Lead, Cadmium, and Arsenic in Raw Cow’s Milk in a Central Andean Area and Risks for the Peruvian Populations
Abstract
:1. Introduction
2. Materials and Methods
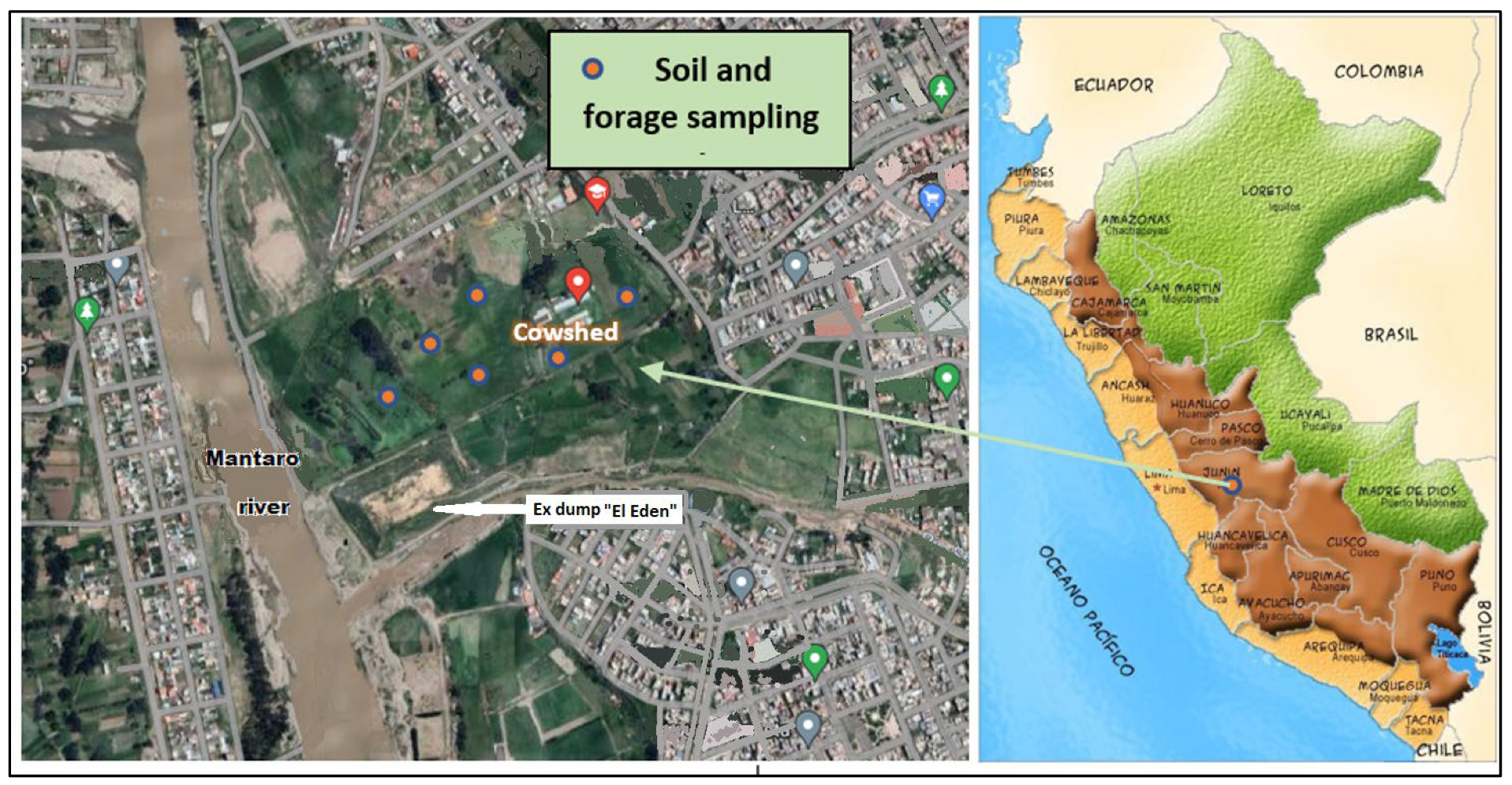
2.1. Study Period and Location
2.2. Animals and Breeding System
2.3. Milk, Soil, and Multi-Species Pasture Sampling and Heavy Metal Analysis
2.4. Estimated Weekly Intake (WI)
2.5. Target Quotient Hazard (TQH)
- Cmetal: metal concentration in milk;
- Wmilk: daily milk intake;
- EF: exposure frequency (365 days per year;
- ED: the exposure period is equivalent to the mean longevity for an adult. For Peru, it is estimated in 76.5 years;
- TA: mean useful life time is 27,922.5 days;
2.6. Hazard Index (HI)
2.7. Data Analysis
3. Results
3.1. Pb, Cd, and As Concentration in the Study Samples
3.2. Weekly Intake (WI), Target Risk Quotient (TQH), and Risk Index (HI)
4. Discussion
4.1. Pb, Cd, and As Concentrations in the Study Samples
4.1.1. Pb, Cd, and As Concentrations in the Soil
4.1.2. Pb, Cd, and As Concentrations in Multi-Species Pasture
4.1.3. Pb, Cd, and As Concentrations in Milk
4.2. Week Intake (WI), Target Hazard Quotient (TQH) and Hazard Index (HI) to Pb, Cd, and As
4.3. Implications of the Intake of Pb, Cd, and As in Mental Health
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Givens, D.I. Milk symposium review: The importance of milk and dairy foods in the diets of infants, adolescents, pregnant women, adults, and the elderly. J. Dairy Sci. 2020, 103, 9681–9699. [Google Scholar] [CrossRef] [PubMed]
- McGuire, S. Department of Agriculture and U.S. Department of Health and Human Services, Dietary Guidelines for Americans 2010. 7th Edition, Washington, DC: U.S. Government Printing Office. Adv. Nutr. 2011, 2, 293–294. [Google Scholar] [CrossRef]
- Statista. Milk Market in Europe—Statistics & Facts. 2020. Available online: https://www.statista.com/topics/3956/milk-market-in-europe/ (accessed on 6 June 2023).
