Pulmonary Toxicity Assessment after a Single Intratracheal Inhalation of Chlorhexidine Aerosol in Mice
Abstract
:1. Introduction
2. Materials and Methods
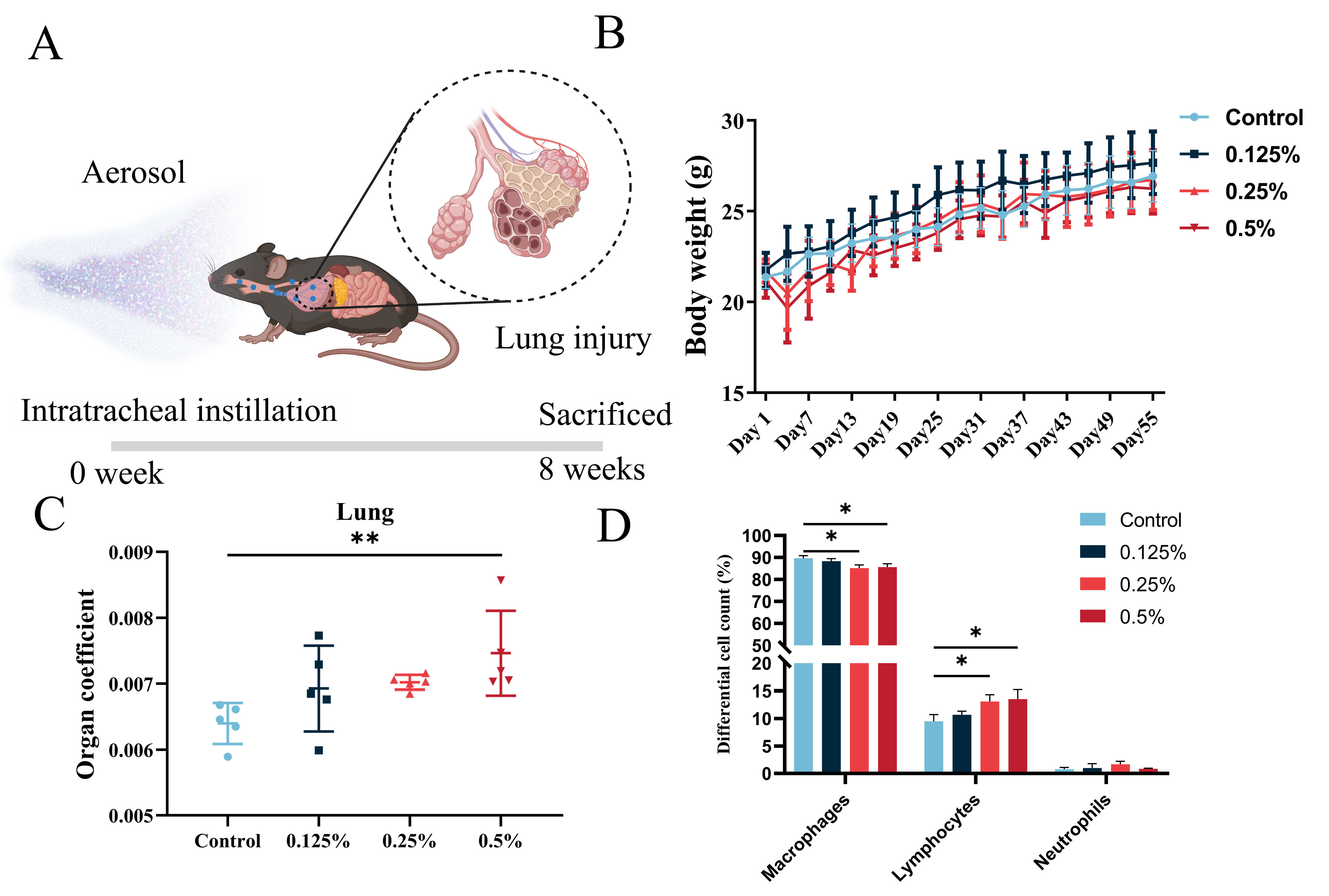
2.1. Animals and Exposure
2.2. Bronchoalveolar Lavage Fluid
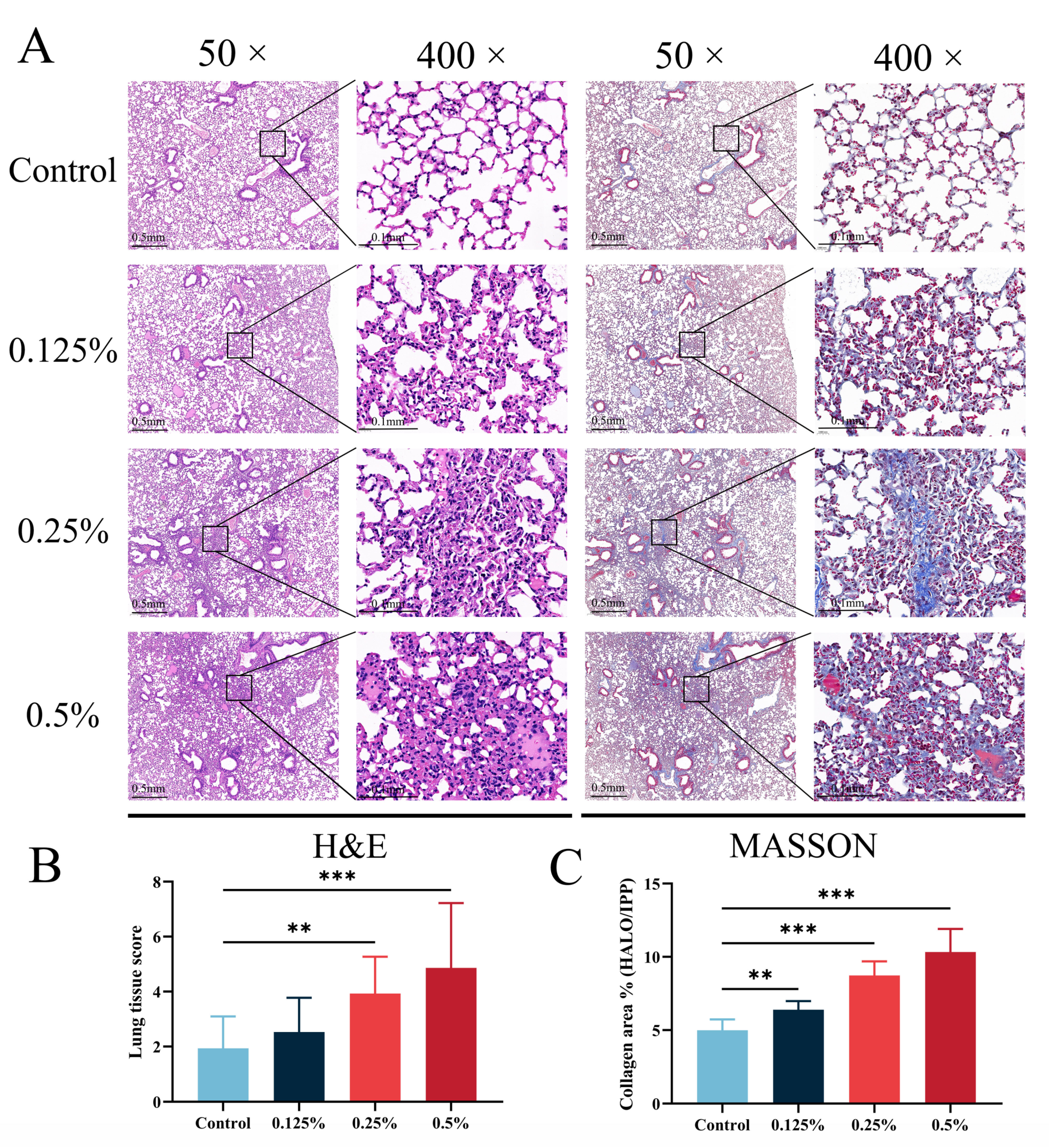
2.3. Histopathological Analysis of Mouse Tissues
2.4. Pulmonary Function Test
2.5. Transcriptome Analysis
2.6. Real-Time Quantitative PCR Analysis
2.7. Statistical Analysis
3. Results
3.1. Benchmark Dose of Mice Mortality Rate after CHG Aerosol Inhalation
3.2. General Toxicological Assessment of CHG Aerosol Inhalation
3.3. CHG Aerosol Inhalation Triggered Lung Histopathological Changes and Collagen Deposition
3.4. CHG Aerosol Inhalation Decreased Lung Function
3.5. Transcriptome Analysis of Mice Lungs after CHG Aerosol Inhalation
3.6. Explore and Verification of Essential Genes for Lung Fibrotic Injury Induced through CHG Aerosol Inhalation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Park, Y.J.; Jeong, M.H.; Bang, I.J.; Kim, H.R.; Chung, K.H. Guanidine-based disinfectants, polyhexamethylene guanidine-phosphate (PHMG-P), polyhexamethylene biguanide (PHMB), and oligo(2-(2-ethoxy)ethoxyethyl guanidinium chloride (PGH) induced epithelial-mesenchymal transition in A549 alveolar epithelial cells. Inhal. Toxicol. 2019, 31, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Oulé, M.K.; Quinn, K.; Dickman, M.; Bernier, A.M.; Rondeau, S.; De Moissac, D.; Boisvert, A.; Diop, L. Akwaton, polyhexamethylene-guanidine hydrochloride-based sporicidal disinfectant: A novel tool to fight bacterial spores and nosocomial infections. J. Med. Microbiol. 2012, 61, 1421–1427. [Google Scholar] [CrossRef] [PubMed]
- Song, J.H.; Ahn, J.; Park, M.Y.; Park, J.; Lee, Y.M.; Myong, J.P.; Koo, J.W.; Lee, J. Health Effects Associated With Humidifier Disinfectant Use: A Systematic Review for Exploration. J. Korean Med. Sci. 2022, 37, e257. [Google Scholar] [CrossRef] [PubMed]
