Amelioration of Hepatotoxic and Neurotoxic Effect of Cartap by Aloe vera in Wistar Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Collection of A. vera Leaf and Phytochemical Extraction
2.3. Animal Care and Experimental Design
2.4. Preparation of Post-Nuclear Supernatant (PNS) in Sucrose Solution
2.5. AChE Extraction from RBC and Rat Brain
2.6. Estimation of Malondialdehyde (MDA) and Glutathione (GSH) Level
2.7. Activity Assay for Superoxide Dismutase (SOD), Catalase (CAT), and Glutathione-S-Transferase (GST)
2.8. Estimation of TNF-α and IL-6
2.9. Calculation for Oxidative Stress Index
2.10. Assay for Transaminases (SGOT and SGPT) and Phosphatases (ALP and ACP)
2.11. Assay for Acetylcholinesterase (AChE) Activity
2.12. Estimation of Total Protein Contents (TPCs)
2.13. Histological Study of Liver
2.14. Statistical Analysis
3. Results
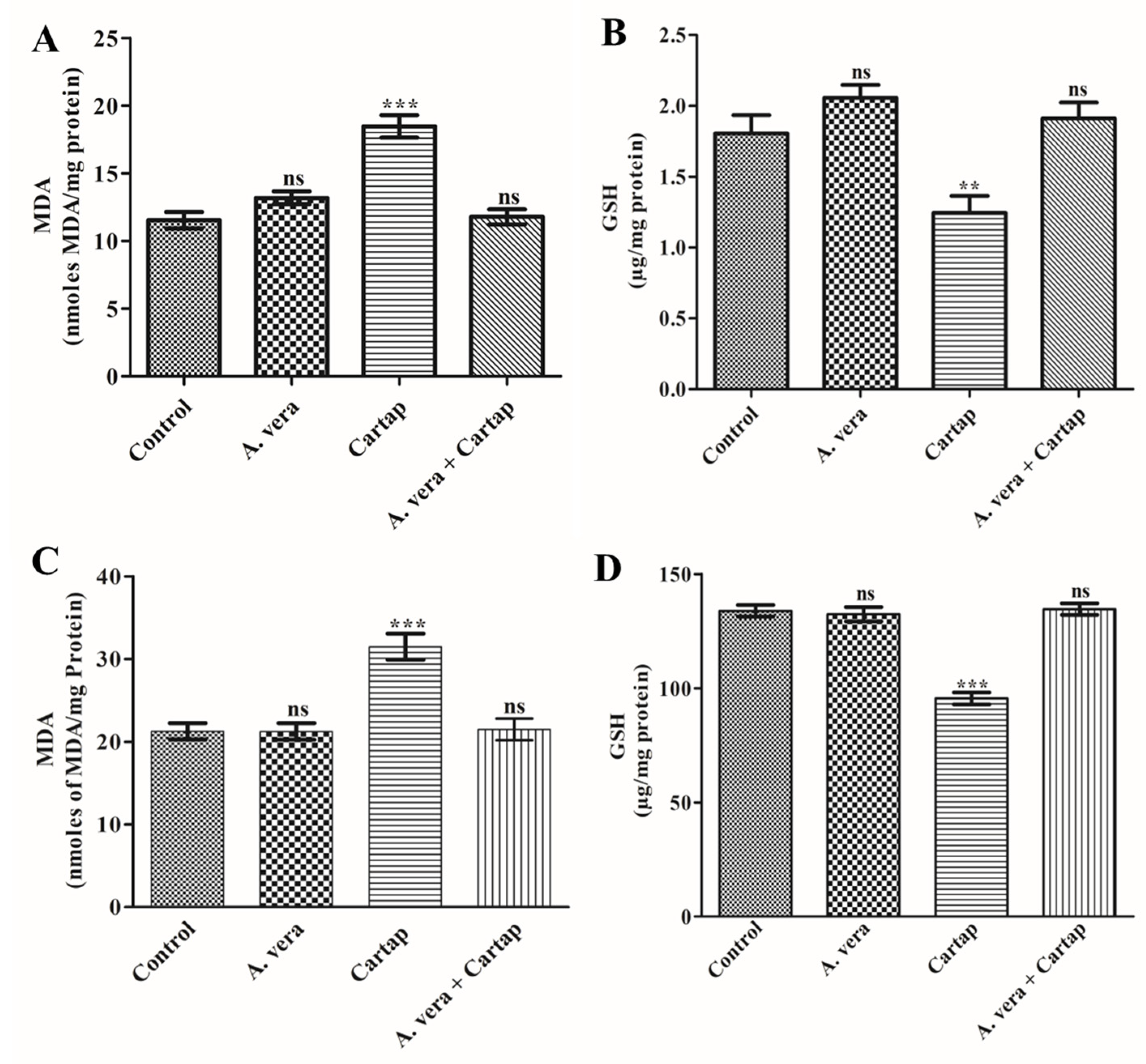
3.1. Effect of Cartap and A. vera on the Levels of MDA and GSH
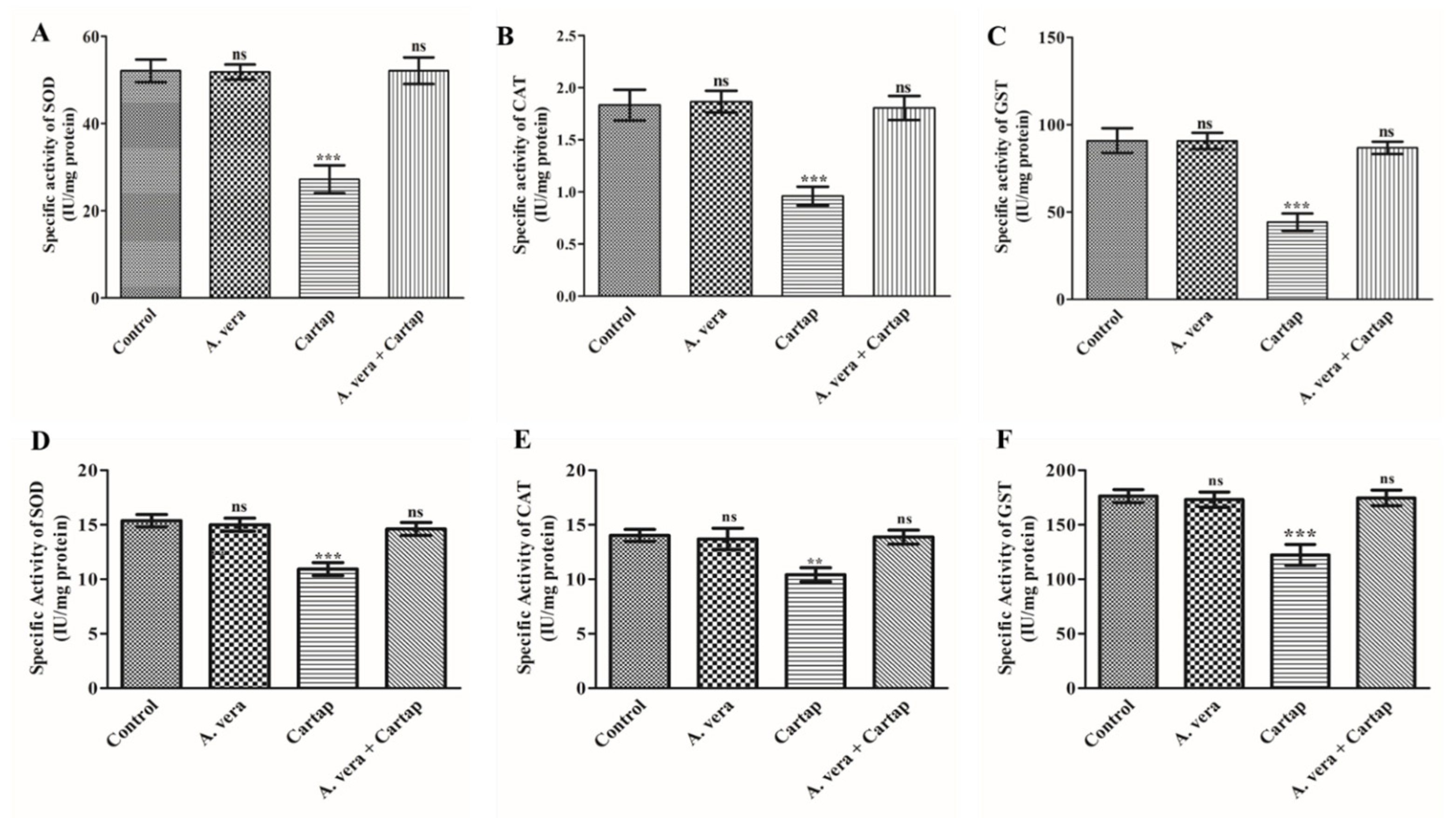
3.2. Impact of Cartap and A. vera on the Activities of SOD, CAT, and GST
3.3. Influence on the Level of P/A Ratio
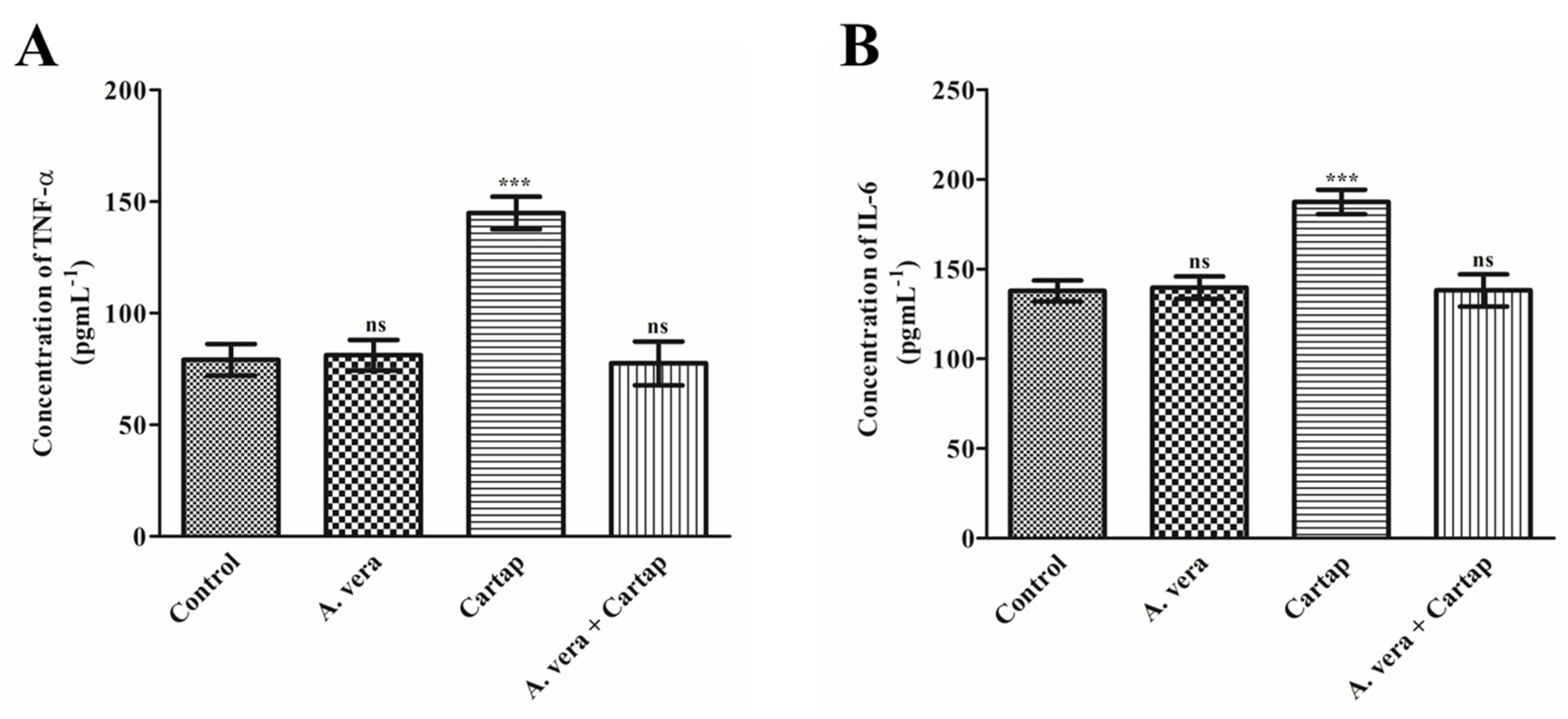
3.4. Effects of Cartap and A. vera on the Concentration of Proinflamatory Cytokines (TNF-α and IL-6)
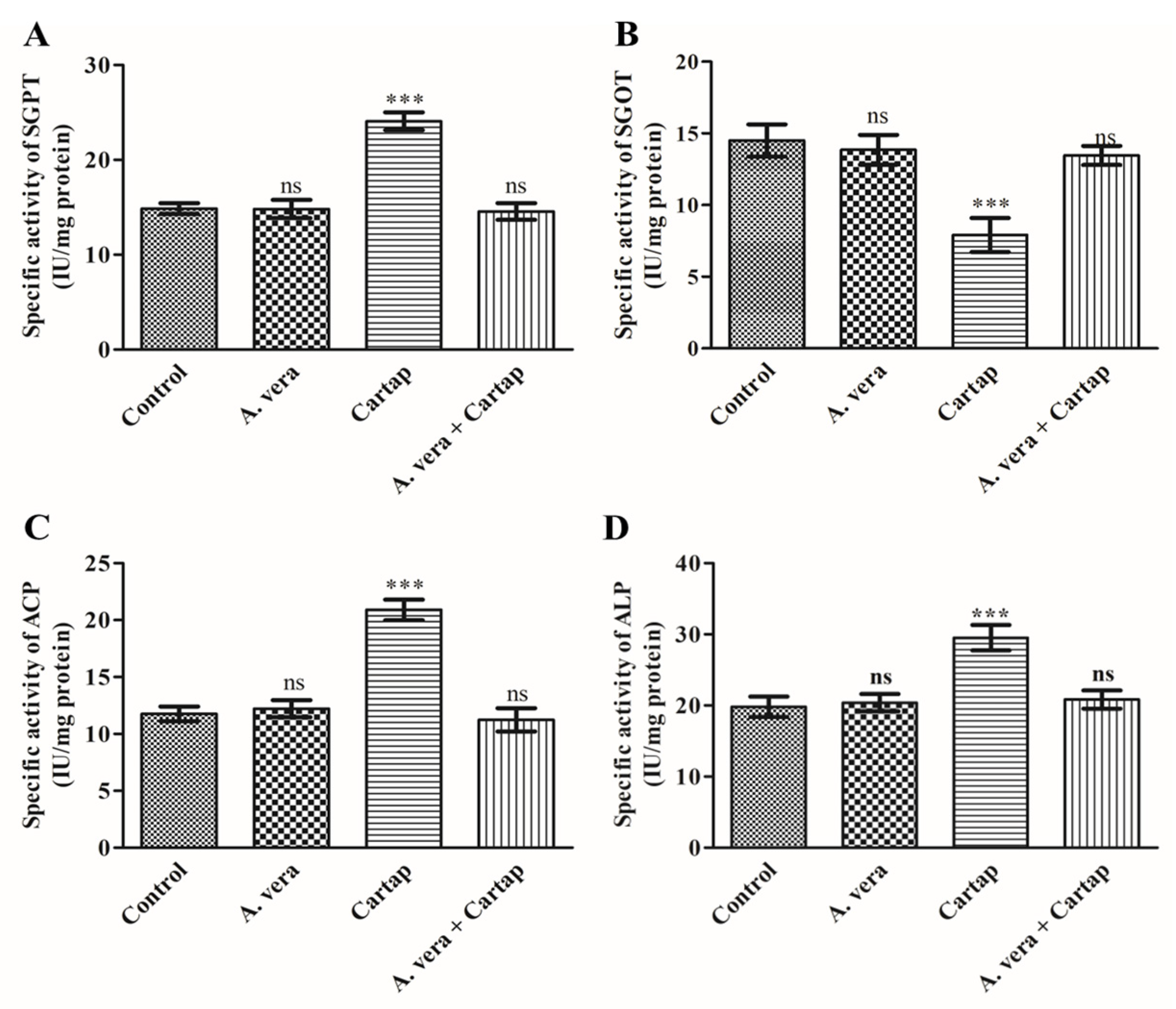
3.5. Activities of Transaminases and Phosphatases against Cartap and A. vera
3.6. Impact of Cartap and A. vera upon AChE Activity in Erythrocytes Membrane and Brain of Rats
3.7. Impact on the Histology of Rat Liver against Cartap and A. vera
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rajak, P.; Roy, S.; Ganguly, A.; Mandi, M.; Dutta, A.; Das, K.; Nanda, S.; Ghanty, S.; Biswas, G. Agricultural pesticides–Friends or foes to biosphere? J. Hazard. Mater. Adv. 2023, 10, 100264. [Google Scholar] [CrossRef]
- Carvalho, F.P. Pesticides, environment, and food safety. Food Energy Secur. 2017, 6, 48–60. [Google Scholar] [CrossRef]
