Polychlorinated Biphenyls (PCBs) Impact Prostatic Collagen Density and Bladder Volume in Young Adult Mice Exposed during in Utero and Lactational Development
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Developmental PCB Exposure
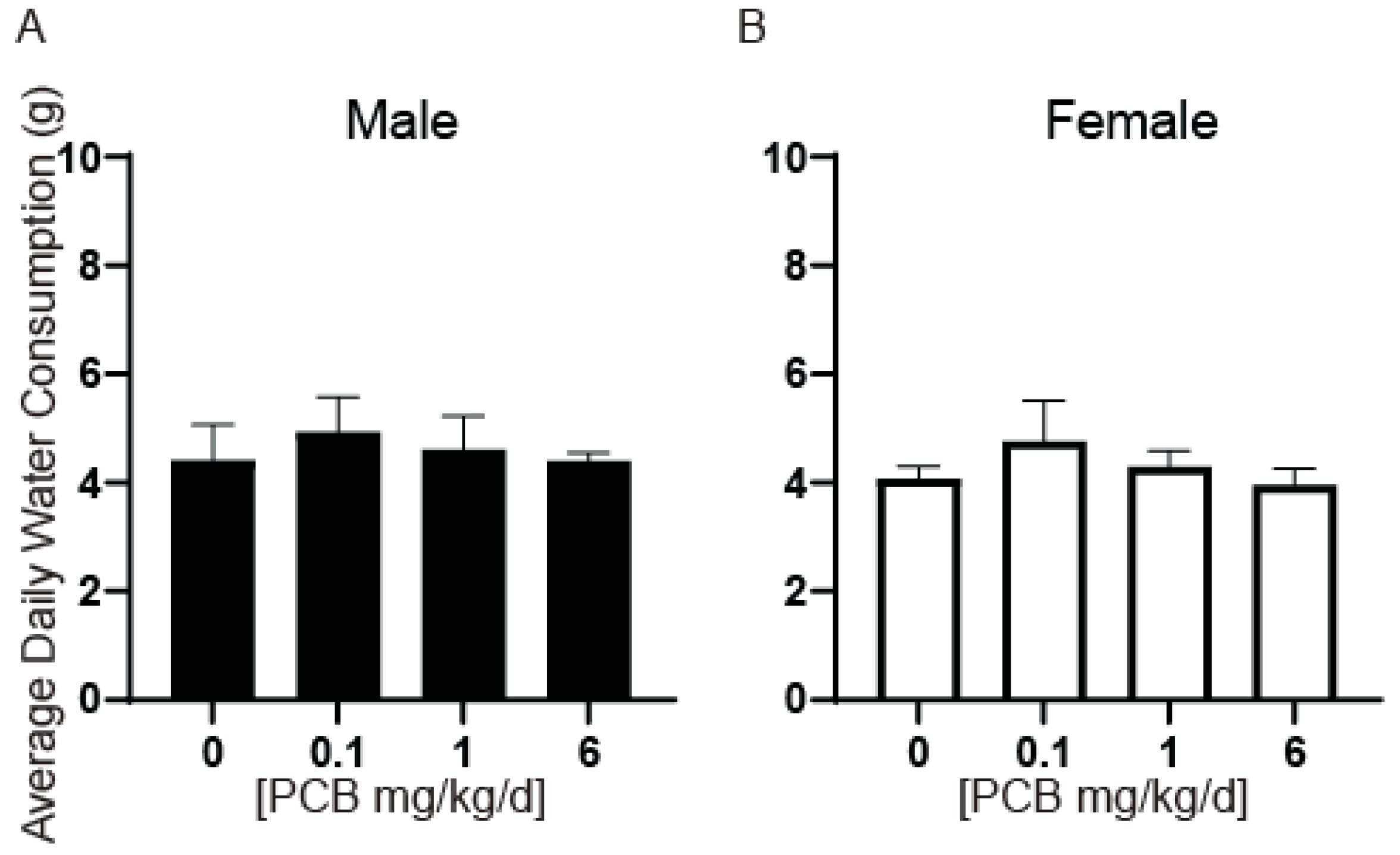
2.3. Water Consumption and Bladder Metrics
2.4. Immunohistochemistry and Tissue Staining
2.5. Creatinine Assays
2.6. Statistics
3. Results
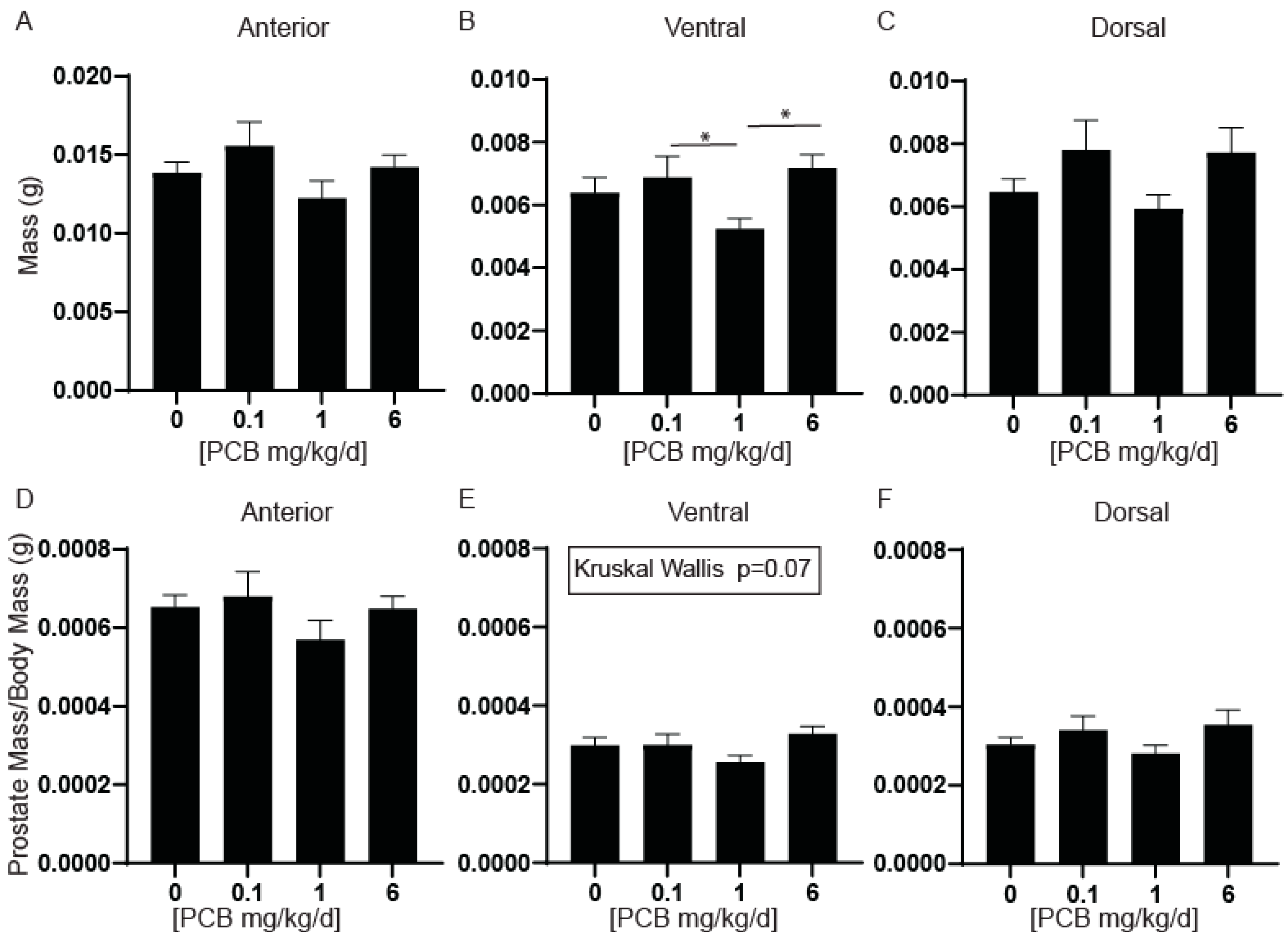
3.1. PCBs Decrease Ventral Prostate Wet Weight in Developmentally Exposed Male Mice
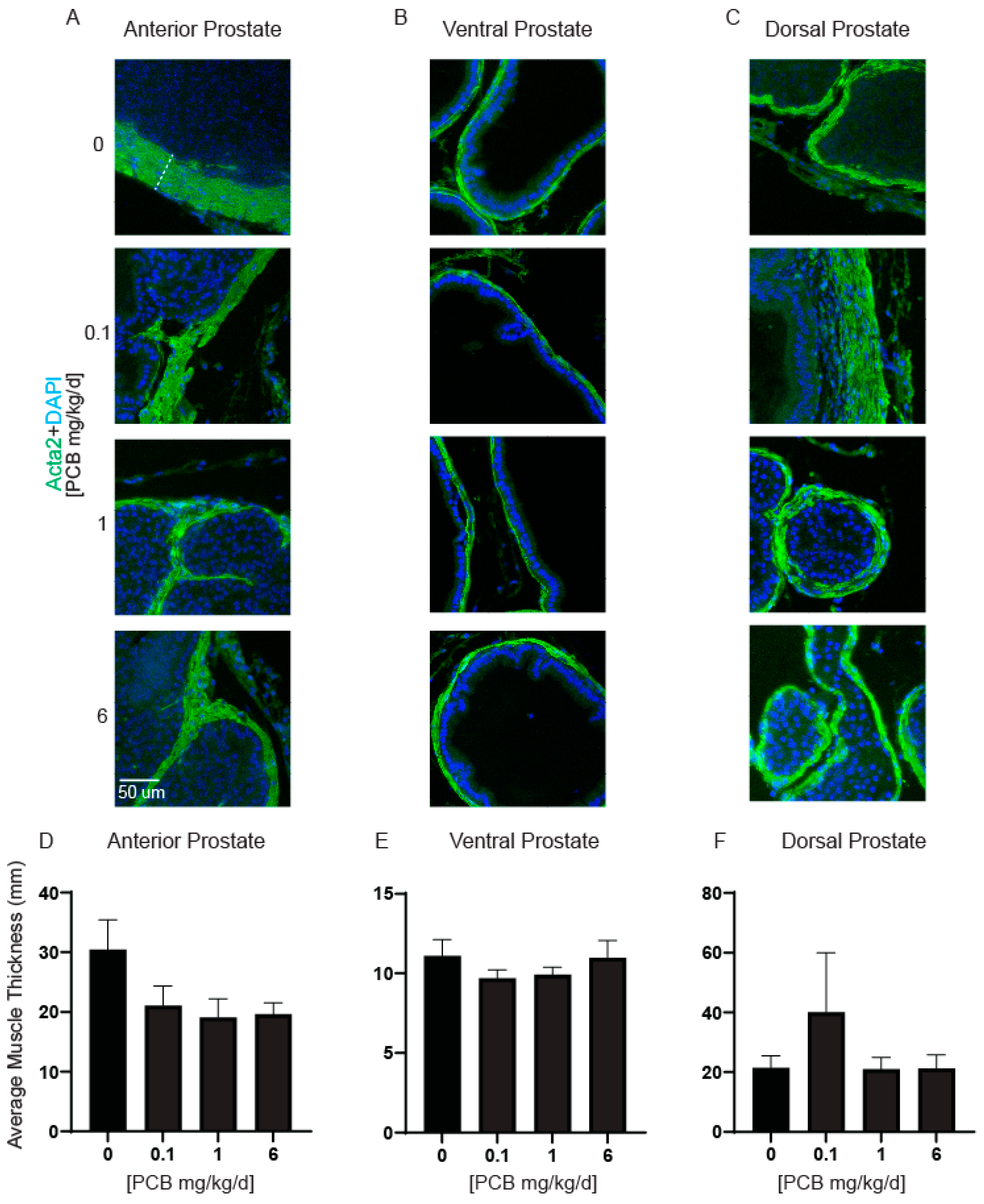
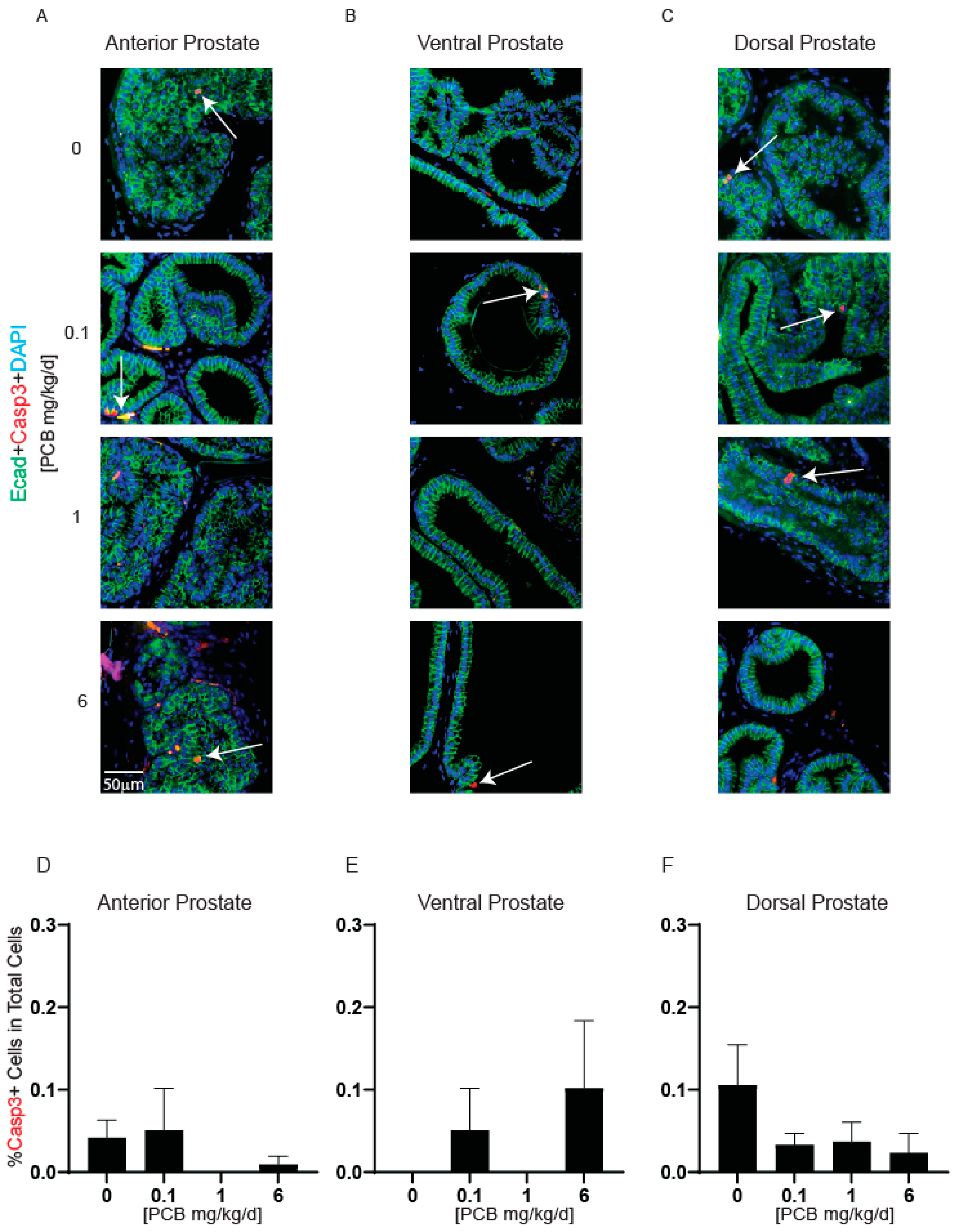
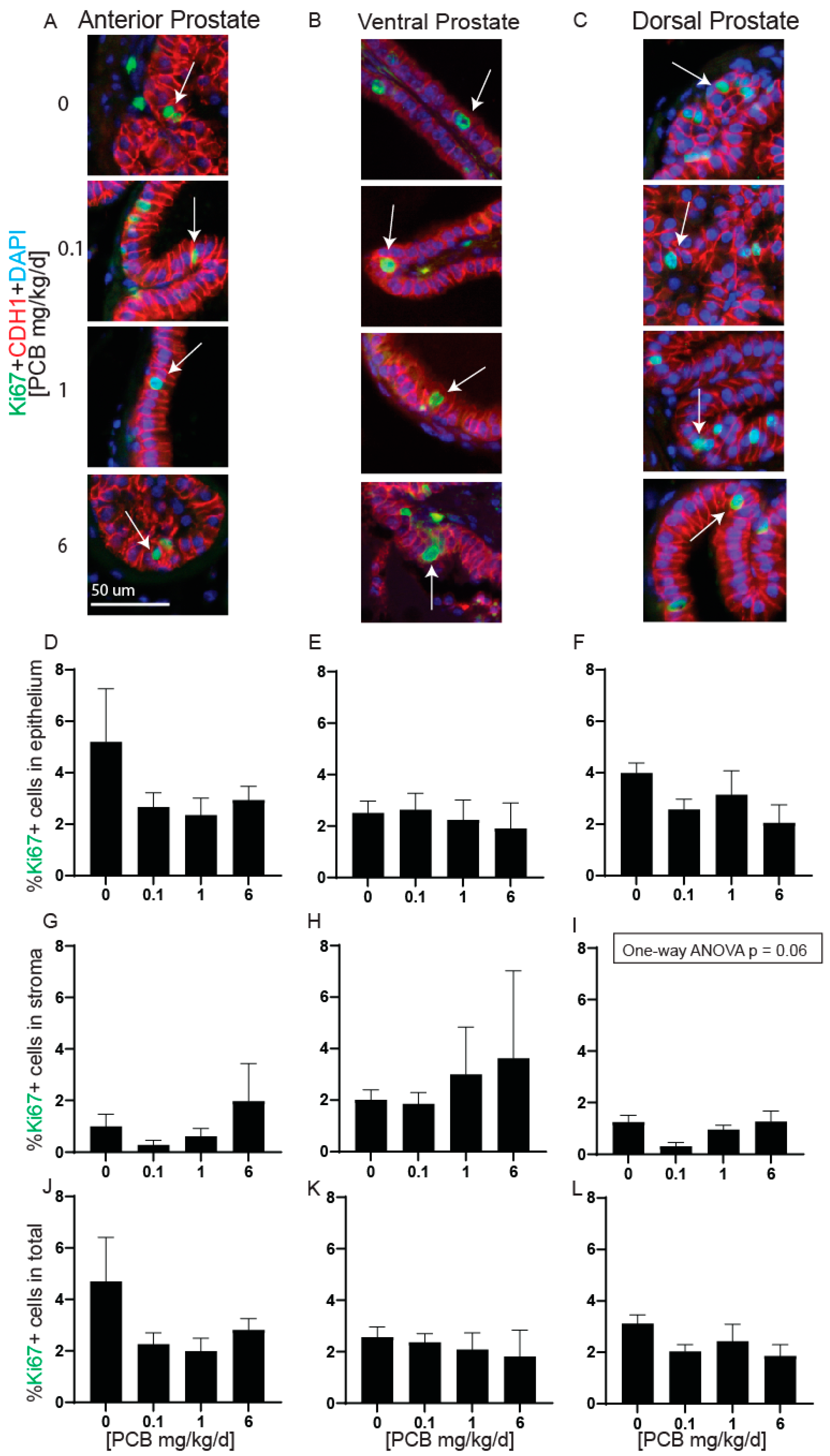
3.2. PCBs Do Not Alter Prostate Smooth Muscle Thickness, Apoptotic Cells, or Proliferating Cells in Developmentally Exposed Male Mice
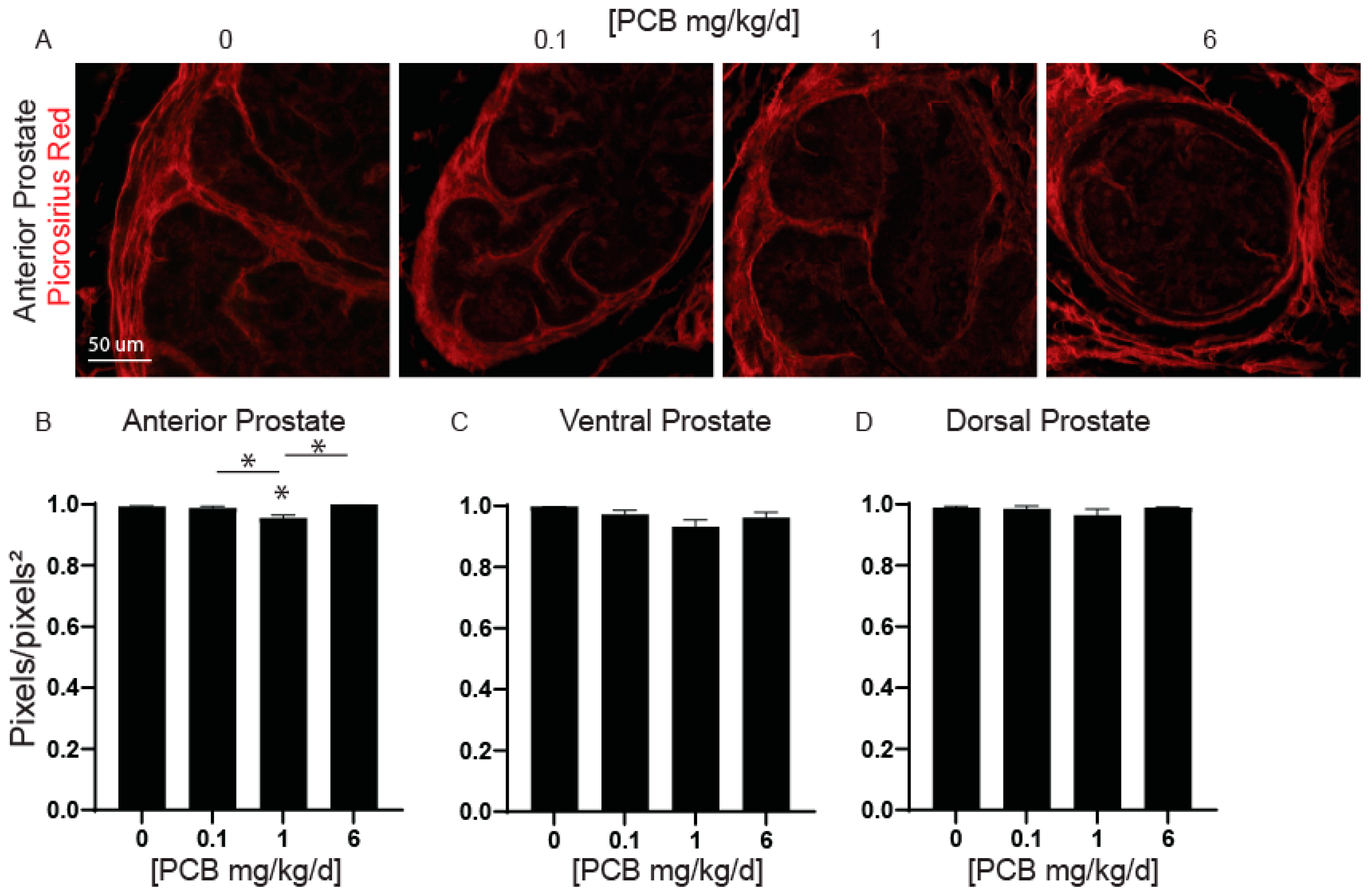
3.3. PCBs Decrease Prostate Collagen Density in the Anterior Prostate in Developmentally Exposed Male Mice
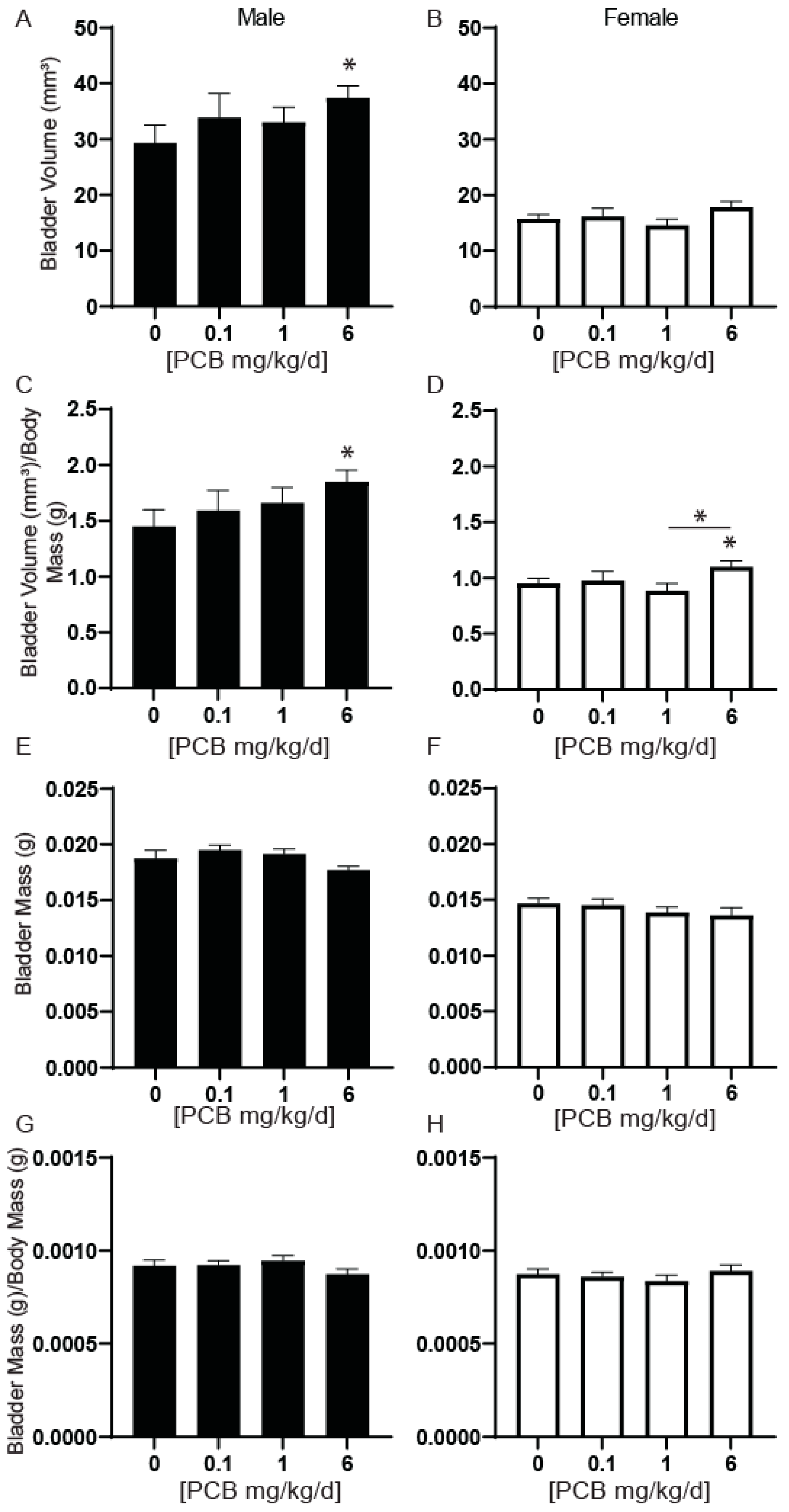
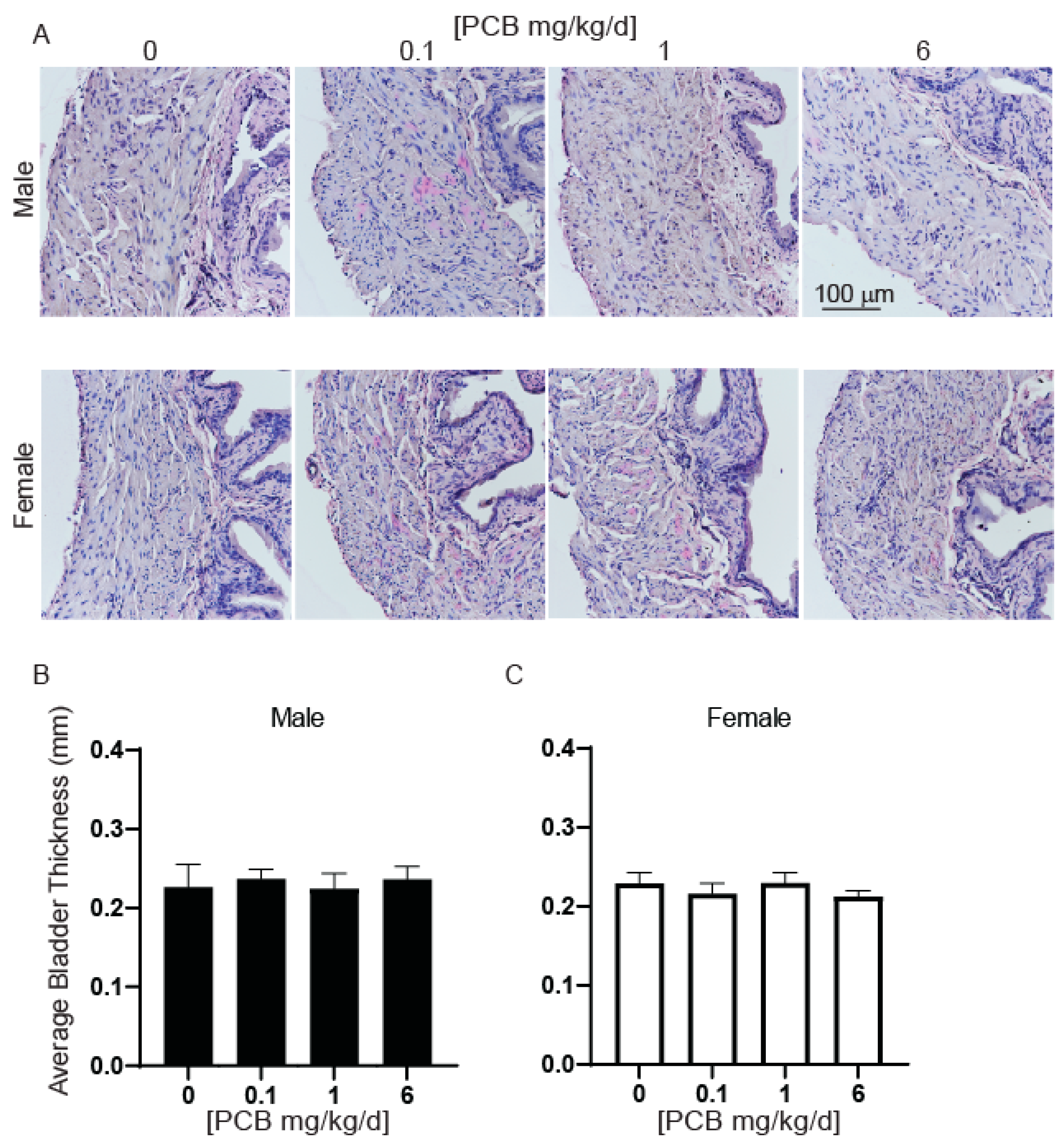
3.4. PCBs Increase Bladder Volume in Developmentally Exposed Mice
3.5. PCBs Increase Serum and Urine Creatinine Concentrations in Developmentally Exposed Male Mice
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rubin, E.B.; Buehler, A.E.; Halpern, S.D. States Worse Than Death Among Hospitalized Patients with Serious Illnesses. JAMA Intern. Med. 2016, 176, 1557–1559. [Google Scholar] [CrossRef] [PubMed]
- Kullmann, F.A.; Birder, L.A.; Andersson, K.E. Translational Research and Functional Changes in Voiding Function in Older Adults. Clin. Geriatr. Med. 2015, 31, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Von Gontard, A.; Equit, M. Comorbidity of ADHD and incontinence in children. Eur. Child Adolesc. Psychiatry 2015, 24, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Von Gontard, A.; Pirrung, M.; Niemczyk, J.; Equit, M. Incontinence in children with autism spectrum disorder. J. Pediatr. Urol. 2015, 11, 264.E1–264.E7. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, G.L.; Marks, J.M.; Ricke, W.A. Benign Prostatic Hyperplasia and Lower Urinary Tract Symptoms: What Is the Role and Significance of Inflammation? Curr. Urol. Rep. 2019, 20, 54. [Google Scholar] [CrossRef]
