Human Health Risk Assessment for Exposure to Heavy Metals via Dietary Intake of Rainbow Trout in the Influence Area of a Smelting Facility Located in Peru
Abstract
1. Introduction
2. Materials and Methods
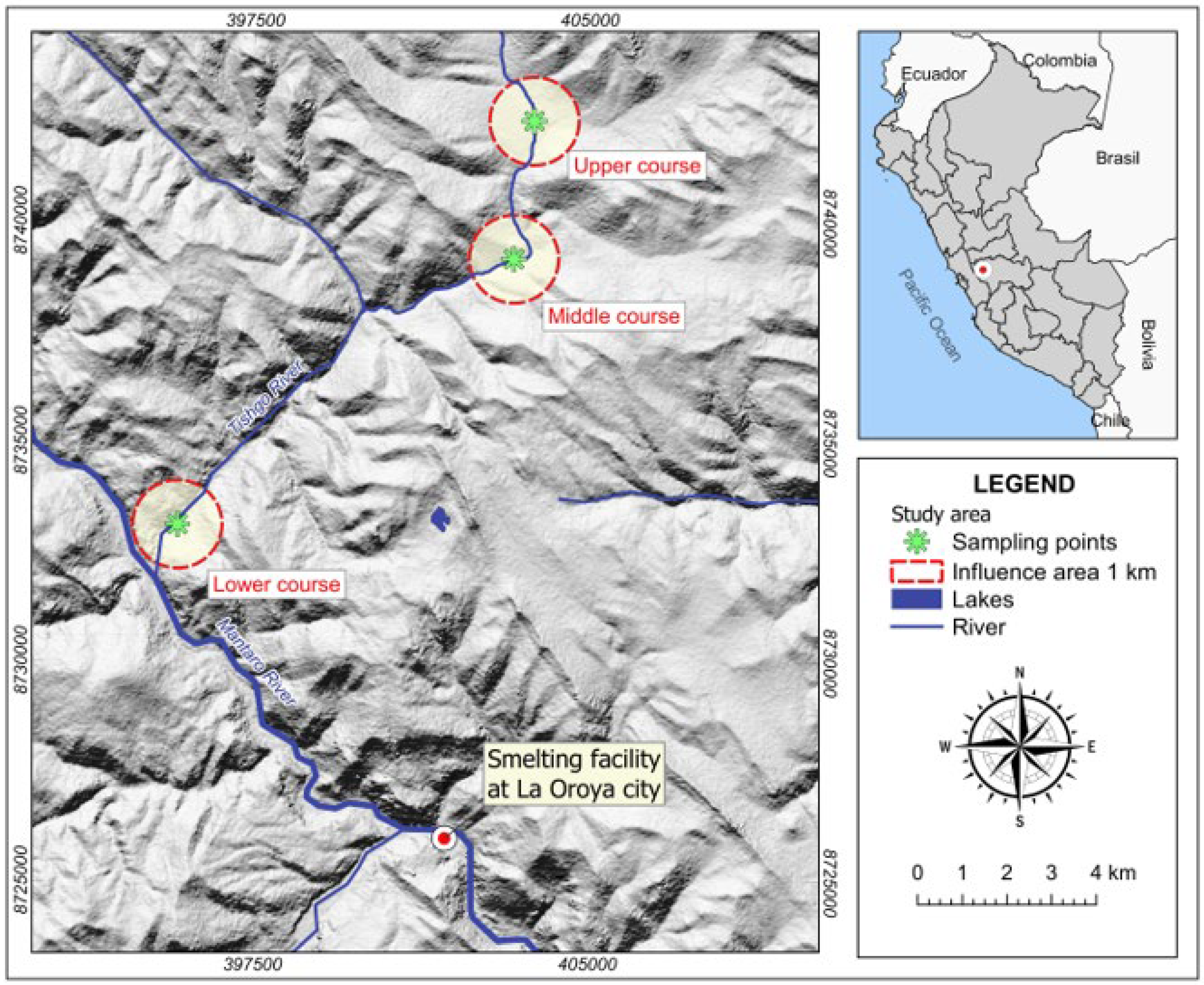
2.1. Study Zone
2.2. Chemical Reagents and Chemical Grade
- Nitric acid (HNO3): Commercial-grade nitric acid with a concentration of 65%.
- Hydrochloric acid (HCl): Analytical grade with a purity exceeding 99.5%.
- Sulfuric acid (H2SO4): Analytical grade with a purity between 95 and 97%.
