Toxicity and Influence of Sublethal Exposure to Sulfoxaflor on the Aphidophagous Predator Hippodamia variegata (Coleoptera: Coccinellidae)
Abstract
1. Introduction
2. Materials and Methods
2.1. Insecticide and Tested Concentrations
2.2. Test Species
Hippodamia Variegata
2.3. Bioassays
Biological Control Agent Bioassays
2.4. Risk Assessment
2.5. Statistical Analysis
3. Results
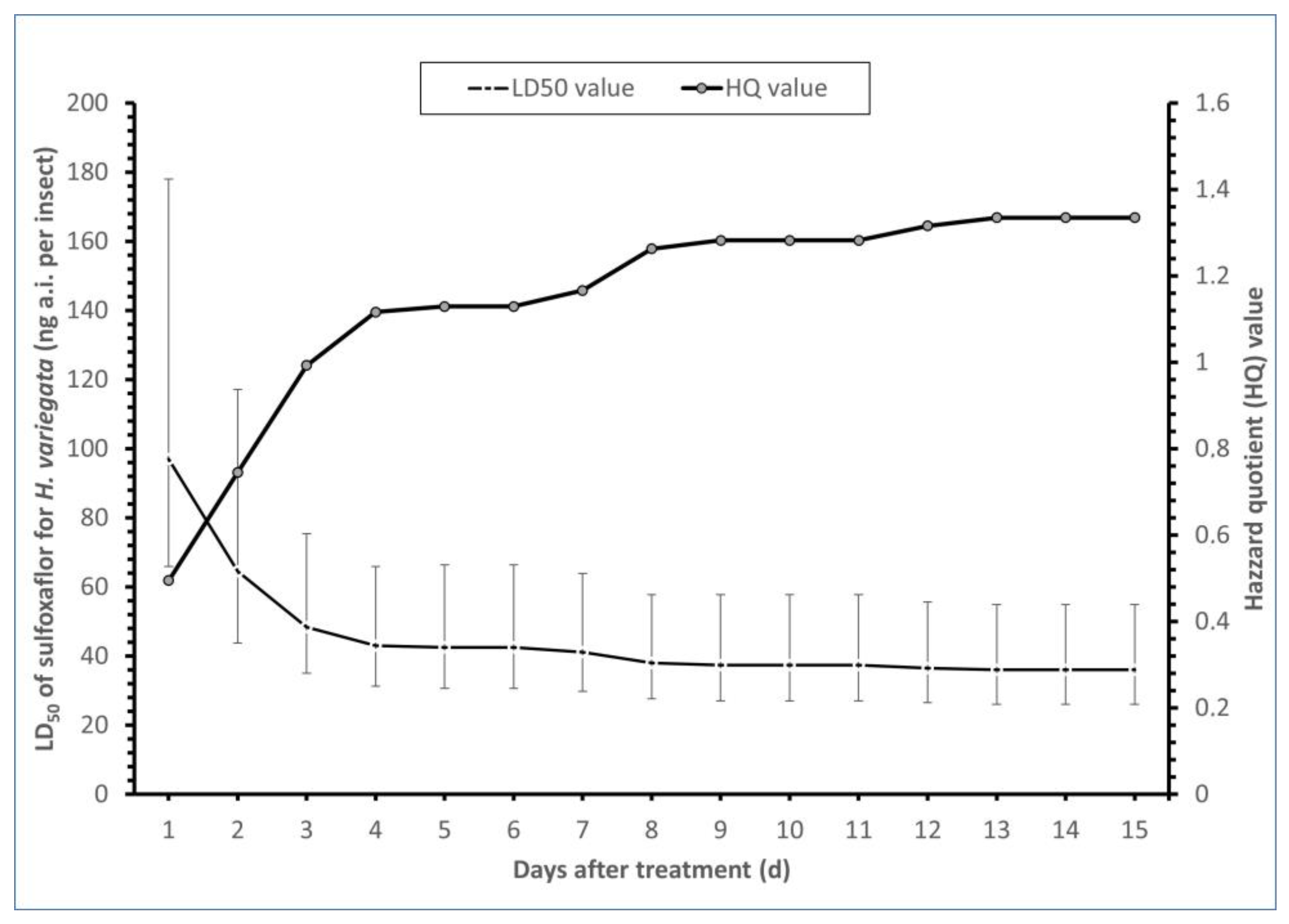
3.1. Toxicity and Influence of Sulfoxaflor on the Survival Rate of H. variegata
3.2. Influence of Sulfoxaflor on the Developmental Time, Female and Male Adult Longevity, and Female Pre-Oviposition Period of H. variegata
3.3. Influence of Sulfoxaflor on Fecundity, Adult Weight, Sex Ratio, Total Effect, and IOBC Toxicity Categories of H. variegata
3.4. Influence of Sulfoxaflor on Population Parameters and Population Projection of H. variegata
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Margaritopoulos, J.T.; Blackman, R.L.; Tsitsipis, J.A.; Sannino, L. Co-existence of different host-adapted forms of the Myzus persicae group (Hemiptera: Aphididae) in southern Italy. Bull. Entomol. Res. 2003, 93, 131–135. [Google Scholar] [CrossRef]
- Blackman, R.L.; Eastop, V.F. Aphids on the World’s Crops: An Identification and Information Guide; John Wiley & Sons Ltd: Hoboken, NJ, USA, 2000. [Google Scholar]
- Bass, C.; Puinean, A.M.; Zimmer, C.T.; Denholm, I.; Field, L.M.; Foster, S.P.; Gutbrod, O.; Nauen, R.; Slater, R.; Williamson, M.S. The evolution of insecticide resistance in the peach potato aphid, Myzus persicae. Insect Biochem. Mol. Biol. 2014, 51, 41–51. [Google Scholar] [CrossRef]
- Bass, C.; Denholm, I.; Williamson, M.S.; Nauen, R. The global status of insect resistance to neonicotinoid insecticides. Pestic. Biochem. Physiol. 2015, 121, 78–87. [Google Scholar] [CrossRef]
- Margaritopoulos, J.T.; Kati, A.N.; Voudouris, C.C.; Skouras, P.J.; Tsitsipis, J.A. Long-term studies on the evolution of resistance of Myzus persicae (Hemiptera: Aphididae) to insecticides in Greece. Bull. Entomol. Res. 2021, 111, 1–16. [Google Scholar] [CrossRef]
- Bass, C.; Nauen, R. The molecular mechanisms of insecticide resistance in aphid crop pests. Insect Biochem. Mol. Biol. 2023, 156, 103937. [Google Scholar] [CrossRef]
- Li, Z.; Li, W.; Qin, W.; Liu, J.; He, Y. Ampicillin enhanced the resistance of Myzus persicae to imidacloprid and cyantraniliprole. Pest Manag. Sci. 2023, 79, 1388–1398. [Google Scholar] [CrossRef]
- Desneux, N.; Fauvergue, X.; Dechaume-Moncharmont, F.X.; Kerhoas, L.; Ballanger, Y.; Kaiser, L. Diaeretiella rapae limits Myzus persicae populations after applications of deltamethrin in oilseed rape. J. Econ. Entomol. 2005, 98, 9–17. [Google Scholar] [CrossRef]
