ATM/Chk2 and ATR/Chk1 Pathways Respond to DNA Damage Induced by Movento® 240SC and Envidor® 240SC Keto-Enol Insecticides in the Germarium of Drosophila melanogaster
Abstract
:1. Introduction
2. Materials and Methods
2.1. Drosophila Melanogaster Strains
2.2. Preparation of Keto-Enol Insecticide Yeast Paste
2.3. Treatment Scheme for Keto-Enol Insecticides Movento® 240SC and Envidor® 240SC
2.4. Dissection of Ovaries
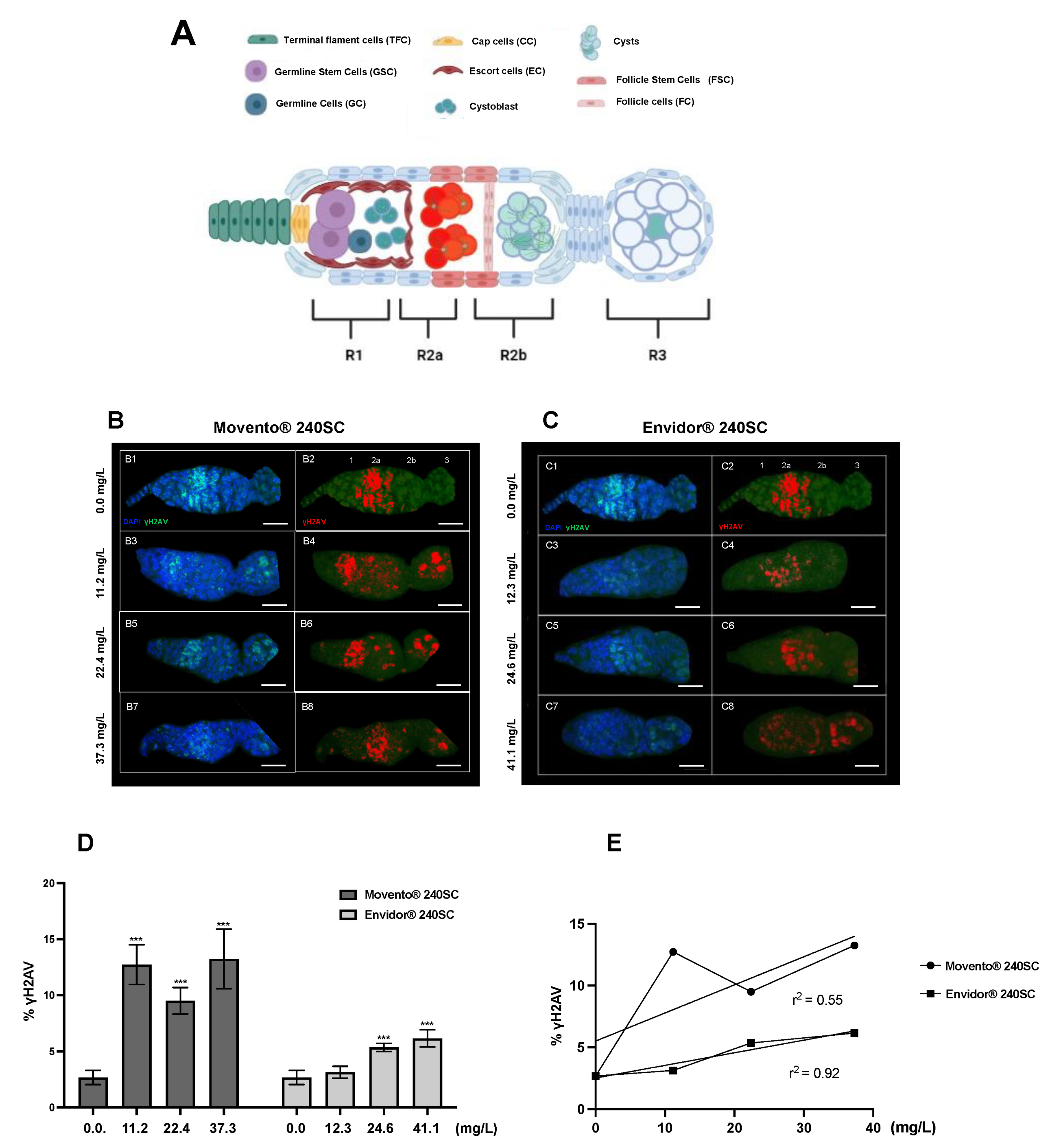
2.5. Expression of γH2AV in the Ovary Germarium of Mutant and Wild-Type D. melanogaster Strains Exposed and Unexposed to Keto-Enol Insecticides by Confocal Immunofluorescence
2.6. Image Processing
2.7. Statistical Analysis
3. Results
3.1. Induction of DNA Damage in the Germarium in the Wild-Type Strain of Drosophila melanogaster (Oregon R) using Keto-Enol Insecticides Movento® 240SC and Envidor® 240SC
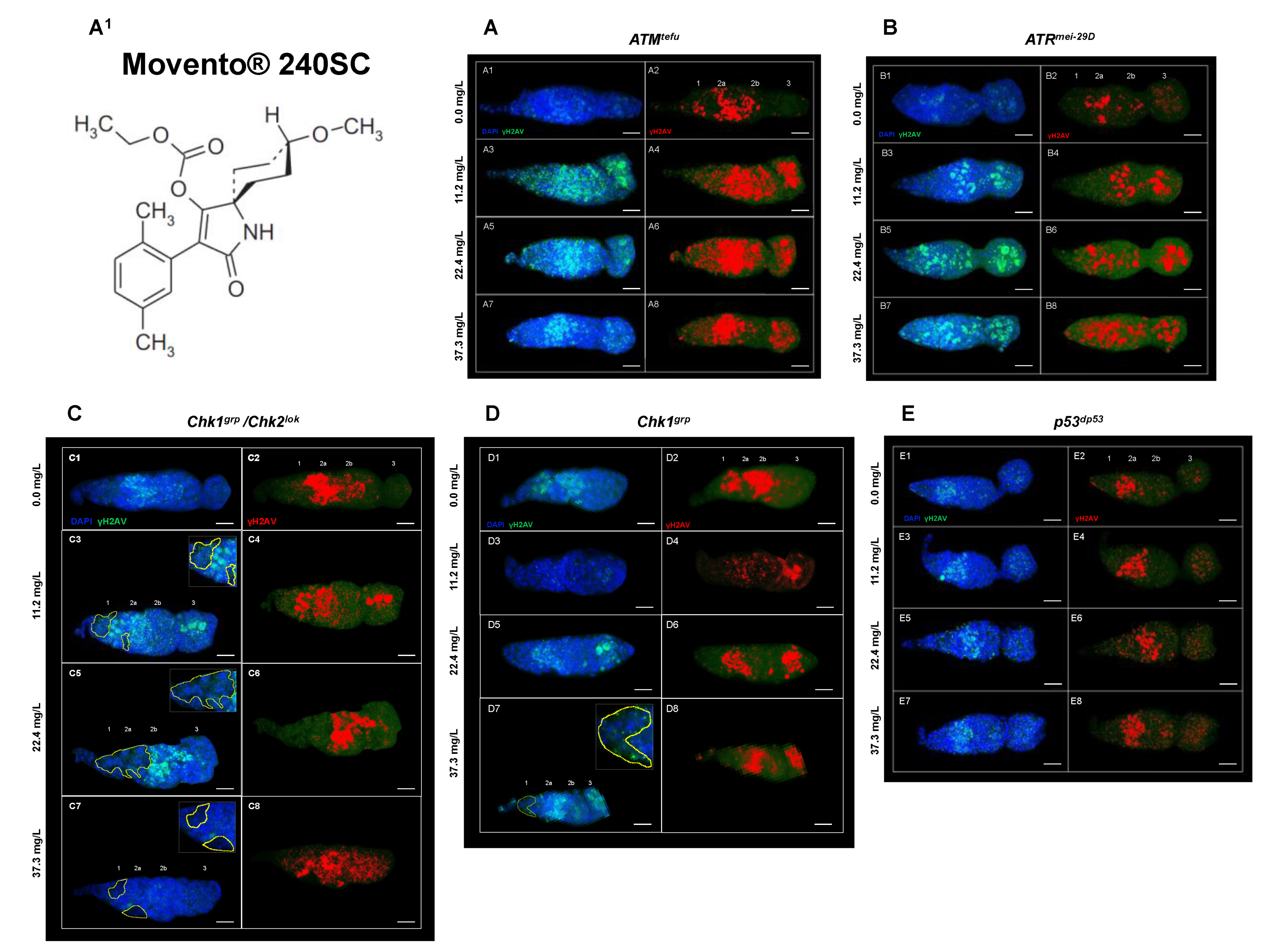
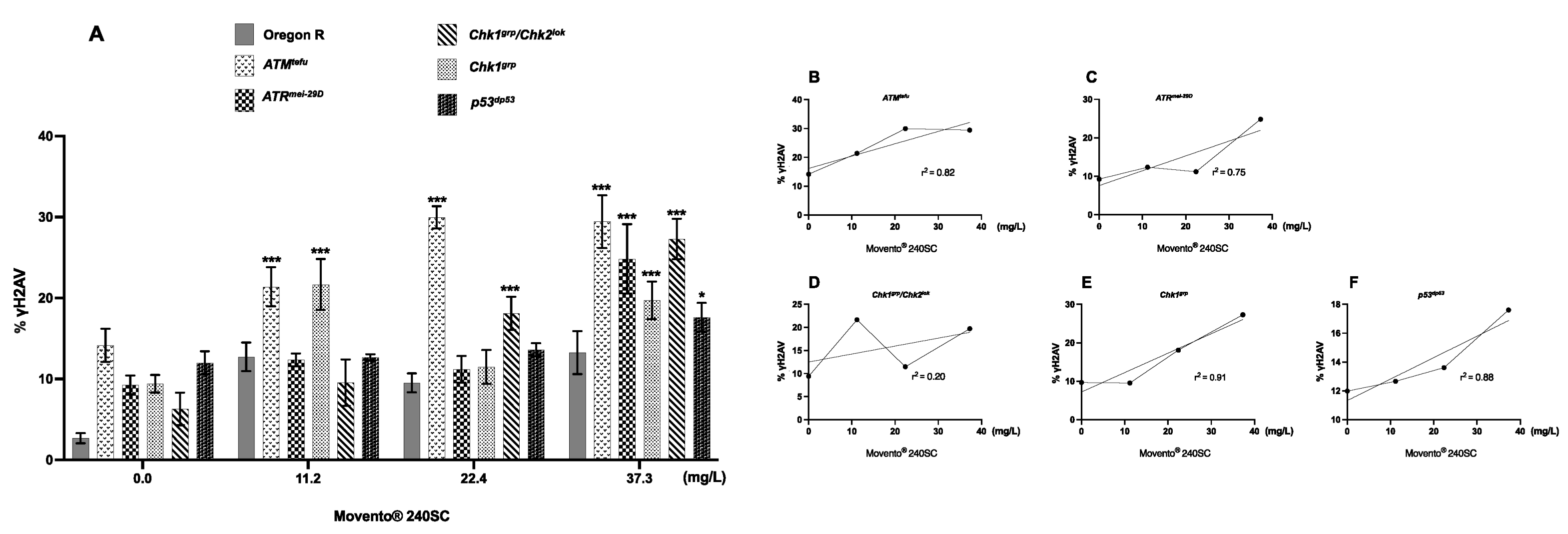
