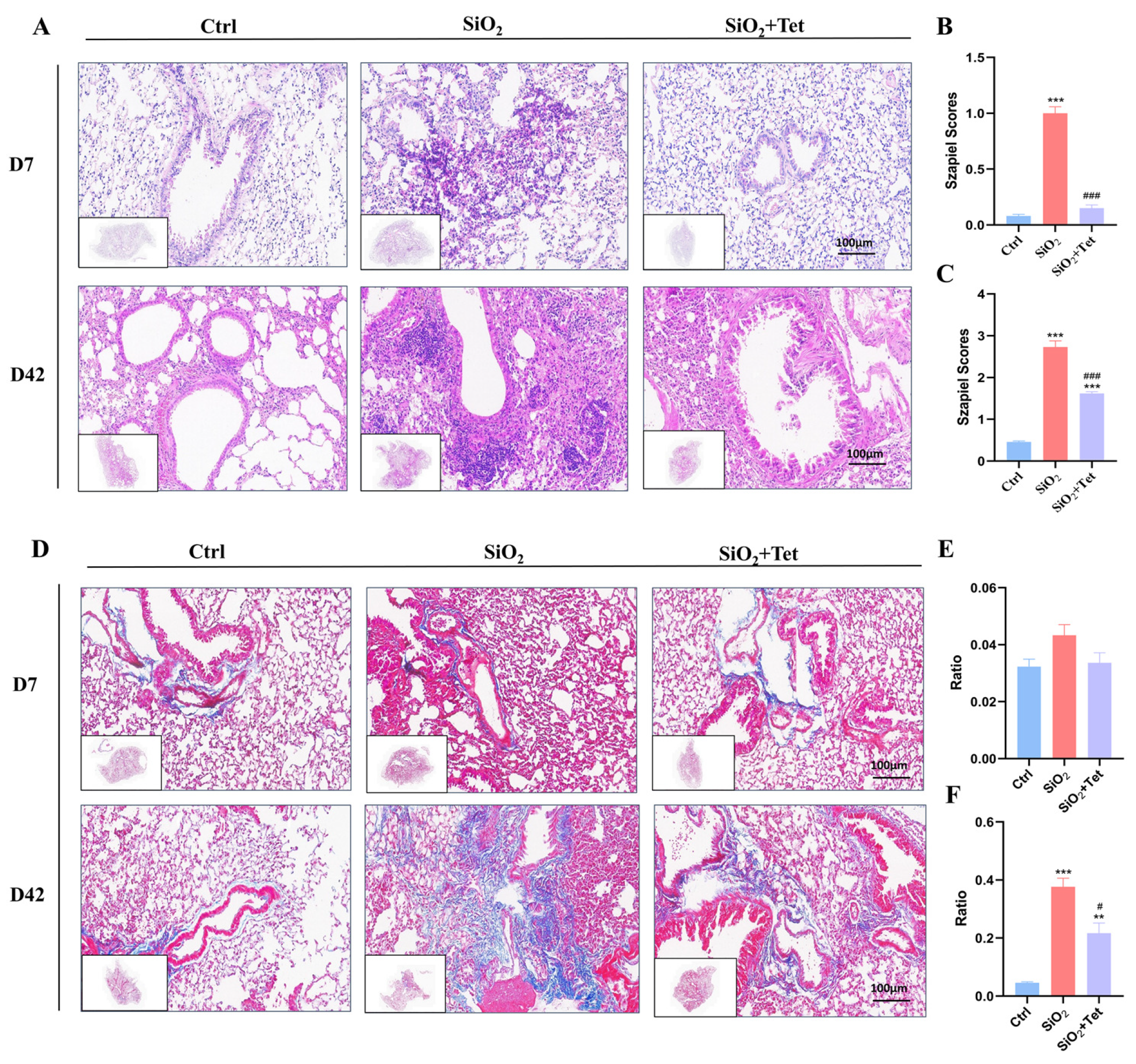
3.2. Tet Could Effectively Alleviate Pulmonary Inflammation and Fibrosis Caused by SiO2 in Mice
3.2.1. Hematoxylin-Eosin (HE) Staining
3.2.2. Masson Staining
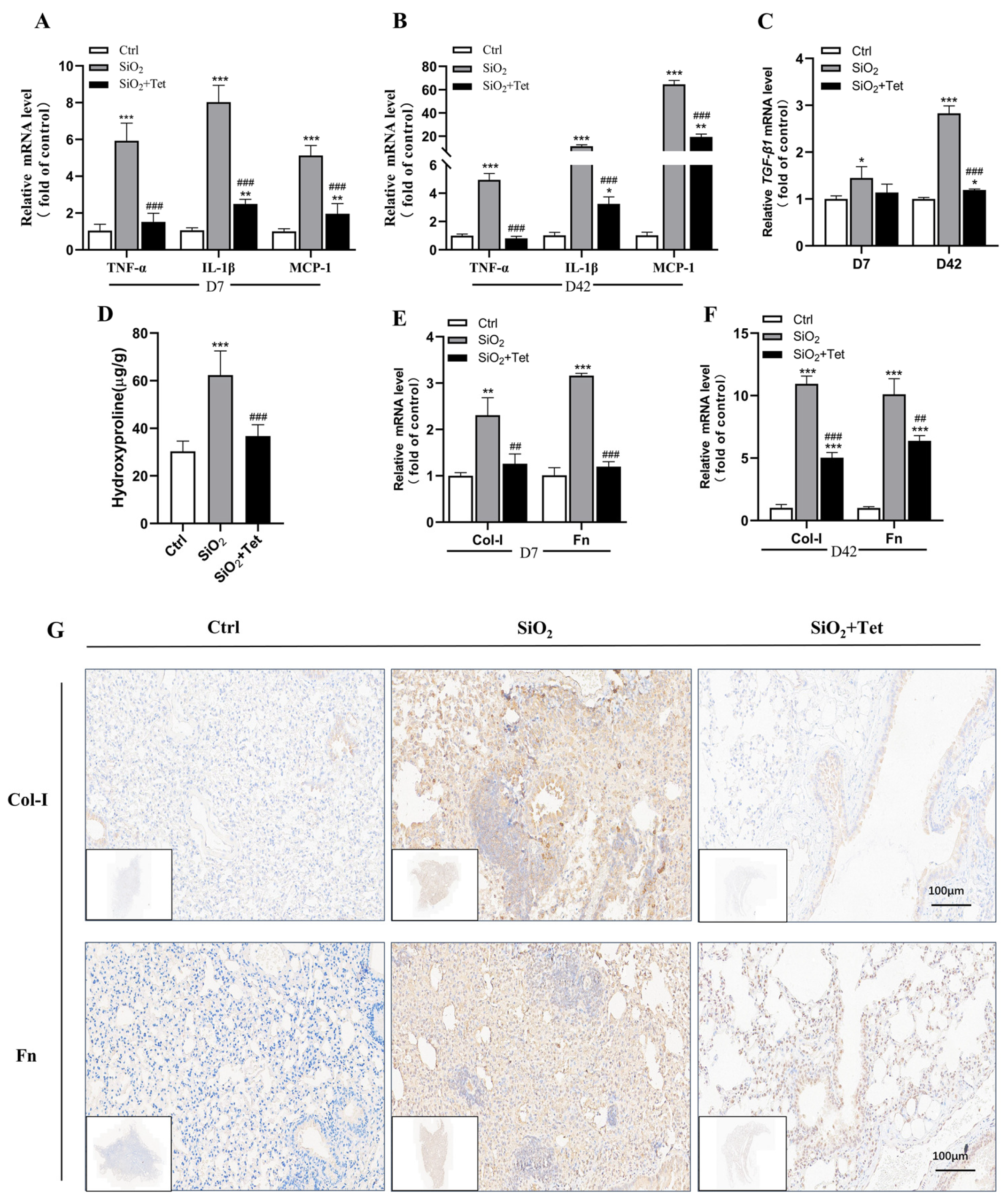
3.3. Tet Could Relieve SiO2-Induced Inflammation by Inhibiting Inflammatory Cytokines TNF-α, IL-1β, MCP-1, and TGF-β1 mRNA in the Lung Tissues of Mice
3.4. Tet Could Alleviate SiO2-Induced Fibrosis by Inhibiting the Expression of Markers of Positive Regulation of Fibrosis—HYP, Col-I, and Fn in Lung Tissue
3.4.1. Effect of Tet Intervention on the HYP Concentration in the Lung Tissues of Mice after 42 Days of SiO2 Exposure
3.4.2. Effect of Tet Intervention on Col-I and Fn in the Lung Tissues of Mice Exposed to SiO2
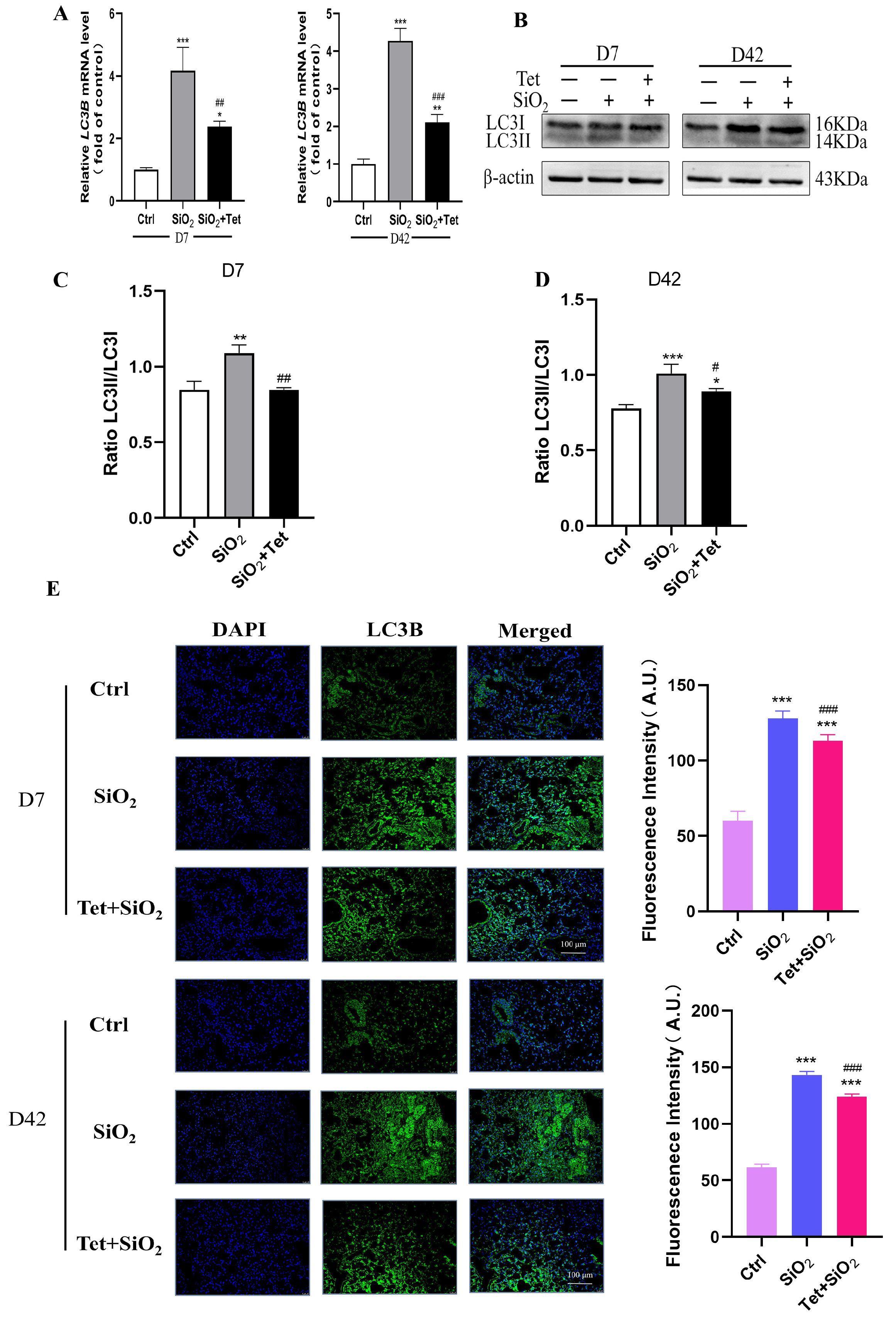
3.5. Tet Could Regulate the Expression of Key Molecules ATG7, LC3B, and P62 in the Autophagy Pathway Induced by Silica in Mouse Lung Tissue
3.5.1. Tet Intervention Increases ATG7 Expression in the Lung Tissues of Mice Exposed to SiO2
3.5.2. Tet Intervention Downregulated the Expression of LC3B in the Lung Tissues of Mice Exposed to SiO2
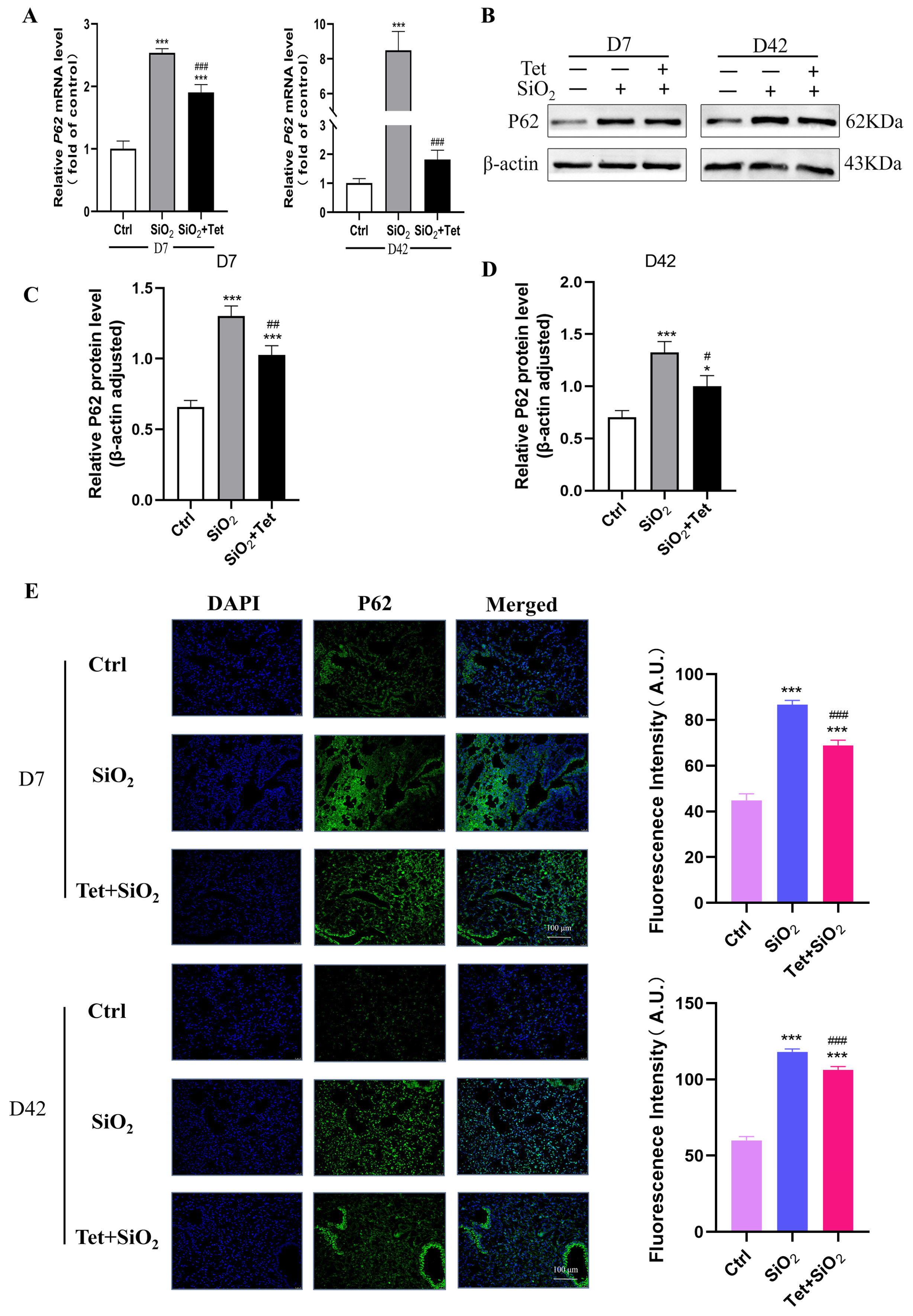
3.5.3. Tet Intervention Decreased the Expression of P62 in the Lung Tissues of Mice Exposed to SiO2
3.6. Tet Promotes the Recovery of Silica-Induced Autophagic Lysosomal System Function
3.6.1. Tet Could Affect the Expression of LAMP1 and CTSB Protein in the Lung Tissue of SiO2-Exposed Mice
3.6.2. Tet Could Affect the Formation of Autophagosomes and Lysosomes in the Lung Tissue of SiO2-Exposed Mice
3.7. Tet Improved SiO2-Induced Apoptosis by Regulating the Release of Apoptotic Factors Bax and Bcl2 in Lung Tissue
