Abstract
Cetaceans are recognized as bioindicators of pollution in oceans. These marine mammals are final trophic chain consumers and easily accumulate pollutants. For example, metals are abundant in oceans and commonly found in the cetacean tissues. Metallothioneins (MTs) are small non-enzyme proteins involved in metal cell regulation and are essential in many cellular processes (cell proliferation, redox balance, etc.). Thus, the MT levels and the concentrations of metals in cetacean tissue are positively correlated. Four types of metallothioneins (MT1, 2, 3, and 4) are found in mammals, which may have a distinct expression in tissues. Surprisingly, only a few genes or mRNA-encoding metallothioneins are characterized in cetaceans; molecular studies are focused on MT quantification, using biochemical methods. Thus, we characterized, in transcriptomic and genomic data, more than 200 complete sequences of metallothioneins (mt1, 2, 3, and 4) in cetacean species to study their structural variability and to propose to the scientific research community Mt genes dataset to develop in future molecular approaches which will study the four types of metallothioneins in diversified organs (brain, gonad, intestine, kidney, stomach, etc.).
1. Introduction
The large number of chemical compounds (medical compounds, Metal Trace Elements, pesticides, plastics, etc.) occur in the marine ecosystem often biodegrade slowly [1] They may come from natural and anthropogenic activities and may be concentrated through the food chain. Dolphins and whales are the final consumers of trophic networks in the marine ecosystem. Some cetaceans filter their food (small crustaceans and fish), whereas others are predators of cephalopods and fish. Thus, a large diversity of pollutants accumulates by biomagnification in cetacean’s tissues, mainly from ingested food, which affects their health [2,3]. A majority of the pollutants are endocrine disturbers or generate cellular oxidative stress [4]. To respond to these negative effects, the organisms synthesize many molecules, playing a role in detoxification processes. Metals are one of the most abundant pollutants in oceans and seas. The organisms, accumulating high metal concentration, synthesize an important metallothionein quantity, and they are non-enzymatic proteins involved in metal detoxification.
1.1. Metals Are Ubiquitous Pollutants in Cetaceans
The metals are highly present in cetacean tissues, and their accumulation appears proportional to the levels in the environment and their prey [5], suggesting that dolphins and whales may be considered to be sentinel (quantitative bioindicator) to reflect the quality of the marine environment [6,7,8,9]. However, many ecological and physiological factors modulate the chance to recover the metals in cetaceans: specie, age, sex, body size, nutritive conditions, and diet [10,11,12].
Non-essential metals (Element Trace Metals, ETMs) may have embryotoxic, nephrotoxic, neurotoxic, and reprotoxic effects, an inducer of immune depression, inducing DNA damage, teratogenic effects, cell proliferation, and oxidative stress [8,13,14,15,16]. Nevertheless, essential metal elements protect against ETM effects. This protective effect could be because essential metals (e.g., Zn) are inducers of the synthesis of metallothioneins (MTs), which are involved in metal detoxification [17]. The metal concentrations in cetaceans are mainly estimated in the kidney and liver because these organs are, respectively, involved in immune response, biotransformation of toxic compounds, and renal filtration; however, some studies are also focused on metal levels in muscle [7,18,19,20,21,22,23,24,25,26,27,28,29]. Unfortunately, it is not possible to compare the metal contaminations determined in distinct cetacean species, because they were collected in different geographical zones and years. In this case, it could be interesting in the future to investigate metal contaminations in more tissues, such as the brain and the digestive tube (esophagus, stomach, intestine, spleen, or the skin), as well as in different species collected in the same locality.
1.2. Metallothionein, a Biomarker in Response to Metal Contaminations
Many publications that studied the metal content in the tissues of cetaceans are focused on the metallothionein concentration because their cellular synthesis is correlated to metal accumulation. MTs’ induction has been considered one of the most important detoxification processes against metal toxicity and is also involved in the regulation of apoptosis and redox balance equilibrium [8,30]. Thus, MTs are considered to be a molecular bioindicator of metal exposure and are used commonly as a tool for biomonitoring programs.
Metallothioneins (MTs) are small non-enzymatic proteins (61–68 amino acids, 6–7 kDa) that are extremely rich in cysteine amino-acids (>30%) [31], which are organized in alternating Cys-Cys, Cys-X-Cys, and Cys-X-X-Cys (X being an amino-acid other than cysteine). Cysteine is implicated in metal complexation [32,33,34]. The MT binding affinity is metal-dependent [35,36]. In mammals, four types of metallothioneins are found: MT1, MT2, MT3, and MT4 [37]. The MT1 and MT2 are expressed in most tissues, whereas MT3 and MT4 (minor isoforms) are expressed in specified tissues [38]. MT3, considered to be a growth-inhibiting factor, is mainly expressed in Central Nervous System but it may be detected in the heart, kidney, and reproductive organs [39]. MT4 is specific to stratified tissues such as the oral epithelium, esophagus, stomach, and skin. Thus, MT1 and MT2 are involved in metal detoxification, homeostasis, and transport, whereas MT3 and MT4 functions are probably involved in tissue differentiation. It is suggested that the metallothionein family evolved by successive duplication genes. Duplicated copies may have accepted an accelerated rate of mutation, under selective pressure, promoting increased gene diversity and following subfunctionalization protein [40].