3.2. Response of ATM, Chk1 and Chk2 in the Germarium with DNA Damage Induced by the Keto-Enol Insecticide Movento® 240SC
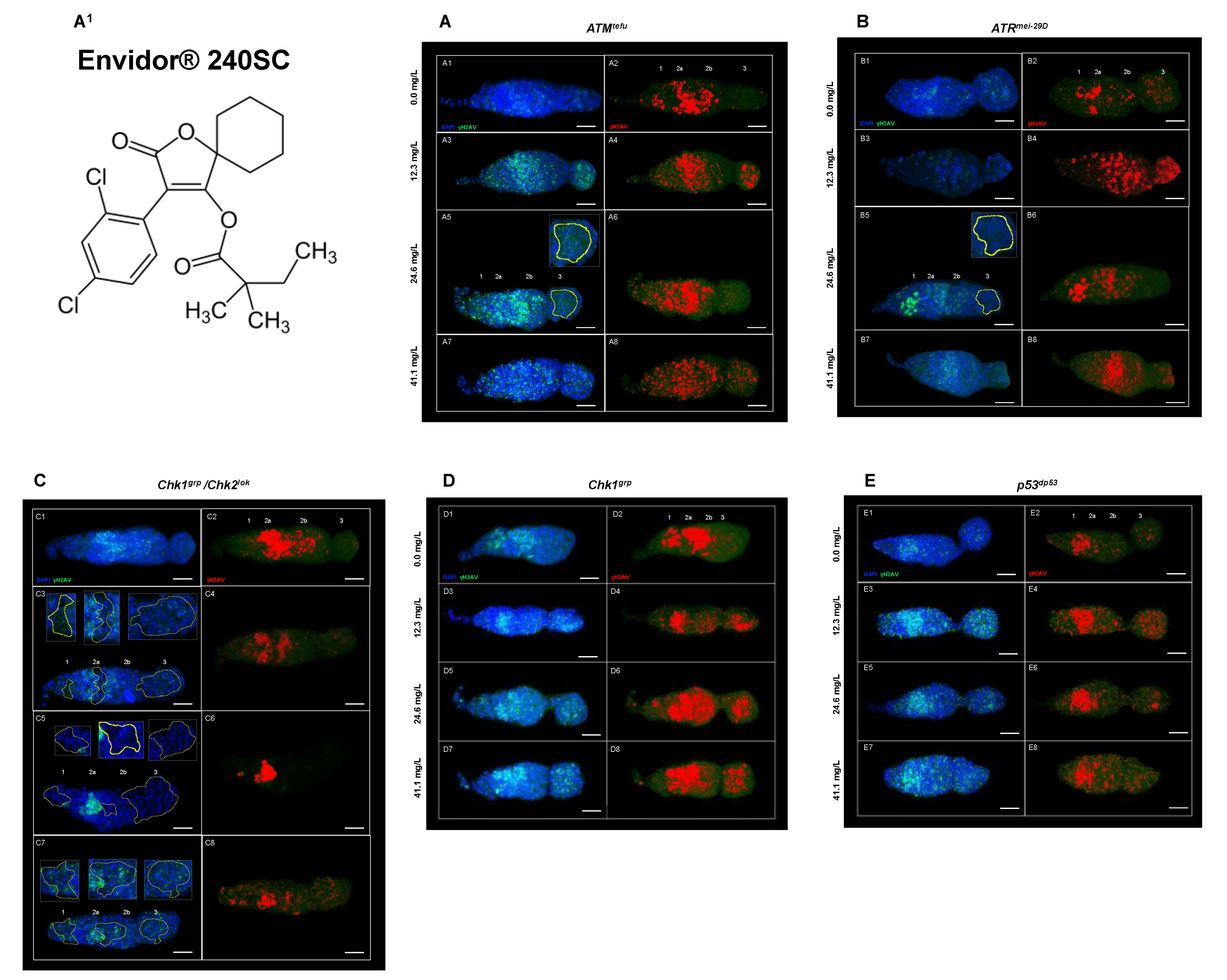
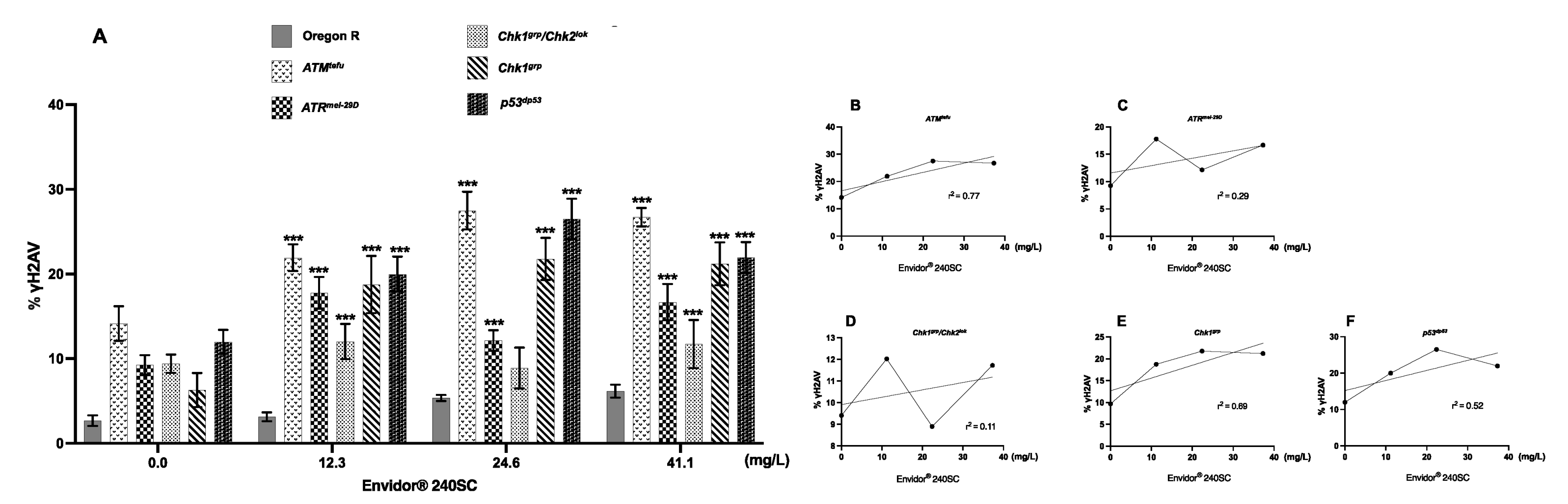
3.3. Response of ATM, ATR, Chk1, Chk1 and p53 in the Germarium of D. melanogaster with DNA Damage Induced by the Keto-Enol Insecticide Envidor® 240 SC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gundogan, K.; Donmez-Altuntas, H.; Hamurcu, Z.; Akbudak, I.H.; Sungur, M.; Bitgen, N.; Baskol, G.; Bayram, F. Evaluation of chromosomal DNA damage, cytotoxicity, cytostasis, oxidative DNA damage and their relationship with endocrine hormones in patients with acute organophosphate poisoning. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2018, 825, 1–7. [Google Scholar] [CrossRef]
- Hilgert Jacobsen-Pereira, C.; Dos Santos, C.R.; Troina Maraslis, F.; Pimentel, L.; Feijó, A.J.L.; Iomara Silva, C.; de Medeiros, G.D.S.; Costa Zeferino, R.; Curi Pedrosa, R.; Weidner Maluf, S. Markers of genotoxicity and oxidative stress in farmers exposed to pesticides. Ecotoxicol. Environ. Saf. 2018, 148, 177–183. [Google Scholar] [CrossRef]