- Domingo, J.L. Concentrations of toxic elements (As, Cd, Hg and Pb) in cow milk: A review of the recent scientific literature. Int. J. Dairy Technol. 2021, 74, 277–285. [Google Scholar] [CrossRef]
- Minkina, T.M.; Mandzhieva, S.S.; Burachevskaya, M.V.; Bauer, T.V.; Sushkova, S.N. Method of determining loosely bound compounds of heavy metals in the soil. Methods X 2018, 5, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zheng, N.; Su, C.; Wang, J.; Soyeurt, H. Relationships between Pb, As, Cr, and Cd in individual cows’ milk and milk composition and heavy metal contents in water, silage, and soil. Environ. Pollut. 2019, 255, 113322. [Google Scholar] [CrossRef] [PubMed]
- Castro-González, N.P.; Calderón-Sánchez, F.; Pérez-Sato, M.; Soní-Guillermo, E.; Reyes-Cervantes, E. Health risk due to chronic heavy metal consumption via cow’s milk produced in Puebla, Mexico, in irrigated wastewater areas. Food Addit. Contam. Part B 2019, 12, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Gao, Y.; Qu, X.; Zhou, X.; Yang, X.; Huang, S.; Han, L.; Zheng, N.; Wang, J. The Occurrence, Pathways, and Risk Assessment of Heavy Metals in Raw Milk from Industrial Areas in China. Toxics 2021, 9, 320. [Google Scholar] [CrossRef]
- Wilcke, W.; Döhler, H. Schwermetalle in der Landwirtschaft (Farming and Heavy Metals); Kuratorium für Technik und Bauwesen in der Landwirtschaft s.V. (KTBL): Darmstadt, Germany, 2016. [Google Scholar]
- ATSDR Agency for Toxic Substances and Disease Registry. ATSDR’s Substance Priority List. 2017. Agency for Toxic Substances and Disease Registry. Available online: https://www.atsdr.cdc.gov/SPL/ (accessed on 12 May 2023).
- Ibrahim, M.I.A.; Mohamed, L.A.; Mahmoud, M.G.; Shaban, K.S.; Fahmy, M.A.; Ebeid, M.H. Potential ecological hazards assessment and prediction of sediment heavy metals pollution along the Gulf of Suez, Egypt. Egypt. J. Aquat. Res. 2019, 45, 329–335. [Google Scholar] [CrossRef]
- Hameed, A.; Akhtar, S.; Amjad, A.; Naeem, I.; Tariq, M. Comparative Assessment of Arsenic Contamination in Raw Milk, Infant Formulas and Breast Milk. Dairy Vet. Sci. J. 2019, 13, 555851. [Google Scholar] [CrossRef]
- Castro, B.J.; Chirinos, P.D.; Tejada de Riveros, D. Plomo en la sangre del cordón umbilical y su impacto sobre el peso. Longitud, hemoglobina y APGAR en zonas con diferente grado de contaminación. Rev. Toxicol. 2012, 29, 100–106. [Google Scholar]
- Castro, J.; López de Romaña, D.; Bedregal, P.; López de Romaña, G.; Chirinos, D. Lead and cadmium in maternal blood and placenta in pregnant women from a mining-smelting zone of Peru and transfer of these metals to their newborns. J. Toxicol. Environ. Health Sci. 2013, 5, 156–165. [Google Scholar] [CrossRef]
- CDC. Childhood Lead Poisoning Prevention. Centers for Disease Control and Prevention. 2022. Available online: https://www.cdc.gov/nceh/lead/news/cdc-updates-blood-lead-reference-value.html (accessed on 17 June 2023).
- Bhattacharya, S. Protective Role of the Essential Trace Elements in the Obviation of Cadmium Toxicity: Glimpses of Mechanisms. Biol. Trace Elem. Res. 2021, 200, 2239–2246. [Google Scholar] [CrossRef] [PubMed]
- Ha, T.T.; Tu, V.V.; Tam, K.B.; Ha, T.H. Accumulation of Arsenic and Heavy Metals in Native and Cultivated Plant Species in a Lead Recycling Area in Vietnam. Minerals 2019, 9, 132. [Google Scholar] [CrossRef]
- Bonomelli, C.; Bonilla, C.; Valenzuela, A. Efecto de la fertilización fosforada sobre el contenido de cadmio en cuatro suelos de Chile. Pesqui. Agropecuária Bras. 2003, 38, 1179–1186. [Google Scholar] [CrossRef]
- Mishra, A.; Oliinyk, P.; Lysiuk, R.; Lenchyk, L.; Singh, S.; Rathod, S.; Antonyak, H.; Darmohray, R.; Dub, N.; Antoniv, O.; et al. Flavonoids and stilbenoids as a promising arsenal for the management of chronic arsenic toxicity. Environ. Toxicol. Pharmacol. 2022, 95, 103970. [Google Scholar] [CrossRef]
- Mukhopadhyay, R.; Rosen, B.P.; Phung, L.; Silver, S. Microbial arsenic: From geocycles to genes and enzymes. FEMS Microbiol. Rev. 2002, 26, 311–325. [Google Scholar] [CrossRef]
- Ren, C.; Zhou, Y.; Liu, W.; Wang, Q. Paradoxical effects of arsenic in the lungs. Environ. Health Prev. Med. 2021, 26, 80. [Google Scholar] [CrossRef]
- Roychoudhury, A.; Krishnamoorthi, S.; Paul, R. Brassica Improvement. In Arsenic Toxicity and Molecular Mechanism of Arsenic Tolerance in Different Members of Brassicaceae; Wani, S., Thakur, A., Jeshima Khan, Y., Eds.; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Chirinos-Peinado, D.; Castro-Bedriñana, J.; García-Olarte, E.; Quispe-Ramos, R.; Gordillo-Espinal, E. Transfer of lead from soil to pasture grass and milk near a metallurgical complex in the Peruvian Andes. Transl. Anim. Sci. 2021, 5, 1–9. [Google Scholar] [CrossRef]
- Data Climate. 2023. Data and Graphs for Weather & Climate in Huancayo. Huancayo Climate: Temperature HUANCAYO & Weather by Month (climate-data.org). Available online: https://en.climate-data.org/south-america/peru/junin/huancayo-3326/ (accessed on 6 June 2023).