- Papachristos, D.P.; Milonas, P.G. Adverse effects of soil applied insecticides on the predatory coccinellid Hippodamia undecimnotata (Coleoptera: Coccinellidae). Biol. Control 2008, 47, 77–81. [Google Scholar] [CrossRef]
- Skouras, P.J.; Brokaki, M.; Stathas, G.J.; Demopoulos, V.; Louloudakis, G.; Margaritopoulos, J.T. Lethal and sub-lethal effects of imidacloprid on the aphidophagous coccinellid Hippodamia variegata. Chemosphere 2019, 229, 392–400. [Google Scholar] [CrossRef]
- Skouras, P.J.; Darras, A.I.; Mprokaki, M.; Demopoulos, V.; Margaritopoulos, J.T.; Delis, C.; Stathas, G.J. Toxicity, Sublethal and Low Dose Effects of Imidacloprid and Deltamethrin on the Aphidophagous Predator Ceratomegilla undecimnotata (Coleoptera: Coccinellidae). Insects 2021, 12, 696. [Google Scholar] [CrossRef]
- Garratt, J.; Kennedy, A. Use of models to assess the reduction in contamination of water bodies by agricultural pesticides through the implementation of policy instruments: A case study of the Voluntary Initiative in the UK. Pest Manag. Sci. 2006, 62, 1138–1149. [Google Scholar] [CrossRef] [PubMed]
- Dara, S.K. The New Integrated Pest Management Paradigm for the Modern Age. J. Integr. Pest Manag. 2019, 10, 12. [Google Scholar] [CrossRef]
- Kontodimas, D.C.; Stathas, G.J. Phenology, fecundity and life table parameters of the predator Hippodamia variegata reared on Dysaphis crataegi. BioControl 2005, 50, 223–233. [Google Scholar] [CrossRef]
- Skouras, P.J.; Margaritopoulos, J.T.; Zarpas, K.D.; Tsitsipis, J.A. Development, growth, feeding and reproduction of Ceratomegilla undecimnotata, Hippodamia variegata and Coccinella septempunctata fed on the tobacco aphid, Myzus persicae nicotianae. Phytoparasitica 2015, 43, 159–169. [Google Scholar] [CrossRef]
- Skouras, P.J.; Stathas, G.J. Development, growth and body weight of Hippodamia variegata fed Aphis fabae in the laboratory. Bull. Insectol. 2015, 68, 193–198. [Google Scholar]
- Watson, G.B.; Olson, M.B.; Beavers, K.W.; Loso, M.R.; Sparks, T.C. Characterization of a nicotinic acetylcholine receptor binding site for sulfoxaflor, a new sulfoximine insecticide for the control of sap-feeding insect pests. Pestic. Biochem. Physiol. 2017, 143, 90–94. [Google Scholar] [CrossRef]
- Sparks, T.C.; Watson, G.B.; Loso, M.R.; Geng, C.; Babcock, J.M.; Thomas, J.D. Sulfoxaflor and the sulfoximine insecticides: Chemistry, mode of action and basis for efficacy on resistant insects. Pestic. Biochem. Physiol. 2013, 107, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Babcock, J.M.; Gerwick, C.B.; Huang, J.X.; Loso, M.R.; Nakamura, G.; Nolting, S.P.; Rogers, R.B.; Sparks, T.C.; Thomas, J.; Watson, G.B.; et al. Biological characterization of sulfoxaflor, a novel insecticide. Pest Manag. Sci. 2011, 67, 328–334. [Google Scholar] [CrossRef]