- Sabarwal, A.; Kumar, K.; Singh, R.P. Hazardous effects of chemical pesticides on human health-Cancer and other associated disorders. Environ. Toxicol. Pharmacol. 2018, 63, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Nauen, R.; Bretschneider, T.; Elbert, A.; Fischer, R.; Tiemann, R. Spirodiclofen and Spiromesifen. J. Pestic. Outlook 2003, 14, 243–245. [Google Scholar] [CrossRef]
- Ouyang, Y.; Montez, G.H.; Liu, L.; Grafton-Cardwell, E.E. Spirodiclofen and Spirotetramat bioassays for monitoring resistance in citrus red mite, Panonychus citri (Acari: Tetranychidae). Pest Manag. Sci. 2012, 68, 781–787. [Google Scholar] [CrossRef]
- Sparks, T.C.; Nauen, R. IRAC: Mode of action classification and insecticide resistance management. Pestic. Biochem. Physiol. 2015, 121, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Bretschneider, T.; Benet-Buchholz, J.; Fischer, R.; Nauen, R. Spirodiclofen and Spiromesifen Novel Acaricidal and Insecticidal Tetronic Acid Derivatives with a New Mode of Action. CHIMIA Int. J. Chem. 2003, 57, 697–701. [Google Scholar] [CrossRef]
- Lümmen, P.; Khajehali, J.; Luther, K.; Van Leeuwen, T. The cyclic keto-enol insecticide spirotetramat inhibits insect and spider mite acetyl-CoA carboxylases by interfering with the carboxyltransferase partial reaction. Insect Biochem. Mol. Biol. 2014, 55, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Inoue, K.; Takahashi, M. Predictive modes of action of pesticides in uterine adenocarcinoma development in rats. J. Toxicol. Pathol. 2015, 28, 207–216. [Google Scholar] [CrossRef]
- FAO. Food and Agriculture Organization, Pesticides-Spirodiklofen Report No: 09. 2019. Available online: http://www.fao.org/fileadmin/templates/agphome/documents/Pests_Pesticides/JMPR/Report09/Spirodiclofen.pdf (accessed on 21 February 2023).