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leung, C.C.; Yu, I.T.; Chen, W. Silicosis. Lancet 2012, 379, 2008–2018. [Google Scholar] [CrossRef]
- Chen, S.; Liu, M.; Xie, F. Global and national burden and trends of mortality and disability-adjusted life years for silicosis, from 1990 to 2019: Results from the Global Burden of Disease study 2019. BMC Pulm. Med. 2022, 22, 240. [Google Scholar] [CrossRef]
- Hoy, R.; Chambers, D.C. Silicosis: An ancient disease in need of a dose of modern medicine. Respirology 2020, 25, 464–465. [Google Scholar] [CrossRef]
- Bart, V.M.T.; Pickering, R.J.; Taylor, P.R.; Ipseiz, N. Macrophage reprogramming for therapy. Immunology 2021, 163, 128–144. [Google Scholar] [CrossRef]
- Adamcakova, J.; Mokra, D. New Insights into Pathomechanisms and Treatment Possibilities for Lung Silicosis. Int. J. Mol. Sci. 2021, 22, 4162. [Google Scholar] [CrossRef]
- Nie, W.; Lan, T.; Yuan, X.; Luo, M.; Shen, G.; Yu, J.; Wei, X. Crystalline silica induces macrophage necrosis and causes subsequent acute pulmonary neutrophilic inflammation. Cell Biol. Toxicol. 2022, 38, 591–609. [Google Scholar] [CrossRef]
- Lv, J.; Xiao, J.; Jia, Q.; Meng, X.; Yang, Z.; Pu, S.; Li, M.; Yu, T.; Zhang, Y.; Wang, H.; et al. Identification of key pathways and genes in the progression of silicosis based on WGCNA. Inhal. Toxicol. 2022, 34, 304–318. [Google Scholar] [CrossRef]
- Niu, Y.; Yang, S.; Hu, X. Activation of canonical inflammasome complex by acute silica exposure in experimental rat model. Toxicol. Res. (Camb) 2022, 11, 162–168. [Google Scholar] [CrossRef]