- Joseph, D.B.; Henry, G.H.; Malewska, A.; Reese, J.C.; Mauck, R.J.; Gahan, J.C.; Hutchinson, R.C.; Malladi, V.S.; Roehrborn, C.G.; Vezina, C.M.; et al. Single-cell analysis of mouse and human prostate reveals novel fibroblasts with specialized distribution and microenvironment interactions. J. Pathol. 2021, 255, 141–154. [Google Scholar] [CrossRef]
- Nicholson, T.M.; Ricke, E.A.; Marker, P.C.; Miano, J.M.; Mayer, R.D.; Timms, B.G.; vom Saal, F.S.; Wood, R.W.; Ricke, W.A. Testosterone and 17beta-estradiol induce glandular prostatic growth, bladder outlet obstruction, and voiding dysfunction in male mice. Endocrinology 2012, 153, 5556–5565. [Google Scholar] [CrossRef]
- Ricke, W.A.; Lee, C.W.; Clapper, T.R.; Schneider, A.J.; Moore, R.W.; Keil, K.P.; Abler, L.L.; Wynder, J.L.; Lopez Alvarado, A.; Beaubrun, I.; et al. In Utero and Lactational TCDD Exposure Increases Susceptibility to Lower Urinary Tract Dysfunction in Adulthood. Toxicol. Sci. Off. J. Soc. Toxicol. 2016, 150, 429–440. [Google Scholar] [CrossRef]
- Taylor, J.A.; Jones, M.B.; Besch-Williford, C.L.; Berendzen, A.F.; Ricke, W.A.; Vom Saal, F.S. Interactive Effects of Perinatal BPA or DES and Adult Testosterone and Estradiol Exposure on Adult Urethral Obstruction and Bladder, Kidney, and Prostate Pathology in Male Mice. Int. J. Mol. Sci. 2020, 21, 3902. [Google Scholar] [CrossRef]
- Turco, A.E.; Oakes, S.R.; Stietz, K.P.K.; Dunham, C.L.; Joseph, D.B.; Chathurvedula, T.S.; Girardi, N.M.; Schneider, A.J.; Gawdzik, J.; Sheftel, C.M.; et al. A neuroanatomical mechanism linking perinatal TCDD exposure to lower urinary tract dysfunction in adulthood. Dis. Model. Mech. 2021, 14, dmm049068. [Google Scholar] [CrossRef]
- Turco, A.E.; Thomas, S.; Crawford, L.K.; Tang, W.; Peterson, R.E.; Li, L.; Ricke, W.A.; Vezina, C.M. In utero and lactational 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) exposure exacerbates urinary dysfunction in hormone-treated C57BL/6J mice through a non-malignant mechanism involving proteomic changes in the prostate that differ from those elicited by testosterone and estradiol. Am. J. Clin. Exp. Urol. 2020, 8, 59–72. [Google Scholar] [PubMed]