2.3. Sampling and Analytical Determination
2.3.1. Analysis of Heavy Metals and Arsenic in Water
2.3.2. Analysis of Heavy Metals and Arsenic in Sediment
2.3.3. Analysis of Heavy Metals and Arsenic in Fish
2.4. Bioconcentration Factor (BCF)
2.5. Biosediment Accumulation Factor (BSAF)
2.6. Risk Assessment for Consumption of Muscle of Rainbow Trout
2.7. Statistical Analysis
3. Results
3.1. Concentration of Heavy Metals and Arsenic
3.2. Bioconcentration Factor of Heavy Metals and Arsenic in Muscle
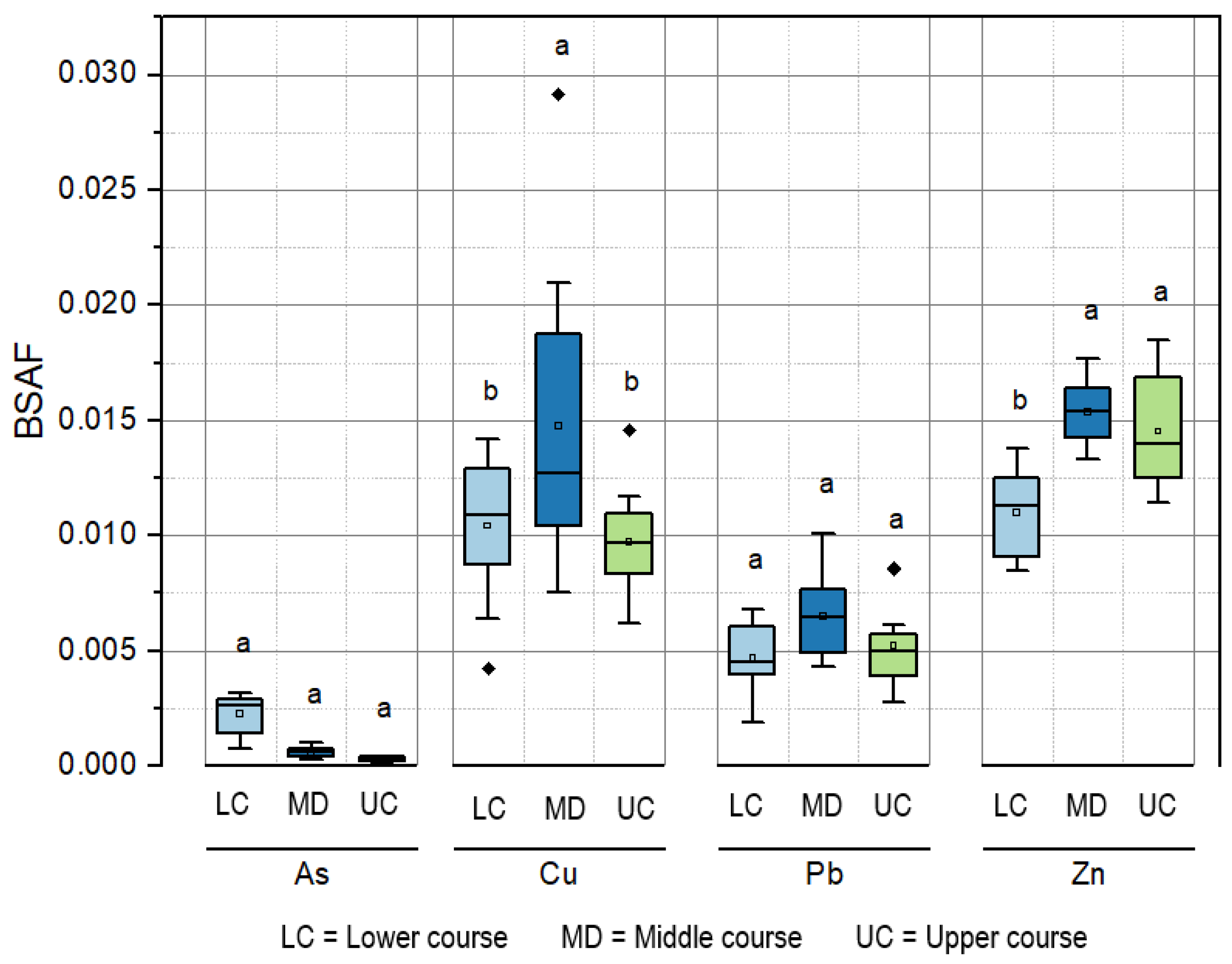
3.3. Biosediment Accumulation Factor (BSAF) of Heavy Metals and Arsenic in Rainbow Trout Muscle
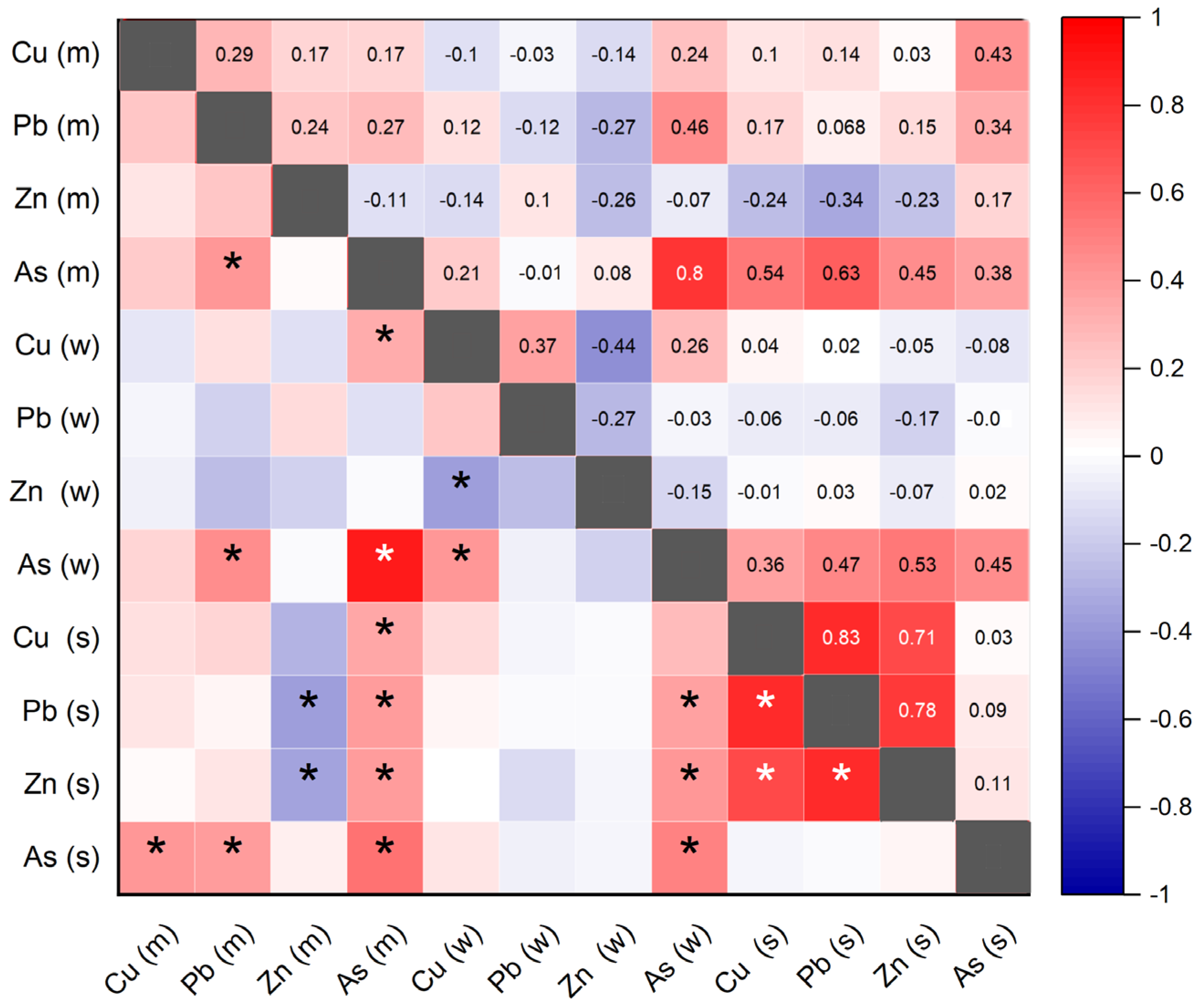
3.4. Relationship between Heavy Metals in Water, Sediment, and Muscle
3.5. Risk Assessments on the Consumption of Rainbow Trout
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Copaja, S.V.; Pérez, C.A.; Vega-Retter, C.; Véliz, D. Heavy Metal Content in Chilean Fish Related to Habitat Use, Tissue Type and River of Origin. Bull. Environ. Contam. Toxicol. 2017, 99, 695–700. [Google Scholar] [CrossRef]
- Marengo, M.; Durieux, E.D.H.; Ternengo, S.; Lejeune, P.; Degrange, E.; Pasqualini, V.; Gobert, S. Comparison of elemental composition in two wild and cultured marine fish and potential risks to human health. Ecotoxicol. Environ. Saf. 2018, 158, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Kara, G.T.; Kara, M.; Bayram, A.; Gündüz, O. Assessment of seasonal and spatial variations of physicochemical parameters and trace elements along a heavily polluted effluent-dominated stream. Environ. Monit. Assess. 2017, 189, 585. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.M.; Ali, M.L.; Islam, M.S.; Rahman, M.Z. Preliminary assessment of heavy metals in water and sediment of Karnaphuli River, Bangladesh. Environ. Nanotechnol. Monit. Manag. 2016, 5, 27–35. [Google Scholar] [CrossRef]
- Kawser Ahmed, M.; Baki, M.A.; Kundu, G.K.; Saiful Islam, M.; Monirul Islam, M.; Muzammel Hossain, M. Human health risks from heavy metals in fish of Buriganga river, Bangladesh. Springerplus 2016, 5, 1697. [Google Scholar] [CrossRef]
- Ahmed, M.K.; Shaheen, N.; Islam, M.S.; Habibullah-Al-Mamun, M.; Islam, S.; Banu, C.P. Trace elements in two staple cereals (rice and wheat) and associated health risk implications in Bangladesh. Environ. Monit. Assess. 2015, 187, 326. [Google Scholar] [CrossRef]
- Mansour, S.A.; Sidky, M.M. Ecotoxicological Studies. 3. Heavy metals contaminating water and fish from Fayoum Governorate, Egypt. Food Chem. 2002, 78, 15–22. [Google Scholar] [CrossRef]
