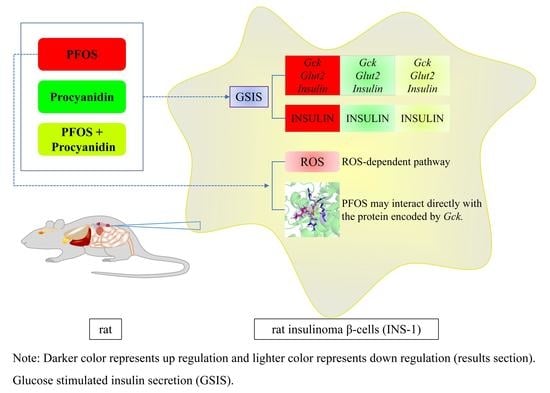
Preliminary Study on the Protective Effects and Molecular Mechanism of Procyanidins against PFOS-Induced Glucose-Stimulated Insulin Secretion Impairment in INS-1 Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials, Reagents and Instruments
2.2. Methods
2.2.1. Cell Culture
2.2.2. Preparation of PFOS and PC Solution
2.2.3. Cell Counting Kit-8 (CCK8) Assay
2.2.4. Exposure Experiment
2.2.5. ELISA Assay
2.2.6. Western Blot
2.2.7. Real-Time Fluorescence Quantitative PCR (RT-qPCR)
2.2.8. ROS Determination
2.3. Statistical Analysis
3. Results
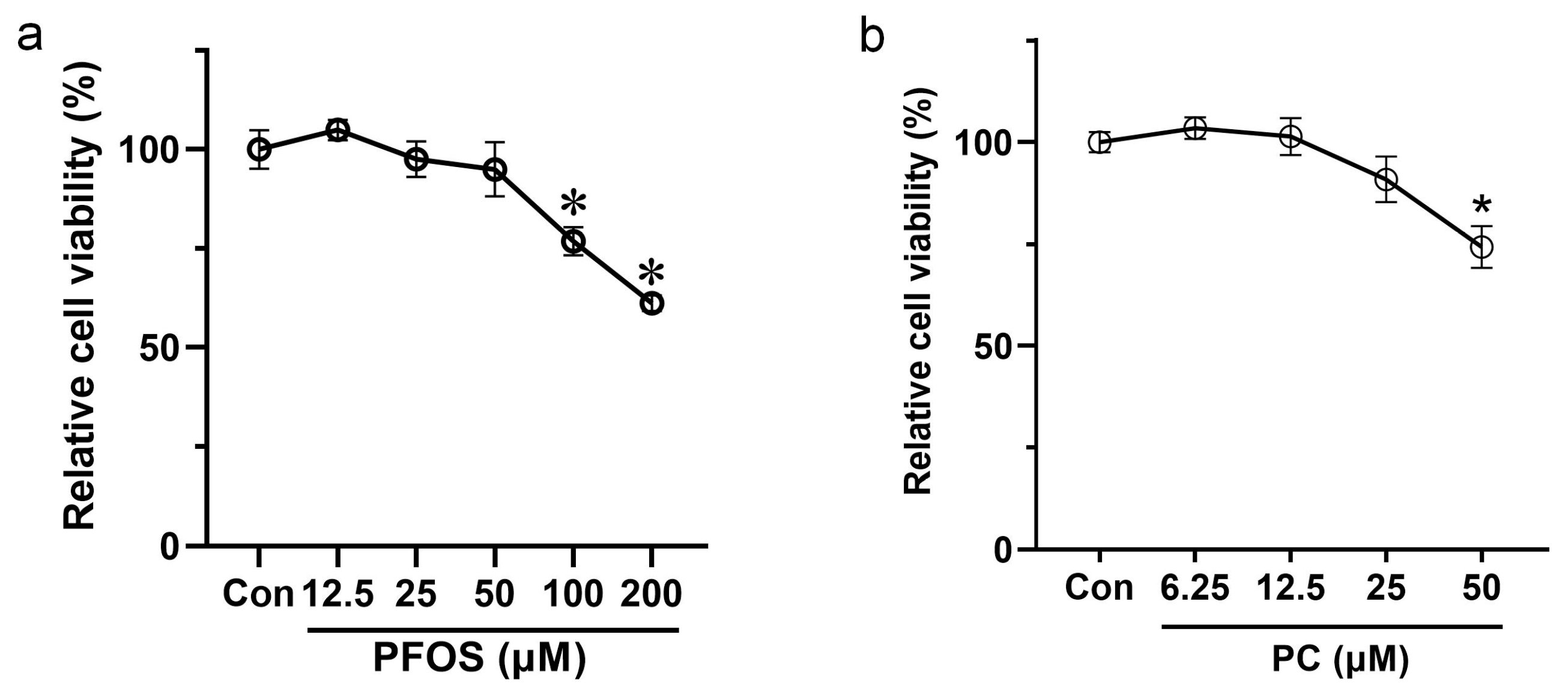
3.1. Effects of PFOS and PC after 48 h Exposure on the Viability of INS-1 Cells
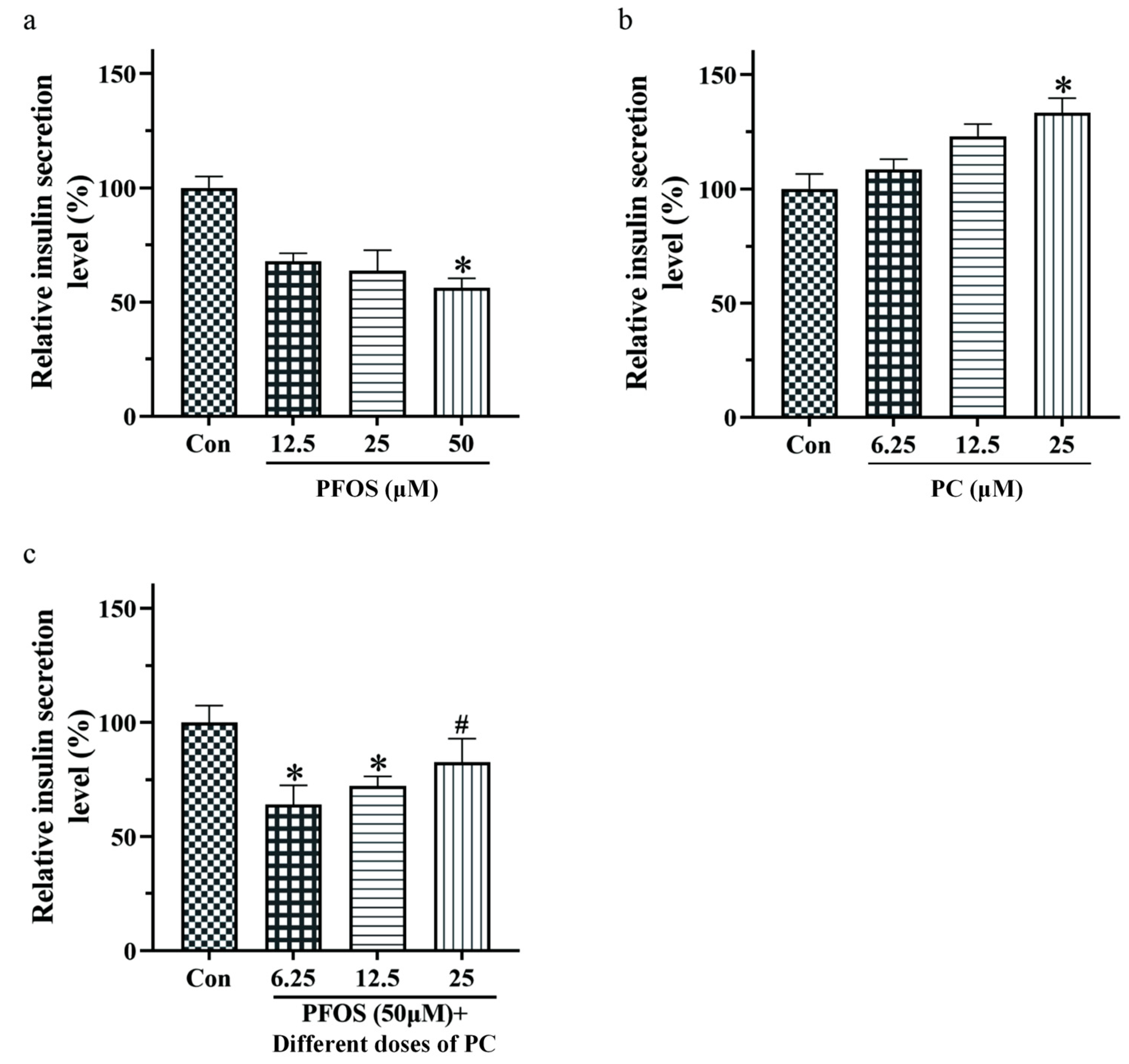
3.2. Effects of PFOS and PC Alone or in Combination after 48 h Exposure on Insulin Secretion of INS-1 Cells under Glucose Stimulation
3.3. Effects of PFOS and PC Alone or in Combination after 48 h Exposure on the Level of Insulin Protein of INS-1 Cells Stimulated by Glucose
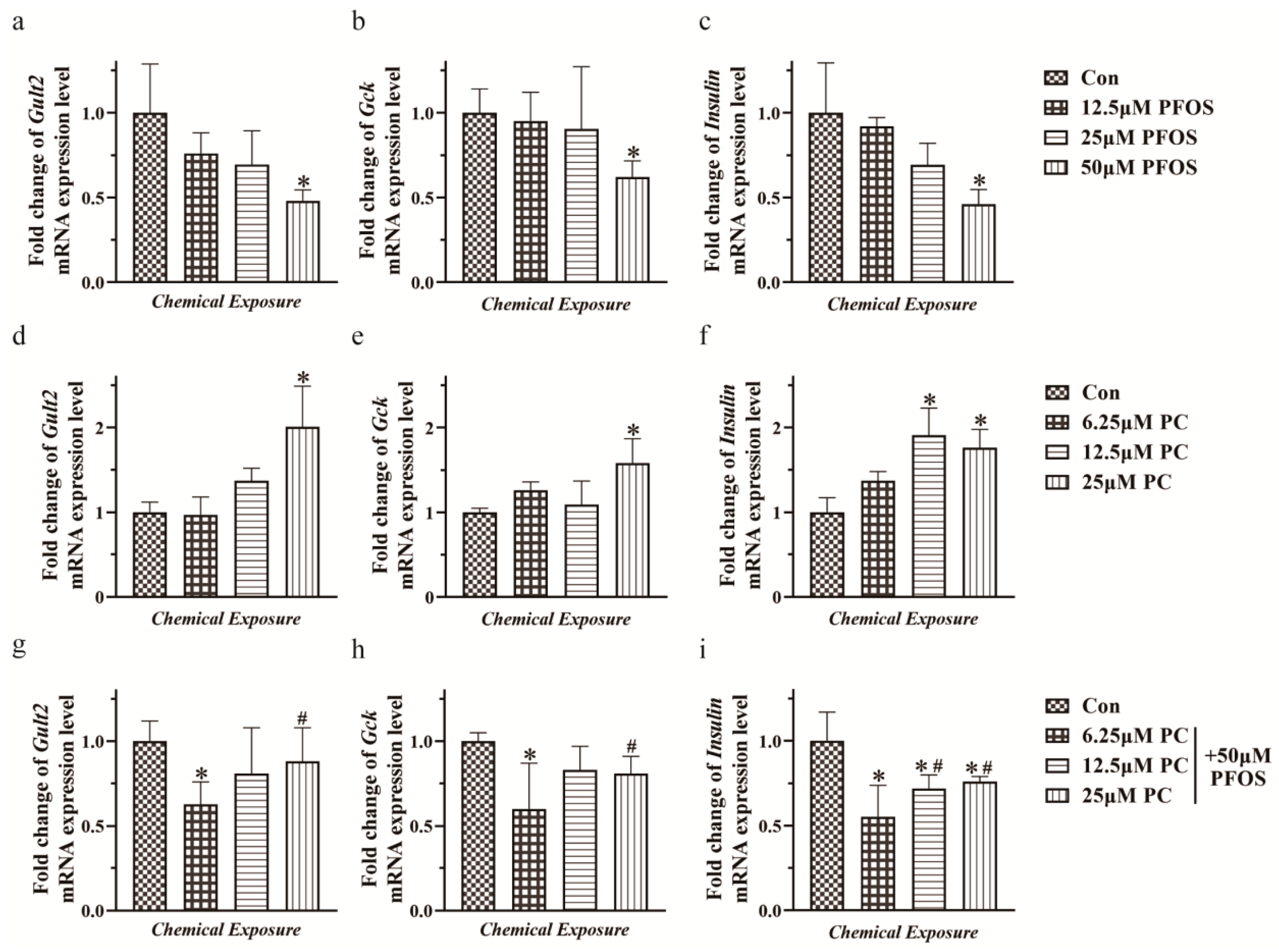
3.4. Relative mRNA Expression Levels of Glut2, Gck, and Insulin in INS-1 Cells Exposed to PFOS and PC Alone or in Combination after 48 h Exposure under Glucose Stimulation
3.5. ROS Levels in INS-1 Cells Exposed to PFOS and/or PC for 12, 24, and 48 h with or without Glucose Stimulation
4. Discussion
5. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Glüge, J.; Scheringer, M.; Cousins, I.T.; DeWitt, J.C.; Goldenman, G.; Herzke, D.; Lohmann, R.; Ng, C.A.; Trier, X.; Wang, Z. An overview of the uses of per- and polyfluoroalkyl substances (PFAS). Environ. Sci. Process. Impacts 2020, 22, 2345–2373. [Google Scholar] [CrossRef] [PubMed]
