CXCL17 Attenuates Diesel Exhaust Emissions Exposure-Induced Lung Damage by Regulating Macrophage Function
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Ethics Considerations
2.2. Exposure Chamber and Characterization
2.3. Study Design
2.4. Respiratory Parameter Evaluation
2.5. Collection of Blood, Bronchoalveolar Lavage Fluid (BALF), and Lungs
2.6. Carbon Content in Airway Macrophages (CCAM) Assay
2.7. Masson Staining and Hematoxylin–Eosin (HE) Staining
2.8. Immunohistochemistry (IHC)
2.9. Western Blot Analysis
2.10. Quantitative Real-Time Polymerase Chain Reaction
2.11. Enzyme-Linked Immunosorbent Assay
2.12. Cell Lines and Culture
2.13. Cell Migration Assay
2.14. Flow Cytometry
2.15. Statistical Analyses
3. Results
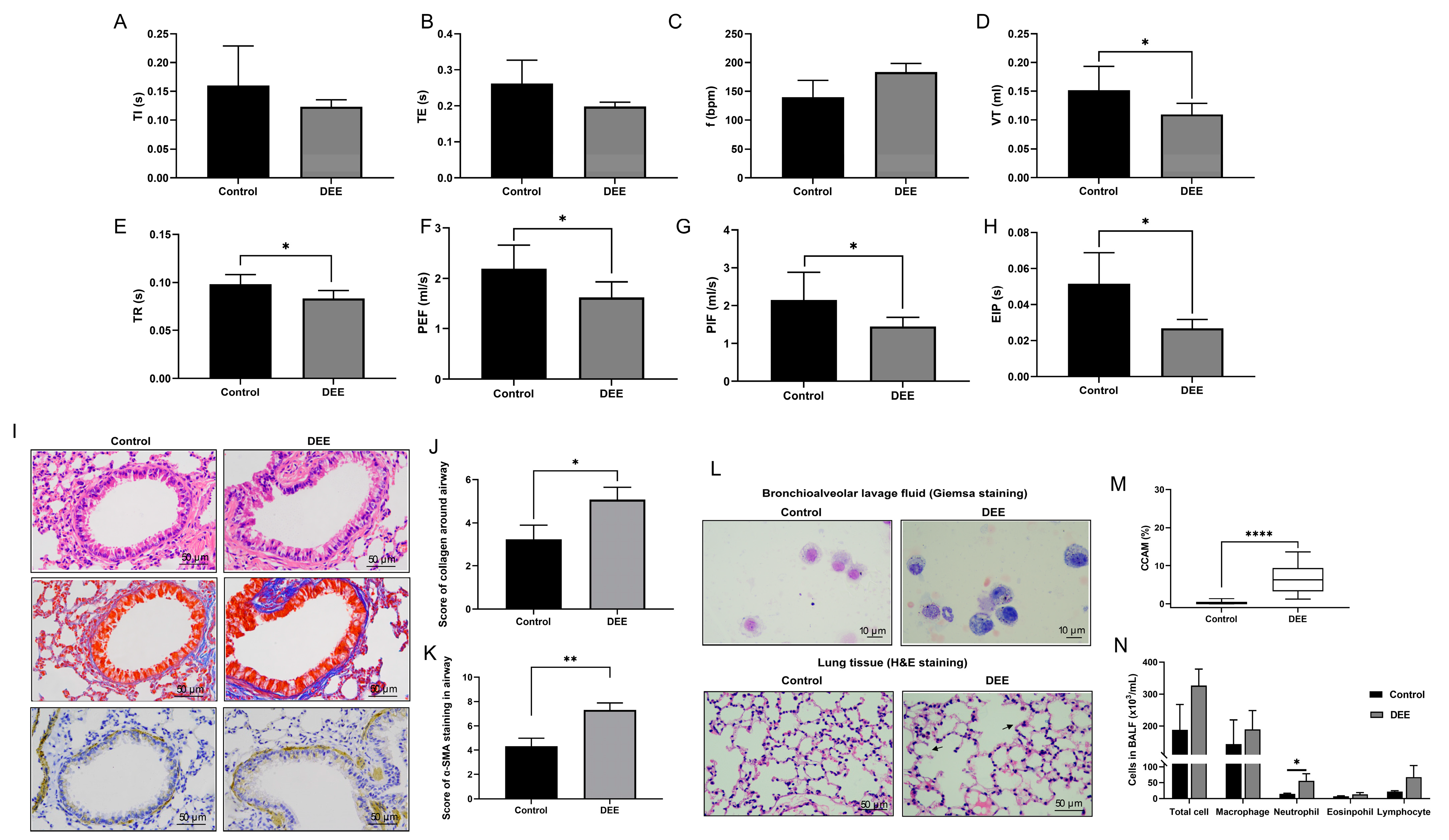
3.1. Effects of Chronic DEE Exposure on Respiratory Parameters, Airway Inflammation, and Airway Injury and Remodeling in Mice
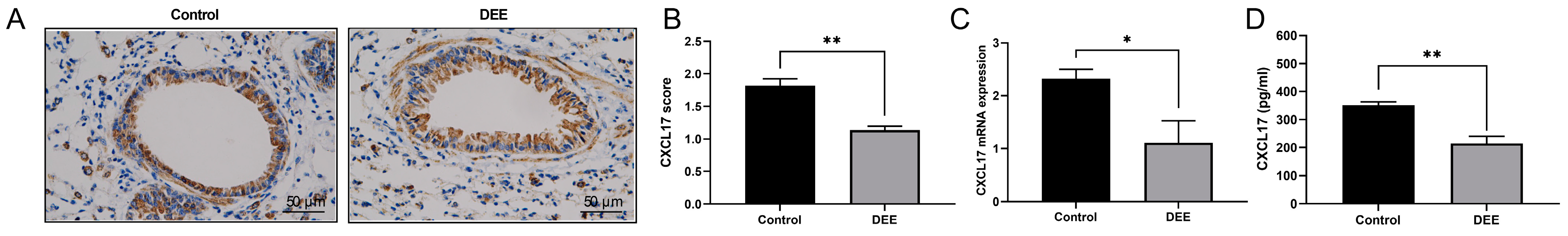
3.2. DEE Exposure Can Downregulate the CXCL17 Expression in Airway Epithelium
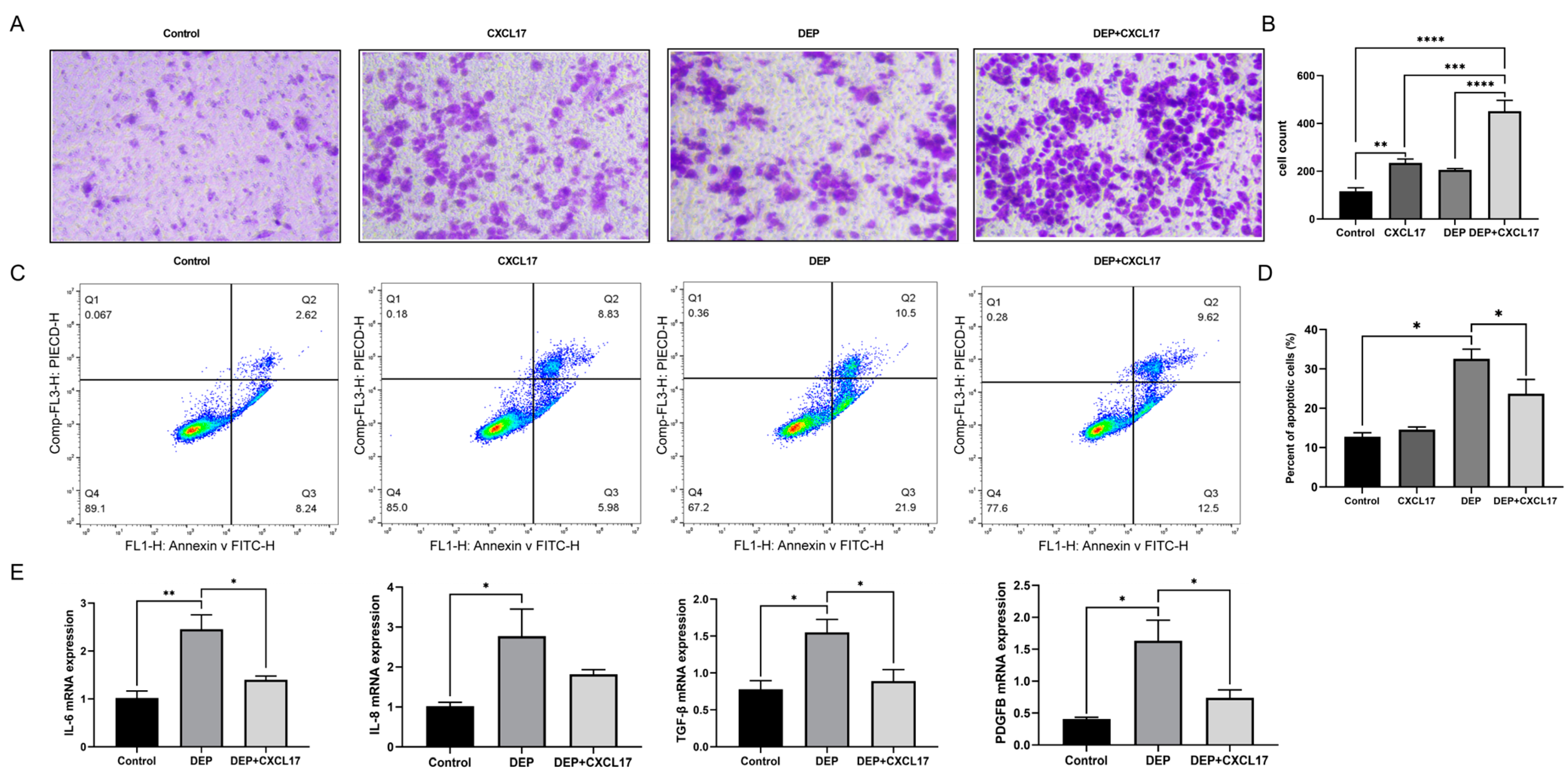
3.3. Effects of DEP on Inflammation and Apoptosis of HBE Cells
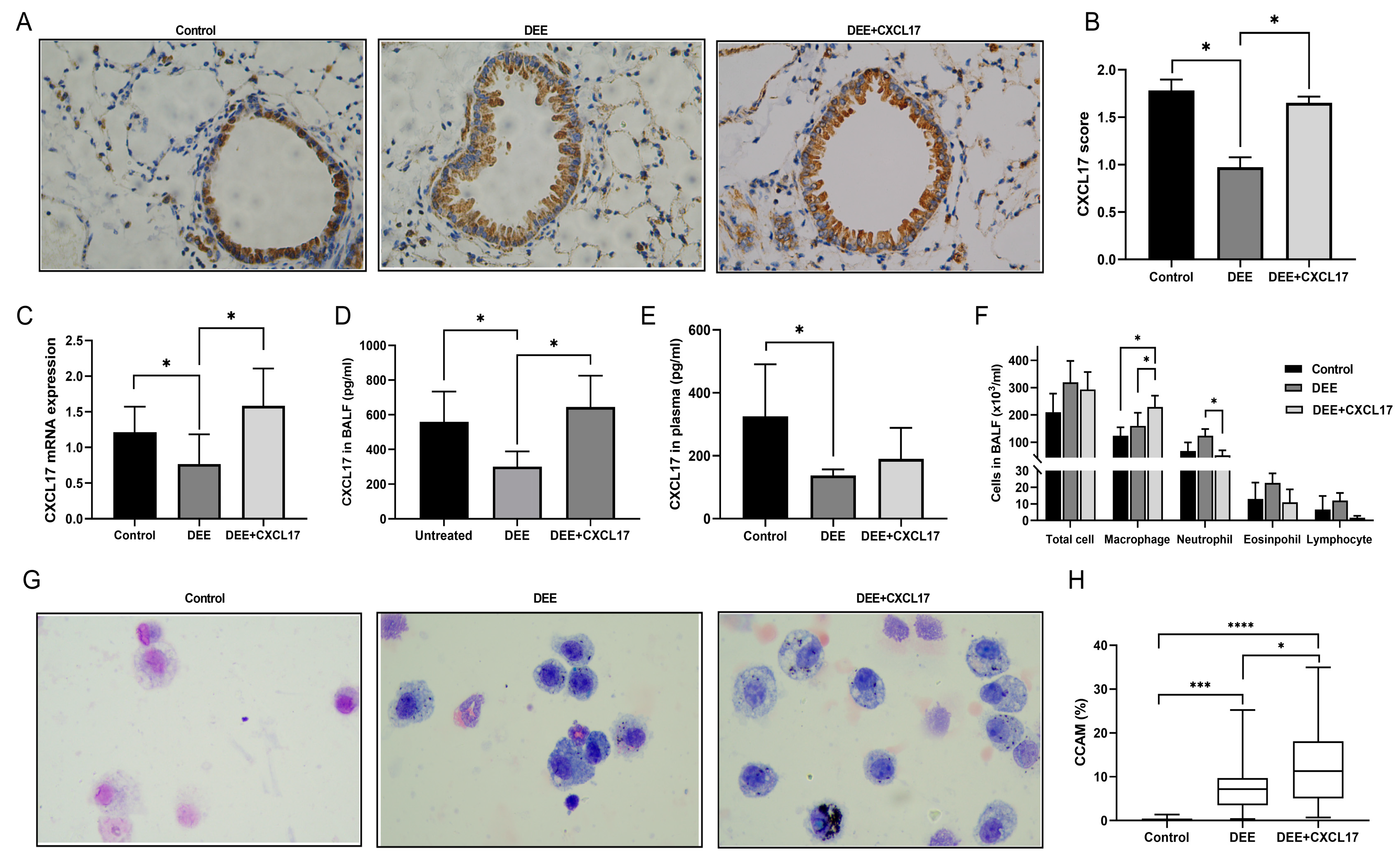
3.4. CXCL17 Overexpression Promotes Alveolar Macrophage Recruitment and Clearance of DEP in Mice
3.5. CXCL17 Overexpression Improved Respiratory Parameters and Alleviated Airway Injury and Airway Remodeling in the DEE-Exposed Mice
3.6. CXCL17-Mediated Macrophage Can Alleviate DEP-Induced HBE Cell Injury
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Air Pollution. Available online: https://www.who.int/airpollution/en/ (accessed on 12 December 2019).
- Zhou, M.; Wang, H.; Zeng, X.; Yin, P.; Zhu, J.; Chen, W.; Li, X.; Wang, L.; Wang, L.; Liu, Y.; et al. Mortality, morbidity, and risk factors in China and its provinces, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 394, 1145–1158. [Google Scholar] [CrossRef] [PubMed]