- Nazir, R.; Khan, M.; Masab, M.; Ur Rehman, H.; Rauf, N.U.; Shahab, S.; Ameer, N.; Sajed, M.; Ullah, M.; Rafeeq, M.; et al. Accumulation of Heavy Metals (Ni, Cu, Cd, Cr, Pb, Zn, Fe) in the soil, water and plants and analysis of physico-chemical parameters of soil and water Collected from Tanda Dam kohat. J. Pharm. Sci. Res. 2015, 7, 89–97. [Google Scholar]
- Xiao, H.; Zang, S.; Guan, Y.; Liu, S.; Gao, Y.; Sun, Q.; Xu, H.; Li, M.; Wang, J.; Pei, X. Assessment of potential risks associated with heavy metal contamination in sediment in Aobaopao Lake, China, determined from sediment cores. Ecotoxicology 2014, 23, 527–537. [Google Scholar] [CrossRef]
- Maurya, P.K.; Malik, D.S.; Yadav, K.K.; Kumar, A.; Kumar, S.; Kamyab, H. Bioaccumulation and potential sources of heavy metal contamination in fish species in River Ganga basin: Possible human health risks evaluation. Toxicol. Rep. 2019, 6, 472–481. [Google Scholar] [CrossRef]
- Wang, J.; Liu, G.; Liu, H.; Lam, P.K.S. Multivariate statistical evaluation of dissolved trace elements and a water quality assessment in the middle reaches of Huaihe River, Anhui, China. Sci. Total Environ. 2017, 583, 421–431. [Google Scholar] [CrossRef]
- Rosales-Rimache, J.; Chavez-Ruiz, M.; Inolopú-Cucche, J.; Rabanal-Sanchez, J.; Rueda-Torres, L.; Sanchez-Holguin, G. Leadcare® II Comparison with Graphite Furnace Atomic Absorption Spectrophotometry for Blood Lead Measurement in Peruvian Highlands. Indian J. Clin. Biochem. 2022, 38, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Waseem, A.; Arshad, J.; Iqbal, F.; Sajjad, A.; Mehmood, Z.; Murtaza, G. Pollution Status of Pakistan: A Retrospective Review on Heavy Metal Contamination of Water, Soil, and Vegetables. Biomed. Res. Int. 2014, 2014, 813206. [Google Scholar] [CrossRef] [PubMed]
- Javed, M.; Usmani, N. An Overview of the Adverse Effects of Heavy Metal Contamination on Fish Health. Proc. Natl. Acad. Sci. India Sect. B.-Biol. Sci. 2019, 89, 389–403. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy Metals Toxicity and the Environment. EXS 2012, 101, 133–164. [Google Scholar] [CrossRef]
- Rahman, M.M.; Asaduzzaman, M.; Naidu, R. Consumption of arsenic and other elements from vegetables and drinking water from an arsenic-contaminated area of Bangladesh. J. Hazard. Mater. 2013, 262, 1056–1063. [Google Scholar] [CrossRef]
