Mono-(2-ethylhexyl) Phthalate (MEHP)-Induced Telomere Structure and Function Disorder Mediates Cell Cycle Dysregulation and Apoptosis via c-Myc and Its Upstream Transcription Factors in a Mouse Spermatogonia-Derived (GC-1) Cell Line
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Antibodies
2.2. Cell Culture and Treatment
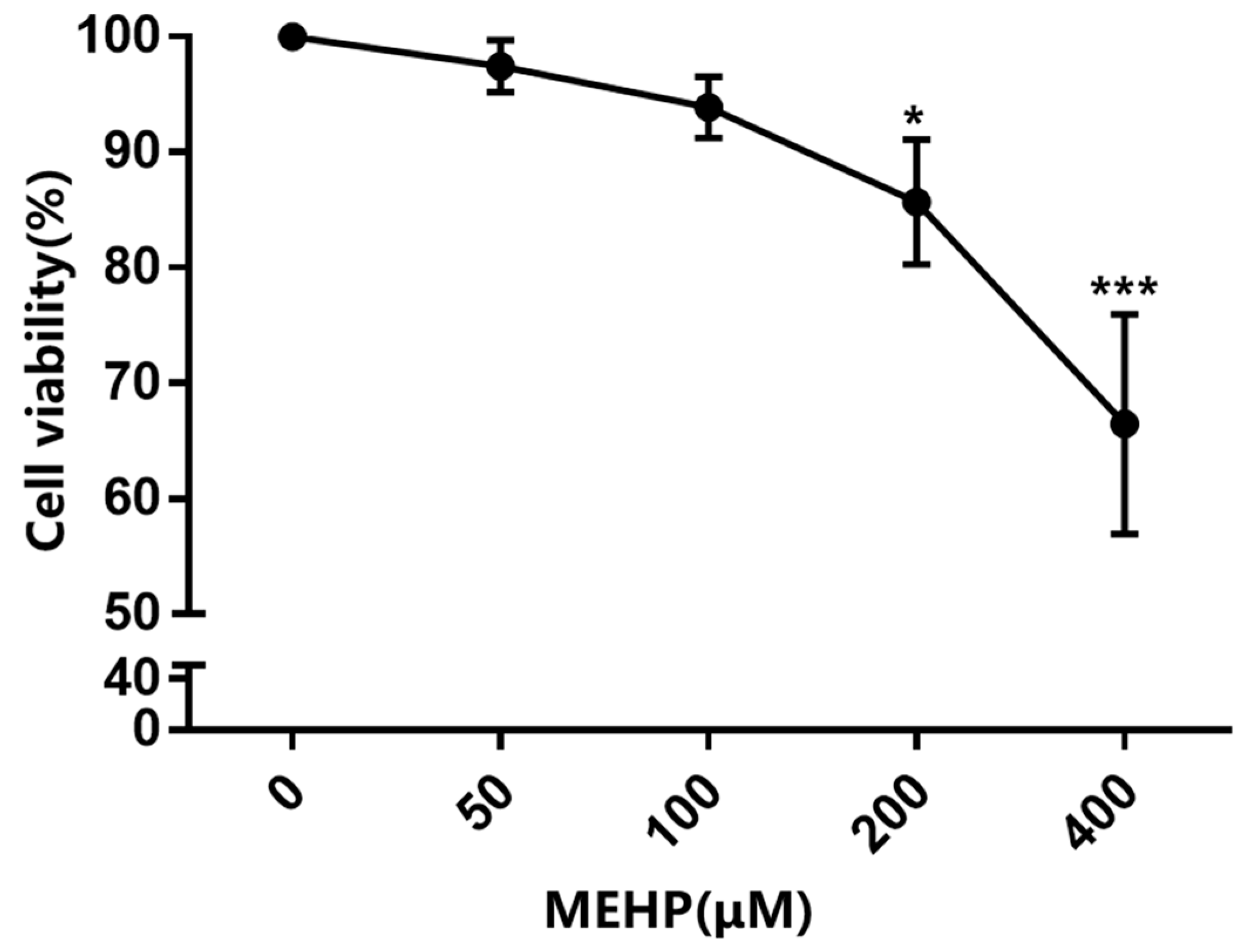
2.3. Cell Viability
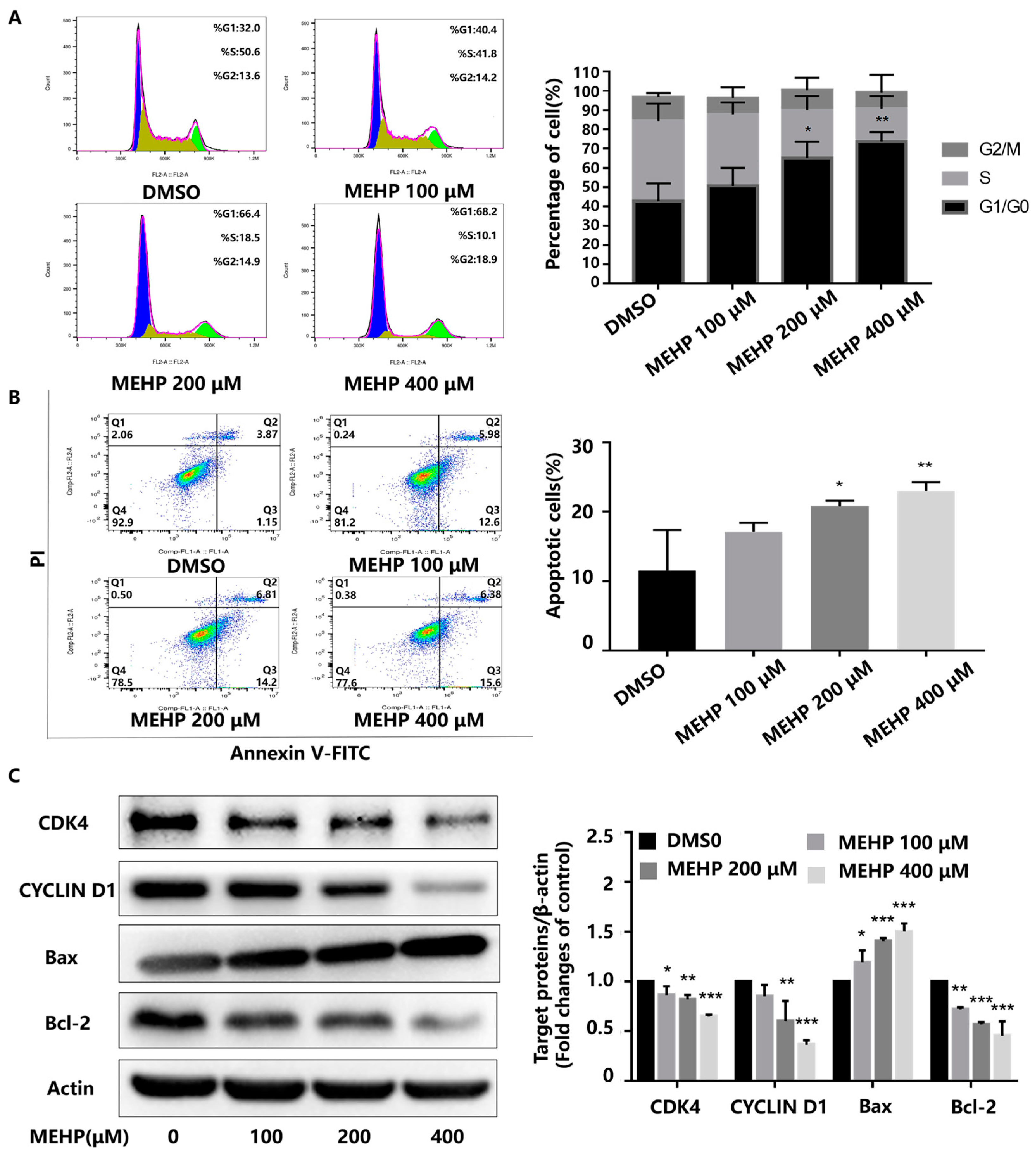
2.4. Cell Cycle Assay
2.5. Apoptosis Assay
2.6. Telomere Length Measurement
2.7. Real-Time Quantitative PCR (RT-qPCR)
2.8. Western Blot Analysis
2.9. Telomerase Activity Measurement
2.10. Statistical Analysis
3. Results
3.1. MEHP Reduces Cell Viability and Induces G0/G1 Phase Cell Cycle Arrest and Apoptosis in GC-1 Cells
3.2. MEHP Induces Telomere Structure and Function Disorder in GC-1 Cells
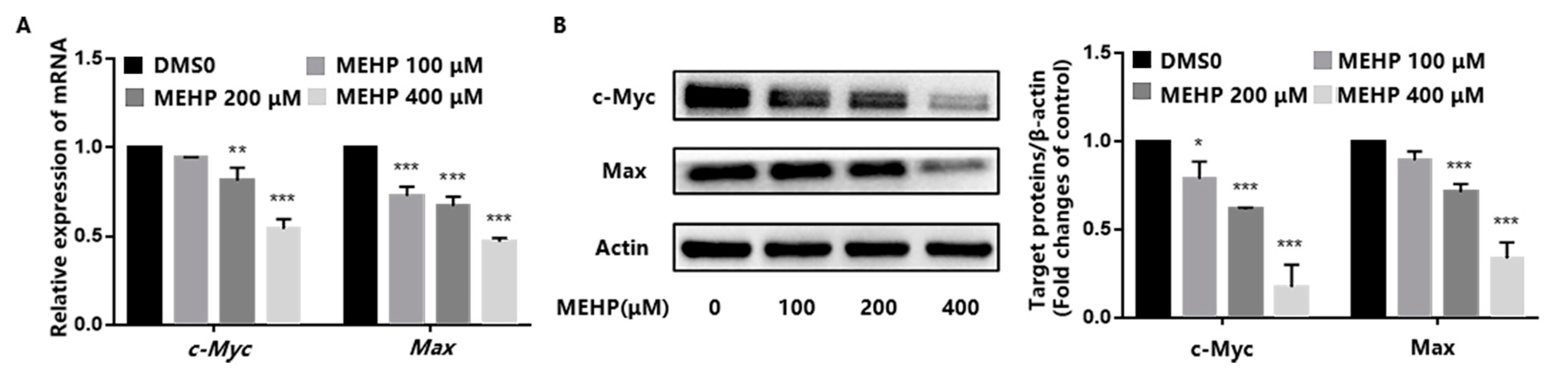
3.3. MEHP Inhibits c-Myc Expression in GC-1 Cells
3.4. Effect of MEHP Exposure on c-Myc Upstream Transcription Factors in GC-1 Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, X.; Fu, H.; Sun, J.; Di, Q.; Xu, Q. N6-methyladenosine modification on Hmbox1 is related to telomere dysfunction in DEHP-induced male reproductive injury. Life Sci. 2022, 309, 121005. [Google Scholar] [CrossRef]
- Cao, Y.; Li, J.; Wu, R.; Lin, H.; Lao, J.Y.; Ruan, Y.; Zhang, K.; Wu, J.; Leung, K.M.Y.; Lam, P.K.S. Phthalate esters in seawater and sediment of the northern South China Sea: Occurrence, distribution, and ecological risks. Sci. Total Environ. 2022, 811, 151412. [Google Scholar] [CrossRef]
- Ji, Y.; Wang, F.; Zhang, L.; Shan, C.; Bai, Z.; Sun, Z.; Liu, L.; Shen, B. A comprehensive assessment of human exposure to phthalates from environmental media and food in Tianjin, China. J. Hazard. Mater. 