- Zhang, J.; Qian, L.; Teng, M.; Mu, X.; Qi, S.; Chen, X.; Zhou, Y.; Cheng, Y.; Pang, S.; Li, X.; et al. The lipid metabolism alteration of three spirocyclic tetramic acids on zebrafish (Danio rerio) embryos. Environ. Pollut. 2019, 248, 715–725. [Google Scholar] [CrossRef]
- Çavuşoğlu, D.; Yalçin, E.; Çavuşoğlu, K.; Acar, A.; Yapar, K. Molecular docking and toxicity assessment of spirodiclofen: Protective role of lycopene. Environ. Sci. Pollut. Res. Int. 2021, 28, 57372–57385. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Jiang, S.; Yu, J.; Zhu, G.; Wu, H.; Mao, C. Effects of Spirotetramat on the acute toxicity, oxidative stress, and lipid peroxidation in Chinese toad (Bufo bufo gargarizans) tadpoles. Environ. Toxicol. Pharmacol. 2014, 37, 1229–1235. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, G.; Yin, P.; Lv, Y.; Yuan, S.; Chen, J.; Wei, B.; Wang, C. Toxicological effects of soil contaminated with spirotetramat to the earthworm Eisenia fetida. Chemosphere 2015, 139, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Qian, L.; Wang, C.; Teng, M.; Duan, M.; Zhou, Y.; Chen, X.; Bo, R.; Wang, C.; Li, X. Dysregulation of endocrine disruption, apoptosis and the transgenerational toxicity induced by spirotetramat. Chemosphere 2020, 240, 124900. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Qian, L.; Wang, C.; Teng, M.; Duan, M.; Chen, X.; Li, X.; Wang, C. UPLC-TOF MS/MS metabolomics analysis of zebrafish metabolism by spirotetramat. Environ. Pollut. 2020, 266 Pt 2, 115310. [Google Scholar] [CrossRef]
- González-Marín, B.; Calderón-Segura, M.E.; González Pérez, A.K.; Moreno Ciénega, L.G. Movento® 240SC (Spirotetramat) and Envidor® 240SC (Spirodiclofen) keto-enol insecticides induce DNA damage in Drosophila melanogaster ovaries. Fundam. Toxicol. Sci. 2021, 8, 81–88. [Google Scholar] [CrossRef]
- Clavel, J. Progress in the epidemiological understanding of gene-environment interactions in major diseases: Cancer. Comptes Rendus Biol. 2007, 330, 306–317. [Google Scholar] [CrossRef]
- Guanggang, X.; Diqiu, L.; Jianzhong, Y.; Jingmin, G.; Huifeng, Z.; Mingan, S.; Liming, T. Carbamate insecticide methomyl confers cytotoxicity through DNA damage induction. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2013, 53, 352–358. [Google Scholar] [CrossRef]
- Jackson, S.P.; Bartek, J. The DNA-damage response in human biology and disease. Nature 2009, 461, 1071–1078. [Google Scholar] [CrossRef]
- Sancar, A.; Lindsey-Boltz, L.A.; Unsal-Kaçmaz, K.; Linn, S. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu. Rev. Biochem. 2004, 73, 39–85. [Google Scholar] [CrossRef]
- Rogakou, E.P.; Pilch, D.R.; Orr, A.H.; Ivanova, V.S.; Bonner, W.M. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J. Biol. Chem. 1998, 273, 5858–5868. [Google Scholar] [CrossRef] [PubMed]
- Rogakou, E.P.; Boon, C.; Redon, C.; Bonner, W.M. Megabase chromatin domains involved in DNA double-strand breaks in vivo. J. Cell Biol. 1999, 146, 905–916. [Google Scholar] [CrossRef] [PubMed]
- Madigan, J.P.; Chotkowski, H.L.; Glaser, R.L. DNA double-strand break-induced phosphorylation of Drosophila histone variant H2Av helps prevent radiation-induced apoptosis. Nucleic Acids Res. 2002, 30, 3698–3705. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Traganos, F.; Darzynkiewicz, Z. DNA damage induced by DNA topoisomerase I- and topoisomerase II-inhibitors detected by histone H2AX phosphorylation in relation to the cell cycle phase and apoptosis. Cell Cycle 2003, 2, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Sedelnikova, O.A.; Horikawa, I.; Zimonjic, D.B.; Popescu, N.C.; Bonner, W.M.; Barrett, J.C. Senescing human cells and ageing mice accumulate DNA lesions with unrepairable double-strand breaks. Nat. Cell Biol. 2004, 6, 168–170. [Google Scholar] [CrossRef]
- Costes, S.V.; Chiolo, I.; Pluth, J.M.; Barcellos-Hoff, M.H.; Jakob, B. Spatiotemporal characterization of ionizing radiation induced DNA damage foci and their relation to chromatin organization. Mutat. Res. 2010, 704, 78–87. [Google Scholar] [CrossRef]
- Zhou, C.; Li, Z.; Diao, H.; Yu, Y.; Zhu, W.; Dai, Y.; Chen, F.F.; Yang, J. DNA damage evaluated by gammaH2AX foci formation by a selective group of chemical/physical stressors. Mutat. Res. 2006, 604, 8–18. [Google Scholar] [CrossRef]
- Watters, G.P.; Smart, D.J.; Harvey, J.S.; Austin, C.A. H2AX phosphorylation as a genotoxicity endpoint. Mutat. Res. 2009, 679, 50–58. [Google Scholar] [CrossRef]