- Tang, F.H.M.; Lenzen, M.; McBratney, A.; Maggi, F. Risk of pesticide pollution at the global scale. Nat. Geosci. 2021, 14, 206–210. [Google Scholar] [CrossRef]
- Popovska-Gorevski, M.; Dubocovich, M.L.; Rajnarayanan, R.V. Carbamate Insecticides Target Human Melatonin Receptors. Chem. Res. Toxicol. 2017, 30, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Wang, M.; Yu, H.; She, Y.; Cao, Z.; Ye, J.; El-Aty, A.M.A.; Hacimuftuoglu, A.; Wang, J.; Lao, S. An Overview on the Mechanisms and Applications of Enzyme Inhibition-Based Methods for Determination of Organophosphate and Carbamate Pesticides. J. Agric. Food Chem. 2020, 68, 7298–7315. [Google Scholar] [CrossRef]
- Mdeni, N.L.; Adeniji, A.O.; Okoh, A.I.; Okoh, O.O. Analytical Evaluation of Carbamate and Organophosphate Pesticides in Human and Environmental Matrices: A Review. Molecules 2022, 27, 618. [Google Scholar] [CrossRef]
- Kim, Y.; Jung, J.; Oh, S.; Choi, K. Aquatic toxicity of cartap and cypermethrin to different life stages of Daphnia magna and Oryzias latipes. J. Environ. Sci. Health Part B 2008, 43, 56–64. [Google Scholar] [CrossRef]
- Gupta, V.K.; Siddiqui, N.J.; Sharma, B. Ameliorative Impact of Aloe vera on Cartap Mediated Toxicity in the Brain of Wistar Rats. Indian J. Clin. Biochem. 2021, 37, 51–59. [Google Scholar] [CrossRef]
- Risher, J.F.; Mink, F.L.; Stara, J.F. The toxicologic effects of the carbamate insecticide aldicarb in mammals: A review. Environ. Health Perspect. 1987, 72, 267–281. [Google Scholar] [CrossRef]
- Gupta, R.C.; Goad, J.T.; Kadel, W.L. In vivo acute effects of carbofuran on protein, lipid, and lipoproteins in rat liver and serum. J. Toxicol. Environ. Health 1994, 42, 451–462. [Google Scholar] [CrossRef]
- Liao, J.-W.; Kang, J.-J.; Jeng, C.-R.; Chang, S.-K.; Kuo, M.-J.; Wang, S.-C.; Liu, M.R.; Pang, V.F. Cartap-induced cytotoxicity in mouse C2C12 myoblast cell line and the roles of calcium ion and oxidative stress on the toxic effects. Toxicology 2006, 219, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Fukuto, T.R. Mechanism of action of organophosphorus and carbamate insecticides. Environ. Health Perspect. 