2014, 279, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Axelsson, J.; Rylander, L.; Rignell-Hydbom, A.; Jonsson, B.A.; Lindh, C.H.; Giwercman, A. Phthalate exposure and reproductive parameters in young men from the general Swedish population. Environ. Int. 2015, 85, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Qin, X.; Xiao, N.; Yao, Y.; Duan, Y.; Cui, X.; Zhang, S.; Luo, H.; Sun, H. Phthalate exposure and semen quality in infertile male population from Tianjin, China: Associations and potential mediation by reproductive hormones. Sci. Total Environ. 2020, 744, 140673. [Google Scholar] [CrossRef]
- Tang, X.; Li, D.; Zhao, T.; Zhu, S.; Gao, X.; Zhou, R.; Deng, F.; Fu, W.; Jia, W.; Liu, G. The inhibition of CFTR in the descended testis of SD rats with unilateral cryptorchidism induced by di-(2-ethylhexyl) phthalate (DEHP). Environ. Sci. Pollut. Res. Int. 2022, 29, 77047–77056. [Google Scholar] [CrossRef]
- XueXia, L.; YaNan, L.; Zi, T.; YuSheng, Z.; ZeLin, W.; Peng, Z.; MeiNa, X.; FuJun, L. Di-2-ethylhexyl phthalate (DEHP) exposure induces sperm quality and functional defects in mice. Chemosphere 2023, 312, 137216. [Google Scholar] [CrossRef]
- Coluzzi, E.; Leone, S.; Sgura, A. Oxidative Stress Induces Telomere Dysfunction and Senescence by Replication Fork Arrest. Cells 2019, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.B.; Luo, S.H.; Luo, Y.; Yuan, B.; Li, Y.H.; Luo, P.C.; Miao, Y.L.; Wang, J.L. Content of seminal plasma plasticizer in the patients with idiopathic asthenozoospermia and its impact on male fertility. Zhonghua Nan Ke Xue 2019, 25, 1097–1101. [Google Scholar]
- Karabulut, G.; Barlas, N. Genotoxic, histologic, immunohistochemical, morphometric and hormonal effects of di-(2-ethylhexyl)-phthalate (DEHP) on reproductive systems in pre-pubertal male rats. Toxicol. Res. 2018, 7, 859–873. [Google Scholar] [CrossRef]
- Allsopp, R.C.; Vaziri, H.; Patterson, C.; Goldstein, S.; Younglai, E.V.; Futcher, A.B.; Greider, C.W.; Harley, C.B. Telomere length predicts replicative capacity of human fibroblasts. Proc. Natl. Acad. Sci. USA 1992, 89, 10114–10118. [Google Scholar] [CrossRef]
- Ju, Z.; Lenhard Rudolph, K. Telomere dysfunction and stem cell ageing. Biochimie 2008, 90, 24–32. [Google Scholar] [CrossRef]
