Spatial Differentiation Characteristics and Evaluation of Cu and Cd in Paddy Soil around a Copper Smelter
Abstract
1. Introduction
2. Materials and Methods
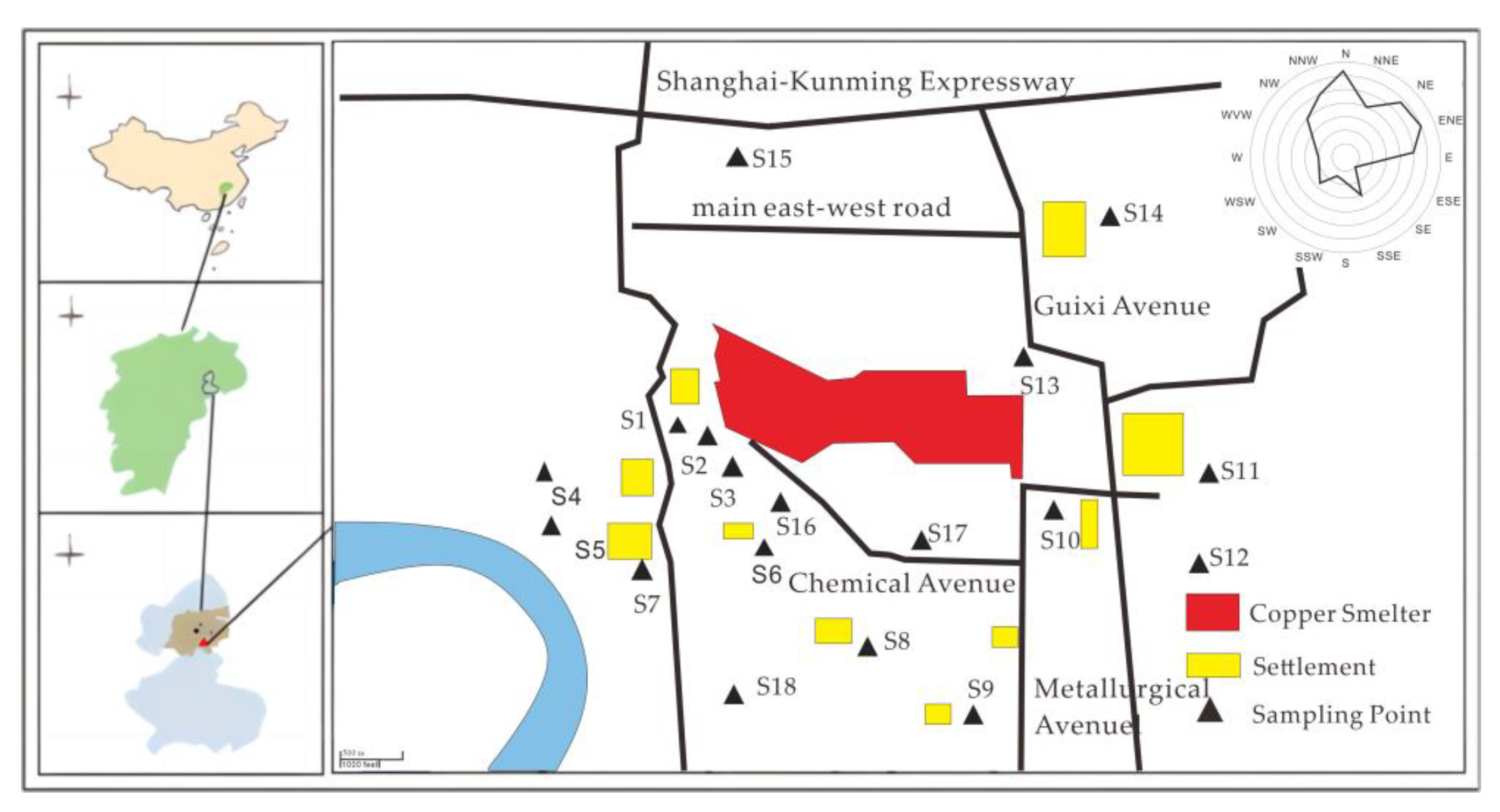
2.1. Study Site
2.2. Soil Sampling
2.3. Sample Analysis
2.4. Methods of Pollution Assessment
2.4.1. Geo-Accumulation Index
2.4.2. Migration Rate (MR)
2.5. Data Analysis
3. Results
3.1. Characteristics of the Soil around a Copper Smelter
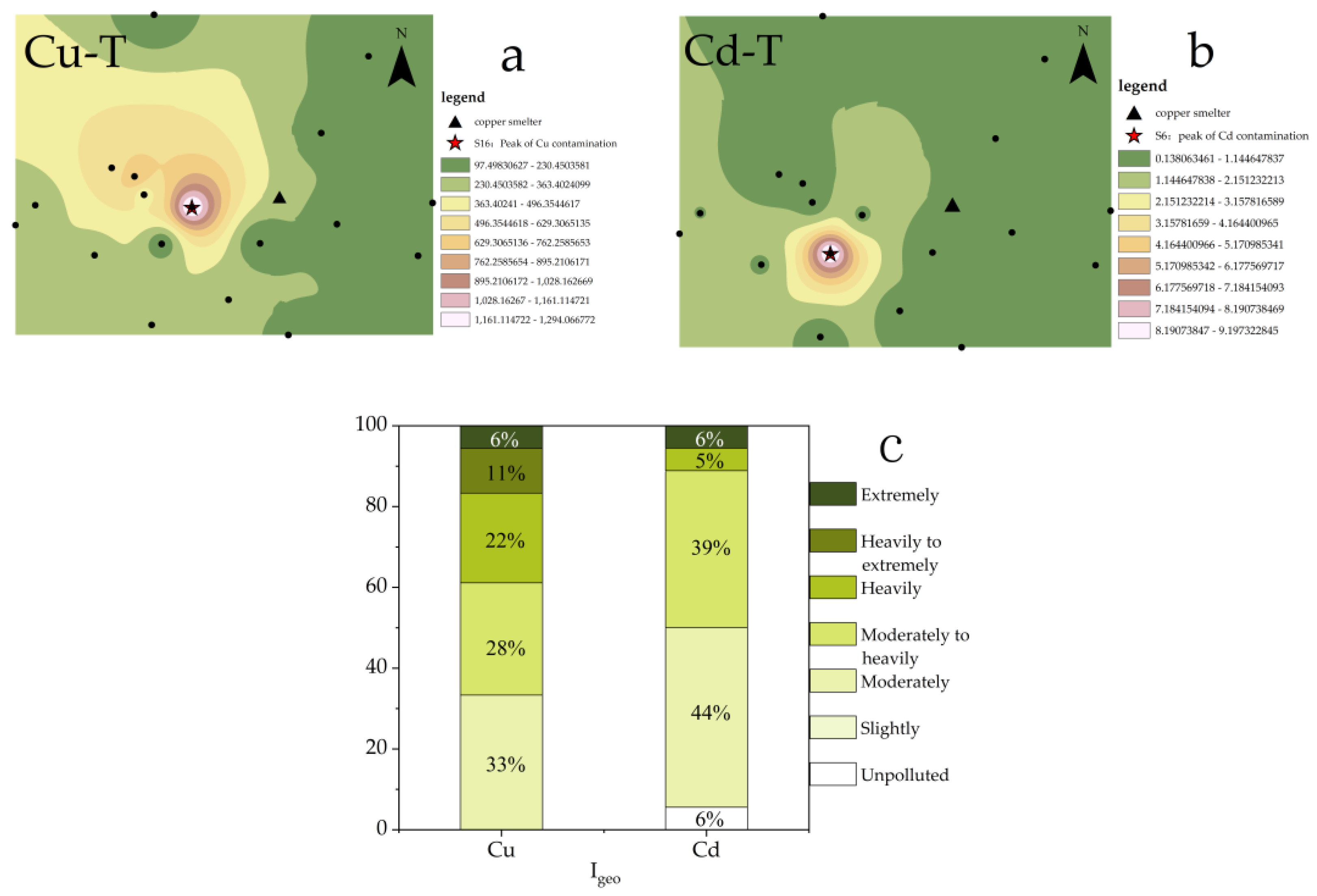
3.2. Distribution and Evaluation of the Total PTEs
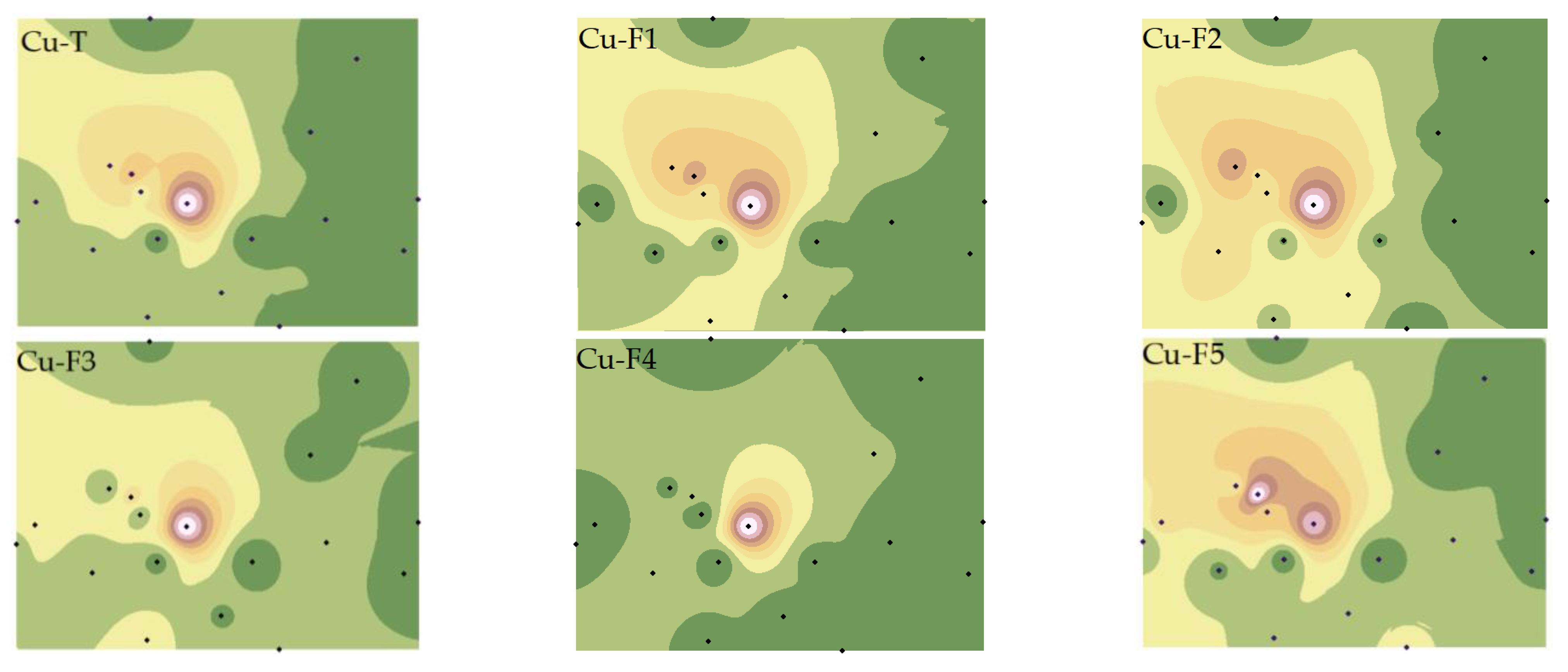
3.3. Spatial Distribution of PTEs’ Speciations
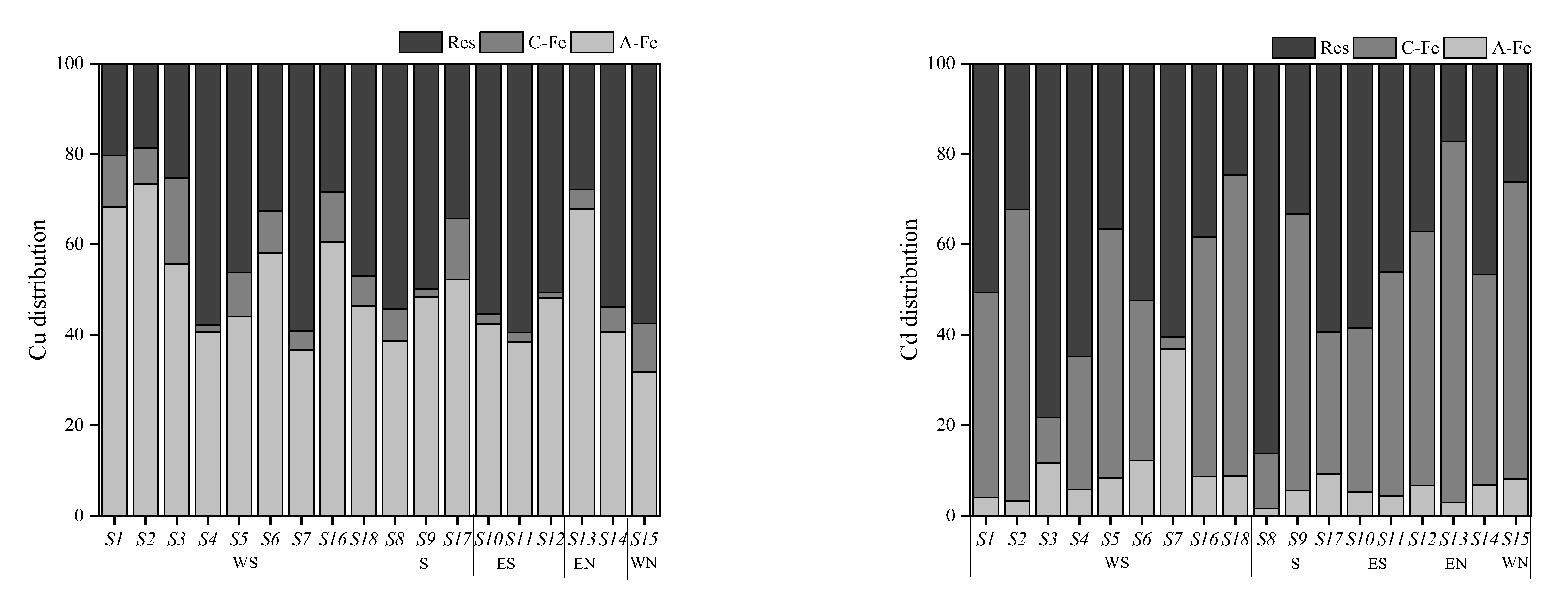
3.4. Distribution of PTEs in Soil Colloid
4. Discussion
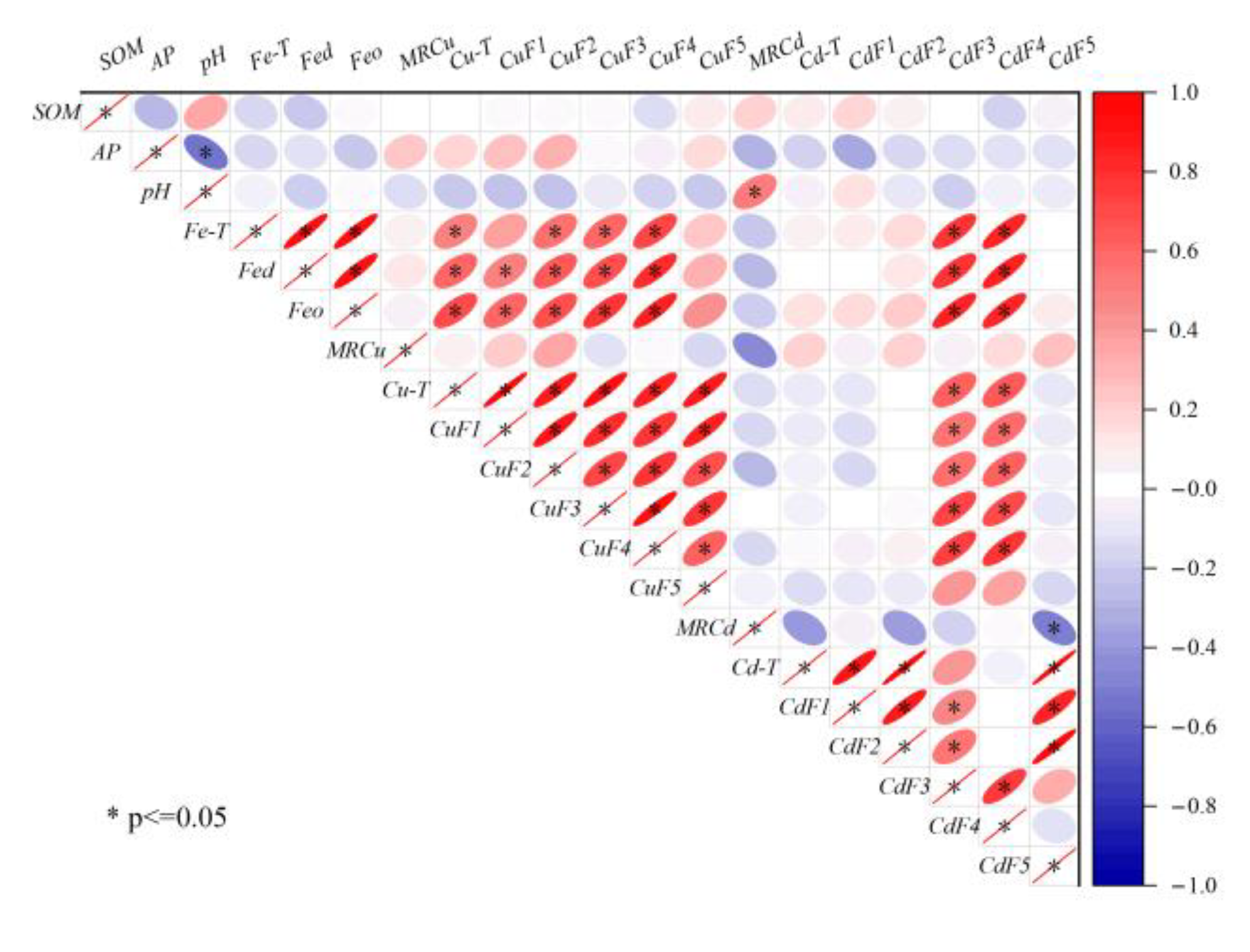
4.1. Factors Affecting the Spatial Heterogeneity of Soil PTEs
4.2. Assessment of PTE Pollution around the Smelter
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hashem, S.; Azam, J.; Vahidreza, J.; Rainer, S. Spatial distribution of copper and other elements in the soils around the Sarcheshmeh copper smelter in southeastern Iran. Atmos. Pollut. Res. 2020, 11, 1681–1691. [Google Scholar]
- Liu, L.; Wu, L.; Luo, Y.; Zhang, C.; Jiang, Y.; Qiu, X. The impact of a copper smelter on adjacent soil zinc and cadmium fractions and soil organic carbon. J. Soil Sediment. 2010, 10, 808–817. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, C. Natural and Human Factors Affect the Distribution of soil heavy metal pollution: A review. Water Air Soil Pollut. 2020, 231, 350. [Google Scholar] [CrossRef]
- Dasharathy, S.; Arjunan, S.; Maliyur, B.A.; Murugasen, V.; Ramachandran, S.; Keshav, R.; Murugan, R. Mutagenic, carcinogenic, and teratogenic effect of heavy metals. Evid.-Based Complement. Altern. Med. 2022, 2022, 8011953. [Google Scholar] [CrossRef] [PubMed]