1990, 87, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Romero, F.J.; Bosch-Morell, F.; Romero, M.J.; Jareño, E.J.; Romero, B.; Marín, N.; Romá, J. Lipid peroxidation products and antioxidants in human disease. Environ. Health Perspect. 1998, 106 (Suppl. 5), 1229–1234. [Google Scholar] [CrossRef] [PubMed]
- Sharma, L.P.; Kadve, M.P.; Lingaraju, M.C.; Telang, A.G. Studies on oral subacute toxicity of cartap in male mice. Drug Chem. Toxicol. 2021, 44, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Kaur, I.; Goyal, D. Cartap hydrochloride induced stress response in Anabaena variabilis ARM 441. Pestic. Biochem. Physiol. 2021, 177, 104904. [Google Scholar] [CrossRef]
- Vivek, C.; Veeraiah, K.; Padmavathi, P.; Rao, H.D.; Bramhachari, P. Acute toxicity and residue analysis of cartap hydrochloride pesticide: Toxicological implications on the fingerlings of fresh water fish Labeo rohita. Biocatal. Agric. Biotechnol. 2016, 7, 193–201. [Google Scholar] [CrossRef]
- Boukhatem, M.N.; Setzer, W.N. Aromatic Herbs, Medicinal Plant-Derived Essential Oils, and Phytochemical Extracts as Potential Therapies for Coronaviruses: Future Perspectives. Plants 2020, 9, 800. [Google Scholar] [CrossRef]
- Ahmad Ganaie, H. Chapter 1—Review of the active principles of medicinal and aromatic plants and their disease fighting properties. In Medicinal and Aromatic Plants; Aftab, T., Hakeem, K.R., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 1–36. [Google Scholar]
- Tao, T.; Liu, M.; Chen, M.; Luo, Y.; Wang, C.; Xu, T.; Jiang, Y.; Guo, Y.; Zhang, J.H. Natural medicine in neuroprotection for ischemic stroke: Challenges and prospective. Pharmacol. Ther. 2020, 216, 107695. [Google Scholar] [CrossRef]
- Gonçalves, S.; Mansinhos, I.; Romano, A. Chapter 11—Aromatic plants: A source of compounds with antioxidant and neuroprotective effects. In Oxidative Stress and Dietary Antioxidants in Neurological Diseases; Martin, C.R., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 155–173. [Google Scholar]