Mammalian MT is composed of two domains separated by a linker. The alpha domain (C-terminal) incorporates four metal cations bound with eleven cysteine residues, and a beta domain (N terminal) includes three metal cations bound to nine cysteines [41]. The biosynthesis of MTs depends mainly on metal accumulation in tissues, even if it may also be produced in response to various other regulator factors, such as glucocorticoids and temperature, depending on the activation of distinct enhancer regions in the promotor [42,43].
1.3. Characterization of Metallothioneins in Cetaceans
The first description of MTs in cetaceans was made by Ridlington et al. [44], who identified metal-binding proteins in the liver of Physeter macrocephalus (sperm whale). In 1986, Kwohn et al. [45] identified two isoforms of MTs (6.8 kDa), including 20–21 cysteine residues (32.7–33.3%), from the kidneys of Stenella coeruleoalba (Striped dolphin). These proteins were revealed as being close to MT1 and MT2 from the horse. Das et al. [46,47] confirmed the existence of MT1 and MT2 in the kidney and liver of Delphinus delphis, Lagenorhynchus albirostris, L. acutus, Phocoena phocoena, and Physeter macrocephalus. Mehra and Bremmer [48] indicated that the MT2 expression may be more prolonged, whereas the MT1 degradation is faster. Parallelly, Caurant et al. [49] showed that mercury (Hg) accumulation in pilot whales (Globicephala melas) was not correlated to metallothionein-like proteins in the liver because it was mainly found in the insoluble fraction. Ikemoto et al. [50] also revealed that the MTs that were identified in hepatic cytosol of Phocoenoides dalli (Dall’s porpoises) were not bound to silver (Ag), but a linear relationship existed between the Cd, Cu, and Zn content and the MTs synthesis. Das et al. [51] and Pedrero et al. [52] confirmed that Hg was mainly found to be complexed to high-molecular-weight proteins (HMWPs), probably as the HgSe form (tiemannite), and not to the MTs. Pollizi et al. [53] investigated the metallothioneins’ induction during ontogeny (fetus, calves, juveniles, and adult) of the coastal Franciscana dolphin Pontoporia blainvillei. They revealed that fetal MT concentrations were higher than in the mothers. The fetal period is characterized by a high metabolic rate during development and growth, and this may explain why high metal concentration is mainly in the liver of the fetus. For example, it may be possible that there is a metal transfer from mother to fetus. Càceres-Saez et al. [54], in relation to the MT/metal ratio, showed that MT/Cd was higher in the liver of Cephalorhynchus commersonii, whereas MT/Hg and MT/Ag were higher in the kidney, revealing a differential tissues accumulation.
Surprisingly, the majority of publications that were cited previously evaluated the MT concentration in tissues by using the spectrophotometric methods (absorbance at 412 nm) described by Elmman [55] or Viarengo et al. [56]. Unfortunately, these spectrophotometric methods did not allow for the discrimination of distinct MT isoforms. Their molecular approach can be explained by the fact that only a few nucleotide sequences of Mts have been well characterized from the genomes and transcriptomes of cetaceans yet. Liu et al. [57] published an innovative study focused on the metallothionein genes. They characterized the Mt2 and Mt4 alleles associated with metal levels in dolphin tissues (kidney, liver, and muscle). They identified two polymorphic sites only in the Mt4 gene which seemed to be associated with Cd, Hg, Mn, and Zn content in Neophocaena asiaeorientalis’s tissues. Many chromosomes, scaffolds, contig, and transcriptomes of cetaceans are available in nucleotide international databases, but any gene annotation is performed.
Our main objective in this study was to constitute an Mt genes dataset to give the opportunity to the scientist community to develop future precise molecular approaches which can be used to evaluate the Mt expression for all genes (Mt1, 2, 3, and 4) in many tissues (such as the brain, esophagus, gonad, heart, skin, stomach, and intestine, which are not integrated into metal content analyses yet). Thus, we decided to identify the metallothionein sequences in all genomic fragments (scaffold, contig, and read), cDNA, and transcriptomes of cetaceans available in international databases.