- Fang, Y.; Sun, X.; Yang, W.; Ma, N.; Xin, Z.; Fu, J.; Liu, X.; Liu, M.; Mariga, A.M.; Zhu, X.; et al. Concentrations and health risks of lead, cadmium, arsenic, and mercury in rice and edible mushrooms in China. Food Chem. 2014, 147, 147–151. [Google Scholar] [CrossRef]
- Islam, M.S.; Ahmed, M.K.; Habibullah-Al-Mamun, M.; Islam, K.N.; Ibrahim, M.; Masunaga, S. Arsenic and lead in foods: A potential threat to human health in Bangladesh. Food Addit. Contam. Part. A Chem. Anal. Control Expo. Risk Assess. 2014, 31, 1982–1992. [Google Scholar] [CrossRef]
- Islam, M.S.; Ahmed, M.K.; Raknuzzaman, M.; Habibullah-Al-Mamun, M.; Islam, M.K. Heavy metal pollution in surface water and sediment: A preliminary assessment of an urban river in a developing country. Ecol. Indic. 2015, 48, 282–291. [Google Scholar] [CrossRef]
- Stankovic, S.; Stankovic, A.R. Bioindicators of Toxic Metals; Springer: Berlin/Heidelberg, Germany, 2013; pp. 151–228. [Google Scholar] [CrossRef]
- Mwakalapa, E.B.; Simukoko, C.K.; Mmochi, A.J.; Mdegela, R.H.; Berg, V.; Müller, M.H.B.; Lyche, J.L.; Polder, A. Heavy metals in farmed and wild milkfish (Chanos chanos) and wild mullet (Mugil cephalus) along the coasts of Tanzania and associated health risk for humans and fish. Chemosphere 2019, 224, 176–186. [Google Scholar] [CrossRef]
- Parang, H.; Esmaeilbeigi, M. Total mercury concentration in the muscle of four mostly consumed fish and associated human health risks for fishermen and non-fishermen families in the Anzali Wetland, Southern Caspian Sea. Reg. Stud. Mar. Sci. 2022, 52, 102270. [Google Scholar] [CrossRef]
- Schiel, D.; Rienitz, O. Final report on CCQM-K70: Determination of Hg in natural water at a concentration level required by the European environmental quality standard (EQS). Metrologia 2011, 48, 08011. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2022; FAO: Roma, Italy, 2022. [Google Scholar] [CrossRef]
- Dietz, R.; Letcher, R.J.; Desforges, J.-P.; Eulaers, I.; Sonne, C.; Wilson, S.; Andersen-Ranberg, E.; Basu, N.; Barst, B.D.; Bustnes, J.O.; et al. Current state of knowledge on biological effects from contaminants on arctic wildlife and fish. Sci. Total Environ. 2019, 696, 133792. [Google Scholar] [CrossRef]
- Habib, M.R.; Hoque, M.; Kabir, J.; Akhter, S.; Rahman, M.S.; Moore, J.; Jolly, Y.N. A comparative study of heavy metal exposure risk from the consumption of some common species of cultured and captured fishes of Bangladesh. J. Food Compos. Anal. 2022, 108, 104455. [Google Scholar] [CrossRef]