- Kennedy, C.L.; Spiegelhoff, A.; Lavery, T.; Wang, K.; Manuel, R.S.; Wang, Z.; Wildermuth, H.; Keil Stietz, K.P. Developmental polychlorinated biphenyl (PCB) exposure alters voiding physiology in young adult male and female mice. Am. J. Clin. Exp. Urol. 2022, 10, 82–97. [Google Scholar] [PubMed]
- Klocke, C.; Lein, P.J. Evidence Implicating Non-Dioxin-Like Congeners as the Key Mediators of Polychlorinated Biphenyl (PCB) Developmental Neurotoxicity. Int. J. Mol. Sci. 2020, 21, 1013. [Google Scholar] [CrossRef] [PubMed]
- Klocke, C.; Sethi, S.; Lein, P.J. The developmental neurotoxicity of legacy vs. contemporary polychlorinated biphenyls (PCBs): Similarities and differences. Environ. Sci. Pollut. Res. Int. 2019, 27, 8885–8896. [Google Scholar] [CrossRef]
- White, S.S.; Birnbaum, L.S. An overview of the effects of dioxins and dioxin-like compounds on vertebrates, as documented in human and ecological epidemiology. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2009, 27, 197–211. [Google Scholar] [CrossRef]
- Labunska, I.; Abdallah, M.A.; Eulaers, I.; Covaci, A.; Tao, F.; Wang, M.; Santillo, D.; Johnston, P.; Harrad, S. Human dietary intake of organohalogen contaminants at e-waste recycling sites in Eastern China. Environ. Int. 2015, 74, 209–220. [Google Scholar] [CrossRef]
- Hu, D.; Hornbuckle, K.C. Inadvertent polychlorinated biphenyls in commercial paint pigments. Environ. Sci. Technol. 2010, 44, 2822–2827. [Google Scholar] [CrossRef]
- Sethi, S.; Chen, X.; Kass, P.H.; Puschner, B. Polychlorinated biphenyl and polybrominated diphenyl ether profiles in serum from cattle, sheep, and goats across California. Chemosphere 2017, 181, 63–73. [Google Scholar] [CrossRef]
- Chen, X.; Lin, Y.; Dang, K.; Puschner, B. Quantification of Polychlorinated Biphenyls and Polybrominated Diphenyl Ethers in Commercial Cows’ Milk from California by Gas Chromatography-Triple Quadruple Mass Spectrometry. PLoS ONE 2017, 12, e0170129. [Google Scholar] [CrossRef] [PubMed]
- Hites, R.A.; Holsen, T.M. Temporal trends of PCBs and DDTs in Great Lakes fish compared to those in air. Sci. Total Environ. 2019, 646, 1413–1418. [Google Scholar] [CrossRef]