- Sánchez-Machado, D.I.; López-Cervantes, J.; Sendón, R.; Sanches-Silva, A. Aloe vera: Ancient knowledge with new frontiers. Trends Food Sci. Technol. 2017, 61, 94–102. [Google Scholar] [CrossRef]
- Hu, Y.; Xu, J.; Hu, Q. Evaluation of Antioxidant Potential of Aloe vera (Aloe barbadensis Miller) Extracts. J. Agric. Food Chem. 2003, 51, 7788–7791. [Google Scholar] [CrossRef]
- López, Z.; Núñez-Jinez, G.; Avalos-Navarro, G.; Rivera, G.; Salazar-Flores, J.; Ramírez, J.A.; Ayil-Gutiérrez, B.A.; Knauth, P. Antioxidant and Cytotoxicological Effects of Aloe vera Food Supplements. J. Food Qual. 2017, 2017, 7636237. [Google Scholar] [CrossRef]
- Gupta, K.V.; Yarla, S.N.; de Lourdes Pereira, M.; Siddiqui, J.N.; Sharma, B. Recent Advances in Ethnopharmacological and Toxicological Properties of Bioactive Compounds from Aloe barbadensis (Miller), Aloe vera. Curr. Bioact. Compd. 2021, 17, 3–24. [Google Scholar] [CrossRef]
- Niehaus, W.G., Jr.; Samuelsson, B. Formation of malonaldehyde from phospholipid arachidonate during microsomal lipid peroxidation. Eur. J. Biochem. 1968, 6, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Marklund, S.; Marklund, G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef]
- Beers, R.F.; Sizer, I.W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 1952, 195, 133–140. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Goldberg, A.F.; Barka, T. Acid phosphatase activity in human blood cells. Nature 1962, 195, 297. [Google Scholar] [CrossRef]
- Reitman, S.; Frankel, S. A Colorimetric Method for the Determination of Serum Glutamic Oxalacetic and Glutamic Pyruvic Transaminases. Am. J. Clin. Pathol. 1957, 28, 56–63. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Gupta, V.K.; Siddiqi, N.J.; Ojha, A.K.; Sharma, B. Hepatoprotective effect of Aloe vera against cartap- and malathion-induced toxicity in Wistar rats. J. Cell. Physiol. 2019, 234, 18329–18343. [Google Scholar] [CrossRef] [PubMed]