- Espinoza-Guillen, J.A.; Alderete-Malpartida, M.B.; Cañari-Cancho, J.H.; Pando-Huerta, D.L.; Vargas-La Rosa, D.F.; Bernabé-Meza, S.J. Immission levels and identification of sulfur dioxide sources in La Oroya city, Peruvian Andes. Environ. Dev. Sustain. 2022. [Google Scholar] [CrossRef]
- Wang, W.-X. Bioaccumulation and Biomonitoring. In Marine Ecotoxicology; Elsevier: Amsterdam, The Netherlands, 2016; pp. 99–119. [Google Scholar] [CrossRef]
- Vallero, D.A. Landmark Cases. In Paradigms Lost; Elsevier: Amsterdam, The Netherlands, 2006; pp. 197–273. [Google Scholar] [CrossRef]
- Nazneen, S.; Mishra, A.K.; Raju, N.J.; Mehmood, G. Coastal macrophytes as bioindicators of trace metals in the Asia’s largest lagoon ecosystem. Mar. Pollut. Bull. 2022, 178, 113576. [Google Scholar] [CrossRef]
- Sanou, A.; Coulibaly, S.; Coulibaly, M.; N’Goran N’dri, S.; Célestin Atse, B. Assessment of heavy metal contamination of fish from a fish farm by bioconcentration and bioaccumulation factors. Egypt. J. Aquat. Biol. Fish. 2021, 25, 821–841. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, X.; Yang, D.; Lei, B.; Zhang, X.; Zhang, X. Evaluation of human health risks posed by carcinogenic and non-carcinogenic multiple contaminants associated with consumption of fish from Taihu Lake, China. Food Chem. Toxicol. 2014, 69, 86–93. [Google Scholar] [CrossRef]
- Bat, L.; Sezgin, M. Heavy Metal Levels in Some Commercial Fish from Sinop Coast of the Black Sea, Turkey. In Proceedings of the Twelfth International Conference on the Mediterranean Coastal Environment MEDCOAST, Varna, Bulgaria, 6–10 October 2015. [Google Scholar]
- Simukoko, C.K.; Mwakalapa, E.B.; Bwalya, P.; Muzandu, K.; Berg, V.; Mutoloki, S.; Polder, A.; Lyche, J.L. Assessment of heavy metals in wild and farmed tilapia (Oreochromis niloticus) on Lake Kariba, Zambia: Implications for human and fish health. Food Addit. Contam. 2021, 39, 74–91. [Google Scholar] [CrossRef]
- Opresko, D.M.; Young, R.A.; Faust, R.A.; Talmage, S.S.; Watson, A.P.; Ross, R.H.; Davidson, K.A.; King, J. Chemical warfare agents: Estimating oral reference doses. Rev. Environ. Contam. Toxicol. 1998, 156, 1–183. [Google Scholar] [CrossRef]
- Saha, S.; Reza, A.H.M.S.; Roy, M.K. Arsenic geochemistry of the sediments of the shallow aquifer and its correlation with the groundwater, Rangpur, Bangladesh. Appl. Water Sci. 2021, 11, 166. [Google Scholar] [CrossRef]
- US Environmental Protection Agency (USEPA). Supplemental Guidance for Assessing Susceptibility from Early-Life Exposure to Carcinogens; US Environmental Protection Agency (USEPA): Washington, DC, USA, 2005.