2. Material and Methods
2.1. Characterization of Metallothionein Sequences inside Available Transcriptomes and Genomes of Cetaceans
In the international database, only fifty MTs sequences were submitted, constituting a disparate dataset (mainly MT1 and MT4), including many MT1-E pseudogene sequences. This limited dataset explains why the metallothionein studies in cetaceans are mainly focused on the MT biosynthesis protein. The typical Mt gene structure includes three exons and two introns in mammals. Two first exons encode to the beta domain of the protein, while the third exon encodes to the alpha domain [58,59].
We screened the Nucleotide collection (nr/nt), Whole-Genome Shotgun Contigs (WGSs), Expressed Sequence Tags (ESTs), and Transcriptome Shotgun Assembly (TSAs) available at NCBI, using the BLASTn program (https://blast.ncbi.nlm.nih.gov/Blast.cgi accessed on 20–27 September 2022), selecting only the cetacean sequences. The intron localizations in genomic metallothionein sequences were determined by comparison with the mRNA of Mt from mammals, and the relevance of encoding sequences was verified by an in silico translation (https://web.expasy.org/translate/ accessed on 20–27 September 2022) and the blast program. The proteins obtained were compared, using the BLASTp program, to other MT sequences of the international database.
2.2. Phylogenetic Analysis of Metallothioneins in Cetaceans
We aligned the metallothionein dataset using the MAFFT algorithm with the default parameters (http://mafft.cbrc.jp/alignment/server/ accessed on 1–10 October 2022). Evolutionary analyses were conducted in MEGA XI (https://www.megasoftware.net/ accessed on 1–10 October 2022). The best evolutionary model for our dataset was determined, and the Maximum Likelihood method was applied [60,61]. A test of phylogeny used was bootstrap; only node values equal to 100 are shown in the figure.
3. Results
A total of more than 200 complete sequences were isolated from 26 species of cetaceans (dolphins and whales) included in the 13 families (Balaenidae, 2; Balaenopteridae, 4; Delphinidae, 6; Eschrichtiidae, 1; Iniidae, 1; Kogiidae, 1; Lipotidae, 1; Monodontidae, 2; Phocoenidae, 3; Physeteridae, 1; Platanistidae, 1; Pontoporiidae, 1; Ziphiidae, 2) (Table 1).

Table 1.
Accession numbers of mitochondrion and metallothionein sequences of cetaceans isolated from NCBI databases and personal data.
To show the total of Mt genes which were characterized in the cetacean species, we built a molecular phylogeny by using the mitochondrion sequences of the 26 species (accession numbers of mitochondrion were indicated in Table 1). The evolutionary history was inferred by using the Maximum Likelihood method and General Time Reversible model. The tree with the highest log likelihood (−129,411.30) is shown. The percentage of trees in which the associated taxa clustered together is shown next to the branches. Initial tree(s) for the heuristic search were obtained automatically by applying Neighbor-Joining and BioNJ algorithms to a matrix of pairwise distances estimated using the Maximum Composite Likelihood (MCL) approach and selecting the topology with superior log likelihood value. A discrete Gamma distribution was used to model the evolutionary rate differences among sites (five categories (+G, parameter = 1.2020)). The rate variation model allowed for some sites to be evolutionarily invariable ([+I], 45.31% sites). This analysis involved 27 nucleotide sequences because the mitochondrial genome of Hippopotamus amphibius (NC_000889) was used as an outgroup. Codon positions included were 1st+2nd+3rd+Noncoding. There was a total of 15,952 positions in the final dataset (Figure 1).
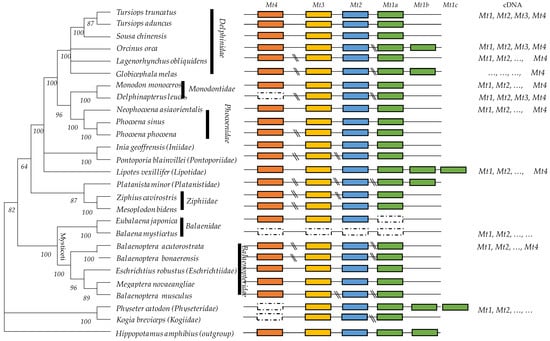
Figure 1.
Genomic organization of metallothioneins in the phylogenetic tree of 26 cetacean species. Functional genes (Mt1, Mt2, Mt3, and Mt4) are indicated in colored rectangles. Phylogenomic analyses were based on mitogenome information and built using the MEGA XI (Maximum Likelihood method and General Time Reversible model, G + I parameters). Bootstrap values are indicated above the branches. The symbol “\\” indicated a large InterGenic Region.