- Meegoda, J.N.; Kewalramani, J.A.; Li, B.; Marsh, R.W. A Review of the applications, environmental release, and remediation technologies of per- and polyfluoroalkyl substances. Int. J. Environ. Res. Public Health 2020, 17, 8117. [Google Scholar] [CrossRef] [PubMed]
- Mokra, K. Endocrine disruptor potential of short- and long-chain perfluoroalkyl substances (PFASs)-a synthesis of current knowledge with proposal of molecular mechanism. Int. J. Mol. Sci. 2021, 22, 2148. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.R. PFAS in soil and groundwater following historical land application of biosolids. Water Res. 2022, 211, 118035. [Google Scholar] [CrossRef]
- Zhang, Z.; Sarkar, D.; Biswas, J.K.; Datta, R. Biodegradation of per- and polyfluoroalkyl substances (PFAS): A review. Bioresour. Technol. 2022, 344 Pt B, 126223. [Google Scholar] [CrossRef]
- Jeddi, M.Z.; Soltanmohammadi, R.; Barbieri, G.; Fabricio, A.S.; Pitter, G.; Dalla Zuanna, T.; Canova, C. To which extent are per-and poly-fluorinated substances associated to metabolic syndrome? Rev. Environ. Health 2021, 37, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Li, G.; Li, Y.; Ying, C.; Chen, J.; Chen, T.; Wei, J.; Lin, Y.; Jiang, Y.; Wang, Y.; et al. Glucose and lipid homeostasis in adult rat is impaired by early-life exposure to perfluorooctane sulfonate. Environ. Toxicol. 2013, 28, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Sun, W.; Sun, H.; Zhang, L. Perfluorooctane sulfonate continual exposure impairs glucose-stimulated insulin secretion via SIRT1-induced upregulation of UCP2 expression. Environ. Pollut. 2021, 278, 116840. [Google Scholar] [CrossRef]