- Granillo, L.; Sethi, S.; Keil, K.P.; Lin, Y.; Ozonoff, S.; Iosif, A.M.; Puschner, B.; Schmidt, R.J. Polychlorinated biphenyls influence on autism spectrum disorder risk in the MARBLES cohort. Environ. Res. 2019, 171, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Sethi, S.; Keil, K.P.; Chen, H.; Hayakawa, K.; Li, X.; Lin, Y.; Lehmler, H.J.; Puschner, B.; Lein, P.J. Detection of 3,3′-Dichlorobiphenyl in Human Maternal Plasma and Its Effects on Axonal and Dendritic Growth in Primary Rat Neurons. Toxicol. Sci. Off. J. Soc. Toxicol. 2017, 158, 401–411. [Google Scholar] [CrossRef]
- Sethi, S.; Morgan, R.K.; Feng, W.; Lin, Y.; Li, X.; Luna, C.; Koch, M.; Bansal, R.; Duffel, M.W.; Puschner, B.; et al. Comparative Analyses of the 12 Most Abundant PCB Congeners Detected in Human Maternal Serum for Activity at the Thyroid Hormone Receptor and Ryanodine Receptor. Environ. Sci. Technol. 2019, 53, 3948–3958. [Google Scholar] [CrossRef] [PubMed]
- Keil Stietz, K.K.; Kennedy, C.L.; Sethi, S.; Valenzuela, A.; Nunez, A.; Wang, K.; Wang, Z.; Wang, P.; Spiegelhoff, A.; Puschner, B.; et al. In utero and lactational PCB exposure drives anatomic changes in the juvenile mouse bladder. Curr. Res. Toxicol. 2021, 2, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, C.L.; Spiegelhoff, A.; Wang, K.; Lavery, T.; Nunez, A.; Manuel, R.; Hillers-Ziemer, L.; Arendt, L.M.; Stietz, K.P.K. The Bladder Is a Novel Target of Developmental Polychlorinated Biphenyl Exposure Linked to Increased Inflammatory Cells in the Bladder of Young Mice. Toxics 2021, 9, 214. [Google Scholar] [CrossRef] [PubMed]
- Keil Stietz, K.P.; Sethi, S.; Klocke, C.R.; de Ruyter, T.E.; Wilson, M.D.; Pessah, I.N.; Lein, P.J. Sex and Genotype Modulate the Dendritic Effects of Developmental Exposure to a Human-Relevant Polychlorinated Biphenyls Mixture in the Juvenile Mouse. Front. Neurosci. 2021, 15, 766802. [Google Scholar] [CrossRef]
- Sethi, S.; Keil Stietz, K.P.; Valenzuela, A.E.; Klocke, C.R.; Silverman, J.L.; Puschner, B.; Pessah, I.N.; Lein, P.J. Developmental Exposure to a Human-Relevant Polychlorinated Biphenyl Mixture Causes Behavioral Phenotypes That Vary by Sex and Genotype in Juvenile Mice Expressing Human Mutations That Modulate Neuronal Calcium. Front. Neurosci. 2021, 15, 766826. [Google Scholar] [CrossRef]