- Counter, C.M.; Hahn, W.C.; Wei, W.; Caddle, S.D.; Beijersbergen, R.L.; Lansdorp, P.M.; Sedivy, J.M.; Weinberg, R.A. Dissociation among in vitro telomerase activity, telomere maintenance, and cellular immortalization. Proc. Natl. Acad. Sci. USA 1998, 95, 14723–14728. [Google Scholar] [CrossRef]
- Zhang, X.; Mar, V.; Zhou, W.; Harrington, L.; Robinson, M.O. Telomere shortening and apoptosis in telomerase-inhibited human tumor cells. Genes Dev. 1999, 13, 2388–2399. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Popov, N.; Hou, M.; Wang, Q.; Björkholm, M.; Gruber, A.; Menkel, A.R.; Henriksson, M. Switch from Myc/Max to Mad1/Max binding and decrease in histone acetylation at the telomerase reverse transcriptase promoter during differentiation of HL60 cells. Proc. Natl. Acad. Sci. USA 2001, 98, 3826–3831. [Google Scholar] [CrossRef] [PubMed]
- Kyo, S.; Takakura, M.; Kanaya, T.; Zhuo, W.; Fujimoto, K.; Nishio, Y.; Orimo, A.; Inoue, M. Estrogen activates telomerase. Cancer Res. 1999, 59, 5917–5921. [Google Scholar] [PubMed]
- Pech, M.F.; Garbuzov, A.; Hasegawa, K.; Sukhwani, M.; Zhang, R.J.; Benayoun, B.A.; Brockman, S.A.; Lin, S.; Brunet, A.; Orwig, K.E.; et al. High telomerase is a hallmark of undifferentiated spermatogonia and is required for maintenance of male germline stem cells. Genes Dev. 2015, 29, 2420–2434. [Google Scholar] [CrossRef] [PubMed]
- McCracken, J.; Baccarelli, A.; Hoxha, M.; Dioni, L.; Melly, S.; Coull, B.; Suh, H.; Vokonas, P.; Schwartz, J. Annual ambient black carbon associated with shorter telomeres in elderly men: Veterans Affairs Normative Aging Study. Environ. Health Perspect. 2010, 118, 1564–1570. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, S.; Schettgen, T.; Beier, F.; Wilop, S.; Quinete, N.; Esser, A.; Masouleh, B.K.; Ferreira, M.S.; Vankann, L.; Uciechowski, P.; et al. Accelerated telomere shortening in peripheral blood lymphocytes after occupational polychlorinated biphenyls exposure. Arch. Toxicol. 2017, 91, 289–300. [Google Scholar] [CrossRef]