- Aminur, R.; Kazuhiro, Y.; Monirul, M.I.; Genta, K. Investigation of efficient adsorption of toxic heavy metals (chromium, lead, cadmium) from aquatic environment using orange peel cellulose as adsorbent. Sustainability 2023, 15, 4470. [Google Scholar]
- Zhang, X.; Zhong, T.; Liu, L.; Ouyang, X. Impact of soil heavy metal pollution on food safety in China. PLoS ONE 2017, 10, e0135182. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, H.; Gao, B.; Xu, D. Improved enrichment factor model for correcting and predicting the evaluation of heavy metals in sediments. Sci. Total Environ. 2021, 755 Pt 1, 142437. [Google Scholar] [CrossRef]
- Salih, M.-A.; Jawdat, A.-J.-M.; Zaki, A.-H. Urban geochemistry assessment using pollution indices: A case study of urban soil in Kirkuk, Iraq. Environ. Earth Sci. 2019, 78, 587. [Google Scholar]
- Ju, T.; Lei, M. Geo-accumulation index method to optimize the evaluation method of polymetallic environment quality: Taking developed agricultural areas as an example. Chin. J. Environ. Sci. 2022, 43, 957–964. [Google Scholar]
- Yan, F.; Liu, C.; Wei, B. Evaluation of heavy metal pollution in the sediment of Poyang Lake based on stochastic geo-accumulation model (SGM). Sci. Total Environ. 2019, 659, 1–6. [Google Scholar]
- Hazzeman, H.; Juen, L.L.; Zaharin, A.A.; Farhanna, M.N.; Ayunie, N.A.; Md, Y.F.; Bakar, S.A.; Mangala, P.S. Geo-accumulation index and contamination factors of heavy metals (Zn and Pb) in urban river sediment. Environ. Geochem. Health 2017, 39, 1259–1271. [Google Scholar]
- Mahanta, M.J.; Bhattacharyya, K.G. Total concentrations, fractionation and mobility of heavy metals in soils of urban area of Guwahati, India. Environ. Monit. Assess. 2011, 173, 221–240. [Google Scholar] [CrossRef]