- Yamamoto, H.; Zhang, S.; Mizushima, N. Autophagy genes in biology and disease. Nat. Rev. Genet. 2023, 24, 382–400. [Google Scholar] [CrossRef]
- Komatsu, M.; Waguri, S.; Ueno, T.; Iwata, J.; Murata, S.; Tanida, I.; Ezaki, J.; Mizushima, N.; Ohsumi, Y.; Uchiyama, Y.; et al. Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J. Cell Biol. 2005, 169, 425–434. [Google Scholar] [CrossRef]
- Wen, J.H.; Li, D.Y.; Liang, S.; Yang, C.; Tang, J.X.; Liu, H.F. Macrophage autophagy in macrophage polarization, chronic inflammation and organ fibrosis. Front. Immunol. 2022, 13, 946832. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Ma, J.; Xie, L.; Li, W.; Yang, M.; Gu, P.; Zhang, Y.; Fan, L.; Wang, D.; Chen, W. Inhibition of Gas6 promotes crystalline silica-induced inflammatory response of macrophages via blocking autophagy flux. Environ. Toxicol. 2022, 37, 1925–1933. [Google Scholar] [CrossRef]
- Li, N.; Shi, F.; Wang, X.; Yang, P.; Sun, K.; Zhang, L.; Hao, X.; Li, X.; Li, J.; Jin, Y. Silica dust exposure induces pulmonary fibrosis through autophagy signaling. Environ. Toxicol. 2021, 36, 1269–1277. [Google Scholar] [CrossRef]
- Tan, S.; Chen, S. The Mechanism and Effect of Autophagy, Apoptosis, and Pyroptosis on the Progression of Silicosis. Int. J. Mol. Sci. 2021, 22, 8110. [Google Scholar] [CrossRef]
- Li, S.X.; Li, C.; Pang, X.R.; Zhang, J.; Yu, G.C.; Yeo, A.J.; Lavin, M.F.; Shao, H.; Jia, Q.; Peng, C. Metformin Attenuates Silica-Induced Pulmonary Fibrosis by Activating Autophagy via the AMPK-mTOR Signaling Pathway. Front. Pharmacol. 2021, 12, 719589. [Google Scholar] [CrossRef]