- Candolfi, M.; Barrett, K.; Campbell, P.; Forster, R.; Grandy, N.; Huet, M.; Lewis, G.; Oomen, P.; Schmuck, R.; Vogt, H. Guidance Document on Regulatory Testing and Risk Assessment Procedures for Plant Protection Products with Non-target Arthropods. In Proceedings of the ESCORT 2 Workshop, Wageningen, The Netherlands, 21–23 March 2001; SETAC: Pensacola, FL, USA, 2001. [Google Scholar]
- Overmeer, W.P.J.; van Zon, A.Q. A standardized method for testing the side effects of pesticides on the predacious mite, Amblyseius potentillae [Acarina: Phytoseiidae]. Entomophaga 1982, 27, 357–363. [Google Scholar] [CrossRef]
- Hassan, S.A. Activities of the IOBC/WPRS working group pesticides and beneficial organisms. IOBC/WPRS Bull 1994, 17, 1–5. [Google Scholar]
- Sparks, T.C.; Nauen, R. IRAC: Mode of action classification and insecticide resistance management. Pestic. Biochem. Physiol. 2015, 121, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Foster, S.; Devine, G.; Devonshire, A. Insecticide Resistance; CABI International: Wallingford, UK, 2017; pp. 426–447. [Google Scholar]
- Jiang, J.; Zhang, Z.; Yu, X.; Ma, D.; Yu, C.; Liu, F.; Mu, W. Influence of lethal and sublethal exposure to clothianidin on the seven-spotted lady beetle, Coccinella septempunctata L. (Coleoptera: Coccinellidae). Ecotoxicol. Environ. Saf. 2018, 161, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Ma, D.; Zhang, Z.; Yu, C.; Liu, F.; Mu, W. Favorable compatibility of nitenpyram with the aphid predator, Coccinella septempunctata L. (Coleoptera: Coccinellidae). Environ. Sci. Pollut. Res. 2018, 25, 27393–27401. [Google Scholar] [CrossRef]
- Yu, C.; Lin, R.; Fu, M.; Zhou, Y.; Zong, F.; Jiang, H.; Lv, N.; Piao, X.; Zhang, J.; Liu, Y.; et al. Impact of imidacloprid on life-cycle development of Coccinella septempunctata in laboratory microcosms. Ecotoxicol. Environ. Saf. 2014, 110, 168–173. [Google Scholar] [CrossRef]
- He, F.; Sun, S.; He, L.; Qin, C.; Li, X.; Zhang, J.; Jiang, X. Responses of Harmonia axyridis (Coleoptera: Coccinellidae) to sulfoxaflor exposure. Ecotoxicol. Environ. Saf. 2020, 187, 109849. [Google Scholar] [CrossRef] [PubMed]
- Yao, F.-L.; Zheng, Y.; Zhao, J.-W.; Desneux, N.; He, Y.-X.; Weng, Q.-Y. Lethal and sublethal effects of thiamethoxam on the whitefly predator Serangium japonicum (Coleoptera: Coccinellidae) through different exposure routes. Chemosphere 2015, 128, 49–55. [Google Scholar] [CrossRef]
- Garzón, A.; Medina, P.; Amor, F.; Viñuela, E.; Budia, F. Toxicity and sublethal effects of six insecticides to last instar larvae and adults of the biocontrol agents Chrysoperla carnea (Stephens) (Neuroptera: Chrysopidae) and Adalia bipunctata (L.) (Coleoptera: Coccinellidae). Chemosphere 2015, 132, 87–93. [Google Scholar] [CrossRef]
- Wanumen, A.C.; Sánchez-Ramos, I.; Viñuela, E.; Medina, P.; Adán, Á. Impact of Feeding on Contaminated Prey on the Life Parameters of Nesidiocoris Tenuis (Hemiptera: Miridae) Adults. J. Insect Sci. 2016, 16, 103. [Google Scholar] [CrossRef]