- Google Maps. Yauris Agricultural Farm. Available online: https://acortar.link/avM5T2 (accessed on 22 February 2023).
- Mapa del Perú. Available online: https://ar.pinterest.com/pin/298996862736799501/ (accessed on 22 February 2023).
- Latimer, G.W. AOAC Official Method 973.35 Lead in Evaporated Milk Atomic Absorption Spectrophotometric Method. In Official Methods of Analysis of AOAC INTERNATIONAL, 20th ed.; AOAC International: Rockville, MD, USA, 2016; Volume 1, p. 2. [Google Scholar]
- Alvira, M.L.; Rada-Mendoza, M.D.P.; Hoyos, S.O.; Villada, C.H. Quantification of arsenic by atomic absorption spectrometry in flexible thermoformed and biodegradable films. Biotecnol. Sect. Agropecu. Agroind. 2012, 10, 157–165. Available online: http://www.scielo.org.co/pdf/bsaa/v10n1/v10n1a18.pdf (accessed on 22 February 2023).
- Martin, A.P.; Turnbull, R.E.; Rissmann, C.W.; Rieger, P. Heavy metal and metalloid concentrations in soils under pasture of Southern New Zealand. Geoderma Reg. 2017, 11, 18–27. [Google Scholar] [CrossRef]
- USEPA. United States Environmental Protection Agency (USEPA) Acid Digestion of Sediments, Sludges, and Soils; United States Environmental Protection Agency, Method 3050B; United States Environmental Protection Agency: Washington, DC, USA, 1996.
- Bidar, G.; Pruvot, C.; Garçon, G.; Verdin, A.; Shirali, P.; Douay, F. Seasonal and annual variations of metal uptake, bioaccumulation, and toxicity in Trifolium repens and Lolium perenne growing in a heavy metal-contaminated field. Environ. Sci. Pollut. Res. 2009, 16, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Ministerio del Ambiente (MINAM). Decreto Supremo No. 011-2017-MINAM. Aprueban Estándares de Calidad Ambiental (ECA) para Suelo (Approval of Environmental Quality Standards (EQS) for Soil). 2017. Available online: https://sinia.minam.gob.pe/download/file/fid/64487 (accessed on 19 June 2023).
- Canadian Council of Ministers of the Environment, CCME. Canadian Soil Quality Guidelines for the Protection of Environmental and Human Health. 2007. Available online: https://publications.gc.ca/collections/Collection/En1-34-9-2005E.pdf (accessed on 1 June 2023).
- Fernández, O.B.; Mullisaca, C.E.; Huanchi, M.L. Nivel de contaminación del suelo con arsénico y metales pesados en Tiquillaca (Perú). Rev. Investig. Altoandin. 2022, 24, 131–138. [Google Scholar] [CrossRef]
- Boularbah, A.; Schwart, C.; Bitton, G.; Aboudrar, W.; Ouhammou, A.; Morel, J.L. Heavy metal contamination from mining sites in South Morocco: 2. Assessment of metal accumulation and toxicity in plants. Chemosphere 2006, 63, 811–817. [Google Scholar] [CrossRef] [PubMed]
- OJEU. Commission Regulation (EU) No 1275/2013. Off. J. Eur. Union 2013, 56, 86–92. Available online: https://acortar.link/OssuRK (accessed on 2 June 2023).
- CENAN-INEI. Estado Nutricional en el Perú. Componente Nutricional ENAHO-CENAN-INS; Ministerio de Salud: Lima, Perú, 2011. Available online: https://bvs.minsa.gob.pe/local/MiNSA/1843.pdf (accessed on 3 June 2023).
- Singh, G.M.; Micha, R.; Khatibzadeh, S.; Shi, P.; Lim, S.; Andrews, K.G.; Engell, R.E.; Ezzati, M.; Mozaffarian, D. Global, regional, and national consumption of sugar-sweetened beverages, fruit juices, and milk: A systematic assessment of beverage intake in 187 countries. PLoS ONE 2015, 10, 0124845. [Google Scholar] [CrossRef]
- USDA. Dairy Update. Country: Peru. United States Department of Agricultura. Foreing Agricultural Service. 2020. Report number: PE2020-0024. Available online: https://www.fas.usda.gov/data/peru-dairy-update (accessed on 2 June 2023).
- Dror, D.K.; Allen, L.H. Dairy product intake in children and adolescents in developed countries: Trends, nutritional contribution, and a review of association with health outcomes. Nutr. Rev. 2013, 72, 68–81. [Google Scholar] [CrossRef]
- Grenov, B.; Larnkjær, A.; Mølgaard, C.; Michaelsen, K.F. Role of Milk and Dairy Products in Growth of the Child. Nestlé Nutr. Inst. Workshop Ser. 2020, 93, 77–90. [Google Scholar] [CrossRef]
- JECFA. Joint FAO/WHO Expert Committee on Food Additives. Evaluation of Certain Food Additives and Contaminants. 73 Report, 2010. Available online: http://apps.who.int/iris/bitstream/handle/10665/44515/WHO_TRS_960_eng.pdf (accessed on 1 June 2023).
- Joint FAO; World Health Organization. Safety Evaluation of Certain Food Additives and Contaminants: Prepared by the Seventy Fourth Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). 2012. Available online: https://www.who.int/publications/i/item/9789241660655 (accessed on 6 June 2023).