- Tan, S.Y.; Zou, H.; Yang, C.; Chen, G.; Chen, S. The study of the impact by atractylenolide-1 on inflammatory cytokine, autophagy and apoptosis in alveolar macrophages of silicosis patients. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 2021, 39, 721–725. [Google Scholar] [CrossRef]
- Tan, S.; Chen, S. Macrophage Autophagy and Silicosis: Current Perspective and Latest Insights. Int. J. Mol. Sci. 2021, 22, 453. [Google Scholar] [CrossRef] [PubMed]
- Man, S.M.; Kanneganti, T.D. Regulation of lysosomal dynamics and autophagy by CTSB/cathepsin B. Autophagy 2016, 12, 2504–2505. [Google Scholar] [CrossRef]
- Eskelinen, E.L. Roles of LAMP-1 and LAMP-2 in lysosome biogenesis and autophagy. Mol. Aspects Med. 2006, 27, 495–502. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Chen, S.; Li, C.; Ban, J.; Wei, Y.; He, Y.; Liu, F.; Chen, Y.; Chen, J. Trehalose Alleviates Crystalline Silica-Induced Pulmonary Fibrosis via Activation of the TFEB-Mediated Autophagy-Lysosomal System in Alveolar Macrophages. Cells 2020, 9, 122. [Google Scholar] [CrossRef]
- Chen, S.; Yuan, J.; Yao, S.; Jin, Y.; Chen, G.; Tian, W.; Xi, J.; Xu, Z.; Weng, D.; Chen, J. Lipopolysaccharides may aggravate apoptosis through accumulation of autophagosomes in alveolar macrophages of human silicosis. Autophagy 2015, 11, 2346–2357. [Google Scholar] [CrossRef]
- Chan, E.W.C.; Wong, S.K.; Chan, H.T. An overview on the chemistry, pharmacology and anticancer properties of tetrandrine and fangchinoline (alkaloids) from Stephania tetrandra roots. J. Integr. Med. 2021, 19, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Luan, F.; He, X.; Zeng, N. Tetrandrine: A review of its anticancer potentials, clinical settings, pharmacokinetics and drug delivery systems. J. Pharm. Pharmacol. 2020, 72, 1491–1512. [Google Scholar] [CrossRef]