- Kalyabina, V.P.; Esimbekova, E.N.; Kopylova, K.V.; Kratasyuk, V.A. Pesticides: Formulants, distribution pathways and effects on human health—A review. Toxicol. Rep. 2021, 8, 1179–1192. [Google Scholar] [CrossRef] [PubMed]
- Abdo, W.; Elmadawy, M.A.; Abdelhiee, E.Y.; Abdel-Kareem, M.A.; Farag, A.; Aboubakr, M.; Ghazy, E.; Fadl, S.E. Protective effect of thymoquinone against lung intoxication induced by malathion inhalation. Sci. Rep. 2021, 11, 2498. [Google Scholar] [CrossRef] [PubMed]
- Sabarwal, A.; Kumar, K.; Singh, R.P. Hazardous effects of chemical pesticides on human health–Cancer and other associated disorders. Environ. Toxicol. Pharmacol. 2018, 63, 103–114. [Google Scholar] [CrossRef]
- Lee, G.-H.; Choi, K.-C. Adverse effects of pesticides on the functions of immune system. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2020, 235, 108789. [Google Scholar] [CrossRef]
- Kim, T.Y.; Lim, M.C.; Lim, J.W.; Woo, M.A. Rolling Circle Amplification-based Copper Nanoparticle Synthesis on Cyclic Olefin Copolymer Substrate and Its Application in Aptasensor. Biotechnol. Bioproc. Eng. 2022, 27, 202–212. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Xia, Z.; Cui, J.Y. Effect of Chronic Cadmium Exposure on Brain and Liver Transporters and Drug-Metabolizing Enzymes in Male and Female Mice Genetically Predisposed to Alzheimer’s Disease. Drug Metab. Dispos. 2022, 50, 1414–1428. [Google Scholar] [CrossRef]
- Di Paolo, M.; Papi, L.; Gori, F.; Turillazzi, E. Natural Products in Neurodegenerative Diseases: A Great Promise but an Ethical Challenge. Int. J. Mol. Sci. 2019, 20, 5170. [Google Scholar] [CrossRef]
- Williams, R.J.; Mohanakumar, K.P.; Beart, P.M. Neuro-nutraceuticals: Natural products nourish the brain but be aware of contrary effects. Neurochem. Int. 2021, 150, 105159. [Google Scholar] [CrossRef]