- Qu, C.; Chen, J.; Mortimer, M.; Wu, Y.; Cai, P.; Huang, Q. Humic acids restrict the transformation and the stabilization of Cd by iron (hydr)oxides. J. Hazard. Mater. 2022, 430, 128365. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, Z.; Chen, Z.; Pan, X.; Luo, J.; Huang, F.; Zhang, X.; Lin, Q. Microbial community characteristics of cadmium speciation transformation in soil after iron-based materials application. Appl. Soil Ecol. 2023, 183, 104745. [Google Scholar] [CrossRef]
- Burachevskaya, M.; Minkina, T.; Mandzhieva, S.; Bauer, T.; Chaplygin, V.; Sushkova, S.; Tsitsuashvili, V.; Popileshko, Y. Chemical partitioning of Zn in soil: Application of two sequential extraction procedures. Geochem. Explor. Environ. Anal. 2019, 19, 93–100. [Google Scholar] [CrossRef]
- Wang, J.J. The Speciations of Heavy Metals in Soils Around a Mining Area in Southern China and the Transformation of Heavy Metal under Reductive Conditions. Master’s Thesis, Huazhong Agricultural University, Wuhan, China, 2018. (In Chinese). [Google Scholar]
- Han, F.X.; Banin, A. Long-term transformations and redistribution of potentially toxic heavy metals in arid-zone soils incubated: I. Under saturated conditions. Water Air Soil Pollut. 1997, 95, 399–423. [Google Scholar]
- Burachevskaya, M.; Minkina, T.; Mandzhieva, S.; Bauer, T.; Chaplygin, V.; Zamulina, I.; Sushkova, S.; Fedorenko, A.; Ghazaryan, K.; Movsesyan, H.; et al. Study of copper, lead, and zinc speciation in the Haplic Chernozem surrounding coal-fired power plant. Appl. Geochem. 2019, 104, 102–108. [Google Scholar] [CrossRef]
- Liang, J.; Zhou, J.; Ma, Y.; Cui, J.; Xu, L. Status and assessment of heavy metals pollution in paddy soil around the spoil area of smeltery. J. Agro-Environ. Sci. 2009, 28, 877–882. (In Chinese) [Google Scholar]
- GB 15618–2018; Soil Environmental Quality-Risk Control Standard for Soil Contamination of Agricultural Land. Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 2018.