- Boland, S.; Baeza-Squiban, A.; Fournier, T.; Houcine, O.; Gendron, M.C.; Chévrier, M.; Jouvenot, G.; Coste, A.; Aubier, M.; Marano, F. Diesel exhaust particles are taken up by human airway epithelial cells in vitro and alter cytokine production. Am. J. Physiol. 1999, 276, L604–L613. [Google Scholar] [CrossRef]
- Steiner, S.; Bisig, C.; Petri-Fink, A.; Rothen-Rutishauser, B. Diesel exhaust: Current knowledge of adverse effects and underlying cellular mechanisms. Arch. Toxicol. 2016, 90, 1541–1553. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Xu, X.; Zhang, C.; Luo, J.; Lv, N.; Shi, L.; Ji, A.; Gao, M.; Chen, F.; Cui, L.; et al. In ovo very early-in-life exposure to diesel exhaust induced cardiopulmonary toxicity in a hatchling chick model. Environ. Pollut. 2020, 264, 114718. [Google Scholar] [CrossRef]
- Kim, B.G.; Lee, P.H.; Lee, S.H.; Kim, Y.E.; Shin, M.Y.; Kang, Y.; Bae, S.H.; Kim, M.J.; Rhim, T.; Park, C.S.; et al. Long-Term Effects of Diesel Exhaust Particles on Airway Inflammation and Remodeling in a Mouse Model. Allergy Asthma Immunol. Res. 2016, 8, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Øvrevik, J.; Refsnes, M.; Låg, M.; Brinchmann, B.C.; Schwarze, P.E.; Holme, J.A. Triggering Mechanisms and Inflammatory Effects of Combustion Exhaust Particles with Implication for Carcinogenesis. Basic Clin. Pharmacol. Toxicol. 2017, 121 (Suppl. S3), 55–62. [Google Scholar] [CrossRef]
- Huang, N.H.; Wang, Q.; Xu, D.Q. Immunological effect of PM2.5 on cytokine production in female Wistar rats. Biomed. Environ. Sci. 2008, 21, 63–68. [Google Scholar] [CrossRef]
- Miyata, R.; van Eeden, S.F. The innate and adaptive immune response induced by alveolar macrophages exposed to ambient particulate matter. Toxicol. Appl. Pharmacol. 2011, 257, 209–226. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.M.; Antonini, J.M.; Barger, M.W.; Butterworth, L.; Roberts, B.R.; Ma, J.K.; Castranova, V.; Ma, J.Y. Diesel exhaust particles suppress macrophage function and slow the pulmonary clearance of Listeria monocytogenes in rats. Environ. Health Perspect. 2001, 109, 515–521. [Google Scholar] [CrossRef]