- Carter, N.S.; Stamper, B.D.; Elbarbry, F.; Nguyen, V.; Lopez, S.; Kawasaki, Y.; Poormohamadian, R.; Roberts, S.C. Natural Products That Target the Arginase in Leishmania Parasites Hold Therapeutic Promise. Microorganisms 2021, 9, 267. [Google Scholar] [CrossRef]
- Kim, H.L.; Choi, B.-K.; Yang, S.H. Terminalia chebula Medicinal Uses: A Review of in vitro and in vivo Studies. Biotechnol. Bioproc. Eng. 2022, 27, 729–739. [Google Scholar] [CrossRef]
- Ji, S.Y.; Lee, H.; Hwangbo, H.; Kim, M.Y.; Kim, D.H.; Park, B.S.; Koo, Y.T.; Kim, J.S.; Lee, K.W.; Ko, J.C. Agarwood Pill Enhances Immune Function in Cyclophosphamide-induced Immunosuppressed Mice. Biotechnol. Bioproc. Eng. 2023, 28, 63–73. [Google Scholar] [CrossRef]
- Wybranowski, T.; Ziomkowska, B.; Cyrankiewicz, M.; Bosek, M.; Pyskir, J.; Napiórkowska, M.; Kruszewski, S. A study of the oxidative processes in human plasma by time-resolved fluorescence spectroscopy. Sci. Rep. 2022, 12, 9012. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Shi, X.-J.; Liu, H.; Mao, X.; Gui, L.-N.; Wang, H.; Cheng, Y. Oxidative stress marker aberrations in children with autism spectrum disorder: A systematic review and meta-analysis of 87 studies (N = 9109). Transl. Psychiatry 2021, 11, 15. [Google Scholar] [CrossRef]
- Wintola, O.A.; Afolayan, A.J. Phytochemical constituents and antioxidant activities of the whole leaf extract of Aloe ferox Mill. Pharmacogn. Mag. 2011, 7, 325–333. [Google Scholar] [CrossRef]
- Werawatganon, D.; Linlawan, S.; Thanapirom, K.; Somanawat, K.; Klaikeaw, N.; Rerknimitr, R.; Siriviriyakul, P. Aloe vera attenuated liver injury in mice with acetaminophen-induced hepatitis. BMC Complement. Altern. Med. 2014, 14, 229. [Google Scholar] [CrossRef]
- Gupta, V.K.; Kumar, A.; Pereira, M.d.L.; Siddiqi, N.J.; Sharma, B. Anti-Inflammatory and Antioxidative Potential of Aloe vera on the Cartap and Malathion Mediated Toxicity in Wistar Rats. Int. J. Environ. Res. Public Health 2020, 17, 5177. [Google Scholar] [CrossRef]
- Halliwell, B. Reactive Oxygen Species and the Central Nervous System. J. Neurochem. 1992, 59, 1609–1623. [Google Scholar] [CrossRef]