- USEPA. Regional Screening Levels (RSLs)—Generic Tables. Regional Screening Level (RSL) Summary Table (TR=1E-06, HQ=0.1). 2020. Available online: https://www.epa.gov/risk/regional-screening-levels-rsls-generic-tables (accessed on 5 June 2023).
- Codex Alimentarius Commission. Report of the 50th Session of the Codex Committee on Food Additives and Contaminants; Codex Alimentarius Commission: Hague, The Netherlands, 2011. [Google Scholar]
- EFSA. EFSA Panel on Contaminants in the Food Chain (CONTAM); Scientific Opinion on tolerable weekly intake for cadmium. EFSA J. 2011, 9, 1975. [Google Scholar] [CrossRef]
- EFSA. Lead dietary exposure in the European population. EFSA J. 2012, 10, 2831. [Google Scholar] [CrossRef]
- EFSA. Cadmium dietary exposure in the European population. EFSA J. 2012, 10, 2551. [Google Scholar] [CrossRef]
- Boudebbouz, A.; Boudalia, S.; Bousbia, A.; Gueroui, Y.; Boussadia, M.I.; Chelaghmia, M.L.; Zebsa, R.; Affoune, A.M.; Symeon, G.K. Determination of Heavy Metal Levels and Health Risk Assessment of Raw Cow Milk in Guelma Region, Algeria. Biol. Trace Elem. Res. 2022, 201, 1704–1716. [Google Scholar] [CrossRef]
- USEPA. Cadmium. United States Environmental Protection Agency. Washington, DC. 1989. Available online: https://www.epa.gov (accessed on 8 June 2023).
- USEPA. Arsenic (Inorganic). United States Environmental Protection Agency. Washington, DC. 1991. Available online: https://www.epa.gov (accessed on 8 June 2023).
- USEPA. USEPA Regional Screening Level (RSL) Summary Table: November 2011. pp 1,2,6. Available online: https://epa-prgs.ornl.gov/chemicals/download/master_sl_table_run_JUN2011.pdf.
- USEPA. EPA Region III Risk-based Concentration (RBC) Table 2008 Region III, 1650 Arch Street, Philadelphia, Pennsylvania 19103. 2012.
- Norouzirad, R.; González-Montaña, J.R.; Martínez-Pastor, F.; Hosseini, H.; Shahrouzian, A.; Khabazkhoob, M.; Malayeri, F.A.; Bandani, H.; Paknejad, M.; Foroughi-Nia, B.; et al. Lead and cadmium levels in raw bovine milk and dietary risk assessment in areas near petroleum extraction industries. Sci. Total Environ. 2018, 635, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Castro, J.; Chirinos, D.; Peñaloza, R. Lead Bioaccumulation in Root and Aerial Part of Natural and Cultivated Pastures in Highly Contaminated Soils in Central Andes of Peru. Adv. Sci. Technol. Eng. 2020, 5, 126–132. [Google Scholar] [CrossRef]
- Kozhanova, N.; Sarsembayeva, N.; Lozowicka, B.; Kozhanov, Z. Seasonal content of heavy metals in the “soil-feed-milk-manure” system in horse husbandry in Kazakhstan. Vet. World 2021, 14, 2947–2956. [Google Scholar] [CrossRef]
- Meter, A.; Atkinson, R.J.; Laliberte, B. Cadmium in Cacao from Latin America and the Caribbean: A Review of Research and Potential Mitigation Solutions; Bioversity International: Rome, Italy, 2019; 73p, Available online: https://scioteca.caf.com/handle/123456789/1506 (accessed on 8 June 2023)ISBN 978-92-9255-135-3.
- McClintock, T.R.; Chen, Y.; Bundschuh, J.; Oliver, J.T.; Navoni, J.; Olmos, V.; Lepori, E.V.; Ahsan, H.; Parvez, F. Arsenic exposure in Latin América: Biomarkers, risk assessments and related health effects. Sci. Total Environ. 2012, 1, 76–91. [Google Scholar] [CrossRef] [PubMed]
- Soto-Benavente, M.; Rodriguez-Achata, L.; Olivera, M.; Arostegui, S.V.; Colina, N.C.; Garate, Q.J. Health risks due to the presence of heavy metals in agricultural products cultivated in areas abandoned by gold mining in the Peruvian Amazon. Sci. Agropecu. 2020, 11, 49–59. [Google Scholar] [CrossRef]
- Zhang, F.; Li, Y.; Yang, M.; Li, E. Content of Heavy Metals in Animal Feeds and Manures from Farms of Different Scales in Northeast China. Int. J. Environ. Res. Public Health 2012, 9, 2658–2668. [Google Scholar] [CrossRef]
- Khan, S.; Cao, Q.; Zheng, Y.M.; Huang, Y.Z.; Zhu, Y.G. Health risks of heavy metals in contaminated soils and food crops irrigated with wastewater in Beijing, China. Environ. Pollut. 2008, 152, 686–692. [Google Scholar] [CrossRef]
- Hou, Q.; Yang, Z.; Ji, J.; Yu, T.; Chen, G.; Li, J.; Xia, X.; Zhang, M.; Yuan, X. Annual net input fluxes of heavy metals of the agro-ecosystem in the Yangtze River delta, China. J. Geochem. Explor. 2014, 139, 68–84. [Google Scholar] [CrossRef]
- Chirinos-Peinado, D.; Castro-Bedriñana, J.; Ríos-Ríos, E.; Mamani-Gamarra, G.; Quijada-Caro, E.; Huacho-Jurado, A.; Nuñez-Rojas, W. Lead and Cadmium Bioaccumulation in Fresh Cow’s Milk in an Intermediate Area of the Central Andes of Peru and Risk to Human Health. Toxics 2022, 10, 317. [Google Scholar] [CrossRef]
- European Union. Commission Regulation (EU) 2015/1005 of 25 June 2015 Amending Regulation (EC) No 1881/2006 as Regards Maximum Levels of Lead in Certain Foodstuffs. Off. J. Eur. Union 2015, 161, 9–12. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32015R1005&from=EN (accessed on 6 June 2023).