- Jaguin, M.; Fardel, O.; Lecureur, V. Exposure to diesel exhaust particle extracts (DEPe) impairs some polarization markers and functions of human macrophages through activation of AhR and Nrf2. PLoS ONE 2015, 10, e0116560. [Google Scholar] [CrossRef]
- Lawal, A.O. Diesel Exhaust Particles and the Induction of Macrophage Activation and Dysfunction. Inflammation 2018, 41, 356–363. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, H.; Sun, L.; Gao, W.; Xiong, Y.; Ma, A.; Liu, X.; Shen, L.; Li, Q.; Yang, H. Manipulation of macrophage polarization by peptide-coated gold nanoparticles and its protective effects on acute lung injury. J. Nanobiotechnology 2020, 18, 38. [Google Scholar] [CrossRef] [PubMed]
- Tu, G.W.; Shi, Y.; Zheng, Y.J.; Ju, M.J.; He, H.Y.; Ma, G.G.; Hao, G.W.; Luo, Z. Glucocorticoid attenuates acute lung injury through induction of type 2 macrophage. J. Transl. Med. 2017, 15, 181. [Google Scholar] [CrossRef] [PubMed]
- Burkhardt, A.M.; Tai, K.P.; Flores-Guiterrez, J.P.; Vilches-Cisneros, N.; Kamdar, K.; Barbosa-Quintana, O.; Valle-Rios, R.; Hevezi, P.A.; Zuñiga, J.; Selman, M.; et al. CXCL17 is a mucosal chemokine elevated in idiopathic pulmonary fibrosis that exhibits broad antimicrobial activity. J. Immunol. 2012, 188, 6399–6406. [Google Scholar] [CrossRef] [PubMed]
- Pisabarro, M.T.; Leung, B.; Kwong, M.; Corpuz, R.; Frantz, G.D.; Chiang, N.; Vandlen, R.; Diehl, L.J.; Skelton, N.; Kim, H.S.; et al. Cutting edge: Novel human dendritic cell- and monocyte-attracting chemokine-like protein identified by fold recognition methods. J. Immunol. 2006, 176, 2069–2073. [Google Scholar] [CrossRef]