- Wang, J.; Shan, Q.; Liang, X.; Guan, F.; Zhang, Z.; Huang, H.; Fang, H. Levels and human health risk assessments of heavy metals in fish tissue obtained from the agricultural heritage rice-fish-farming system in China. J. Hazard. Mater. 2020, 386, 121627. [Google Scholar] [CrossRef] [PubMed]
- Noman, A.; Feng, W.; Zhu, G.; Hossain, M.B.; Chen, Y.; Zhang, H.; Sun, J. Bioaccumulation and potential human health risks of metals in commercially important fishes and shellfishes from Hangzhou Bay, China. Sci. Rep. 2022, 12, 4634. [Google Scholar] [CrossRef] [PubMed]
- OEHHA. Appendix A: Hot Spots Unit Risk and Cancer Potency Values Updated October 2020; Office of Environmental Health Hazard Assessment: Oakland, CA, USA, 2020.
- US EPA. Arsenic, Inorganic CASRN 7440-38-2 | DTXSID4023886|IRIS|US EPA, ORD. 1991. Available online: https://cfpub.epa.gov/ncea/iris2/chemicalLanding.cfm?substance_nmbr=278 (accessed on 26 September 2022).
- R Core Team. R: The R Project for Statistical Computing. USA. 2022. Available online: https://www.r-project.org/ (accessed on 26 September 2022).
- Fahimah, N.; Oginawati, K.; Suharyant. Fate and spatial distribution of Pb, Cd, Cu and Zn in the water column and in the surface sediment of Indonesian Estuary (Citarum River Estuary). E3S Web Conf. 2020, 148, 07007. [Google Scholar] [CrossRef]
- Ofman, P.; Puchlik, M.; Simson, G.; Krasowska, M.; Struk-Sokołowska, J. Impact assessment of treated wastewater on water quality of the receiver using the Wilcoxon test. E3S Web Conf. 2017, 22, 8. [Google Scholar] [CrossRef]
- Salgado, D.; Awad, G. Metodología para el análisis estratégico cuantitativo en proyectos a partir del análisis de riesgos. Estud. Gerenc. 2022, 38, 424–435. [Google Scholar] [CrossRef]
- FAO/WHO. Report of the 34th Session of the Codex Committee on Food Additives and Contaminants; FAO/WHO: The Hague, The Netherlands, 2002. [Google Scholar]
- FSANZ. Food Standards Australia and New Zealand—Australia New Zealand Food Standards Code—Standard 1.4.1—Contaminants and Natural Toxicants. 2013. Available online: https://www.legislation.gov.au/Details/F2013C00140/ (accessed on 27 September 2022).
- USEPA. National Primary Drinking Water Regulations|US EPA. 2022. Available online: https://www.epa.gov/ground-water-and-drinking-water/national-primary-drinking-water-regulations (accessed on 27 September 2022).
- CEQGs. Canadian Sediment Quality Guidelines for the Protection of Aquatic Life. 2022. Available online: https://ccme.ca/en/current-activities/canadian-environmental-quality-guidelines (accessed on 27 September 2022).
- Custodio, M.; Peñaloza, R.; Espinoza, C.; Peralta-Ortiz, T.; Ordinola-Zapata, A.; Sánchez-Suárez, H.; Vieyra-Peña, E. Data on the concentration of heavy metals and metalloids in lotic water of the Mantaro river watershed and human risk assessment, Peru. Data Brief 2020, 30, 105493. [Google Scholar] [CrossRef]
- Tapia, P.M. Diatoms as bioindicators of pollution in the Mantaro River, Central Andes, Peru. Int. J. Environ. Health 2008, 2, 82–91. [Google Scholar] [CrossRef]
- Monna, F.; Camizuli, E.; Revelli, P.; Biville, C.; Thomas, C.; Losno, R.; Scheifler, R.; Bruguier, O.; Baron, S.; Chateau, C.; et al. Wild Brown Trout Affected by Historical Mining in the Cévennes National Park, France. Environ. Sci. Technol. 2011, 45, 6823–6830. [Google Scholar] [CrossRef]
- Holloway, P.C.; Etsell, T.H.; Murland, A.L. Roasting of la Oroya zinc ferrite with Na2CO3. Metall. Mater. Trans. B Process Metall. Mater. Process. Sci. 2007, 38, 781–791. [Google Scholar] [CrossRef]
- Barker, I.L.; Jacobi, J.S.; Wadia, B.H. Some notes on Oroya copper slags. JOM 1957, 9, 774–780. [Google Scholar] [CrossRef]
- Chamveha, P.; Kattiyapon, C.; Chuachuensuk, A.; Authayanun, S.; Arpornwichanop, A. Performance Analysis of a Smelting Reactor for Copper Production Process. Ind. Eng. Chem. Res. 2008, 48, 1120–1125. [Google Scholar] [CrossRef]
- Carn, S.A.; Krueger, A.J.; Krotkov, N.A.; Yang, K.; Levelt, P.F. Sulfur dioxide emissions from Peruvian copper smelters detected by the Ozone Monitoring Instrument. Geophys. Res. Lett. 2007, 34. [Google Scholar] [CrossRef]
- Bridge, G. Contested terrain: Mining and the Environment. Annu. Rev. Environ. Resour. 2004, 29, 205–259. [Google Scholar] [CrossRef]
- Das, S.; Kim, G.W.; Hwang, H.Y.; Verma, P.P.; Kim, P.J. Cropping with slag to address soil, environment, and food security. Front. Microbiol. 2019, 10, 1320. [Google Scholar] [CrossRef]
- Zinabu, E.; Kelderman, P.; van der Kwast, J.; Irvine, K. Monitoring river water and sediments within a changing Ethiopian catchment to support sustainable development. Environ. Monit. Assess. 2019, 191, 455. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention, National Center for Environmental Health, Division of Emergency and Environmental Health Services. Development of an Integrated Intervention Plan to Reduce Exposure to Lead and Other Contaminants in the Mining Center of La Oroya, Perú. 2005. Available online: https://pdf.usaid.gov/pdf_docs/pnadd579.pdf (accessed on 9 August 2023).