- He, F.; Sun, S.; Tan, H.; Sun, X.; Shang, D.; Yao, C.; Qin, C.; Ji, S.; Li, X.; Zhang, J.; et al. Compatibility of chlorantraniliprole with the generalist predator Coccinella septempunctata L. (Coleoptera: Coccinellidae) based toxicity, life-cycle development and population parameters in laboratory microcosms. Chemosphere 2019, 225, 182–190. [Google Scholar] [CrossRef]
- Aita, R.C.; Tran, A.K.; Koch, R.L. Susceptibility of First Instar Hippodamia convergens (Coleoptera: Coccinellidae) and Chrysoperla rufilabris (Neuroptera: Chrysopidae) to the Insecticide Sulfoxaflor. J. Fla. Entomol. 2020, 103, 191–196. [Google Scholar] [CrossRef]
- Barbosa, P.R.R.; Michaud, J.P.; Bain, C.L.; Torres, J.B. Toxicity of three aphicides to the generalist predators Chrysoperla carnea (Neuroptera: Chrysopidae) and Orius insidiosus (Hemiptera: Anthocoridae). Ecotoxicology 2017, 26, 589–599. [Google Scholar] [CrossRef]
- Dai, C.; Ricupero, M.; Puglisi, R.; Lu, Y.; Desneux, N.; Biondi, A.; Zappalà, L. Can contamination by major systemic insecticides affect the voracity of the harlequin ladybird? Chemosphere 2020, 256, 126986. [Google Scholar] [CrossRef]
- Skouras, P.J.; Stathas, G.J.; Voudouris, C.C.; Darras, A.I.; Tsitsipis, J.A.; Margaritopoulos, J.T. Effect of synthetic insecticides on the larvae of Coccinella septempunctata from Greek populations. Phytoparasitica 2017, 45, 165–173. [Google Scholar] [CrossRef]
- Dai, C.; Ricupero, M.; Wang, Z.; Desneux, N.; Biondi, A.; Lu, Y. Transgenerational Effects of a Neonicotinoid and a Novel Sulfoximine Insecticide on the Harlequin Ladybird. Insects 2021, 12, 681. [Google Scholar] [CrossRef]
- Ju, D.; Liu, Y.-X.; Liu, X.; Dewer, Y.; Mota-Sanchez, D.; Yang, X.-Q. Exposure to lambda-cyhalothrin and abamectin drives sublethal and transgenerational effects on the development and reproduction of Cydia pomonella. Ecotoxicol. Environ. Saf. 2023, 252, 114581. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Zhao, J.; Guo, X.; Chen, H.; Qu, M.; Zhai, W.; Desneux, N.; Biondi, A.; Zhang, F.; Wang, S. Sublethal effects of imidacloprid on the predatory seven-spot ladybird beetle Coccinella septempunctata. Ecotoxicology 2016, 25, 1782–1793. [Google Scholar] [CrossRef]
- Wang, L.; Zhai, Y.; Zhu, J.; Wang, Q.; Ji, X.; Wang, W.; Yuan, H.; Rui, C.; Cui, L. Sulfoxaflor adversely influences the biological characteristics of Coccinella septempunctata by suppressing vitellogenin expression and predation activity. J. Hazard. Mater. 2023, 447, 130787. [Google Scholar] [CrossRef]
- Chi, H. TWOSEX-MSChart: A Computer Program for the Age-Stage, Two-Sex Life Table Analysis; National Chung Hsing University: Taichung, Taiwan, 2021; Available online: http://140.120.197.173/Ecology/ (accessed on 1 November 2022).