- Song, J.A.; Park, H.J.; Yang, M.J.; Jung, K.J.; Yang, H.S.; Song, C.W.; Lee, K. Polyhexamethyleneguanidine phosphate induces severe lung inflammation, fibrosis, and thymic atrophy. Food Chem. Toxicol. 2014, 69, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Jeong, M.H.; Jeon, M.S.; Kim, G.E.; Kim, H.R. Polyhexamethylene Guanidine Phosphate Induces Apoptosis through Endoplasmic Reticulum Stress in Lung Epithelial Cells. Int. J. Mol. Sci. 2021, 22, 1215. [Google Scholar] [CrossRef] [PubMed]
- Pallotto, C.; Fiorio, M.; De Angelis, V.; Ripoli, A.; Franciosini, E.; Quondam Girolamo, L.; Volpi, F.; Iorio, P.; Francisci, D.; Tascini, C.; et al. Daily bathing with 4% chlorhexidine gluconate in intensive care settings: A randomized controlled trial. Clin. Microbiol. Infect. 2019, 25, 705–710. [Google Scholar] [CrossRef]
- D’Journo, X.B.; Falcoz, P.E.; Alifano, M.; Le Rochais, J.P.; D’Annoville, T.; Massard, G.; Regnard, J.F.; Icard, P.; Marty-Ane, C.; Trousse, D.; et al. Oropharyngeal and nasopharyngeal decontamination with chlorhexidine gluconate in lung cancer surgery: A randomized clinical trial. Intensive Care Med. 2018, 44, 578–587. [Google Scholar] [CrossRef]
- Chamsai, B.; Soodvilai, S.; Opanasopit, P.; Samprasit, W. Topical Film-Forming Chlorhexidine Gluconate Sprays for Antiseptic Application. Pharmaceutics 2022, 14, 1124. [Google Scholar] [CrossRef]
- Kwon, D.; Kwon, J.T.; Lim, Y.M.; Shim, I.; Kim, E.; Lee, D.H.; Yoon, B.I.; Kim, P.; Kim, H.M. Inhalation toxicity of benzalkonium chloride and triethylene glycol mixture in rats. Toxicol. Appl. Pharmacol. 2019, 378, 114609. [Google Scholar] [CrossRef]
- Takanabe, Y.; Maruoka, Y.; Kondo, J.; Yagi, S.; Chikazu, D.; Okamoto, R.; Saitoh, M. Dispersion of Aerosols Generated during Dental Therapy. Int. J. Environ. Res. Public Health 2021, 18, 11279. [Google Scholar] [CrossRef]
- Junor, B.J.R.; Briggs, J.D.; Forwell, M.A.; Dobbie, J.W.; Henderson, I. Sclerosing Peritonitis -The Contribution of Chlorhexidine in Alcohol. Perit. Dial. Int. 1985, 5, 101–104. [Google Scholar] [CrossRef]
- Brezovec, N.; Kojc, N.; Erman, A.; Hladnik, M.; Stergar, J.; Milanič, M.; Tomšič, M.; Čučnik, S.; Sodin-Šemrl, S.; Perše, M.; et al. Molecular and Cellular Markers in Chlorhexidine-Induced Peritoneal Fibrosis in Mice. Biomedicines 2022, 10, 2726. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, H.; Kasahara, M.; Mori, K.; Ogawa, Y.; Kuwabara, T.; Imamaki, H.; Kawanishi, T.; Koga, K.; Ishii, A.; Kato, Y.; et al. Pleiotrophin triggers inflammation and increased peritoneal permeability leading to peritoneal fibrosis. Kidney Int. 2012, 81, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Zhang, S.; Yang, Y.; Lu, M.; Wang, Y.; Zhang, T.; Tang, M.; Takeshita, H. Acute pulmonary toxic effects of chlorhexidine (CHX) following an intratracheal instillation in rats. Hum. Exp. Toxicol. 2011, 30, 1795–1803. [Google Scholar] [CrossRef]
- Orito, K.; Hashida, M.; Hirata, K.; Kurokawa, A.; Shirai, M.; Akahori, F. Effects of single intratracheal exposure to chlorhexidine gluconate on the rat lung. Drug Chem. Toxicol. 2006, 29, 1–9. [Google Scholar] [CrossRef]
- Mikawa, K.; Nishina, K.; Takao, Y.; Obara, H. ONO-1714, a nitric oxide synthase inhibitor, attenuates endotoxin-induced acute lung injury in rabbits. Anesth. Analg. 2003, 97, 1751–1755. [Google Scholar] [CrossRef]
- Vanoirbeek, J.A.; Rinaldi, M.; De Vooght, V.; Haenen, S.; Bobic, S.; Gayan-Ramirez, G.; Hoet, P.H.; Verbeken, E.; Decramer, M.; Nemery, B.; et al. Noninvasive and invasive pulmonary function in mouse models of obstructive and restrictive respiratory diseases. Am. J. Respir. Cell Mol. Biol. 2010, 42, 96–104. [Google Scholar] [CrossRef]
- Mafra, C.S.; Carrijo-Carvalho, L.C.; Chudzinski-Tavassi, A.M.; Taguchi, F.M.; Foronda, A.S.; Carvalho, F.R.; de Freitas, D. Antimicrobial action of biguanides on the viability of Acanthamoeba cysts and assessment of cell toxicity. Investig. Ophthalmol. Vis. Sci. 2013, 54, 6363–6372. [Google Scholar] [CrossRef]
- Böhle, S.; Röhner, E.; Zippelius, T.; Jacob, B.; Matziolis, G.; Rohe, S. Cytotoxic effect of sodium hypochlorite (Lavanox 0.08%) and chlorhexidine gluconate (Irrisept 0.05%) on human osteoblasts. Eur. J. Orthop. Surg. Traumatol. 2022, 32, 81–89. [Google Scholar] [CrossRef]
- Röhner, E.; Jacob, B.; Böhle, S.; Rohe, S.; Löffler, B.; Matziolis, G.; Zippelius, T. Sodium hypochlorite is more effective than chlorhexidine for eradication of bacterial biofilm of staphylococci and Pseudomonas aeruginosa. Knee Surg. Sports Traumatol. Arthrosc. 2020, 28, 3912–3918. [Google Scholar] [CrossRef]