- Tomankova, T.; Kriegova, E.; Liu, M. Chemokine receptors and their therapeutic opportunities in diseased lung: Far beyond leukocyte trafficking. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 308, L603–L618. [Google Scholar] [CrossRef]
- Zlotnik, A.; Yoshie, O. The chemokine superfamily revisited. Immunity 2012, 36, 705–716. [Google Scholar] [CrossRef]
- Choreño-Parra, J.A.; Thirunavukkarasu, S.; Zúñiga, J.; Khader, S.A. The protective and pathogenic roles of CXCL17 in human health and disease: Potential in respiratory medicine. Cytokine Growth Factor Rev. 2020, 53, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Burkhardt, A.M.; Maravillas-Montero, J.L.; Carnevale, C.D.; Vilches-Cisneros, N.; Flores, J.P.; Hevezi, P.A.; Zlotnik, A. CXCL17 is a major chemotactic factor for lung macrophages. J. Immunol. 2014, 193, 1468–1474. [Google Scholar] [CrossRef]
- Saito, Y.; Azuma, A.; Kudo, S.; Takizawa, H.; Sugawara, I. Long-term inhalation of diesel exhaust affects cytokine expression in murine lung tissues: Comparison between low- and high-dose diesel exhaust exposure. Exp. Lung Res. 2002, 28, 493–506. [Google Scholar] [CrossRef]
- Singh, N.; Arora, N. Diesel exhaust exposure in mice induces pulmonary fibrosis by TGF-β/Smad3 signaling pathway. Sci. Total Environ. 2022, 807, 150623. [Google Scholar] [CrossRef]
- Bai, Y.; Brugha, R.E.; Jacobs, L.; Grigg, J.; Nawrot, T.S.; Nemery, B. Carbon loading in airway macrophages as a biomarker for individual exposure to particulate matter air pollution—A critical review. Environ. Int. 2015, 74, 32–41. [Google Scholar] [CrossRef]
- Frankenberger, M.; Menzel, M.; Betz, R.; Kassner, G.; Weber, N.; Kohlhäufl, M.; Häussinger, K.; Ziegler-Heitbrock, L. Characterization of a population of small macrophages in induced sputum of patients with chronic obstructive pulmonary disease and healthy volunteers. Clin. Exp. Immunol. 2004, 138, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.J.; Zhang, G.S.; Zhang, X.; Qiu, Z.W.; Wang, P.L.; Li, Y.W.; Li, W.; Xie, Q.M.; Ke, Y.H.; Lee, J.J.; et al. Protein tyrosine phosphatase SHP2 regulates TGF-β1 production in airway epithelia and asthmatic airway remodeling in mice. Allergy 2012, 67, 1547–1556. [Google Scholar] [CrossRef] [PubMed]