- He, X.; Liu, Y.; Xu, B.; Gu, L.; Tang, W. PFOA is associated with diabetes and metabolic alteration in US men: National Health and Nutrition Examination Survey 2003–2012. Sci. Total Environ. 2018, 625, 566–574. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.S.; Wen, L.L.; Chu, P.L.; Lin, C.Y. Association among total serum isomers of perfluorinated chemicals, glucose homeostasis, lipid profiles, serum protein and metabolic syndrome in adults: NHANES, 2013–2014. Environ. Pollut. 2018, 232, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, O.; Ficorilli, J.; Shaw, J.; Echeverri, F.; Schwede, F.; Chepurny, O.G.; Leech, C.A.; Holz, G.G. Intra-islet glucagon confers β-cell glucose competence for first-phase insulin secretion and favors GLP-1R stimulation by exogenous glucagon. J. Biol. Chem. 2021, 298, 101484. [Google Scholar] [CrossRef]
- Mittendorfer, B.; Patterson, B.W.; Smith, G.I.; Yoshino, M.; Klein, S. β cell function and plasma insulin clearance in people with obesity and different glycemic status. J. Clin. Investig. 2021, 132, e154068. [Google Scholar] [CrossRef] [PubMed]
- Boland, B.B.; Rhodes, C.J.; Grimsby, J.S. The dynamic plasticity of insulin production in β-cells. Mol. Metab. 2017, 6, 958–973. [Google Scholar] [CrossRef] [PubMed]
- Luca, S.V.; Macovei, I.; Bujor, A.; Miron, A.; Skalicka-Woźniak, K.; Aprotosoaie, A.C.; Trifan, A. Bioactivity of dietary polyphenols: The role of metabolites. Crit. Rev. Food Sci. Nutr. 2020, 60, 626–659. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and human health: The role of bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomas-Barberan, F.A. The effects of polyphenols and other bioactives on human health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef]