We built a phylogenetic tree based on the encoding nucleotide (mRNA, gene) sequences of metallothionein characterized in cetaceans, using also the MEGA XI (Maximum Likelihood method and Kimura two-parameter model and tree with the highest log likelihood: −2304.11, +G, parameter = 0.7943, 219 positions in the final dataset). This analysis allowed us to determine the cluster of Mt genes.
We showed that there is a unique copy of Mt4, Mt3, and Mt2 genes in cetacean genomes but successive duplicated Mt1 copies (Mt1a, Mt1b, and Mt1c). The length of the InterGenic Regions (IGRs) inside the metallothionein cluster (Mt4-Mt3-Mt2-Mt1) was calculated (Table 2). The IGR (Mt4/Mt3) is highest (19,583–36,109 bp). The IGR (Mt3-Mt2) ranges from 7129 to 7571 bp, IGR (Mt2-Mt1) from 2064 to 5310 bp, and the IGR between distinct Mt1 copies varying approximately within 3000 bp (Table 2). This information is primordial to people whose genes amplify the successive Mt genes by PCR. To design specific primers for long PCR, people may report to Table 2, where they will find the accession number of the contig, scaffold or gene for each species where we identified the distinct Mt isoforms. The intron and exon sizes were also determined (Table 3 and Table 4). High stability of exon lengths was noted between the species and for each gene: Exon I (28–31 bp), Exon II (66 bp), and Exon III (92 bp), except for the Mt3, which showed the highest exon III (104–107 bp) (Table 4). The intron length was highly variable. The Mt4 appeared to be the longest gene (±4500 bp).

Table 2.
Length (base pairs, bp) of InterGenic Region (IGR) between metallothionein genes (Mt1, Mt2, Mt3, and Mt4) in cetacean species.

Table 3.
Exon length (base pairs, bp) for metallothionein genes (Mt1, Mt2, Mt3, and Mt4) in cetacean species.

Table 4.
Intron length (base pairs, bp) for Metallothionein genes (Mt1, Mt2, Mt3, and Mt4) in cetacean species.
Phylogenetic analyses based on 213 nucleotide metallothionein sequences (encoding part: ATG-TAA/TAG) identified in this study clearly showed four clusters (Mt1, Mt2, Mt3, and Mt4) (Figure 2). It is noted that the intron-free Mt2 genes identified constitute a specific cluster, whereas the intron-free Mt1 genes are dispatched (Figure 2). The isoforms Mt1, Mt2, and Mt3 are more closed than Mt4. MT1 and MT2 are synthesized in many tissues, whereas MT3 is mainly mentioned in regard to the Central Nervous System and MT4 in stratified tissues. It is possible to suggest that these phylogenetic relationships may be explained by successive duplicates of the ancestral gene of metallothionein, which gave Mt1 and Mt2, then Mt3, and, more recently, Mt4.
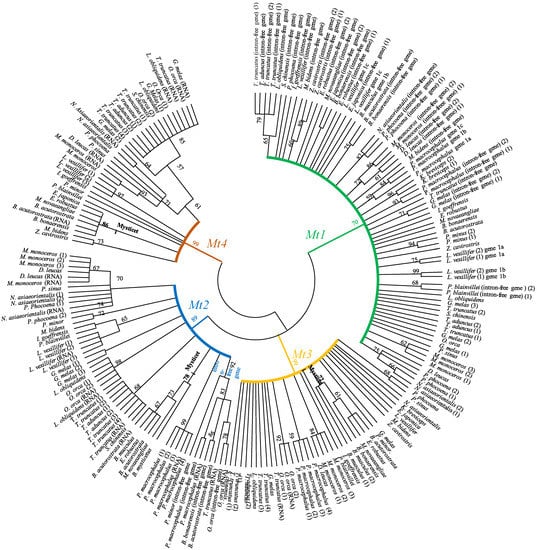
Figure 2.
Phylogenetic tree of metallothioneins (Mts) in cetaceans. The relationships among the Mts genes are estimated using the Kimura 2-parameter model, a tree with the highest log likelihood: −2304.11, +G, parameter = 0.7943, 219 positions in the final dataset. Numbers above the nodes correspond to bootstrap values. Branches in green, blue, yellow, and brown indicate Mt1, Mt2, Mt3, and Mt4, respectively.
4. Conclusions
This study revealing the identification of more than 200 sequences of metallothioneins in genomes and transcriptomes sequences of 26 cetacean species constitutes a novel tool to develop a gene expression inside distinct tissues not used yet (brain, esophagus, gonad, heart, stomach, intestine, etc.) and in the skin. Now, using our indication, it is possible for people to design specific primers to develop a study of the metallothionein gene expression in cetaceans. It will increase our knowledge of the involvement of these molecular biomarkers in the detoxification responses of cetaceans against marine pollution. For example, we will analyze the gene expression of four Mt in distinct tissues (brain, intestine, kidney, and liver) of Globicephala melas to estimate if there is a differential response. Parallelly, another publication focused on the evolution of metallothionein in marine mammals, based on the structural analysis, positive selection events, and annotation errors of some Mt sequences available in the nucleotide database, will be written.