- ATSDR. The ATSDR 2019 Substance Priority List; Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2020. Available online: https://www.atsdr.cdc.gov/spl/ (accessed on 8 June 2023).
- FAO-Codex Alimentarius Commission. The Secretariat of the Joint FAO/WHO Food Standards Program, 25th ed.; FAO: Rome, Italy, 2013.
- Boudebbouz, A.; Boudalia, S.; Bousbia, A.; Habila, S.; Boussadia, M.I.; Gueroui, Y. Heavy metals levels in raw cow milk and health risk assessment across the globe: A systematic review. Sci. Total Environ. 2021, 751, 141830. [Google Scholar] [CrossRef]
- Diacono, E.; Faye, B.; Meldebekova, A.; Konuspayeva, G. Plant, water and milk pollution in Kazakhstan. In Impact of Pollution on Animal Products; Faye, B., Sinyavskiy, Y., Eds.; NATO Science for Peace and Security Series Series C: Environmental Security; Springer: Dordrecht, The Netherlands, 2008; pp. 107–116. [Google Scholar] [CrossRef]
- Licata, P.; Trombetta, D.; Cristani, M.; Giofrè, F.; Martino, D.; Calò, M.; Naccari, F. Levels of “toxic” and “essential” metals in samples of bovine milk from various dairy farms in Calabria, Italy. Environ. Int. 2004, 30, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Pavlovic, I.; Sikiric, M.; Havranek, J.L.; Plavljanic, N.; Brajenovic, N. Lead and cadmium levels in raw cow’s milk from an industrialised Croatian region determined by electrothermal atomic absorption spectrometry. Czech J. Anim. Sci. 2004, 49, 164–168. [Google Scholar] [CrossRef]
- Bilandžić, N.; Okić, M.; Sedak, M.; Solomun, B.; Varenina, I.; Knežević, Z.; Benić, M. Trace element levels in raw milk from northern and southern regions of Croatia. Food Chem. 2011, 127, 63–66. [Google Scholar] [CrossRef]
- Meshref, A.M.S.; Moselhy, W.A.; Hassan, N.E. Heavy metals and trace elements levels in milk and milk products. J. Food Meas. Charact. 2014, 8, 381–388. [Google Scholar] [CrossRef]
- Arianejad, M.; Alizadeh, M.; Bahrami, A.; Arefhoseini, S.R. Levels of Some Heavy Metals in Raw Cow’s Milk from Selected Milk Production Sites in Iran: Is There any Health Concern? Health Promot. Perspect. 2015, 5, 176–182. [Google Scholar] [CrossRef]
- Eleboudi, A.A.; El-Makarem, H.A.; Hadour, H.H.A. Heavy metals residues in some dairy products. Alexandria. J. Vet. Sci. 2017, 52, 334–346. [Google Scholar] [CrossRef]
- Pérez-Carrera, A.; Fernández-Cirelli, A. Arsenic concentration in water and bovine milk in Cordoba, Argentina. Preliminary results. J. Dairy Res. 2005, 72, 122–124. [Google Scholar] [CrossRef]
- Najarnezhad, V.; Akbarabadi, M. Heavy metals in raw cow and ewe milk from north-east Iran. Food Addit. Contam. Part B 2013, 6, 158–162. [Google Scholar] [CrossRef]
- Shahbazi, Y.; Ahmadi, F.; Fakhari, F. Voltammetric determination of Pb, Cd, Zn, Cu, and Se in milk and dairy products collected from Iran: An emphasis on permissible limits and risk assessment of exposure to heavy metals. Food Chem. 2016, 192, 1060–1067. [Google Scholar] [CrossRef]
- Bilandžić, N.; Sedak, M.; Čalopek, B.; Luburić, D.B.; Kolanović, B.S.; Varenina, I.; Đokić, M.; Kmetić, I.; Murati, T. Lead concentrations in raw cow and goat milk collected in rural areas of Croatia from 2010 to 2014. Bull. Environ. Contam. Toxicol. 2016, 96, 645–649. [Google Scholar] [CrossRef] [PubMed]
- Saribal, D. ICP-MS analysis of trace element concentrations in cow’s milk samples from supermarkets in Istanbul, Turkey. Biol. Trace Elem. Res. 2020, 193, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Pšenková, M.; Toman, R.; Tančin, V. Concentrations of toxic metals and essential elements in raw cow milk from areas with potentially undisturbed and highly disturbed environment in Slovakia. Environ. Sci. Pollut. Res. 2020, 27, 26763–26772. [Google Scholar] [CrossRef] [PubMed]
- Patra, R.C.; Swarup, D.; Kumar, P.; Nandi, D.; Naresh, R.; Ali, S.L. Milk trace elements in lactating cows environmentally exposed to higher level of lead and cadmium around different industrial units. Sci. Total Environ. 2008, 404, 36–43. [Google Scholar] [CrossRef]
- De Castro, C.S.; Arruda, A.F.; Da Cunha, L.R.; De Souza, J.R.; Braga, J.W.; Dorea, J.G. Toxic metals (Pb and Cd) and their respective antagonists (Ca and Zn) in infant formulas and milk marketed in Brasilia, Brazil. Int. J. Environ. Res. Public Health 2010, 7, 4062–4077. [Google Scholar] [CrossRef] [PubMed]
- Malhat, F.; Hagag, M.; Saber, A.; Fayz, A.E. Contamination of cow’s milk by heavy metal in Egypt. Bull. Environ. Contam. Toxicol. 2012, 88, 611–613. [Google Scholar] [CrossRef]
- Su, C.; Liu, H.; Qu, X.; Zhou, X.; Gao, Y.; Yang, H.; Zheng, N.; Wang, J. Heavy Metals in Raw Milk and Dietary Exposure Assessment in the Vicinity of Leather-Processing Plants. Biol. Trace Elem. Res. 2020, 199, 3303–3311. [Google Scholar] [CrossRef] [PubMed]