- Long, A.-H.; Liu, J.-J.; Ni, C.-Y.; Huang, G.-F.; Tang, H.-Y. Assessment on the characteristic of heavy metals contaminated farmland soil around Guixi Smeltery Jiangxi Province. Chin. J. Soil Sci. 2006, 37, 1212–1217. (In Chinese) [Google Scholar]
- Zhou, J.; Cui, H.-B. Engineering and prospect for remediating large-scale arable land contaminated by heavy metals with “demonstration project of soil remediation on the periphery of Guixi smelter” as example. Bull. Chin. Acad. Sci. 2014, 29, 272, 336–343. (In Chinese) [Google Scholar]
- Zhang, J.; Sun, H.; Xie, L.; Hou, D. Changes in the ecological risk of heavy metals after soil remediation in a typical Brownfield: A case study of Guixi smelter in Jiangxi Province. Acta Ecol. Sin. 2017, 37, 6128–6137. [Google Scholar]
- HJ/T 166–2004; The Technical Specification for Soil Environmental Monitoring. Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 2004.
- Bao, S.D. Soil and Agricultural Chemistry Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2005. (In Chinese) [Google Scholar]
- Zhang, Z.-W.; Zhu, Z.-X.; Fu, W.-L.; Wen, Z.-L. Morphology of soil iron oxides and its correlation with soil-forming process and forming conditions in a karst mountain. Environ. Sci. 2012, 33, 2013–2020. [Google Scholar]
- Liu, Z.; Zhou, M.; Liao, W.; Liu, J.; Luo, C.; Lu, C.; Chen, Z.; Zhu, H. Fertilizer-holding performance of graphene on soil colloids based on double electric layer theory. Materials 2023, 16, 2578. [Google Scholar] [CrossRef]
- Li, X.; Li, L.; Zhou, Z.; Li, T.; An, J.; Zhang, S.; Xu, X.; Pu, Y.; Wang, G.; Jia, Y.; et al. Soil potentially toxic element pollution at different urbanization intensities: Quantitative source apportionment and source-oriented health risk assessment. Environ. Earth Sci. 2023, 251, 114550. [Google Scholar] [CrossRef]
- National Soil Survey Office. Soil Species of China; China Agriculture Press: Beijing, China, 1993; Volume 1. (In Chinese) [Google Scholar]