Author Contributions
Conceptualization, L.P., V.L., and F.C.; validation, F.C. and V.L.; writing—original draft preparation, V.L.; writing—review and editing, M.S.G. and F.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
All metallothionein sequences characterized in this study are available according to the accession number indicated in Table 1.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Page-Karjian, A.; Lo, C.F.; Ritchie, B.; Harms, C.A.; Rotstein, D.S.; Han, S.; Hassan, S.M.; Lehner, A.F.; Buchweitz, J.P.; Thayer, V.G.; et al. Anthropogenic Contaminants and Histopathological Findings in Stranded Cetaceans in the Southeastern United States, 2012–2018. Front. Mar. Sci. 2020, 7, 630. [Google Scholar] [CrossRef]
- Fossi, M.C.; Marsili, L. The use of non-destructive biomarkers in the study of marine mammals. Biomarkers 1997, 2, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Weijs, L.; Zaccaroni, A. Toxicology of marine mammals: News developments on opportunities. Arch. Environ. Contam. Toxicol. 2016, 70, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Baulch, S.; Perry, C. Evaluating the impacts of marine debris on cetaceans. Mar. Pollut. Bull. 2014, 80, 210–221. [Google Scholar] [CrossRef] [PubMed]
- Kehring, H.A.; Hauser-Davis, R.A.; Seixas, T.G.; Pinheiro, A.B.; Paula, A.; Di Beneditto, M. Mercury species, selenium, metallothioneins and glutathione in two dolphins from the southeastern Brazilian coast: Mercury detoxification and physiological differences in diving capacity. Environ. Pollut. 2016, 213, 785–792. [Google Scholar] [CrossRef]
- Kaschner, K.; Tittensor, D.P.; Ready, J.; Gerrodette, T.; Worm, B. Current and future patterns of global marine mammal biodiversity. PLoS ONE 2011, 6, e19653. [Google Scholar] [CrossRef]
- Càceres-Saez, I.; Ribeiro Guevara, S.; Dellabianca, N.A.; Goodall, N.P.; Cappozzo, H.L. Heavy metals and essential elements in Commerson’s dolphins (Cephalorhynchus c. commersonii) from the southwestern South Atlantic Ocean. Environ. Monit. Assess. 2013, 185, 5375–5386. [Google Scholar] [CrossRef]
- Wang, W.-C.; Mao, H.; Ma, D.-D.; Yang, W.-X. Characteristics, functions, and applications of metallothionein in aquatic vertebrates. Front. Mar. Sci. 2014, 1, 34. [Google Scholar] [CrossRef]
- Durante, C.A.; Reis, B.M.M.; Azevedo, A.; Crespo, E.A.; Lailson-Brito, J. Trace elements in trophic webs from South Atlantic: The use of cetaceans as sentinels. Mar. Pollut. Bull. 2020, 150, 110674. [Google Scholar] [CrossRef]
- Dorneles, P.R.; Lailson-Brito, J.; Dos Santos, R.A.; Silva da Costa, P.A.; Malm, O.; Azevedo, A.F.; Machado Torres, J.P. Cephalopods and cetaceans as indicators of offshore bioavailability of cadmium off Central South Brazil Bight. Environ. Pollut. 2007, 148, 352–359. [Google Scholar] [CrossRef]
- Lahaye, V.; Bustamante, P.; Dabin, W.; Van Canneyt, O.; Dhermain, F.; Cesarini, C.; Pierce, G.J.; Caurant, F. New insights from age determination on toxic element accumulation in striped and bottlenose dolphins from Atlantic and Mediterranean waters. Mar. Pollut. Bull. 2006, 52, 1219–1230. [Google Scholar] [CrossRef] [PubMed]
- Seixas, T.G.; Kehrig, H.A.; Fillman, G.; Di Beneditto, A.P.M.; Souza, C.M.; Secchi, E.R.; Moreira, I.; Malm, O. Ecological and biological determinants of trace elements accumulation in liver and kidney of Pontoporia blainvillei. Sci. Total Environ. 2007, 385, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Jihen, E.H.; Imed, M.; Fatima, H.; Abdelhamid, K. Protective effects of selenium (Se) and zinc (Zn) on cadmium (Cd) toxicity in the liver and kidney of the rat: Histology and Cd accumulation. Food Chem. Toxicol. 2008, 46, 3522–3527. [Google Scholar] [CrossRef] [PubMed]