- Hershman, J.M.; France, B.; Hon, K.; Damoiseaux, R. Direct quantification of gamma H2AX by cell-based high throughput screening for evaluation of genotoxicity of pesticides in a human thyroid cell lines. Environ. Mol. Mutagen. 2017, 58, 522–528. [Google Scholar] [CrossRef]
- Song, Y.H.; Mirey, G.; Betson, M.; Haber, D.A.; Settleman, J. The Drosophila ATM ortholog, dATM, mediates the response to ionizing radiation and to spontaneous DNA damage during development. Curr. Biol. 2004, 14, 1354–1359. [Google Scholar] [CrossRef]
- Maréchal, A.; Zou, L. DNA damage sensing by the ATM and ATR kinases. Cold Spring Harb. Perspect. Biol. 2013, 5, a012716. [Google Scholar] [CrossRef]
- Brodsky, M.H.; Weinert, B.T.; Tsang, G.; Rong, Y.S.; McGinnis, N.M.; Golic, K.G.; Rio, D.C.; Rubin, G.M. Drosophila melanogaster MNK/Chk2 and p53 regulate multiple DNA repair and apoptotic pathways following DNA damage. Mol. Cell. Biol. 2004, 24, 1219–1231. [Google Scholar] [CrossRef]
- de Vries, H.I.; Uyetake, L.; Lemstra, W.; Brunsting, J.F.; Su, T.T.; Kampinga, H.H.; Sibon, O.C. Grp/DChk1 is required for G2-M checkpoint activation in Drosophila S2 cells, whereas Dmnk/DChk2 is dispensable. J. Cell Sci. 2005, 118 Pt 9, 1833–1842. [Google Scholar] [CrossRef]
- Khan, C.; Muliyil, S.; Rao, B.J. Genome Damage Sensing Leads to Tissue Homeostasis in Drosophila. Int. Rev. Cell Mol. Biol. 2019, 345, 173–224. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Khadpe, J.; Hu, B.; Iliakis, G.; Wang, Y. An overactivated ATR/CHK1 pathway is responsible for the prolonged G2 accumulation in irradiated AT cells. J. Biol. Chem. 2003, 278, 30869–30874. [Google Scholar] [CrossRef] [PubMed]
- Shiloh, Y.; Ziv, Y. The ATM protein: The importance of being active. J. Cell Biol. 2012, 198, 273–275. [Google Scholar] [CrossRef] [PubMed]
- Shiloh, Y.; Ziv, Y. The ATM protein kinase: Regulating the cellular response to genotoxic stress, and more. Nature reviews. Mol. Cell Biol. 2013, 14, 197–210. [Google Scholar] [CrossRef]
- Brodsky, M.H.; Nordstrom, W.; Tsang, G.; Kwan, E.; Rubin, G.M.; Abrams, J.M. Drosophila p53 binds a damage response element at the reaper locus. Cell 2000, 101, 103–113. [Google Scholar] [CrossRef]
- Ollmann, M.; Young, L.M.; Di Como, C.J.; Karim, F.; Belvin, M.; Robertson, S.; Whittaker, K.; Demsky, M.; Fisher, W.W.; Buchman, A.; et al. Drosophila p53 is a structural and functional homolog of the tumor suppressor p53. Cell 2000, 101, 91–101. [Google Scholar] [CrossRef]
- Rajak, P.; Dutta, M.; Roy, S. Altered differential hemocyte count in 3rd instar larvae of Drosophila melanogaster as a response to chronic exposure of Acephate. Interdiscip. Toxicol. 2015, 8, 84–88. [Google Scholar] [CrossRef]
- Mirzoyan, Z.; Sollazzo, M.; Allocca, M.; Valenza, A.M.; Grifoni, D.; Bellosta, P. Drosophila melanogaster: A Model Organism to Study Cancer. Front. Genet. 2019, 10, 51. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.M.; Beck, B.D.; Rhomberg, L.R. Historical perspective on the role of cell proliferation in carcinogenesis for DNA-reactive and non-DNA-reactive carcinogens: Arsenic as an example. Toxicology 2021, 456, 152783. [Google Scholar] [CrossRef] [PubMed]
- Sekelsky, J.J.; Brodsky, M.H.; Burtis, K.C. DNA repair in Drosophila: Insights from the Drosophila genome sequence. J. Cell Biol. 2000, 150, F31–F36. [Google Scholar] [CrossRef] [PubMed]
- Sekelsky, J. DNA Repair in Drosophila: Mutagens, Models, and Missing Genes. Genetics 2017, 205, 471–490. [Google Scholar] [CrossRef]
- Baonza, A.; Tur-Gracia, S.; Pérez-Aguilera, M.; Estella, C. Regulation and coordination of the different DNA damage responses in Drosophila. Front. Cell Dev. Biol. 2022, 10, 993257. [Google Scholar] [CrossRef]
- Jang, J.K.; Sherizen, D.E.; Bhagat, R.; Manheim, E.A.; McKim, K.S. Relationship of DNA double-strand breaks to synapsis in Drosophila. J. Cell Sci. 2003, 116 Pt 15, 3069–3077. [Google Scholar] [CrossRef]
- Hsu, H.J.; Drummond-Barbosa, D. Insulin signals control the competence of the Drosophila female germline stem cell niche to respond to Notch ligands. Dev. Biol. 2011, 350, 290–300. [Google Scholar] [CrossRef]
- Hatkevich, T.; Miller, D.E.; Turcotte, C.A.; Miller, M.C.; Sekelsky, J. A pathway for error-free non-homologous end joining of resected meiotic double-strand breaks. Nucleic Acids Res. 2021, 49, 879–890. [Google Scholar] [CrossRef]