- Blankson, E.R.; Klerks, P.L. The effect of bioturbation by Lumbriculus variegatus on transport and distribution of lead in a freshwater microcosm. Environ. Toxicol. Chem. 2016, 35, 1123–1129. [Google Scholar] [CrossRef]
- Wahyuningsih, S.; Fatimatuzzahroh, F.; Gitarama, A.M. Distribution and estimation of heavy metal (pb) contamination levels in the water and sediment bondet estuary, cirebon. J. Ilmu Perikan. Dan Sumberd. Perair. 2021, 9, 923–936. [Google Scholar] [CrossRef]
- Wan, D.; Zhang, N.; Chen, W.; Cai, P.; Zheng, L.; Huang, Q. Organic matter facilitates the binding of Pb to iron oxides in a subtropical contaminated soil. Environ. Sci. Pollut. Res. 2018, 25, 32130–32139. [Google Scholar] [CrossRef]
- Ndungu, K.; Zurbrick, C.M.; Stammerjohn, S.; Severmann, S.; Sherrell, R.M.; Flegal, A.R. Lead Sources to the Amundsen Sea, West Antarctica. Environ. Sci. Technol. 2016, 50, 6233–6239. [Google Scholar] [CrossRef]
- Tye, A.M.; Chenery, S.; Cave, M.R.; Price, R. Using 206/207Pb isotope ratios to estimate phosphorus sources in historical sediments of a lowland river system. J. Soils Sediments 2021, 21, 613–626. [Google Scholar] [CrossRef]
- Zhu, W.H.; Huang, T.L.; Chai, B.B.; Yang, P.; Yao, J.L. Influence of the environmental conditions on the fractionation of heavy metals in the Fenhe reservoir sediment. Geochem. J. 2010, 44, 399–410. [Google Scholar] [CrossRef][Green Version]
- Sofuoglu, S.C.; Kavcar, P. An exposure and risk assessment for fluoride and trace metals in black tea. J. Hazard. Mater. 2008, 158, 392–400. [Google Scholar] [CrossRef]
- Barone, G.; Storelli, A.; Garofalo, R.; Mallamaci, R.; Storelli, M.M. Residual Levels of Mercury, Cadmium, Lead and Arsenic in Some Commercially Key Species from Italian Coasts (Adriatic Sea): Focus on Human Health. Toxics 2022, 10, 223. [Google Scholar] [CrossRef] [PubMed]
- Tkachenko, H.; Kasiyan, O.; Kamiński, P.; Kurhaluk, N. Assessing the human health risk of Baltic Sea sea trout (Salmo trutta L.) Consumption. Fish. Aquat. Life 2022, 30, 27–43. [Google Scholar] [CrossRef]
- Kenšová, R.; Kružíková, K.; Havránek, J.; Haruštiakov, D.; Svobodová, Z. Distribution of mercury in rainbow trout tissues at embryo-larval and Juvenile stages. Sci. World J. 2012, 2012, 652496. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Streit, B. Bioaccumulation of contaminants in fish. EXS 1998, 86, 353–387. [Google Scholar] [CrossRef]
- Surana, R.; Gadhia, M.; Ansari, E. Accumulation of lead in the muscle of brackish water fish (Boleopthalmus dussumieri). J. Appl. Nat. Sci. 2015, 7, 662–665. [Google Scholar] [CrossRef]
- Stefan, B.; Pirjol, N.; Negreanu Pirjol, T.; Popoviciu, D.R. Copper, Manganese and Zinc Bioaccumulation in Some Common Poaceae Species Along Romanian Black Sea Coast. Rev. Chim. SRL 2017, 68, 2488–2491. [Google Scholar] [CrossRef]
- Khemaissia, H.; Jelassi, R.; Ghemari, C.; Raimond, M.; Souty-Grosset, C.; Nasri-Ammar, K. Evaluation of trace element contamination using Armadillo officinalis Duméril, 1816 (Crustacea, Isopoda) as a tool: An ultrastructural study. Microsc. Res. Techn. 2019, 82, 2014–2025. [Google Scholar] [CrossRef]