- Vijaya Padma, V.; Sowmya, P.; Arun Felix, T.; Baskaran, R.; Poornima, P. Protective effect of gallic acid against lindane induced toxicity in experimental rats. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2011, 49, 991–998. [Google Scholar] [CrossRef]
- Jaiswal, S.K.; Gupta, V.K.; Ansari, D.; Siddiqi, N.J.; Sharma, B. Vitamin C acts as a hepatoprotectant in carbofuran treated rat liver slices in vitro. Toxicol. Rep. 2017, 4, 265–273. [Google Scholar] [CrossRef]
- Selmi, S.; Rtibi, K.; Grami, D.; Sebai, H.; Marzouki, L. Malathion, an organophosphate insecticide, provokes metabolic, histopathologic and molecular disorders in liver and kidney in prepubertal male mice. Toxicol. Rep. 2018, 5, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chu, D.; Kalantar-Zadeh, K.; George, J.; Young, H.A.; Liu, G. Cytokines: From Clinical Significance to Quantification. Adv. Sci. 2021, 8, 2004433. [Google Scholar] [CrossRef] [PubMed]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, K.L.; Jäger, A.K.; Viljoen, A.M.; van Wyk, B.E. Cyclooxygenase inhibitory activity of Aloe species. S. Afr. J. Bot. 2002, 68, 47–50. [Google Scholar] [CrossRef]
- Duansak, D.; Somboonwong, J.; Patumraj, S. Effects of Aloe vera on leukocyte adhesion and TNF-alpha and IL-6 levels in burn wounded rats. Clin. Hemorheol. Microcirc. 2003, 29, 239–246. [Google Scholar]
- Woźniak, A.; Paduch, R. Aloe vera extract activity on human corneal cells. Pharm. Biol. 2011, 50, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Palanivelu, V.; Vijayavel, K.; Balasubramanian, S.E.; Balasubramanian, M.P. Influence of insecticidal derivative (cartap hydrochloride) from the marine polycheate on certain enzyme systems of the freshwater fish Oreochromis mossambicus. J. Env. Biol. 2005, 26, 191–195. [Google Scholar]