- Elgaml, S.A.; Khalil, R.; Hashis, E.A.; El-Murr, A. Protective effects of selenium and alpha-tocopherol against lead-induced hepatic and renal toxicity in Oreochromis niloticus. J. Aquac. Res. Dev. 2015, 6. [Google Scholar] [CrossRef]
- Botté, A.; Seguin, C.; Nahrgang, J.; Zaidi, M.; Guery, J.; Leignel, V. Lead in the marine environment: Concentrations and effects on invertebrates. Ecotoxicology 2022, 31, 194–207. [Google Scholar] [CrossRef] [PubMed]
- Rogalska, J.; Pilat-Marcikiewicz, B.; Brozka, M.M. Protective effective of zinc against cadmium hepatotoxicity depends on this bioelement intake and level of cadmium exposure: A study in a rat model. Chem. Biol. Interact. 2011, 193, 191–203. [Google Scholar] [CrossRef]
- Monteiro, F.; Lemos, L.S.; Fulgêncio de Moura, J.; Chàvez Rocha, R.C.; Moreira, I.; Di Beneditto, A.P.; Kehrig, H.A.; Bordon, I.C.A.C.; Siciliano, S.; Saint’Pierre, T.D.; et al. Subcellular metal distribution and metallothionein associations in rough-toothed dolphins (Steno bredanesis) from Southeastern Brazil. Mar. Pollut. Bull. 2019, 14, 263–273. [Google Scholar] [CrossRef]
- Méndez-Fernandez, P.; Spitz, J.; Dars, C.; Dabin, W.; Mahfouz, C.; André, J.-M.; Chouvelon, T.; Authier, M.; Caurant, F. Two cetacean species reveal different long-term trends for toxic trace elements in European Atlantic French waters. Chemosphere 2022, 294, 133676. [Google Scholar] [CrossRef]
- Law, R.J.; Fleman, C.F.; Hopkins, A.D.; Baker, J.R.; Harwood, J.; Jackson, D.B.; Kennedy, S.; Maritn, A.R.; Morris, R.J. Concentrations of trace metals in the livers of marine mammals (seals, porpoises and dolphins) from waters around the British Isles. Mar. Pollut. Bull. 1991, 22, 183–191. [Google Scholar] [CrossRef]
- Romero, M.B.; Polizzi, P.; Chiodi, L.; Robles, A.; Gerpe, M. Metals as chemical tracers to discriminate ecological populations of threatened Franciscana dolphins (Pontoporia blainvillei) from Argentina. Environ. Sci. Pollut. Res. 2017, 24, 3940–3950. [Google Scholar] [CrossRef]
- Lailson-Brito, J.; Cruz, R.; Domeles, P.R.; Andrade, L.; Azevedo, A.d.F.; Fragoso, A.B.; Vidal, L.G.; Costa, M.B.; Bisi, T.L.; Almeida, R.; et al. Mercury-Selenium relationship in liver of Guiana dolphin: The possible role of kupffer cells in the detoxification process by tiemannite formation. PLoS ONE 2012, 7, e42162. [Google Scholar] [CrossRef] [PubMed]
- Roditi-Elasar, M.; Kerem, D.; Hornung, H.; Kress, N.; Shoham-Frider, E.; Goffman, O.; Spanier, E. Heavy metal levels in bottlenose and striped dolphins off the Mediterranean coast of Israel. Mar. Pollut. Bull. 2003, 46, 491–521. [Google Scholar] [CrossRef] [PubMed]
- Decataldo, A.; Di Leo, A.; Giandomenico, S.; Cardellicchio, N. Association of metals (mercury, cadmium and zinc) with metallothionein-like proteins in storage organs of stranded dolphins from the Mediterranean Sea (Southern Italy). J. Environ. Monit. 2004, 6, 361–367. [Google Scholar] [CrossRef]
- Hansen, A.M.K.; Bryan, C.E.; West, K.; Jensen, B.A. Trace Element Concentrations in Liver of 16 Species of Cetaceans Stranded on Pacific Islands from 1997 through 2013. Arch. Environ. Contam. Toxicol. 2016, 70, 75–95. [Google Scholar] [CrossRef] [PubMed]
- Jirillo, E.; Caccavo, D.; Magrone, T.; Piccigallo, E.; Amati, L.; Lembo, A.; Kalis, C.; Gumenscheimer, M. The role of the liver in the response to LPS: Experimental and clinical findings. J. Endotoxin. Res. 2002, 8, 319–327. [Google Scholar] [CrossRef]
- Zhou, J.L.; Salvador, S.M.; Liu, Y.P.; Sequeira, M. Heavy metals in the tissues of common dolphins (Delphinus delphis) stranded on the Portuguese coast. Sci. Total Environ. 2001, 273, 61–76. [Google Scholar] [CrossRef] [PubMed]