- Wang, X.; Li, L.; Guo, N.; Xin, Z.; Li, X.; Sun, X.; Li, Y. A comprehensive exploration on pollution characteristics and ecological risks of heavy metals in surface paddy soils around a large copper smelter, southeast China. Sustainability 2021, 13, 13359. [Google Scholar] [CrossRef]
- Zhao, Z.; Shi, Y.; Mao, X.; Li, H. Characteristics assessment and source analysis of heavy metal pollution in soil surrounding a smelter. Chin. J. Inorg. Anal. Chem. 2020, 10, 22–27. (In Chinese) [Google Scholar]
- Sun, B.; Cao, Y. Spatial variation and affecting factors of Cu and Cd pollution in paddy soil in hilly region. J. Agro-Environ. Sci. 2006, 25, 922–928. (In Chinese) [Google Scholar]
- Xiao, H.-Y.; Jiang, S.-Y.; Wu, D.-S.; Zhou, W.-B. Risk element (As, Cd, Cu, Pb, and Zn) contamination of soils and edible vegetables in the vicinity of Guixi smelter, south China. Soil Sediment Contam. 2011, 20, 592–604. [Google Scholar] [CrossRef]
- Chowdhury, M.A.R.; Singer, D.M. Trace metal enrichment in the colloidal fraction in soils developing on abandoned mine spoils. Minerals 2022, 12, 1290. [Google Scholar] [CrossRef]
- Rahman, M.S.; Ahmed, Z.; Seefat, S.M.; Alam, R.; Islam, A.; Choudhury, T.R.; Begum, B.A.; Idris, A.M. Assessment of heavy metal contamination in sediment at the newly established tannery industrial Estate in Bangladesh: A case study. Environ. Chem. Ecotoxicol. 2022, 4, 1–12. [Google Scholar] [CrossRef]
- Tang, X.; Wu, Y.; Han, L.; Lan, Z.; Rong, X. Characteristics of heavy metal migration in farmland. Environ. Earth Sci. 2022, 81, 338. [Google Scholar] [CrossRef]
- Hu, B.; Guo, P.; Wu, Y.; Deng, J.; Su, H.; Li, Y.; Nan, Y. Study of soil physicochemical properties and heavy metals of a mangrove restoration wetland. J. Clean. Prod. 2021, 291, 125965. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, H.; Wei, Q.; Farooq, U.; Zhang, Q.; Lu, T.; Wang, X.; Chen, W.; Qi, Z. Mobility of water-soluble aerosol organic matters (WSAOMs) and their effects on soil colloid-mediated transport of heavy metal ions in saturated porous media. J. Hazard. Mater. 2022, 440, 129733. [Google Scholar] [CrossRef]