- Ross, E.M.; Rajan, M.P.; Wesley, S.G. Milk minerals in cow milk with special reference to elevated calcium and its radiological implications. Radiat. Prot. Environ. 2012, 35, 64–68. [Google Scholar] [CrossRef]
- Amer, A.A.E.; El-Makarem, H.S.A.; El-Maghraby, M.A.; Abou-Alella, S.A. Lead, cadmium, and aluminum in raw bovine milk: Residue level, estimated intake, and fate during artisanal dairy manufacture. J. Adv. Vet. Anim. Res. 2021, 8, 454–464. [Google Scholar] [CrossRef]
- Sharifi, S.; Sohrabvandi, S.; Mofid, V.; Javanmardi, F.; Khanniri, E.; Mortazavian, A.M. The assessment of lead concentration in raw milk collected from some major dairy farms in Iran and evaluation of associated health risk. J. Environ. Health Sci. Eng. 2022, 20, 181–186. [Google Scholar] [CrossRef]
- Yan, M.; Niu, C.; Li, X.; Wang, F.; Jiang, S.; Li, K.; Yao, Z. Heavy metal levels in milk and dairy products and health risk assessment: A systematic review of studies in China. Sci. Total Environ. 2022, 851, 158161. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Prasad, S.; Yadav, K.K.; Shrivastava, M.; Gupta, N.; Nagar, S.; Bach, Q.V.; Kamyab, H.; Khan, S.A.; Yadav, S.; et al. Hazardous heavy metals contamination of vegetables and food chain: Role of sustainable remediation approaches—A review. Environ. Res. 2019, 179, 108792. [Google Scholar] [CrossRef] [PubMed]
- Ayala, J.; Romero, H. Presencia de metales pesados (arsénico y mercurio) en leche de vaca al sur de Ecuador. La Granja. Rev. Cien. Vida 2013, 17, 36–46. [Google Scholar] [CrossRef]
- Song, S.; Liu, N.; Wang, G.; Wang, Y.; Zhang, X.; Zhao, X.; Chang, H.; Yu, Z.; Liu, X. Sex Specificity in the Mixed Effects of Blood Heavy Metals and Cognitive Function on Elderly: Evidence from NHANES. Nutrients 2023, 5, 2874. [Google Scholar] [CrossRef]
- Ramírez, O.D.; González, E.D.F.; Blanco, A.T.; Pineda, B.; Gómez, M.S.; Marcial, Q.J.; Carrillo, M.P.; Pérez de la Cruz, V. Cognitive Impairment Induced by Lead Exposure during Lifespan: Mechanisms of Lead Neurotoxicity. Toxics 2021, 9, 23. [Google Scholar] [CrossRef]
- Zaw, Y.H.; Taneepanichskul, N. Blood heavy metals and brain-derived neurotrophic factor in the first trimester of pregnancy among migrant workers. PLoS ONE 2019, 14, e0218409. [Google Scholar] [CrossRef] [PubMed]
- Skogheim, T.S.; Weyde, K.V.; Engel, S.M.; Aase, H.; Surén, P.; Øie, G.M.; Biele, G.; Reichborn-Kjennerud, T.; Caspersen, I.H.; Hornig, M.; et al. Metal and essential element concentrations during pregnancy and associations with autism spectrum disorder and attention-deficit/hyperactivity disorder in children. Environ. Int. 2021, 152, 106468. [Google Scholar] [CrossRef]
- Dórea, J.G. Environmental exposure to low-level lead (Pb) co-occurring with other neurotoxicants in early life and neurodevelopment of children. Environ. Res. 2019, 177, 108641. [Google Scholar] [CrossRef] [PubMed]
- Jaga, K.; Dharmani, C. The Interrelation between Organophosphate Toxicity and the Epidemiology of Depression and Suicide. Rev. Environ. Health 2007, 22, 57–73. [Google Scholar] [CrossRef]
- Ayuso-Álvarez, A.; Simón, L.; Nuñez, O.; Rodríguez-Blázquez, C.; Martín-Méndez, I.; Bel-Lán, A.; López-Abente, G.; Merlo, J.; Fernandez-Navarro, P.; Galán, I. Association between Heavy Metals and Metalloids in Topsoil and Mental Health in the Adult Population of Spain. Environ. Res. 2019, 179, 108784. [Google Scholar] [CrossRef]
- Berk, M.; Williams, L.J.; Andreazza, A.C.; Pasco, J.A.; Dodd, S.; Jacka, F.N.; Moylan, S.; Reiner, E.J.; Magalhaes, P.V. Pop, heavy metal and the blues: Secondary analysis of persistent organic pollutants (POP), heavy metals and depressive symptoms in the NHANES National Epidemiological Survey. BMJ Open 2014, 4, e005142. [Google Scholar] [CrossRef]
- Theorell, T.; Hammarström, A.; Aronsson, G.; Träskman Bendz, L.; Grape, T.; Hogstedt, C.; Marteinsdottir, I.; Skoog, I.; Hall, C. A Systematic Review Including Meta-Analysis of Work Environment and Depressive Symptoms. BMC Public Health 2015, 15, 738. [Google Scholar] [CrossRef] [PubMed]
- Jurczak, A.; Brodowska, A.; Szkup, M.; Prokopowicz, A.; Karakiewicz, B.; Łój, B.; Kotwas, A.; Brodowska, A.; Grochans, E. Influence of Pb and Cd Levels in Whole Blood of Postmenopausal Women on the Incidence of Anxiety and Depressive Symptoms. Ann. Agric. Environ. Med. 2018, 25, 219–223. [Google Scholar] [CrossRef] [PubMed]
- USP. Official from December 1, 2017. The United States Pharmacopeia Convention. Available online: https://www.usp.org/sites/default/files/usp/document/our-work/chemical-medicines/key-issues/232-40-35-1s.pdf (accessed on 12 December 2022).