- Plant, P.J.; North, M.L.; Ward, A.; Ward, M.; Khanna, N.; Correa, J.; Scott, J.A.; Batt, J. Hypertrophic airway smooth muscle mass correlates with increased airway responsiveness in a murine model of asthma. Am. J. Respir. Cell Mol. Biol. 2012, 46, 532–540. [Google Scholar] [CrossRef]
- Ryu, M.H.; Afshar, T.; Li, H.; Wooding, D.J.; Orach, J.; Zhou, J.S.; Murphy, S.; Lau, K.S.K.; Schwartz, C.; Yuen, A.C.Y.; et al. Impact of Exposure to Diesel Exhaust on Inflammation Markers and Proteases in Former Smokers with Chronic Obstructive Pulmonary Disease: A Randomized, Double-blinded, Crossover Study. Am. J. Respir. Crit. Care Med. 2022, 205, 1046–1052. [Google Scholar] [CrossRef]
- Thurston, G.D.; Balmes, J.R.; Garcia, E.; Gilliland, F.D.; Rice, M.B.; Schikowski, T.; Van Winkle, L.S.; Annesi-Maesano, I.; Burchard, E.G.; Carlsten, C.; et al. Outdoor Air Pollution and New-Onset Airway Disease. An Official American Thoracic Society Workshop Report. Ann. Am. Thorac. Soc. 2020, 17, 387–398. [Google Scholar] [CrossRef]
- Tiotiu, A.I.; Novakova, P.; Nedeva, D.; Chong-Neto, H.J.; Novakova, S.; Steiropoulos, P.; Kowal, K. Impact of Air Pollution on Asthma Outcomes. Int. J. Environ. Res. Public Health 2020, 17, 6212. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, J.; Ma, Q.; Tang, J.; Jiang, M.; Cao, X.; Lin, L.; Kong, N.; Yu, S.; Sood, A.; et al. Chronic exposure to diesel exhaust may cause small airway wall thickening without lumen narrowing: A quantitative computerized tomography study in Chinese diesel engine testers. Part. Fibre Toxicol. 2021, 18, 14. [Google Scholar] [CrossRef] [PubMed]