- Tran, H.T.T.; Herz, C.; Lamy, E. Long-term exposure to “low-dose” bisphenol A decreases mitochondrial DNA copy number, and accelerates telomere shortening in human CD8 + T cells. Sci. Rep. 2020, 10, 15786. [Google Scholar] [CrossRef]
- Wai, K.M.; Umezaki, M.; Kosaka, S.; Mar, O.; Umemura, M.; Fillman, T.; Watanabe, C. Impact of prenatal heavy metal exposure on newborn leucocyte telomere length: A birth-cohort study. Environ. Pollut. 2018, 243, 1414–1421. [Google Scholar] [CrossRef] [PubMed]
- Reig-Viader, R.; Garcia-Caldés, M.; Ruiz-Herrera, A. Telomere homeostasis in mammalian germ cells: A review. Chromosoma 2016, 125, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Ling, X.; Yang, W.; Zou, P.; Zhang, G.; Wang, Z.; Zhang, X.; Chen, H.; Peng, K.; Han, F.; Liu, J.; et al. TERT regulates telomere-related senescence and apoptosis through DNA damage response in male germ cells exposed to BPDE in vitro and to B[a]P in vivo. Environ. Pollut. 2018, 235, 836–849. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, J.; Zhou, G.; Sang, Y.; Zhang, Y.; Jing, L.; Shi, Z.; Zhou, X.; Sun, Z. BDE-209 and DBDPE induce male reproductive toxicity through telomere-related cell senescence and apoptosis in SD rat. Environ. Int. 2021, 146, 106307. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Li, L.; Hao, G.; Li, B.; Yang, S.; Wang, N.; Liang, J.; Sun, H.; Ma, S.; Yan, L.; et al. Sperm mtDNA copy number, telomere length, and seminal spermatogenic cells in relation to ambient air pollution: Results of a cross-sectional study in Jing-Jin-Ji region of China. J. Hazard. Mater. 2021, 406, 124308. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Song, X.K.; Cui, Q.H.; Chen, L.J. Effect of fluoride on expression of telomerase reverse transcriptase expression and proliferating cell nuclear antigen in germ cells of rats’ testes. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 2007, 25, 96–99. [Google Scholar] [PubMed]