- Oboh, G.; Ademiluyi, A.O.; Akinyemi, A.J.; Henle, T.; Saliu, J.A.; Schwarzenbolz, U. Inhibitory effect of polyphenol-rich extracts of jute leaf (Corchorus olitorius) on key enzyme linked to type 2 diabetes (α-amylase and α-glucosidase) and hypertension (angiotensin I converting) in vitro. J. Funct. Foods 2012, 4, 450–458. [Google Scholar] [CrossRef]
- Valencia-Hernandez, L.J.; Wong-Paz, J.E.; Ascacio-Valdes, J.A.; Chavez-Gonzalez, M.L.; Contreras-Esquivel, J.C.; Aguilar, C.N. Procyanidins: From agro-industrial waste to food as bioactive molecules. Foods 2021, 10, 3152. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, H.; Yang, E.; Li, J.; Dong, L. Berberine decreases intestinal GLUT2 translocation and reduces intestinal glucose absorption in mice. Int. J. Mol. Sci. 2021, 23, 327. [Google Scholar] [CrossRef]
- Bahl, V.; Lee, M.C.; Perez, A.; Glaser, B.; Kaestner, K.H. Genetic activation of α cell glucokinase in mice causes enhanced glucose-suppression of glucagon secretion during normal and diabetic states. Mol. Metab. 2021, 49, 101193. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.T.; Zhao, Y.G.; Leung, P.Y.; Wong, C.K. Perinatal exposure to perfluorooctane sulfonate affects glucose metabolism in adult offspring. PLoS ONE 2014, 9, e87137. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.C.; Zhu, B.Q.; Wang, Y.Q.; He, Q.Z. ROS-triggered autophagy is involved in PFOS-induced apoptosis of human embryo liver L-02 cells. BioMed Res. Int. 2021, 2021, 6625952. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Ren, X.; Zhao, L.; Guo, L. Exposure to perfluorooctane sulfonate reduced cell viability and insulin release capacity of beta cells. J. Environ. Sci. 2022, 115, 162–172. [Google Scholar] [CrossRef]