- Filho, P.S.; Nunes, L.; da Rosa, N.; Betemps, G.; Pereira, R. Comparison among native floating aquatic macrophytes for bioconcentration of heavy metals. Ecotoxicol. Environ. Contam. 2015, 10, 1–6. [Google Scholar] [CrossRef]
- Zhou, H.; Greig, A.; You, C.-F.; Lai, Z.; Tang, J.; Guan, Y.; Yuan, D. Arsenic in a Speleothem from Central China: Stadial-Interstadial Variations and Implications. Environ. Sci. Technol. 2011, 45, 1278–1283. [Google Scholar] [CrossRef] [PubMed]
- Eiche, E.; Kramar, U.; Berg, M.; Berner, Z.; Norra, S.; Neumann, T. Geochemical changes in individual sediment grains during sequential arsenic extractions. Water Res. 2010, 44, 5545–5555. [Google Scholar] [CrossRef] [PubMed]
- Kligerman, S.; White, C. Epidemiology of lung cancer in women: Risk factors, survival, and screening. Am. J. Roentgenol. 2011, 196, 287–295. [Google Scholar] [CrossRef] [PubMed]


| Factor | Location | As | Cu | Pb | Zn |
|---|---|---|---|---|---|
| Muscle | Lower course | 0.11 ± 0.03 | 0.21 ± 0.11 | 0.24 ± 0.05 | 2.51 ± 0.08 |
| Middle course | 0.02 ± 0.01 | 0.2 ± 0.11 | 0.19 ± 0.05 | 2.57 ± 0.09 | |
| Upper course | 0.01 ± 0.003 | 0.15 ± 0.15 | 0.17 ± 0.04 | 2.51 ± 0.07 | |
| ML | |||||
| Water | Lower course | 0.03 * ± 0.005 | 0.02 ± 0.012 | 0.03 * ± 0.009 | 0.05 ± 0.031 |
| Middle course | 0.02 * ± 0.003 | 0.02 ± 0.016 | 0.03 * ± 0.008 | 0.07 ± 0.025 | |
| Upper course | 0.01 ± 0.003 | 0.02 ± 0.017 | 0.03 * ± 0.01 | 0.06 ± 0.028 | |
| ML | |||||
| Sediment | Lower course | 40.25 * ± 3.66 | 22.24 ± 2.32 | 47.68 * ± 9.97 | 231.04 * ± 39.08 |
| Middle course | 42.44 * ± 3.41 | 15.22 ± 1.41 | 31.75 ± 2.92 | 169.83 * ± 14.54 | |
| Upper course | 32.53 * ± 2.74 | 18.39 ± 2.85 | 36.62 * ± 7.2 | 180.11 * ± 24.3 | |
| ML—ISQGs |
| Sector | THQ-Cu | THQ-Pb | THQ-Zn | THQ-As | HI |
|---|---|---|---|---|---|
| Lower course | 0.0004 | 0.1954 | 0.0007 | 0.0299 | 0.2264 |
| Middle course | 0.0004 | 0.1547 | 0.0007 | 0.0054 | 0.1613 |
| Upper course | 0.0003 | 0.1384 | 0.0007 | 0.0027 | 0.1421 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peñaloza, R.; Custodio, M.; Cacciuttolo, C.; Chanamé, F.; Cano, D.; Solorzano, F. Human Health Risk Assessment for Exposure to Heavy Metals via Dietary Intake of Rainbow Trout in the Influence Area of a Smelting Facility Located in Peru. Toxics 2023, 11, 764. https://doi.org/10.3390/toxics11090764
Peñaloza R, Custodio M, Cacciuttolo C, Chanamé F, Cano D, Solorzano F. Human Health Risk Assessment for Exposure to Heavy Metals via Dietary Intake of Rainbow Trout in the Influence Area of a Smelting Facility Located in Peru. Toxics. 2023; 11(9):764. https://doi.org/10.3390/toxics11090764
Chicago/Turabian StylePeñaloza, Richard, María Custodio, Carlos Cacciuttolo, Fernán Chanamé, Deyvis Cano, and Fernando Solorzano. 2023. "Human Health Risk Assessment for Exposure to Heavy Metals via Dietary Intake of Rainbow Trout in the Influence Area of a Smelting Facility Located in Peru" Toxics 11, no. 9: 764. https://doi.org/10.3390/toxics11090764
APA StylePeñaloza, R., Custodio, M., Cacciuttolo, C., Chanamé, F., Cano, D., & Solorzano, F. (2023). Human Health Risk Assessment for Exposure to Heavy Metals via Dietary Intake of Rainbow Trout in the Influence Area of a Smelting Facility Located in Peru. Toxics, 11(9), 764. https://doi.org/10.3390/toxics11090764