- Wang, S.; Fu, J.L.; Hao, H.F.; Jiao, Y.N.; Li, P.P.; Han, S.Y. Metabolic reprogramming by traditional Chinese medicine and its role in effective cancer therapy. Pharmacol. Res. 2021, 170, 105728. [Google Scholar] [CrossRef]
- Mir, R.H.; Shah, A.J.; Mohi-Ud-Din, R.; Pottoo, F.H.; Dar, M.A.; Jachak, S.M.; Masoodi, M.H. Natural Anti-inflammatory Compounds as Drug Candidates in Alzheimer’s Disease. Curr. Med. Chem. 2021, 28, 4799–4825. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Qi, J.; Li, H.; Xing, Z. Clinical efficacy of acetylcysteine combined with tetrandrine tablets on patients with silicosis and its effect on exercise tolerance and pulmonary function. Exp. Ther. Med. 2020, 20, 1285–1290. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Song, P.; Wang, Y.; Chen, Y. Clinical efficacy of acetylcysteine combined with tetrandrine tablets in the treatment of silicosis and the effect on serum IL-6 and TNF-α. Exp. Ther. Med. 2019, 18, 3383–3388. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Zhang, S.; Li, J.; Fang, H. Effects of tetrandrine combined with acetylcysteine on exercise tolerance, pulmonary function and serum TNF-β1 and MMP-7 in silicosis patients. Exp. Ther. Med. 2020, 19, 2195–2201. [Google Scholar] [CrossRef]
- Wu, W.H.; Feng, Y.H.; Min, C.Y.; Zhou, S.W.; Chen, Z.D.; Huang, L.M.; Yang, W.L.; Yang, G.H.; Li, J.; Shi, J.; et al. Clinical efficacy of tetrandrine in artificial stone-associated silicosis: A retrospective cohort study. Front. Med. 2023, 10, 1107967. [Google Scholar] [CrossRef]