- Sonne, C.; Xia, C.; Lam, S.S. Ban fluorinated organic substances to spark green alternatives. Eco-Environ. Health 2022, 1, 105–106. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, H.-M.; Wu, M.-Y.; Shi, X.-C.; Liu, K.-L.; Zhang, Y.-C.; Zhang, Y.-F.; Li, H.-M. Preliminary Study on the Protective Effects and Molecular Mechanism of Procyanidins against PFOS-Induced Glucose-Stimulated Insulin Secretion Impairment in INS-1 Cells. Toxics 2023, 11, 174. https://doi.org/10.3390/toxics11020174
Xu H-M, Wu M-Y, Shi X-C, Liu K-L, Zhang Y-C, Zhang Y-F, Li H-M. Preliminary Study on the Protective Effects and Molecular Mechanism of Procyanidins against PFOS-Induced Glucose-Stimulated Insulin Secretion Impairment in INS-1 Cells. Toxics. 2023; 11(2):174. https://doi.org/10.3390/toxics11020174
Chicago/Turabian StyleXu, Hai-Ming, Meng-Yu Wu, Xin-Chen Shi, Ke-Liang Liu, Ying-Chi Zhang, Yin-Feng Zhang, and Hong-Mei Li. 2023. "Preliminary Study on the Protective Effects and Molecular Mechanism of Procyanidins against PFOS-Induced Glucose-Stimulated Insulin Secretion Impairment in INS-1 Cells" Toxics 11, no. 2: 174. https://doi.org/10.3390/toxics11020174
APA StyleXu, H.-M., Wu, M.-Y., Shi, X.-C., Liu, K.-L., Zhang, Y.-C., Zhang, Y.-F., & Li, H.-M. (2023). Preliminary Study on the Protective Effects and Molecular Mechanism of Procyanidins against PFOS-Induced Glucose-Stimulated Insulin Secretion Impairment in INS-1 Cells. Toxics, 11(2), 174. https://doi.org/10.3390/toxics11020174