- Arabaci, T.; Türkez, H.; Çanakçi, C.F.; Özgöz, M. Assessment of cytogenetic and cytotoxic effects of chlorhexidine digluconate on cultured human lymphocytes. Acta Odontol. Scand. 2013, 71, 1255–1260. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Kuan, Y.H.; Lee, S.S.; Huang, F.M.; Chang, Y.C. Cytotoxicity and genotoxicity of chlorhexidine on macrophages in vitro. Environ. Toxicol. 2014, 29, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Zhang, S.; Tang, M.; Zhang, T.; Wang, Y.; Hieda, Y.; Takeshita, H. Comparative study on toxic effects induced by oral or intravascular administration of commonly used disinfectants and surfactants in rats. J. Appl. Toxicol. 2012, 32, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, J.; Du, C.; Jiang, Y.; Zhang, W.; Wang, S.; Zhu, X.; Gao, J.; Zhang, X.; Ren, D.; et al. Polyhexamethylene guanidine aerosol triggers pulmonary fibrosis concomitant with elevated surface tension via inhibiting pulmonary surfactant. J. Hazard. Mater. 2021, 420, 126642. [Google Scholar] [CrossRef]
- Zhu, X.; Kong, X.; Ma, S.; Liu, R.; Li, X.; Gao, S.; Ren, D.; Zheng, Y.; Tang, J. TGFβ/Smad mediated the polyhexamethyleneguanide areosol-induced irreversible pulmonary fibrosis in subchronic inhalation exposure. Inhal. Toxicol. 2020, 32, 419–430. [Google Scholar] [CrossRef]
- Takahashi, F.; Takahashi, K.; Okazaki, T.; Maeda, K.; Ienaga, H.; Maeda, M.; Kon, S.; Uede, T.; Fukuchi, Y. Role of osteopontin in the pathogenesis of bleomycin-induced pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2001, 24, 264–271. [Google Scholar] [CrossRef]
- Chan, T.Y. Poisoning due to Savlon (cetrimide) liquid. Hum. Exp. Toxicol. 1994, 13, 681–682. [Google Scholar] [CrossRef]
- Ishigami, S.; Hase, S.; Nakashima, H.; Yamada, H.; Dohgomori, H.; Natsugoe, S.; Aikou, T. Intravenous chlorhexidine gluconate causing acute respiratory distress syndrome. J. Toxicol. Clin. Toxicol. 2001, 39, 77–80. [Google Scholar] [CrossRef]
- De Langhe, E.; Cailotto, F.; De Vooght, V.; Aznar-Lopez, C.; Vanoirbeek, J.A.; Luyten, F.P.; Lories, R.J. Enhanced endogenous bone morphogenetic protein signaling protects against bleomycin induced pulmonary fibrosis. Respir. Res. 2015, 16, 38. [Google Scholar] [CrossRef]