- Bendtsen, K.M.; Brostrøm, A.; Koivisto, A.J.; Koponen, I.; Berthing, T.; Bertram, N.; Kling, K.I.; Dal Maso, M.; Kangasniemi, O.; Poikkimäki, M.; et al. Airport emission particles: Exposure characterization and toxicity following intratracheal instillation in mice. Part. Fibre Toxicol. 2019, 16, 23. [Google Scholar] [CrossRef]
- Beentjes, D.; Shears, R.K.; French, N.; Neill, D.R.; Kadioglu, A. Mechanistic Insights into the Impact of Air Pollution on Pneumococcal Pathogenesis and Transmission. Am. J. Respir. Crit. Care Med. 2022, 206, 1070–1080. [Google Scholar] [CrossRef]
- Champion, J.A.; Mitragotri, S. Shape induced inhibition of phagocytosis of polymer particles. Pharm. Res. 2009, 26, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Xie, W.; Zhou, L. Mucosal chemokine CXCL17: What is known and not known. Scand J. Immunol. 2021, 93, e12965. [Google Scholar] [CrossRef] [PubMed]
- Dawson, R.E.; Jenkins, B.J.; Saad, M.I. IL-6 family cytokines in respiratory health and disease. Cytokine 2021, 143, 155520. [Google Scholar] [CrossRef] [PubMed]
- Rincon, M.; Irvin, C.G. Role of IL-6 in asthma and other inflammatory pulmonary diseases. Int. J. Biol. Sci. 2012, 8, 1281–1290. [Google Scholar] [CrossRef]
- Ha, H.; Debnath, B.; Neamati, N. Role of the CXCL8-CXCR1/2 Axis in Cancer and Inflammatory Diseases. Theranostics 2017, 7, 1543–1588. [Google Scholar] [CrossRef]
- Grubek-Jaworska, H.; Paplińska, M.; Hermanowicz-Salamon, J.; Białek-Gosk, K.; Dąbrowska, M.; Grabczak, E.; Domagała-Kulawik, J.; Stępień, J.; Chazan, R. IL-6 and IL-13 in induced sputum of COPD and asthma patients: Correlation with respiratory tests. Respiration 2012, 84, 101–107. [Google Scholar] [CrossRef]
- Zhang, X.; Zheng, H.; Zhang, H.; Ma, W.; Wang, F.; Liu, C.; He, S. Increased interleukin (IL)-8 and decreased IL-17 production in chronic obstructive pulmonary disease (COPD) provoked by cigarette smoke. Cytokine 2011, 56, 717–725. [Google Scholar] [CrossRef]