- Chi, H.; Liu, H. Two new methods for the study of insect population ecology. Bull. Inst. Zool. Acad. Sin. 1985, 24, 225–240. [Google Scholar]
- Chi, H. Life-Table Analysis Incorporating Both Sexes and Variable Development Rates Among Individuals. Environ. Entomol. 1988, 17, 26–34. [Google Scholar] [CrossRef]
- Akköprü, E.P.; Atlıhan, R.; Okut, H.; Chi, H. Demographic assessment of plant cultivar resistance to insect pests: A case study of the dusky-veined walnut aphid (Hemiptera: Callaphididae) on five walnut cultivars. J. Econ. Entomol. 2015, 108, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Chi, H. TIMING-MSChart: A Computer Program for the Population Projection Based on Age-Stage, Two-Sex Life Table; National Chung Hsing University: Taichung, Taiwan, 2020. [Google Scholar]


| Treatment | Dose Used (ng a.i. per Insect) | Proportion of Female (%) | Fresh Mass of Adults (mg) | Cumulative Mortality (%) | Fecundity (Eggs/Female) | Total Effect (E *) | IOBC Toxicity Category * |
|---|---|---|---|---|---|---|---|
| Control | - | 56.61 ± 8.36 a | 10.20 ± 0.25 a | 05.00 ± 2.88 e | 804.72 ± 56.99 a | - | - |
| Sulfoxaflor | 3 | 56.33 ± 0.78 a | 10.02 ± 0.27 ab | 08.33 ± 1.67 e | 575.61 ± 53.65 ab | 11.32 | 1 |
| 6 | 48.89 ± 1.11 a | 09.47 ± 0.29 abc | 25.00 ± 2.89 d | 532.14 ± 44.12 ab | 30.39 | 2 | |
| 12 | 47.44 ± 1.28 a | 09.34 ± 0.34 abc | 36.67 ± 1.67 c | 486.61 ± 58.78 b | 45.29 | 2 | |
| 24 | 43.94 ± 4.01 a | 08.74 ± 0.30 bc | 43.33 ± 1.67 bc | 431.33 ± 66.21 b | 55.91 | 2 | |
| 48 | 47.04 ± 15.40 a | 08.44 ± 0.33 c | 53.33 ± 1.67 b | 435.23 ± 65.73 b | 61.00 | 2 | |
| 96 | 52.38 ± 4.12 a | 08.31 ± 0.35 c | 65.00 ± 2.89 a | 392.00 ± 72.90 b | 73.72 | 2 |
| Treatment | Dose Used (ng a.i. per Insect) | Duration of Different Life Stages (d) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2nd Instar | 3rd Instar | 4th Instar | 2nd to 4th Instar | Pupae | Female Adult Longevity (d) | APOPa | TPOPb | Male Adult Longevity (d) | ||
| Control | - | 1.65 ± 0.064 b | 1.79 ± 0.070 c | 3.46 ± 0.087 b | 6.89 ± 0.111 b | 3.70 ± 0.075 b | 60.31 ± 2.84 a | 2.03 ± 0.18 d | 17.72 ± 0.29 d | 43.84 ± 3.03 a |
| Sulfoxaflor | 3 | 2.05 ± 0.048 a | 1.82 ± 0.059 bc | 3.89 ± 0.106 ab | 7.76 ± 0.104 a | 4.07 ± 0.100 ab | 46.94 ± 2.82 ab | 2.32 ± 0.21 cd | 19.29 ± 0.29 c | 41.04 ± 3.09 a |
| 6 | 2.04 ± 0.044 a | 2.07 ± 0.074 abc | 3.89 ± 0.079 ab | 8.00 ± 0.119 a | 4.09 ± 0.083 ab | 45.23 ± 4.29 ab | 2.46 ± 0.24 cd | 19.77 ± 0.35 bc | 38.83 ± 3.16 a | |
| 12 | 1.97 ± 0.070 a | 2.13 ± 0.086 ab | 3.87 ± 0.094 ab | 7.97 ± 0.144 a | 4.08 ± 0.087 ab | 44.44 ± 4.32 ab | 3.06 ± 0.36 bcd | 20.44 ±0.38 abc | 34.95 ± 3.38 a | |