- Yang, D.; Kim, K.H.; Phimister, A.; Bachstetter, A.D.; Ward, T.R.; Stackman, R.W.; Mervis, R.F.; Wisniewski, A.B.; Klein, S.L.; Kodavanti, P.R.; et al. Developmental exposure to polychlorinated biphenyls interferes with experience-dependent dendritic plasticity and ryanodine receptor expression in weanling rats. Environ. Health Perspect. 2009, 117, 426–435. [Google Scholar] [CrossRef]
- Kania-Korwel, I.; Lukasiewicz, T.; Barnhart, C.D.; Stamou, M.; Chung, H.; Kelly, K.M.; Bandiera, S.; Lein, P.J.; Lehmler, H.J. Editor’s Highlight: Congener-Specific Disposition of Chiral Polychlorinated Biphenyls in Lactating Mice and Their Offspring: Implications for PCB Developmental Neurotoxicity. Toxicol. Sci. Off. J. Soc. Toxicol. 2017, 158, 101–115. [Google Scholar] [CrossRef]
- Abler, L.L.; Keil, K.P.; Mehta, V.; Joshi, P.S.; Schmitz, C.T.; Vezina, C.M. A high-resolution molecular atlas of the fetal mouse lower urogenital tract. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2011, 240, 2364–2377. [Google Scholar] [CrossRef]
- Wegner, K.A.; Cadena, M.T.; Trevena, R.; Turco, A.E.; Gottschalk, A.; Halberg, R.B.; Guo, J.; McMahon, J.A.; McMahon, A.P.; Vezina, C.M. An immunohistochemical identification key for cell types in adult mouse prostatic and urethral tissue sections. PLoS ONE 2017, 12, e0188413. [Google Scholar] [CrossRef]
- Wegner, K.A.; Abler, L.L.; Oakes, S.R.; Mehta, G.S.; Ritter, K.E.; Hill, W.G.; Zwaans, B.M.; Lamb, L.E.; Wang, Z.; Bjorling, D.E.; et al. Void spot assay procedural optimization and software for rapid and objective quantification of rodent voiding function, including overlapping urine spots. Am. J. Physiol. Ren. Physiol. 2018, 315, F1067–F1080. [Google Scholar] [CrossRef] [PubMed]
- Cunha, G.R. Mesenchymal-epithelial interactions during androgen-induced development of the prostate. Prog. Clin. Biol. Res. 1985, 171, 15–24. [Google Scholar]
- Cunha, G.R.; Donjacour, A.A. Mesenchymal-epithelial interactions in the growth and development of the prostate. Cancer Treat. Res. 1989, 46, 159–175. [Google Scholar]