- He, B.; Wang, Y.; Li, S.; Zhao, Y.; Ma, X.; Wang, W.; Li, X.; Zhang, Y. A cross–sectional survey of preschool children: Exploring heavy metal exposure, neurotransmitters, and neurobehavioural relationships and mediation effects. Ecotoxicol. Environ. Saf. 2021, 220, 112391. [Google Scholar] [CrossRef] [PubMed]
- Astete, J.; Gastañaga, M.C.; Fiestas, V.; Oblitas, T.; Sabastizagal, I.; Lucero, M.; Abadíe, J.M.; Muñoz, M.E.; Valverde, A.; Suarez, M. Comunicable Diseases, Mental Health and Exposure to Environmental Pollutants in Population Living Near Las Bambas Mining Project before Exploitation Phase, Peru 2006. Rev. Peru. Med. Exp. Salud Publica 2010, 27, 512–519. Available online: http://www.scielo.org.pe/pdf/rins/v27n4/a04v27n4.pdf (accessed on 8 June 2023). [CrossRef]
- Vega, J.; De Coll, J.; Lermo, J.; Escobar, J.; Díaz, M.; Castro, J. Niveles Intelectuales y Ansiedad en Niños con Intoxicación Plúmbica Crónica. Colegio “María Reiche” Callao-Perú, 2002. An. Fac. Med. 2005, 66, 142–147. Available online: http://www.scielo.org.pe/pdf/afm/v66n2/a08v66n2.pdf (accessed on 8 June 2023). [CrossRef]





| Pb | Cd | As | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Soil | Sproud | Milk | Soil | Sproud | Milk | Soil | Sproud | Milk | |
| Mean | 652.35 a | 23.17 b | 0.062 c | 7.09 a | 0.250 b | 0.014 c | 6.29 a | 0.063 b | 0.030 c |
| SD | 192.50 | 10.02 | 0.008 | 5.31 | 0.57 | 0.005 | 4.98 | 0.09 | 0.028 |
| Min | 339.69 | 9.32 | 0.047 | 1.73 | 0.002 | 0.008 | 3.49 | 0.01 | 0.009 |
| Max | 879.00 | 34.00 | 0.077 | 19.59 | 1.40 | 0.026 | 16.41 | 0.34 | 0.096 |
| MPL | 70 | 30 | 0.02 | 1.4 | 1.0 | 0.003 | 12 | 2.0 | 0.014 |
| T, % | 100.00 | 3.55 | 0.27 | 100.00 | 3.52 | 5.60 | 100.00 | 1.00 | 47.62 |
| B, % | 100.00 | 0.01 | 100.00 | 0.20 | 100.00 | 0.48 | |||
| Age (Year) | Minimum Milk Intake | Medium Milk Intake | Maximum Milk Intake | TWI Pb | TWI Cd | TWI As | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WI Pb | WI Cd | WI As | WI Pb | WI Cd | WI As | WI Pb | WI Cd | WI As | ||||
| 2 | 174.16 | 40.32 | 85.12 | 217.70 | 50.40 | 106.40 | 261.24 | 60.48 | 127.68 | 310 | 72 | 4 |
| 3 | 174.16 | 40.32 | 85.12 | 217.70 | 50.40 | 106.40 | 261.24 | 60.48 | 127.68 | 360 | 84 | 4 |
| 4 | 174.16 | 40.32 | 85.12 | 217.70 | 50.40 | 106.40 | 261.24 | 60.48 | 127.68 | 403 | 93 | 5 |
| 5 | 174.16 | 40.32 | 85.12 | 217.70 | 50.40 | 106.40 | 261.24 | 60.48 | 127.68 | 448 | 104 | 5 |
| 6 | 208.99 | 48.38 | 102.14 | 261.24 | 60.48 | 127.68 | 313.49 | 72.58 | 153.22 | 503 | 117 | 6 |
| 7 | 208.99 | 48.38 | 102.14 | 261.24 | 60.48 | 127.68 | 313.49 | 72.58 | 153.22 | 550 | 128 | 7 |
| 8 | 208.99 | 48.38 | 102.14 | 261.24 | 60.48 | 127.68 | 313.49 | 72.58 | 153.22 | 615 | 143 | 7 |
| 9 | 208.99 | 48.38 | 102.14 | 261.24 | 60.48 | 127.68 | 313.49 | 72.58 | 153.22 | 670 | 155 | 8 |
| 10 | 208.99 | 48.38 | 102.14 | 261.24 | 60.48 | 127.68 | 313.49 | 72.58 | 153.22 | 740 | 172 | 9 |
| 11 | 208.99 | 48.38 | 102.14 | 261.24 | 60.48 | 127.68 | 313.49 | 72.58 | 153.22 | 818 | 190 | 10 |
| 12 | 217.70 | 50.40 | 106.40 | 261.24 | 60.48 | 127.68 | 313.49 | 72.58 | 153.22 | 910 | 211 | 11 |
| : | ||||||||||||
| 81 | 52.25 | 12.10 | 25.54 | 80.55 | 18.65 | 39.37 | 121.04 | 28.02 | 59.16 | 1478 | 343 | 18 |
| 82 | 52.25 | 12.10 | 25.54 | 80.55 | 18.65 | 39.37 | 121.04 | 28.02 | 59.16 | 1475 | 342 | 18 |
| 83 | 52.25 | 12.10 | 25.54 | 80.55 | 18.65 | 39.37 | 121.04 | 28.02 | 59.16 | 1368 | 317 | 16 |
| 84 | 52.25 | 12.10 | 25.54 | 80.55 | 18.65 | 39.37 | 121.04 | 28.02 | 59.16 | 1415 | 328 | 17 |