- Andersen, S.L.; Bergstralh, D.T.; Kohl, K.P.; LaRocque, J.R.; Moore, C.B.; Sekelsky, J. Drosophila MUS312 and the vertebrate ortholog BTBD12 interact with DNA structure-specific endonucleases in DNA repair and recombination. Mol. Cell 2009, 35, 128–135. [Google Scholar] [CrossRef]
- Hernando, J.; Alvarez, L.; Ferreiro, J.A.; Sancho, I.; Comendador, M.A.; Sierra, L.M. Female germ cell mutagenicity of model chemicals in Drosophila melanogaster: Mechanistic information and analysis of repair systems. Mutat. Res. 2004, 545, 59–72. [Google Scholar] [CrossRef]
- LaRocque, J.R.; Jaklevic, B.; Su, T.T.; Sekelsky, J. Drosophila ATR in double-strand break repair. Genetics 2007, 175, 1023–1033. [Google Scholar] [CrossRef]
- Laurençon, A.; Purdy, A.; Sekelsky, J.; Hawley, R.S.; Su, T.T. Phenotypic analysis of separation-of-function alleles of MEI-41, Drosophila ATM/ATR. Genetics 2003, 164, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Huang, Q.; Lu, M.; Zhang, L.; Yang, Z.; Zong, M.; Tao, L. The organophosphate insecticide chlorpyrifos confers its genotoxic effects by inducing DNA damage and cell apoptosis. Chemosphere 2015, 135, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Siddique, H.R.; Sharma, A.; Gupta, S.C.; Murthy, R.C.; Dhawan, A.; Saxena, D.K.; Chowdhuri, D.K. DNA damage induced by industrial solid waste leachates in Drosophila melanogaster: A mechanistic approach. Environ. Mol. Mutagen. 2008, 49, 206–216. [Google Scholar] [CrossRef]
- Tanaka, T.; Huang, X.; Halicka, H.D.; Zhao, H.; Traganos, F.; Albino, A.P.; Dai, W.; Darzynkiewicz, Z. Cytometry of ATM activation and histone H2AX phosphorylation to estimate extent of DNA damage induced by exogenous agents. Cytom. Part A J. Int. Soc. Anal. Cytol. 2007, 71, 648–661. [Google Scholar] [CrossRef] [PubMed]
- Dewey, E.B.; Parra, A.S.; Johnston, C.A. Loss of the spectraplakin gene Short stop induces a DNA damage response in Drosophila epithelia. Sci. Rep. 2020, 10, 20165. [Google Scholar] [CrossRef]
- Gorski, M.M.; Romeijn, R.J.; Eeken, J.C.; de Jong, A.W.; van Veen, B.L.; Szuhai, K.; Mullenders, L.H.; Ferro, W.; Pastink, A. Disruption of Drosophila Rad50 causes pupal lethality, the accumulation of DNA double-strand breaks and the induction of apoptosis in third instar larvae. DNA Repair 2004, 3, 603–615. [Google Scholar] [CrossRef]
- Gaschler, M.M.; Stockwell, B.R. Lipid peroxidation in cell death. Biochem. Biophys. Res. Commun. 2017, 482, 419–425. [Google Scholar] [CrossRef]
- Wu, H.; Rao, Q.; Zheng, J.; Mao, C.; Sun, Y.; Gu, D.; Wang, M.; Liu, X. Biochemical and histological alterations in adult zebrafish (Danio rerio) ovary following exposure to the tetronic acid insecticide spirotetramat. Ecotoxicol. Environ. Saf. 2018, 164, 149–154. [Google Scholar] [CrossRef]
- Shiloh, Y. ATM and ATR: Networking cellular responses to DNA damage. Curr. Opin. Genet. Dev. 2001, 11, 71–77. [Google Scholar] [CrossRef]
- Abraham, R.T. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev. 2001, 15, 2177–2196. [Google Scholar] [CrossRef]
- Bartek, J.; Lukas, J. DNA damage checkpoints: From initiation to recovery or adaptation. Curr. Opin. Cell Biol. 2007, 19, 238–245. [Google Scholar] [CrossRef]
- Tan, Y.; Raychaudhuri, P.; Costa, R.H. Chk2 mediates stabilization of the FoxM1 transcription factor to stimulate expression of DNA repair genes. Mol. Cell. Biol. 2007, 27, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- Evans-Anderson, H.J.; Alfieri, C.M.; Yutzey, K.E. Regulation of cardiomyocyte proliferation and myocardial growth during development by FOXO transcription factors. Circ. Res. 2008, 102, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Paik, J.H.; Kollipara, R.; Chu, G.; Ji, H.; Xiao, Y.; Ding, Z.; Miao, L.; Tothova, Z.; Horner, J.W.; Carrasco, D.R.; et al. FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell 2007, 128, 309–323. [Google Scholar] [CrossRef]
- Sengupta, A.; Molkentin, J.D.; Paik, J.H.; DePinho, R.A.; Yutzey, K.E. FoxO transcription factors promote cardiomyocyte survival upon induction of oxidative stress. J. Biol. Chem. 2011, 286, 7468–7478. [Google Scholar] [CrossRef]
- Kafshgiri, S.K.; Parivar, K.; Baharara, J.; Kerachian, M.A.; Hayati Roodbari, N. Movento influences development of granulosa cells and ovarian follicles and FoxO1 and Vnn1 gene expression in BALB/c mice. Iran. J. Basic Med. Sci. 2016, 19, 1209–1215. [Google Scholar] [PubMed]