- Cunha, G.R.; Donjacour, A.A.; Cooke, P.S.; Mee, S.; Bigsby, R.M.; Higgins, S.J.; Sugimura, Y. The endocrinology and developmental biology of the prostate. Endocr. Rev. 1987, 8, 338–362. [Google Scholar] [CrossRef]
- Sugimura, Y.; Cunha, G.R.; Donjacour, A.A. Morphogenesis of ductal networks in the mouse prostate. Biol. Reprod. 1986, 34, 961–971. [Google Scholar] [CrossRef] [PubMed]
- Cunha, G.R.; Vezina, C.M.; Isaacson, D.; Ricke, W.A.; Timms, B.G.; Cao, M.; Franco, O.; Baskin, L.S. Development of the human prostate. Differ. Res. Biol. Divers. 2018, 103, 24–45. [Google Scholar] [CrossRef]
- Lanning, L.L.; Creasy, D.M.; Chapin, R.E.; Mann, P.C.; Barlow, N.J.; Regan, K.S.; Goodman, D.G. Recommended approaches for the evaluation of testicular and epididymal toxicity. Toxicol. Pathol. 2002, 30, 507–520. [Google Scholar] [CrossRef] [PubMed]
- Yokota, S.; Miyaso, H.; Hirai, T.; Suga, K.; Wakayama, T.; Taquahashi, Y.; Kitajima, S. Development of a non-invasive method for testicular toxicity evaluation using a novel compact magnetic resonance imaging system. J. Toxicol. Sci. 2023, 48, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Kuriyama, S.N.; Chahoud, I. In utero exposure to low-dose 2,3′,4,4′,5-pentachlorobiphenyl (PCB 118) impairs male fertility and alters neurobehavior in rat offspring. Toxicology 2004, 202, 185–197. [Google Scholar] [CrossRef]
- Keil, K.P.; Abler, L.L.; Altmann, H.M.; Wang, Z.; Wang, P.; Ricke, W.A.; Bjorling, D.E.; Vezina, C.M. Impact of a folic acid-enriched diet on urinary tract function in mice treated with testosterone and estradiol. Am. J. Physiol. Ren. Physiol. 2015, 308, F1431–F1443. [Google Scholar] [CrossRef]
- Yang, D.; Lein, P.J. Polychlorinated biphenyls increase apoptosis in the developing rat brain. Curr. Neurobiol. 2010, 1, 70–76. [Google Scholar]
- Ghosh, S.; De, S.; Chen, Y.; Sutton, D.C.; Ayorinde, F.O.; Dutta, S.K. Polychlorinated biphenyls (PCB-153) and (PCB-77) absorption in human liver (HepG2) and kidney (HK2) cells in vitro: PCB levels and cell death. Environ. Int. 2010, 36, 893–900. [Google Scholar] [CrossRef]
- Sager, D.B. Effect of postnatal exposure to polychlorinated biphenyls on adult male reproductive function. Environ. Res. 1983, 31, 76–94. [Google Scholar] [CrossRef]