- Bhagya, N.; Chandrashekar, K.R. Autophagy and cancer: Can tetrandrine be a potent anticancer drug in the near future? Biomed. Pharmacother. 2022, 148, 112727. [Google Scholar] [CrossRef]
- Wang, H.; Liu, T.; Li, L.; Wang, Q.; Yu, C.; Liu, X.; Li, W. Tetrandrine is a potent cell autophagy agonist via activated intracellular reactive oxygen species. Cell Biosci. 2015, 5, 4. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhong, W.; Zhang, J.; Chen, W.; Lu, Y.; Qiao, Y.; Zeng, Z.; Huang, H.; Cai, S.; Dong, H. Tetrandrine Modulates Rheb-mTOR Signaling-Mediated Selective Autophagy and Protects Pulmonary Fibrosis. Front. Pharmacol. 2021, 12, 739220. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Wang, R.; Wu, M.Y.; Shan, J.; Zhang, Y.C.; Xu, H.M. Study on the molecular mechanisms of tetrandrine against pulmonary fibrosis based on network pharmacology, molecular docking and experimental verification. Heliyon 2022, 8, e10201. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Singh, R. Protective effects of intranasal curcumin on silica-induced lung damage. Cytokine 2022, 157, 155949. [Google Scholar] [CrossRef]
- Li, B.; Mu, M.; Sun, Q.; Cao, H.; Liu, Q.; Liu, J.; Zhang, J.; Xu, K.; Hu, D.; Tao, X.; et al. A suitable silicosis mouse model was constructed by repeated inhalation of silica dust via nose. Toxicol. Lett. 2021, 353, 1–12. [Google Scholar] [CrossRef]
- Szapiel, S.V.; Elson, N.A.; Fulmer, J.D.; Hunninghake, G.W.; Crystal, R.G. Bleomycin-induced interstitial pulmonary disease in the nude, athymic mouse. Am. Rev. Respir. Dis. 1979, 120, 893–899. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Puyo, C.A.; Earhart, A.; Staten, N.; Prince, O.A.; Haug, C.; Kollef, M.; Awad, M. Endotracheal intubation results in acute tracheal damage induced by mtDNA/TLR9/NF-κB activity. J. Leukoc. Biol. 2019, 105, 577–587. [Google Scholar] [CrossRef]