- Wu, K.J.; Grandori, C.; Amacker, M.; Simon-Vermot, N.; Polack, A.; Lingner, J.; Dalla-Favera, R. Direct activation of TERT transcription by c-MYC. Nat. Genet. 1999, 21, 220–224. [Google Scholar] [CrossRef]
- Cerni, C. Telomeres, telomerase, and myc. An update. Mutat. Res. 2000, 462, 31–47. [Google Scholar] [CrossRef]
- Albro, P.W.; Chae, K.; Philpot, R.; Corbett, J.T.; Schroeder, J.; Jordan, S. In vitro metabolism of mono-2-ethylhexyl phthalate by microsomal enzymes. Similarity to omega- and (omega-1) oxidation of fatty acids. Drug Metab. Dispos. 1984, 12, 742–748. [Google Scholar]
- Hanioka, N.; Isobe, T.; Kinashi, Y.; Tanaka-Kagawa, T.; Jinno, H. Hepatic and intestinal glucuronidation of mono(2-ethylhexyl) phthalate, an active metabolite of di(2-ethylhexyl) phthalate, in humans, dogs, rats, and mice: An in vitro analysis using microsomal fractions. Arch. Toxicol. 2016, 90, 1651–1657. [Google Scholar] [CrossRef]
- Park, J.D.; Habeebu, S.S.; Klaassen, C.D. Testicular toxicity of di-(2-ethylhexyl)phthalate in young Sprague-Dawley rats. Toxicology 2002, 171, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Hoyer, B.B.; Lenters, V.; Giwercman, A.; Jonsson, B.A.G.; Toft, G.; Hougaard, K.S.; Bonde, J.P.E.; Specht, I.O. Impact of Di-2-Ethylhexyl Phthalate Metabolites on Male Reproductive Function: A Systematic Review of Human Evidence. Curr. Environ. Health Rep. 2018, 5, 20–33. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, N.B.; Olsson, M. How telomere dynamics are influenced by the balance between mitochondrial efficiency, reactive oxygen species production and DNA damage. Mol. Ecol. 2022, 31, 6040–6052. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Liu, B.; Wu, M.; Zhang, L.; Wang, L.; Zhang, B.; Xiong, C.; Li, Y.; Cao, Z.; Wang, Y.; et al. Prenatal Exposure to Phthalates and Newborn Telomere Length: A Birth Cohort Study in Wuhan, China. Environ. Health Perspect. 2019, 127, 87007. [Google Scholar] [CrossRef]