- Tanaka, K.I.; Azuma, A.; Miyazaki, Y.; Sato, K.; Mizushima, T. Effects of lecithinized superoxide dismutase and/or pirfenidone against bleomycin-induced pulmonary fibrosis. Chest 2012, 142, 1011–1019. [Google Scholar] [CrossRef]
- Horowitz, J.C.; Thannickal, V.J. Mechanisms for the Resolution of Organ Fibrosis. Physiology 2019, 34, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N. Transforming growth factor-β in tissue fibrosis. J. Exp. Med. 2020, 217, e20190103. [Google Scholar] [CrossRef] [PubMed]
- Weiskirchen, R.; Weiskirchen, S.; Tacke, F. Organ and tissue fibrosis: Molecular signals, cellular mechanisms and translational implications. Mol. Aspects Med. 2019, 65, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Xu, W.; Chen, H.; Warburton, D.; Dong, R.; Qian, B.; Selman, M.; Gauldie, J.; Kolb, M.; Shi, W. A novel profibrotic mechanism mediated by TGFβ-stimulated collagen prolyl hydroxylase expression in fibrotic lung mesenchymal cells. J. Pathol. 2015, 236, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Yang, I.V.; Coldren, C.D.; Leach, S.M.; Seibold, M.A.; Murphy, E.; Lin, J.; Rosen, R.; Neidermyer, A.J.; McKean, D.F.; Groshong, S.D.; et al. Expression of cilium-associated genes defines novel molecular subtypes of idiopathic pulmonary fibrosis. Thorax 2013, 68, 1114–1121. [Google Scholar] [CrossRef]
- Lipinski, J.H.; Moore, B.B.; O’Dwyer, D.N. The evolving role of the lung microbiome in pulmonary fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 319, L675–L682. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, Z.N.; Xu, A.R.; Fang, Z.F.; Chen, S.Y.; Hou, X.T.; Zhou, Z.Q.; Lin, H.M.; Xie, J.X.; Tang, X.X.; et al. Mucus Hypersecretion and Ciliary Impairment in Conducting Airway Contribute to Alveolar Mucus Plugging in Idiopathic Pulmonary Fibrosis. Front. Cell Dev. Biol. 2021, 9, 810842. [Google Scholar] [CrossRef]
- Bingle, C.D.; Araujo, B.; Wallace, W.A.; Hirani, N.; Bingle, L. What is top of the charts? BPIFB1/LPLUNC1 localises to the bronchiolised epithelium in the honeycomb cysts in UIP. Thorax 2013, 68, 1167–1168. [Google Scholar] [CrossRef]