- Saldivar, J.C.; Cortez, D.; Cimprich, K.A. The essential kinase ATR: Ensuring faithful duplication of a challenging genome. Nature reviews. Mol. Cell Biol. 2017, 18, 622–636. [Google Scholar] [CrossRef]
- Byun, T.S.; Pacek, M.; Yee, M.C.; Walter, J.C.; Cimprich, K.A. Functional uncoupling of MCM helicase and DNA polymerase activities activates the ATR-dependent checkpoint. Genes Dev. 2005, 19, 1040–1052. [Google Scholar] [CrossRef]
- Zou, L.; Elledge, S.J. Sensing DNA damage through ATRIP recognition of RPA-ssDNA complexes. Science 2003, 300, 1542–1548. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.; Powell, S.N.; Iliakis, G.; Wang, Y. ATR affecting cell radiosensitivity is dependent on homologous recombination repair but independent of nonhomologous end joining. Cancer Res. 2004, 64, 7139–7143. [Google Scholar] [CrossRef] [PubMed]
- Roos, W.P.; Kaina, B. DNA damage-induced cell death: From specific DNA lesions to the DNA damage response and apoptosis. Cancer Lett. 2013, 332, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Kastan, M.B.; Bartek, J. Cell-cycle checkpoints and cancer. Nature 2004, 432, 316–323. [Google Scholar] [CrossRef]
- Smith, J.; Tho, L.M.; Xu, N.; Gillespie, D.A. The ATM-Chk2 and ATR-Chk1 pathways in DNA damage signaling and cancer. Adv. Cancer Res. 2010, 108, 73–112. [Google Scholar] [CrossRef]
- Chehab, N.H.; Malikzay, A.; Appel, M.; Halazonetis, T.D. Chk2/hCds1 functions as a DNA damage checkpoint in G(1) by stabilizing p53. Genes Dev. 2000, 14, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Shieh, S.Y.; Ahn, J.; Tamai, K.; Taya, Y.; Prives, C. The human homologs of checkpoint kinases Chk1 and Cds1 (Chk2) phosphorylate p53 at multiple DNA damage-inducible sites. Genes Dev. 2000, 14, 289–300. [Google Scholar] [CrossRef]
- Chen, Z.; Trotman, L.C.; Shaffer, D.; Lin, H.K.; Dotan, Z.A.; Niki, M.; Koutcher, J.A.; Scher, H.I.; Ludwig, T.; Gerald, W.; et al. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature 2005, 436, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Stevens, C.; Smith, L.; La Thangue, N.B. Chk2 activates E2F-1 in response to DNA damage. Nat. Cell Biol. 2003, 5, 401–409. [Google Scholar] [CrossRef]

| Genotype in the Text | Genotype 1 | Characteristic |
|---|---|---|
| Oregon R | + | Wild-type strain competent in all DNA damage response mechanisms. |
| ATMtefu | w; tefu e6 [be] | Mutant deficient in the protein kinase tefu (telomere fusion), homologue of ATM in mammals. |
| ATRmei−29D | w; mei-4129D/y{UASp41} mei-4129D; p{mtα}/+ | Mutant deficient in the protein kinase mei-41 (meiotic-41), ortholog of ATR in mammals. |
| Chk1grp/Chk2lok | w/+; grp209 lok30/grpZ5170 lok30 | Mutant deficient in the protein kinases: grp (grappes) and lok (localized ovarian kinase), respectively orthologs of Chk1 and Chk2 in mammals. |
| Chk1grp | w/+; grp209/grpZ5170 | Mutant deficient in the protein kinase grp (grapes), ortholog of Chk1 in mammals. |
| p53dp53 | y1 w1118; p535A−1−4 | Mutant deficient in the dp53 protein (tumor suppressor), ortholog of p53 in mammals. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Marín, B.; Calderón-Segura, M.E.; Sekelsky, J. ATM/Chk2 and ATR/Chk1 Pathways Respond to DNA Damage Induced by Movento® 240SC and Envidor® 240SC Keto-Enol Insecticides in the Germarium of Drosophila melanogaster. Toxics 2023, 11, 754. https://doi.org/10.3390/toxics11090754
González-Marín B, Calderón-Segura ME, Sekelsky J. ATM/Chk2 and ATR/Chk1 Pathways Respond to DNA Damage Induced by Movento® 240SC and Envidor® 240SC Keto-Enol Insecticides in the Germarium of Drosophila melanogaster. Toxics. 2023; 11(9):754. https://doi.org/10.3390/toxics11090754
Chicago/Turabian StyleGonzález-Marín, Berenyce, María Elena Calderón-Segura, and Jeff Sekelsky. 2023. "ATM/Chk2 and ATR/Chk1 Pathways Respond to DNA Damage Induced by Movento® 240SC and Envidor® 240SC Keto-Enol Insecticides in the Germarium of Drosophila melanogaster" Toxics 11, no. 9: 754. https://doi.org/10.3390/toxics11090754
APA StyleGonzález-Marín, B., Calderón-Segura, M. E., & Sekelsky, J. (2023). ATM/Chk2 and ATR/Chk1 Pathways Respond to DNA Damage Induced by Movento® 240SC and Envidor® 240SC Keto-Enol Insecticides in the Germarium of Drosophila melanogaster. Toxics, 11(9), 754. https://doi.org/10.3390/toxics11090754