- Huang, H.; Huang, X.; Zeng, K.; Deng, F.; Lin, C.; Huang, W. Interleukin-6 is a Strong Predictor of the Frequency of COPD Exacerbation Within 1 Year. Int. J. Chron Obs. Pulmon Dis. 2021, 16, 2945–2951. [Google Scholar] [CrossRef]
- Hao, D.; Li, Y.; Shi, J.; Jiang, J. Baicalin alleviates chronic obstructive pulmonary disease through regulation of HSP72-mediated JNK pathway. Mol. Med. 2021, 27, 53. [Google Scholar] [CrossRef]
- Zhu, L.; Xu, F.; Kang, X.; Zhou, J.; Yao, Q.; Lin, Y.; Zhang, W. The antioxidant N-acetylcysteine promotes immune response and inhibits epithelial-mesenchymal transition to alleviate pulmonary fibrosis in chronic obstructive pulmonary disease by suppressing the VWF/p38 MAPK axis. Mol. Med. 2021, 27, 97. [Google Scholar] [CrossRef]
- Ristovski, Z.D.; Miljevic, B.; Surawski, N.C.; Morawska, L.; Fong, K.M.; Goh, F.; Yang, I.A. Respiratory health effects of diesel particulate matter. Respirology 2012, 17, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Taxell, P.; Santonen, T. Diesel Engine Exhaust: Basis for Occupational Exposure Limit Value. Toxicol. Sci. 2017, 158, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.; Li, L.; Yao, W.; Qin, W.; Hao, C.; Lu, L. Influence of Silica Exposure for Lung Silicosis Rat. Dis. Markers 2021, 2021, 6268091. [Google Scholar] [CrossRef]
- Shahabi, R.; Dehghani, M.; Javad Moosavi, S.A.; Shahabi, B.; Poordakan, O.; Sadeghi, M.; Aryan, L.; Ghasempoor, A.; Aghanasiri, F.; Mohseni, M.; et al. The effect of nanoparticles on pulmonary fibrosis: A systematic review and Meta-analysis of preclinical studies. Arch. Environ. Occup. Health 2022, 77, 684–694. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.Z.; Sun, H.; Chen, J.H. Histone deacetylases 3 deletion restrains PM2.5-induced mice lung injury by regulating NF-κB and TGF-β/Smad2/3 signaling pathways. Biomed. Pharmacother. 2017, 85, 756–762. [Google Scholar] [CrossRef]