- Boon, K.; Bailey, N.W.; Yang, J.; Steel, M.P.; Groshong, S.; Kervitsky, D.; Brown, K.K.; Schwarz, M.I.; Schwartz, D.A. Molecular phenotypes distinguish patients with relatively stable from progressive idiopathic pulmonary fibrosis (IPF). PLoS ONE 2009, 4, e5134. [Google Scholar] [CrossRef]
- Herrera, J.A.; Dingle, L.A.; Monetero, M.A.; Venkateswaran, R.V.; Blaikley, J.F.; Granato, F.; Pearson, S.; Lawless, C.; Thornton, D.J. Morphologically intact airways in lung fibrosis have an abnormal proteome. Respir. Res. 2023, 24, 99. [Google Scholar] [CrossRef]




| Model Name | BMD a | BMDL b | BMDU c | p-Value | AIC d |
|---|---|---|---|---|---|
| Gamma | 0.112 | 0.067 | 0.202 | 0.433 | 93.635 |
| Logistic | 0.211 | 0.162 | 0.276 | 0.099 | 98.604 |
| Log-Logistic | 0.124 | 0.040 | 0.204 | 0.462 | 93.333 |
| Log-Probit | 0.135 | 0.050 | 0.209 | 0.485 | 93.052 |
| Multistage | 0.094 | 0.055 | 0.178 | 0.450 | 93.790 |
| Probit | 0.200 | 0.155 | 0.259 | 0.107 | 98.172 |
| Weibull | 0.106 | 0.067 | 0.192 | 0.438 | 93.689 |
| Quantal-Linear | 0.090 | 0.066 | 0.126 | 0.628 | 91.809 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Jiang, X.; Li, X.; Sun, H.; Wang, M.; Zhang, W.; Li, H.; Wang, H.; Zhuang, M.; Zhang, L.; et al. Pulmonary Toxicity Assessment after a Single Intratracheal Inhalation of Chlorhexidine Aerosol in Mice. Toxics 2023, 11, 910. https://doi.org/10.3390/toxics11110910
Zhang J, Jiang X, Li X, Sun H, Wang M, Zhang W, Li H, Wang H, Zhuang M, Zhang L, et al. Pulmonary Toxicity Assessment after a Single Intratracheal Inhalation of Chlorhexidine Aerosol in Mice. Toxics. 2023; 11(11):910. https://doi.org/10.3390/toxics11110910
Chicago/Turabian StyleZhang, Jianzhong, Xinmin Jiang, Xin Li, He Sun, Mingyue Wang, Wanjun Zhang, Haonan Li, Hongmei Wang, Min Zhuang, Lin Zhang, and et al. 2023. "Pulmonary Toxicity Assessment after a Single Intratracheal Inhalation of Chlorhexidine Aerosol in Mice" Toxics 11, no. 11: 910. https://doi.org/10.3390/toxics11110910
APA StyleZhang, J., Jiang, X., Li, X., Sun, H., Wang, M., Zhang, W., Li, H., Wang, H., Zhuang, M., Zhang, L., Lu, L., & Tang, J. (2023). Pulmonary Toxicity Assessment after a Single Intratracheal Inhalation of Chlorhexidine Aerosol in Mice. Toxics, 11(11), 910. https://doi.org/10.3390/toxics11110910