- Huaux, F. New developments in the understanding of immunology in silicosis. Curr. Opin. Allergy Clin. Immunol. 2007, 7, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wei, S.; Chu, K.; Li, Q.; Zhou, Y.; Ma, Y.; Xue, L.; Tian, H.; Tao, S. Upregulation of autophagy in M2 macrophage by vitamin D alleviates crystalline silica-induced pulmonary inflammatory damage. Ecotoxicol. Environ. Saf. 2021, 225, 112730. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, Y.; Qiu, T.; Liu, W.; Yao, P. Autophagy, an important therapeutic target for pulmonary fibrosis diseases. Clin. Chim. Acta 2020, 502, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, Z.; Yu, C.; Zeng, C.; Xu, X.; Wu, G.; Huang, Z.; Li, W. Tetrandrine antagonizes acute megakaryoblastic leukaemia growth by forcing autophagy-mediated differentiation. Br. J. Pharmacol. 2017, 174, 4308–4328. [Google Scholar] [CrossRef] [PubMed]
- Li, L.C.; Kan, L.D. Traditional Chinese medicine for pulmonary fibrosis therapy: Progress and future prospects. J. Ethnopharmacol. 2017, 198, 45–63. [Google Scholar] [CrossRef]
- Komatsu, M.; Wang, Q.J.; Holstein, G.R.; Friedrich, V.L., Jr.; Iwata, J.; Kominami, E.; Chait, B.T.; Tanaka, K.; Yue, Z. Essential role for autophagy protein Atg7 in the maintenance of axonal homeostasis and the prevention of axonal degeneration. Proc. Natl. Acad. Sci. USA 2007, 104, 14489–14494. [Google Scholar] [CrossRef]