- Shaikh, S.B.; Prabhakar Bhandary, Y. Effect of curcumin on IL-17A mediated pulmonary AMPK kinase/cyclooxygenase-2 expressions via activation of NFκB in bleomycin-induced acute lung injury in vivo. Int. Immunopharmacol. 2020, 85, 106676. [Google Scholar] [CrossRef]
- Wollin, L.; Maillet, I.; Quesniaux, V.; Holweg, A.; Ryffel, B. Antifibrotic and anti-inflammatory activity of the tyrosine kinase inhibitor nintedanib in experimental models of lung fibrosis. J. Pharmacol. Exp. Ther. 2014, 349, 209–220. [Google Scholar] [CrossRef]
- Lin, X.; Lin, W.; Zhuang, Y.; Gao, F. Angiotensin-Converting Enzyme 2 Inhibits Lipopolysaccharide-Caused Lung Fibrosis via Downregulating the Transforming Growth Factor β-1/Smad2/Smad3 Pathway. J. Pharmacol. Exp. Ther. 2022, 381, 236–246. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, Y.; Mu, C.; Wang, J.; Wang, Y.; Hu, W.; Zhu, W.; Yu, X.; Hao, W.; Zheng, Y.; Li, Q.; et al. CXCL17 Attenuates Diesel Exhaust Emissions Exposure-Induced Lung Damage by Regulating Macrophage Function. Toxics 2023, 11, 646. https://doi.org/10.3390/toxics11080646
Yin Y, Mu C, Wang J, Wang Y, Hu W, Zhu W, Yu X, Hao W, Zheng Y, Li Q, et al. CXCL17 Attenuates Diesel Exhaust Emissions Exposure-Induced Lung Damage by Regulating Macrophage Function. Toxics. 2023; 11(8):646. https://doi.org/10.3390/toxics11080646
Chicago/Turabian StyleYin, Yize, Chaohui Mu, Jiahui Wang, Yixuan Wang, Wenmin Hu, Wenjing Zhu, Xinjuan Yu, Wanming Hao, Yuxin Zheng, Qinghai Li, and et al. 2023. "CXCL17 Attenuates Diesel Exhaust Emissions Exposure-Induced Lung Damage by Regulating Macrophage Function" Toxics 11, no. 8: 646. https://doi.org/10.3390/toxics11080646
APA StyleYin, Y., Mu, C., Wang, J., Wang, Y., Hu, W., Zhu, W., Yu, X., Hao, W., Zheng, Y., Li, Q., & Han, W. (2023). CXCL17 Attenuates Diesel Exhaust Emissions Exposure-Induced Lung Damage by Regulating Macrophage Function. Toxics, 11(8), 646. https://doi.org/10.3390/toxics11080646