- Scinicariello, F.; Feroe, A.G.; Attanasio, R. Urinary Phthalates and Leukocyte Telomere Length: An Analysis of NHANES 1999-2002. EBioMedicine 2016, 6, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Tan, Y.; Qiu, X.; Luo, H.; Li, Y.; Li, R.; Yang, X. Sperm telomere length as a novel biomarker of male infertility and embryonic development: A systematic review and meta-analysis. Front. Endocrinol. 2022, 13, 1079966. [Google Scholar] [CrossRef] [PubMed]
- Ling, X.; Zhang, G.; Chen, Q.; Yang, H.; Sun, L.; Zhou, N.; Wang, Z.; Zou, P.; Wang, X.; Cui, Z.; et al. Shorter sperm telomere length in association with exposure to polycyclic aromatic hydrocarbons: Results from the MARHCS cohort study in Chongqing, China and in vivo animal experiments. Environ. Int. 2016, 95, 79–85. [Google Scholar] [CrossRef]
- Ling, X.; Zhang, G.; Sun, L.; Wang, Z.; Zou, P.; Gao, J.; Peng, K.; Chen, Q.; Yang, H.; Zhou, N.; et al. Polycyclic aromatic hydrocarbons exposure decreased sperm mitochondrial DNA copy number: A cross-sectional study (MARHCS) in Chongqing, China. Environ. Pollut. 2017, 220, 680–687. [Google Scholar] [CrossRef]
- Eisenhauer, K.M.; Gerstein, R.M.; Chiu, C.P.; Conti, M.; Hsueh, A.J. Telomerase activity in female and male rat germ cells undergoing meiosis and in early embryos. Biol. Reprod. 1997, 56, 1120–1125. [Google Scholar] [CrossRef]
- Nagasawa, K.; Imura-Kishi, K.; Uchida, A.; Hiramatsu, R.; Kurohmaru, M.; Kanai, Y. Regionally distinct patterns of STAT3 phosphorylation in the seminiferous epithelia of mouse testes. Mol. Reprod. Dev. 2018, 85, 262–270. [Google Scholar] [CrossRef]
- Jonak, C.R.; Lainez, N.M.; Boehm, U.; Coss, D. GnRH Receptor Expression and Reproductive Function Depend on JUN in GnRH Receptor–Expressing Cells. Endocrinology 2018, 159, 1496–1510. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Hernandez, A.; Lilienthal, I.; Fukuda, N.; Galjart, N.; Hoog, C. CTCF contributes in a critical way to spermatogenesis and male fertility. Sci. Rep. 2016, 6, 28355. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, C.S.; Cooke, P.S. Endocrine disruption through membrane estrogen receptors and novel pathways leading to rapid toxicological and epigenetic effects. J. Steroid Biochem. Mol. Biol. 2019, 187, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Hess, R.A. Disruption of estrogen receptor signaling and similar pathways in the efferent ductules and initial segment of the epididymis. Spermatogenesis 2014, 4, e979103. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, F.; Guo, C.; Wang, L.; Zhang, G.; Wang, J.; Chen, W.; Cui, K.; Tan, Y.; Zhou, Z. Mono-(2-ethylhexyl) Phthalate (MEHP)-Induced Telomere Structure and Function Disorder Mediates Cell Cycle Dysregulation and Apoptosis via c-Myc and Its Upstream Transcription Factors in a Mouse Spermatogonia-Derived (GC-1) Cell Line. Toxics 2023, 11, 448. https://doi.org/10.3390/toxics11050448
Zhou F, Guo C, Wang L, Zhang G, Wang J, Chen W, Cui K, Tan Y, Zhou Z. Mono-(2-ethylhexyl) Phthalate (MEHP)-Induced Telomere Structure and Function Disorder Mediates Cell Cycle Dysregulation and Apoptosis via c-Myc and Its Upstream Transcription Factors in a Mouse Spermatogonia-Derived (GC-1) Cell Line. Toxics. 2023; 11(5):448. https://doi.org/10.3390/toxics11050448
Chicago/Turabian StyleZhou, Fangji, Chengwei Guo, Lingqiao Wang, Guowei Zhang, Jia Wang, Weiyan Chen, Ke Cui, Yao Tan, and Ziyuan Zhou. 2023. "Mono-(2-ethylhexyl) Phthalate (MEHP)-Induced Telomere Structure and Function Disorder Mediates Cell Cycle Dysregulation and Apoptosis via c-Myc and Its Upstream Transcription Factors in a Mouse Spermatogonia-Derived (GC-1) Cell Line" Toxics 11, no. 5: 448. https://doi.org/10.3390/toxics11050448
APA StyleZhou, F., Guo, C., Wang, L., Zhang, G., Wang, J., Chen, W., Cui, K., Tan, Y., & Zhou, Z. (2023). Mono-(2-ethylhexyl) Phthalate (MEHP)-Induced Telomere Structure and Function Disorder Mediates Cell Cycle Dysregulation and Apoptosis via c-Myc and Its Upstream Transcription Factors in a Mouse Spermatogonia-Derived (GC-1) Cell Line. Toxics, 11(5), 448. https://doi.org/10.3390/toxics11050448