| 85 | 52.25 | 12.10 | 25.54 | 80.55 | 18.65 | 39.37 | 121.04 | 28.02 | 59.16 | 1390 | 322 | 17 |
| Age (Year) | Milk Intake, kg/d | Minimum Milk Intake | Milk Intake, kg/d | Medium Milk Intake | Milk Intake, kg/d | Maximum Milk Intake | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TQH Pb | TQH Cd | TQH As | HI | TQH Pb | TQH Cd | TQH As | HI | TQH Pb | TQH Cd | TQH As | HI | ||||
| 2 | 0.40 | 0.28 | 0.24 | 1.13 | 1.65 | 0.50 | 0.35 | 0.30 | 1.41 | 2.06 | 0.60 | 0.42 | 0.36 | 1.69 | 2.47 |
| 3 | 0.40 | 0.24 | 0.20 | 0.97 | 1.42 | 0.50 | 0.30 | 0.26 | 1.22 | 1.77 | 0.60 | 0.36 | 0.31 | 1.46 | 2.13 |
| 4 | 0.40 | 0.21 | 0.18 | 0.86 | 1.25 | 0.50 | 0.27 | 0.23 | 1.08 | 1.57 | 0.60 | 0.32 | 0.27 | 1.29 | 1.88 |
| 5 | 0.40 | 0.19 | 0.16 | 0.77 | 1.12 | 0.50 | 0.24 | 0.20 | 1.05 | 1.49 | 0.60 | 0.29 | 0.24 | 1.15 | 1.68 |
| 6 | 0.48 | 0.20 | 0.17 | 0.82 | 1.20 | 0.60 | 0.25 | 0.22 | 1.03 | 1.50 | 0.72 | 0.31 | 0.26 | 1.23 | 1.80 |
| 7 | 0.48 | 0.19 | 0.16 | 0.75 | 1.09 | 0.60 | 0.23 | 0.20 | 0.93 | 1.36 | 0.72 | 0.28 | 0.24 | 1.12 | 1.64 |
| 8 | 0.48 | 0.17 | 0.14 | 0.67 | 0.97 | 0.60 | 0.21 | 0.18 | 0.83 | 1.22 | 0.72 | 0.25 | 0.21 | 1.00 | 1.46 |
| 9 | 0.48 | 0.15 | 0.13 | 0.60 | 0.87 | 0.60 | 0.19 | 0.16 | 0.75 | 1.09 | 0.72 | 0.22 | 0.19 | 0.90 | 1.31 |
| 10 | 0.48 | 0.13 | 0.11 | 0.54 | 0.78 | 0.60 | 0.17 | 0.14 | 0.67 | 0.98 | 0.72 | 0.20 | 0.17 | 0.81 | 1.17 |
| 11 | 0.48 | 0.12 | 0.10 | 0.47 | 0.68 | 0.60 | 0.14 | 0.12 | 0.58 | 0.85 | 0.72 | 0.17 | 0.15 | 0.70 | 1.02 |
| 12 | 0.50 | 0.11 | 0.09 | 0.42 | 0.62 | 0.60 | 0.13 | 0.11 | 0.51 | 0.74 | 0.72 | 0.15 | 0.13 | 0.61 | 0.89 |
| : | |||||||||||||||
| 81 | 0.12 | 0.02 | 0.02 | 0.09 | 0.13 | 0.19 | 0.04 | 0.03 | 0.14 | 0.21 | 0.28 | 0.05 | 0.05 | 0.21 | 0.31 |
| 82 | 0.12 | 0.02 | 0.02 | 0.09 | 0.13 | 0.19 | 0.04 | 0.03 | 0.14 | 0.21 | 0.28 | 0.05 | 0.05 | 0.21 | 0.31 |
| 83 | 0.12 | 0.02 | 0.02 | 0.09 | 0.13 | 0.19 | 0.03 | 0.03 | 0.13 | 0.19 | 0.28 | 0.05 | 0.04 | 0.20 | 0.29 |
| 84 | 0.12 | 0.02 | 0.02 | 0.09 | 0.13 | 0.19 | 0.03 | 0.03 | 0.14 | 0.20 | 0.28 | 0.05 | 0.04 | 0.21 | 0.31 |
| 85 | 0.12 | 0.02 | 0.02 | 0.09 | 0.13 | 0.19 | 0.03 | 0.03 | 0.14 | 0.20 | 0.28 | 0.05 | 0.04 | 0.21 | 0.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro-Bedriñana, J.; Chirinos-Peinado, D.; Ríos-Ríos, E.; Castro-Chirinos, G.; Chagua-Rodríguez, P.; De La Cruz-Calderón, G. Lead, Cadmium, and Arsenic in Raw Cow’s Milk in a Central Andean Area and Risks for the Peruvian Populations. Toxics 2023, 11, 809. https://doi.org/10.3390/toxics11100809
Castro-Bedriñana J, Chirinos-Peinado D, Ríos-Ríos E, Castro-Chirinos G, Chagua-Rodríguez P, De La Cruz-Calderón G. Lead, Cadmium, and Arsenic in Raw Cow’s Milk in a Central Andean Area and Risks for the Peruvian Populations. Toxics. 2023; 11(10):809. https://doi.org/10.3390/toxics11100809
Chicago/Turabian StyleCastro-Bedriñana, Jorge, Doris Chirinos-Peinado, Elva Ríos-Ríos, Gianfranco Castro-Chirinos, Perfecto Chagua-Rodríguez, and Gina De La Cruz-Calderón. 2023. "Lead, Cadmium, and Arsenic in Raw Cow’s Milk in a Central Andean Area and Risks for the Peruvian Populations" Toxics 11, no. 10: 809. https://doi.org/10.3390/toxics11100809
APA StyleCastro-Bedriñana, J., Chirinos-Peinado, D., Ríos-Ríos, E., Castro-Chirinos, G., Chagua-Rodríguez, P., & De La Cruz-Calderón, G. (2023). Lead, Cadmium, and Arsenic in Raw Cow’s Milk in a Central Andean Area and Risks for the Peruvian Populations. Toxics, 11(10), 809. https://doi.org/10.3390/toxics11100809