- Zhao, W.; Gu, C.; Ying, H.; Feng, X.; Zhu, M.; Wang, M.; Tan, W.; Wang, X. Fraction distribution of heavy metals and its relationship with iron in polluted farmland soils around distinct mining area. Appl. Geochem. 2021, 130, 104969. [Google Scholar] [CrossRef]
- Oral, R.; Pagano, G.; Siciliano, A.; Toscanesi, M.; Gravina, M.; Nunzio, A.D.; Palumbo, A.; Thomas, P.J.; Tommasi, F.; Burić, P.; et al. Soil pollution and toxicity in an area affected by emissions from a bauxite processing plant and a power plant in Gardanne (southern France). Ecotoxicol. Environ. Saf. 2019, 170, 55–61. [Google Scholar] [CrossRef]
- Vasarevičius, S.; Danila, V.; Januševičius, T. Immobilisation of cadmium, copper, lead, and nickel in soil using nano zerovalent iron particles: Ageing effect on heavy metal retention. Water Air Soil Pollut. 2020, 231, 496. [Google Scholar] [CrossRef]
- Yang, J.; Liu, J.; Dynes, J.J.; Peak, D.; Regier, T.; Wang, J.; Zhu, S.; Shi, J.; Tse, J.S. Speciation and distribution of copper in a mining soil using multiple synchrotron-based bulk and microscopic techniques. Environ. Sci. Pollut. Res. 2013, 21, 2943–2954. [Google Scholar] [CrossRef]
- Shi, M.; Min, X.; Ke, Y.; Lin, Z.; Yang, Z.; Wang, S.; Peng, N.; Yan, X.; Luo, S.; Wu, J.; et al. Recent progress in understanding the mechanism of heavy metals retention by iron (oxyhydr)oxides. Sci. Total Environ. 2021, 752, 141930. [Google Scholar] [CrossRef]
- Shen, Q.; Demisie, W.; Zhang, S.; Zhang, M. The association of heavy metals with iron oxides in the aggregates of naturally enriched soil. Bull. Environ. Contam. Toxicol. 2020, 104, 144–148. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, H.; Cui, Z. Migration and speciation of heavy metal in salinized mine tailings affected by iron mining. Water Sci. Techenol. 2017, 76, 1867–1874. [Google Scholar] [CrossRef]