- Du, S.; Li, C.; Lu, Y.; Lei, X.; Zhang, Y.; Li, S.; Liu, F.; Chen, Y.; Weng, D.; Chen, J. Dioscin Alleviates Crystalline Silica-Induced Pulmonary Inflammation and Fibrosis through Promoting Alveolar Macrophage Autophagy. Theranostics 2019, 9, 1878–1892. [Google Scholar] [CrossRef]
- Bjørkøy, G.; Lamark, T.; Pankiv, S.; Øvervatn, A.; Brech, A.; Johansen, T. Monitoring autophagic degradation of p62/SQSTM1. Methods Enzymol. 2009, 452, 181–197. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Yoshimori, T.; Levine, B. Methods in mammalian autophagy research. Cell 2010, 140, 313–326. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Cheng, B.; Lin, Y.-J.; Wang, R.; Xuan, J.; Xu, H.-M. Preliminary Study on the Effect and Molecular Mechanism of Tetrandrine in Alleviating Pulmonary Inflammation and Fibrosis Induced by Silicon Dioxide. Toxics 2023, 11, 765. https://doi.org/10.3390/toxics11090765
Wang Y, Cheng B, Lin Y-J, Wang R, Xuan J, Xu H-M. Preliminary Study on the Effect and Molecular Mechanism of Tetrandrine in Alleviating Pulmonary Inflammation and Fibrosis Induced by Silicon Dioxide. Toxics. 2023; 11(9):765. https://doi.org/10.3390/toxics11090765
Chicago/Turabian StyleWang, Yi, Bin Cheng, Yu-Jia Lin, Rui Wang, Jie Xuan, and Hai-Ming Xu. 2023. "Preliminary Study on the Effect and Molecular Mechanism of Tetrandrine in Alleviating Pulmonary Inflammation and Fibrosis Induced by Silicon Dioxide" Toxics 11, no. 9: 765. https://doi.org/10.3390/toxics11090765
APA StyleWang, Y., Cheng, B., Lin, Y.-J., Wang, R., Xuan, J., & Xu, H.-M. (2023). Preliminary Study on the Effect and Molecular Mechanism of Tetrandrine in Alleviating Pulmonary Inflammation and Fibrosis Induced by Silicon Dioxide. Toxics, 11(9), 765. https://doi.org/10.3390/toxics11090765