- Venkataraman, P.; Sridhar, M.; Dhanammal, S.; Vijayababu, M.R.; Srinivasan, N.; Arunakaran, J. Antioxidant role of zinc in PCB (Aroclor 1254) exposed ventral prostate of albino rats. J. Nutr. Biochem. 2004, 15, 608–613. [Google Scholar] [CrossRef]
- Venkataraman, P.; Sridhar, M.; Dhanammal, S.; Vijayababu, M.R.; Arunkumar, A.; Srinivasan, N.; Arunakaran, J. Effects of vitamin supplementation on PCB (Aroclor 1254)-induced changes in ventral prostatic androgen and estrogen receptors. Endocr. Res. 2004, 30, 469–480. [Google Scholar] [CrossRef]
- Ruetten, H.; Sandhu, J.; Mueller, B.; Wang, P.; Zhang, H.L.; Wegner, K.A.; Cadena, M.; Sandhu, S.; Abler, L.L.; Zhu, J.; et al. A uropathogenic E. coli UTI89 model of prostatic inflammation and collagen accumulation for use in studying aberrant collagen production in the prostate. Am. J. Physiol. Ren. Physiol. 2021, 320, F31–F46. [Google Scholar] [CrossRef]
- Ruetten, H.; Wehber, M.; Murphy, M.; Cole, C.; Sandhu, S.; Oakes, S.; Bjorling, D.; Waller, K., 3rd; Viviano, K.; Vezina, C. A retrospective review of canine benign prostatic hyperplasia with and without prostatitis. Clin. Theriogenol. 2021, 13, 360–366. [Google Scholar]
- Bell-Cohn, A.; Mazur, D.J.; Hall, C.; Schaeffer, A.J.; Thumbikat, P. Uropathogenic Escherichia coli-induced fibrosis, leading to lower urinary tract symptoms, is associated with type 2 cytokine signaling. Am. J. Physiol. Ren. Physiol. 2019, 316, F682–F692. [Google Scholar] [CrossRef]
- Osei, E.T.; Mostaço-Guidolin, L.B.; Hsieh, A.; Warner, S.M.; Al-Fouadi, M.; Wang, M.; Cole, D.J.; Maksym, G.N.; Hallstrand, T.S.; Timens, W.; et al. Epithelial-interleukin-1 inhibits collagen formation by airway fibroblasts: Implications for asthma. Sci. Rep. 2020, 10, 8721. [Google Scholar] [CrossRef]
- Tuttle, T.G.; Lujan, H.L.; Tykocki, N.R.; DiCarlo, S.E.; Roccabianca, S. Remodeling of extracellular matrix in the urinary bladder of paraplegic rats results in increased compliance and delayed fiber recruitment 16 weeks after spinal cord injury. Acta Biomater. 2022, 141, 280–289. [Google Scholar] [CrossRef] [PubMed]


| Primary Antibodies | Source | Company | Catalog # | Dilution | |