- Maya, E.; Juan, S.-L.-P.; Vincent, N.; Kristin, B.; Scott, F. Organic compounds alter the preference and rates of heavy metal adsorption on ferrihydrite. Sci. Total Environ. 2021, 750, 141485. [Google Scholar]
- Ye, Y.; Li, Y.; Cao, Z.; Liu, S.; Zhao, Y. Experimental and numerical study on Cu and Cd migration in different functional-area soils under simulated rainfall conditions. Environ. Res. 2021, 208, 112239. [Google Scholar] [CrossRef]
- Cupara, N.; Nikolić, I.; Đurović, D.; Milašević, I.; Medin, D.; Krivokapić, S. Heavy metal assessment in agricultural soils and vegetables in the vicinity of industrial pollutants in the Pljevlja municipality (Montenegro): Ecological and health risk approach. Environ. Monit. Assess. 2022, 194, 819. [Google Scholar] [CrossRef]


| Fraction | Extractants | Extraction Procedures |
|---|---|---|
| exchangeable and carbonate fraction (F1) | 16 mL NaAc–HAc (1 M) | 25 °C, 6 h shaking |
| readily reducible iron and manganese oxide fraction (F2) | 20 mL NH2OH–HCl (0.04 M) | 96 °C, 6 h shaking in the dark |
| organic fraction (F3) | 3 mL HNO3 (0.02 M) + 5 mL H2O2 (30%), pH = 2; 5 mL 3.2 M CH3COONH4 | 5 h in a water basin at 85 °C; cool, add CH3COONH4 and shake for 30 min, 25 °C |
| crystalline iron oxide fraction (F4) | 10 mL DCB + Na2S2O4 | 15 min in a water basin at 80 °C, repeat twice |
| residual fraction (F5) | 2 mL HCl/6 mL HNO3/2 mL HF | digestion |
| Class | Index | Pollution Level |
|---|---|---|
| 0 | Igeo ≤ 0 | Unpolluted |
| 1 | 0 < Igeo < 1 | Slightly polluted |
| 2 | 1 < Igeo < 2 | Moderately polluted |
| 3 | 2 < Igeo < 3 | Moderately to heavily polluted |
| 4 | 3 < Igeo < 4 | Heavily polluted |
| 5 | 4 < Igeo < 5 | Heavily to extremely polluted |