- Machovsky-Capuska, G.E.; von Haeften, G.; Romero, M.A.; Rodriguez, D.H.; Gerpe, M.S. Linking cadmium and mercury accumulation to nutritional intake in common dolphins (Delphinus delphis) from Patagonia, Argentina. Environ. Pollut. 2020, 263, 114480. [Google Scholar] [CrossRef]
- Lischka, A.; Betty, E.L.; Braid, H.E.; Pook, C.J.; Gaw, S.; Bolstad, K.S.R. Trace element concentrations, including Cd and Hg, in long-finned pilot whales (Globicephala melas edwardii) mass stranded on the New Zealand coast. Mar. Pollut. Bull. 2021, 165, 112084. [Google Scholar] [CrossRef]
- Holsbeek, L.; Joiris, C.R.; Debacker, V.; Ali, I.B.; Roose, P.; Nellissen, J.-P.; Gobert, S.; Bouqueneau, J.-M.; Bossicart, M. Heavy metals, organochlorines and polycyclic aromatic hydrocarbons in sperm whales stranded in the southern North Sea during the 1994/1995 winter. Mar. Pollut. Bull. 1999, 38, 304–313. [Google Scholar] [CrossRef]
- Roesijadi, G. Metallothionein induction as a measure of response to metal exposure in aquatic animals. Environ. Health Perspect. 1994, 102, 91–95. [Google Scholar] [CrossRef]
- Roesijadi, G. Metallothioneins in metal regulation and toxicity in aquatic animals. Aquat. Toxicol. 1992, 22, 81–114. [Google Scholar] [CrossRef]
- Palmiter, R.D. The elusive function of metallothioneins. Proc. Natl. Acad. Sci. USA 1998, 95, 8428–8430. [Google Scholar] [CrossRef] [PubMed]
- Kägi, J.H.R.; Vasàk, M.; Lerch, K.; Gilg, D.E.O.; Hunziker, P.; Werner, R.; Good, B.; Good, M. Structure of mammalian metallothionein. Environ. Health Perspect. 1984, 54, 93–103. [Google Scholar] [PubMed]
- Kägi, J.; Vallee, B.L. Metallothionein: A cadmium- and zinc-containing protein from equine renal cortex. J. Biol. Chem. 1960, 235, 3460–3465. [Google Scholar] [CrossRef]
- Nielson, K.B.; Atkin, C.L.; Winge, D.R. Distinct metal-binding configurations in metallothionein. J. Biol. Chem. 1985, 260, 5342–5350. [Google Scholar] [CrossRef]
- Hamer, D.H. Metallothionein. Annu. Rev. Biochem. 1986, 55, 913–951. [Google Scholar] [CrossRef]
- Moffatt, P.; Denizeau, F. Metallothionein in physiological and physiopathological processes. Drug Metab. Rev. 1997, 29, 261–307. [Google Scholar] [CrossRef]
- Sakulsak, N. Metallothionein: An overview on its metal homeostatic regulation in mammals. Int. J. Morphol. 2012, 30, 1007–1012. [Google Scholar] [CrossRef]
- Ruttkay-Nedesky, B.; Nejdl, L.; Gumulec, J.; Zitka, O.; Masarik, M.; Eckschlager, T.; Stiborova, M.; Adam, V.; Kizek, R. The role of metallothionein in oxidative stress. Int. J. Mol. Sci. 2013, 1, 6044–6066. [Google Scholar] [CrossRef]
- Serén, N.; Glaberman, S.; Carretero, M.A.; Chiari, Y. Molecular evolution and functional divergence of the metallothionein gene family in vertebrates. J. Mol. Evol. 2014, 78, 217–233. [Google Scholar] [CrossRef]
- Coyle, P.; Philcox, J.C.; Carey, L.C.; Rofe, A.M. Metallothionein: The multipurpose protein. Cell. Mol. Life. Sci. 2002, 59, 627–647. [Google Scholar] [CrossRef] [PubMed]
- Roesijadi, G. Metallothionein and its role in toxic metal regulation. Comp. Biochem. Physiol. 1996, 113, 117–123. [Google Scholar] [CrossRef]
- Arizono, K.; Peterson, K.L.; Brady, F.O. Inhibitors of Ca2+ channels, calmodulin and protein kinases prevent A23187 and other inductions of metallothionein mRNA in EC, rat hepatoma cells. Life Sci. 1993, 53, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Ridlington, J.W.; Chapman, D.C.; Goeger, D.E.; Whanger, P.D. Metallothionein and Cu-chelatin: Characterization of metal-binding proteins from tissues of four marine animals. Comp. Biochem. Physiol. Part B Comp. Biochem. 1981, 70, 93–104. [Google Scholar] [CrossRef]