| E-Cadherin (CDH1) | Rabbit | Cell Signaling Technology | 3195s | 1:250 | |
| E-Cadherin (CDH1) | Mouse | BD Transduction Labs (via Fisher) | 610181 | 1:250 | |
| Cleaved caspase 3 (Casp3) | Rabbit | Cell Signaling Technology | 9661S | 1:200 | |
| Ki67 | Rabbit | Abcam | ab15580 | 1:200 | |
| Smooth Muscle Actin (Acta2) | Goat | Fisher Scientific | PA518292 | 1:250 | |
| Secondary Antibodies | Pairing | ||||
| Anti-Mouse Alexa Fluor 594 | Donkey | Jackson ImmunoResearch | 715-545-150 | 1:250 | CDH1 |
| Anti-Rabbit Alexa Fluor 488 | Donkey | Jackson ImmunoResearch | 711-545-152 | 1:250 | CDH1, Ki67 |
| Anti-Rabbit Alexa Fluor 594 | Goat | Jackson ImmunoResearch | 111-585-144 | 1:250 | Casp3 |
| Anti-Goat Alexa Fluor 488 | Donkey | Jackson ImmunoResearch | 705-545-003 | 1:250 | Acta2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spiegelhoff, A.; Wang, K.; Ridlon, M.; Lavery, T.; Kennedy, C.L.; George, S.; Stietz, K.P.K. Polychlorinated Biphenyls (PCBs) Impact Prostatic Collagen Density and Bladder Volume in Young Adult Mice Exposed during in Utero and Lactational Development. Toxics 2023, 11, 609. https://doi.org/10.3390/toxics11070609
Spiegelhoff A, Wang K, Ridlon M, Lavery T, Kennedy CL, George S, Stietz KPK. Polychlorinated Biphenyls (PCBs) Impact Prostatic Collagen Density and Bladder Volume in Young Adult Mice Exposed during in Utero and Lactational Development. Toxics. 2023; 11(7):609. https://doi.org/10.3390/toxics11070609
Chicago/Turabian StyleSpiegelhoff, Audrey, Kathy Wang, Monica Ridlon, Thomas Lavery, Conner L. Kennedy, Serena George, and Kimberly P. Keil Stietz. 2023. "Polychlorinated Biphenyls (PCBs) Impact Prostatic Collagen Density and Bladder Volume in Young Adult Mice Exposed during in Utero and Lactational Development" Toxics 11, no. 7: 609. https://doi.org/10.3390/toxics11070609
APA StyleSpiegelhoff, A., Wang, K., Ridlon, M., Lavery, T., Kennedy, C. L., George, S., & Stietz, K. P. K. (2023). Polychlorinated Biphenyls (PCBs) Impact Prostatic Collagen Density and Bladder Volume in Young Adult Mice Exposed during in Utero and Lactational Development. Toxics, 11(7), 609. https://doi.org/10.3390/toxics11070609