| 6 | Igeo > 5 | Extremely polluted |
| Items | Maximum | Minimum | Mean | SD | Skewness | Kurt. | CV | K–S Test |
|---|---|---|---|---|---|---|---|---|
| pH | 5.16 | 4.04 | 4.78 | 0.29 | −1.53 | 2.24 | 0.06 | Non-normal |
| SOM (%) | 4.43 | 1.09 | 2.27 | 0.67 | 1.84 | 6.48 | 0.30 | Approximately normal |
| AP (mg·kg−1) | 123.29 | 0.75 | 31.17 | 30.71 | 2.18 | 4.66 | 0.99 | Approximately log-normal |
| Fe-T (g·kg−1) | 64.34 | 10.37 | 23.74 | 15.37 | 1.82 | 2.33 | 0.65 | Non-normal |
| Fed (g·kg−1) | 31.47 | 4.87 | 9.95 | 7.01 | 2.08 | 4.43 | 0.70 | Non-normal |
| Feo (g·kg−1) | 22.64 | 0.79 | 4.73 | 5.43 | 2.52 | 6.79 | 1.15 | Non-normal |
| Cu (mg·kg−1) | 1294.63 | 97.47 | 317.70 | 301.13 | 2.39 | 6.29 | 0.95 | Approximately log-normal |
| Cd (mg·kg−1) | 9.06 | 0.14 | 1.14 | 2.04 | 4.09 | 17.09 | 1.79 | Approximately log-normal |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, Y.; Xi, L.; Wu, Y.; Chen, Y.; Guo, X.; Shi, H.; Cai, S. Spatial Differentiation Characteristics and Evaluation of Cu and Cd in Paddy Soil around a Copper Smelter. Toxics 2023, 11, 647. https://doi.org/10.3390/toxics11080647
Ding Y, Xi L, Wu Y, Chen Y, Guo X, Shi H, Cai S. Spatial Differentiation Characteristics and Evaluation of Cu and Cd in Paddy Soil around a Copper Smelter. Toxics. 2023; 11(8):647. https://doi.org/10.3390/toxics11080647
Chicago/Turabian StyleDing, Yuan, Li Xi, Yujing Wu, Yihong Chen, Xiaoping Guo, Hong Shi, and Shuo Cai. 2023. "Spatial Differentiation Characteristics and Evaluation of Cu and Cd in Paddy Soil around a Copper Smelter" Toxics 11, no. 8: 647. https://doi.org/10.3390/toxics11080647
APA StyleDing, Y., Xi, L., Wu, Y., Chen, Y., Guo, X., Shi, H., & Cai, S. (2023). Spatial Differentiation Characteristics and Evaluation of Cu and Cd in Paddy Soil around a Copper Smelter. Toxics, 11(8), 647. https://doi.org/10.3390/toxics11080647