- Jing, X.; Du, L.-M.; Wu, H.; Wu, W.-Y.; Chang, Y.-X. Determination of Pesticide Residue Cartap Using a Sensitive Fluorescent Probe. J. Integr. Agric. 2012, 11, 1861–1870. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Wang, Y.; Li, X.; Wang, S.; Wang, Z. Recent advance on carbamate-based cholinesterase inhibitors as potential multifunctional agents against Alzheimer’s disease. Eur. J. Med. Chem. 2022, 240, 114606. [Google Scholar] [CrossRef]
- Shah, Z.U.; Parveen, S. Oxidative, biochemical and histopathological alterations in fishes from pesticide contaminated river Ganga, India. Sci. Rep. 2022, 12, 3628. [Google Scholar] [CrossRef]
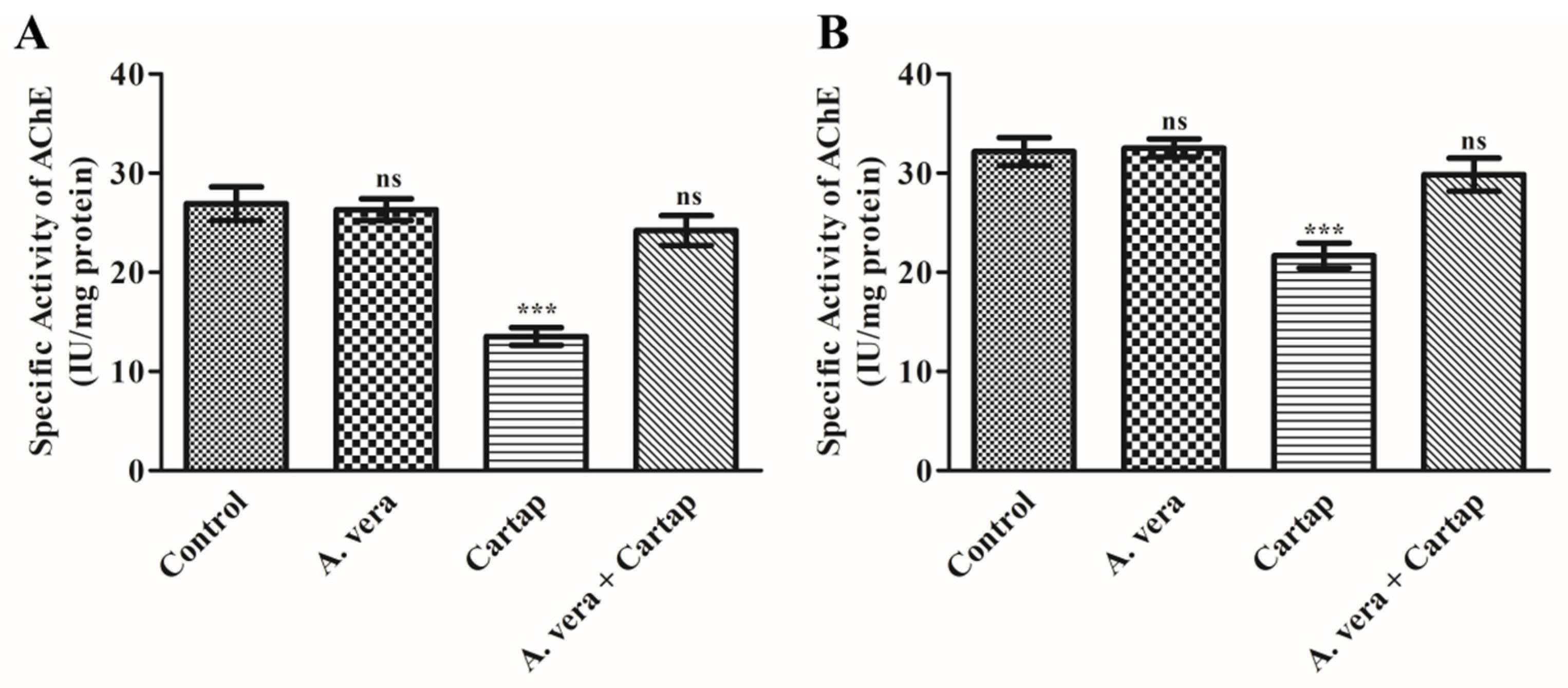
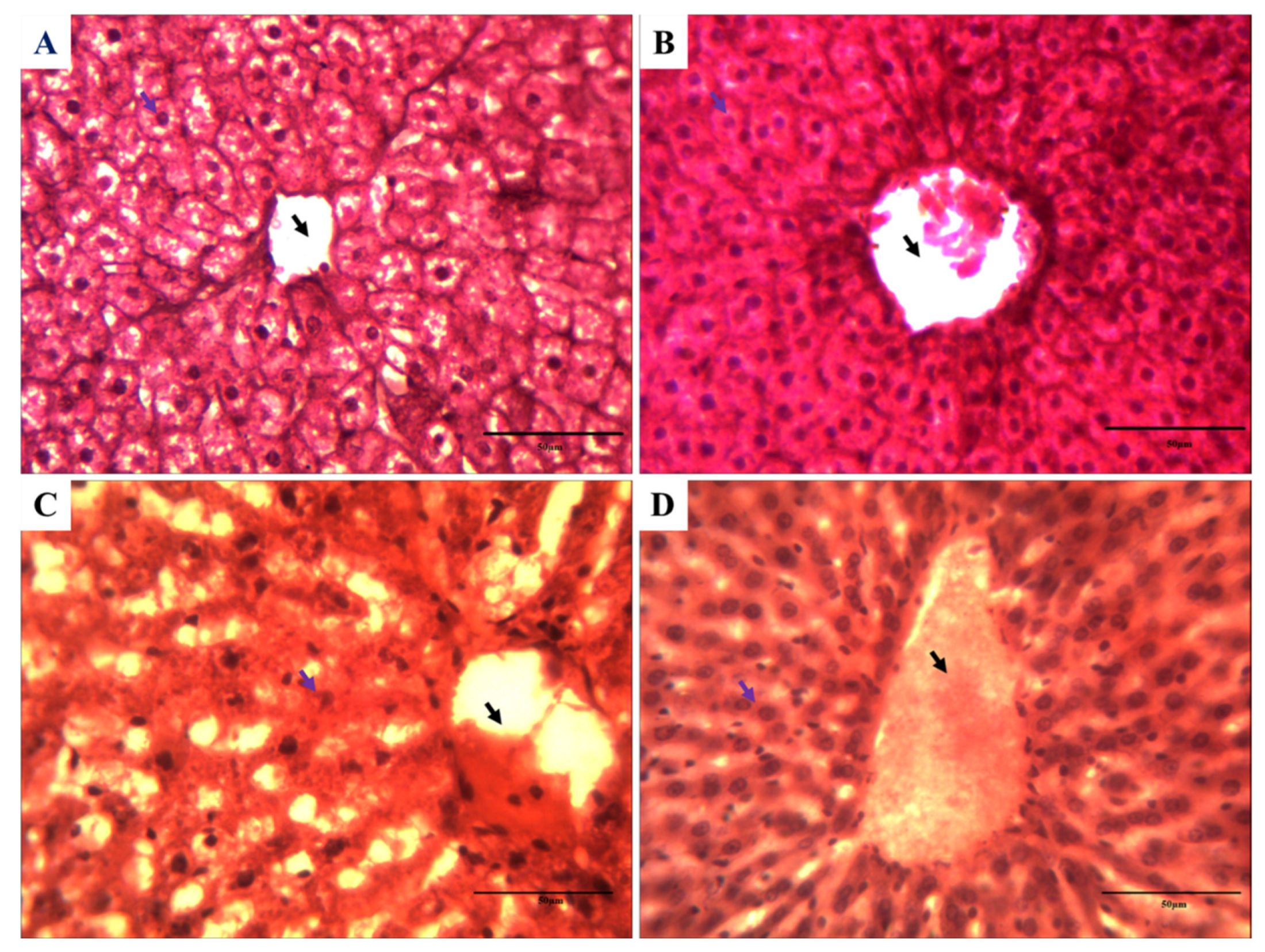
| Parameter | Control | A. vera | Cartap | A. vera + Cartap |
|---|---|---|---|---|
| (P/A)Liver | 0.146 | 0.147 (+0.68%) | 0.434 (+197.26%) | 0.152 (+4.109%) |
| (P/A)Brain | 0.0560 | 0.0596 (+6.42%) | 0.1285 (+129.46%) | 0.0580 (+3.57%) |
| Parameters | Control | A. vera | Cartap | A. vera + Cartap |
|---|---|---|---|---|
| DH | _ | _ | +++ | _ |
| DHT | _ | _ | ++++ | + |
| NH | _ | _ | +++ | _ |
| CCV | _ | _ | +++ | _ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, V.K.; Park, U.; Siddiqi, N.J.; Huh, Y.S.; Sharma, B. Amelioration of Hepatotoxic and Neurotoxic Effect of Cartap by Aloe vera in Wistar Rats. Toxics 2023, 11, 472. https://doi.org/10.3390/toxics11050472
Gupta VK, Park U, Siddiqi NJ, Huh YS, Sharma B. Amelioration of Hepatotoxic and Neurotoxic Effect of Cartap by Aloe vera in Wistar Rats. Toxics. 2023; 11(5):472. https://doi.org/10.3390/toxics11050472
Chicago/Turabian StyleGupta, Vivek Kumar, Uichang Park, Nikhat J. Siddiqi, Yun Suk Huh, and Bechan Sharma. 2023. "Amelioration of Hepatotoxic and Neurotoxic Effect of Cartap by Aloe vera in Wistar Rats" Toxics 11, no. 5: 472. https://doi.org/10.3390/toxics11050472
APA StyleGupta, V. K., Park, U., Siddiqi, N. J., Huh, Y. S., & Sharma, B. (2023). Amelioration of Hepatotoxic and Neurotoxic Effect of Cartap by Aloe vera in Wistar Rats. Toxics, 11(5), 472. https://doi.org/10.3390/toxics11050472