| 24 | 1.88 ± 0.082 ab | 2.06 ± 0.072 abc | 3.74 ± 0.088 ab | 7.68 ± 0.132 a | 4.12 ± 0.101 a | 43.73 ± 4.32 ab | 3.47 ± 0.31 abc | 20.53 ± 0.42 abc | 36.47 ± 3.47 a | |
| 48 | 1.68 ± 0.090 b | 2.07 ± 0.102 abc | 3.86 ± 0.112 ab | 7.61 ± 0.165 a | 4.21 ± 0.079 a | 41.08 ± 6.68 b | 4.31 ± 0.54 ab | 21.62 ± 0.45 a | 34.27 ± 3.91 a | |
| 96 | 1.62 ± 0.129 b | 2.19 ± 0.088 a | 3.95 ± 0.129 a | 7.76 ± 0.217 a | 4.14 ± 0.104 a | 41.18 ± 5.19 b | 4.55 ± 0.68 a | 21.54 ± 0.49 ab | 27.80 ± 4.79 a | |
| Treatment | Dose Used (ng a.i. per Insect) | Life Table Parameters | |||
|---|---|---|---|---|---|
| Net Reproduction Rate (R0) (Female Female−1) | Intrinsic Rate of Increase (r) (Female Female−1d−1) | Finite Rate of Increase (λ) (Female Female−1d−1) | Mean Generation Time (T) (d) | ||
| Control | - | 362.69 ± 53.94 a | 0.2053 ± 0.0086 a | 1.2279 ± 0.0105 a | 28.71 ± 0.74 b |
| Sulfoxaflor | 3 | 251.32 ± 40.99 ab | 0.1869 ± 0.0073 ab | 1.2055 ± 0.0088 ab | 29.57 ± 0.56 a |
| 6 | 164.89 ± 31.67 bc | 0.1774 ± 0.0083 bc | 1.1941 ± 0.0099 bc | 28.78 ± 0.68 b | |
| 12 | 123.37 ± 29.03 cd | 0.1658 ± 0.0099 bcd | 1.1804 ± 0.0117 bcd | 29.04 ± 0.66 b | |
| 24 | 91.13 ± 24.90 cd | 0.1542 ± 0.0113 cd | 1.1667 ± 0.0131 cd | 29.26 ± 0.79 a | |
| 48 | 79.69 ± 23.76 d | 0.1400 ± 0.0105 d | 1.1503 ± 0.0120 d | 31.27 ± 0.68 a | |
| 96 | 60.73 ± 20.03 d | 0.1339 ± 0.0141 d | 1.1433 ± 0.0160 d | 30.67 ± 1.45 ab | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skouras, P.J.; Karanastasi, E.; Demopoulos, V.; Mprokaki, M.; Stathas, G.J.; Margaritopoulos, J.T. Toxicity and Influence of Sublethal Exposure to Sulfoxaflor on the Aphidophagous Predator Hippodamia variegata (Coleoptera: Coccinellidae). Toxics 2023, 11, 533. https://doi.org/10.3390/toxics11060533
Skouras PJ, Karanastasi E, Demopoulos V, Mprokaki M, Stathas GJ, Margaritopoulos JT. Toxicity and Influence of Sublethal Exposure to Sulfoxaflor on the Aphidophagous Predator Hippodamia variegata (Coleoptera: Coccinellidae). Toxics. 2023; 11(6):533. https://doi.org/10.3390/toxics11060533
Chicago/Turabian StyleSkouras, Panagiotis J., Eirini Karanastasi, Vasilis Demopoulos, Marina Mprokaki, George J. Stathas, and John T. Margaritopoulos. 2023. "Toxicity and Influence of Sublethal Exposure to Sulfoxaflor on the Aphidophagous Predator Hippodamia variegata (Coleoptera: Coccinellidae)" Toxics 11, no. 6: 533. https://doi.org/10.3390/toxics11060533
APA StyleSkouras, P. J., Karanastasi, E., Demopoulos, V., Mprokaki, M., Stathas, G. J., & Margaritopoulos, J. T. (2023). Toxicity and Influence of Sublethal Exposure to Sulfoxaflor on the Aphidophagous Predator Hippodamia variegata (Coleoptera: Coccinellidae). Toxics, 11(6), 533. https://doi.org/10.3390/toxics11060533