- Kwohn, Y.-T.; Yamazaki, S.; Okubo, A.; Yoshimura, E.; Tatsukawa, R.; Toda, S. Isolation and characterization of metallothionein from kidney of striped dolphins Stenella coeruleoalba. Agric. Biol. Chem. 1986, 50, 2881–2885. [Google Scholar]
- Das, K.; Jacob, V.; Debacker, V.; Millerioux, G.; Biondo, R.; Gerardin, N.; Bouquegneau, J.-M. Characterization and potential role of the metallothioneins in marine mammals. Comp. Biochem. Physiol. Part A 2000, 126, S1–S163. [Google Scholar] [CrossRef]
- Das, K.; Jacob, V.; Bouquegneau, J.M. White-sided dolphin metallothioneins: Purification, characterization and potential role. Comp. Biochem. Physiol. Part C 2002, 131, 245–251. [Google Scholar] [CrossRef]
- Mehra, P.K.; Brenmer, I. Studies on the metabolism of rat liver copper-metabolism. Biochem. J. 1985, 227, 903–908. [Google Scholar] [CrossRef]
- Caurant, F.; Navarro, M.; Amiard, J.C. Mercury in pilot whales: Possible limits to the detoxification process. Sci. Total Environ. 1996, 186, 95–104. [Google Scholar] [CrossRef]
- Ikemoto, T.; Kunito, T.; Anan, Y.; Tanaka, H.; Baba, N.; Miyazari, N.; Tanabe, S. Association of heavy metals with metallothionein and other proteins in hepatic cytosol of marine mammals and seabirds. Environ. Toxicol. Chem. 2004, 23, 2008–2016. [Google Scholar] [CrossRef]
- Das, K.; De Groof, A.; Jauniaux, T.; Bouquegneau, J.-M. Zn, Cu, Cd and Hg binding to metallothioneins in harbor porpoises Phocoena phocoena from the Southern North Sea. BMC Ecol. 2006, 6, 2. [Google Scholar] [CrossRef] [PubMed]
- Pedrero, Z.; Ouerdane, L.; Mounicou, S.; Lobinski, R.; Monperrrus, M.; Amouroux, D. Identification of mercury and other metals complexes with Metallothioneins in Dolphin liver by hydrophilic interaction liquid chromatography with the parallel detection by ICP MS and electrospray hybrid linear/orbital trap MS/MS. Metallomics 2012, 4, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Pollizzi, P.S.; Romero, M.B.; Chiodi Boudet, L.N.; Das, K.; DEnuncio, P.E.; Rodriguez, D.H.; Gerpe, M.S. Metallothioneins pattern during ontogeny of coastal dolphin, Pontoporia blainvillei, from Argentina. Mar. Pollut. Bull. 2014, 80, 275–281. [Google Scholar] [CrossRef]
- Càceres-Saez, I.; Polizzi, P.; Romero, B.; Dellabianca, N.A.; Guevara, S.R.; Goodall, R.N.P.; Cappozzo, H.L.; Gerpe, M. Hepatic and renal metallothionein concentrations in Comerson’s dolphins (Cephalorhynchus commersonii) from Tierra del Fuego, South Atlantic Ocean. Mar. Pollut. Bull. 2016, 108, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Viarengo, A.; Ponzano, E.; Dondero, F.; Fabbri, R. A simple spectrophotometric method for metallothionein evaluation in marine organisms: An application to Mediterranean and Antarctic molluscs. Mar. Environ. Res. 1997, 44, 69–84. [Google Scholar] [CrossRef]
- Liu, J.; Chen, B.; Jefferson, T.A.; Wang, H.; Yang, G. Trace element concentrations, risks and their correlation with metallothionein genes polymorphism: A case study of narrow-ridged finless porpoises (Neophocaena asiaeorientalis) in the East China Sea. Sci. Total Environ. 2017, 575, 628–638. [Google Scholar] [CrossRef] [PubMed]
- Vasàk, M.; Meloni, G. Chemistry and biology of mammalian metallothioneins. J. Biol. Inorg. Chem. 2011, 16, 1067–1078. [Google Scholar] [CrossRef]
- Hidalgo, J.; Chung, R.; Penkowa, M.; Vasàk, M. Structure and function of vertebrate metallothioneins. Met. Ions Life Sci. 2009, 5, 279–317. [Google Scholar]
- Nei, M.; Kumar, S. Molecular Evolution and Phylogenetics; Oxford University Press: New York, NY, USA, 2000. [Google Scholar]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).