A Review on Per- and Polyfluoroalkyl Substances in Pregnant Women: Maternal Exposure, Placental Transfer, and Relevant Model Simulation
Abstract
1. Introduction
2. Methodology of Literature Sources
3. PFASs in Pregnant Women
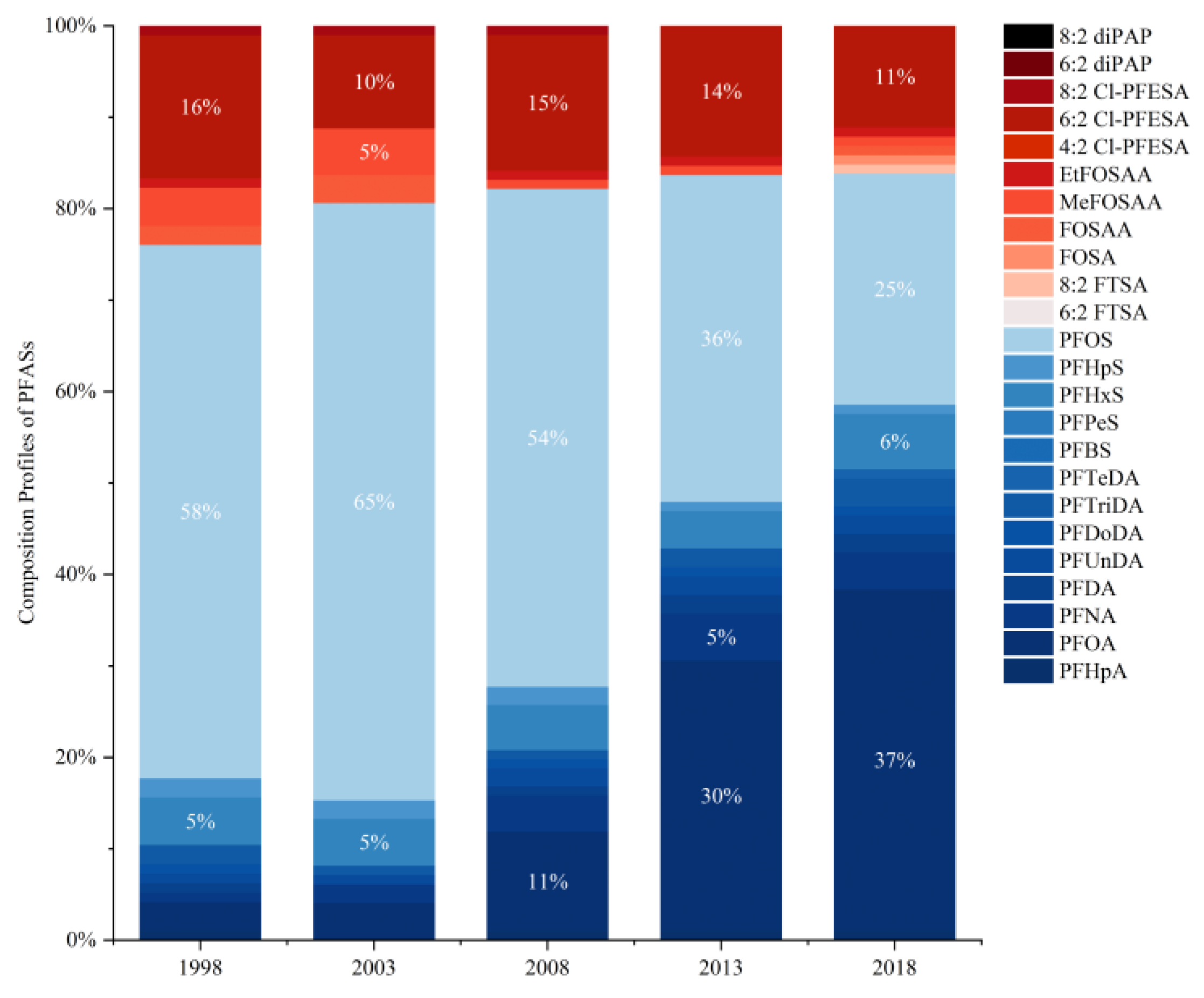
3.1. Maternal Exposure to PFASs
3.2. Placental Transfer of PFASs
3.3. Binding of PFASs to Proteins
3.4. Simulation through Machine Learning
4. Conclusions and Future Research Emphases
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Organisation for Economic Co-operation and Development. Toward A New Comprehensive Global Database of Per–and Polyfluoroalkyl Substances (PFASs): Summary Report on Updating the OECD 2007 List of Per–and Polyfluoroalkyl Substances (PFASs); Organisation for Economic Cooperation and Development (OECD): Paris, France, 2018. [Google Scholar]
- Kissa, E. Fluorinated Surfactants and Repellents, 2nd ed.; Marcel Dekker: New York, NY, USA, 2001. [Google Scholar]
- Houde, M.; Martin, J.W.; Letcher, R.J.; Solomon, K.R.; Muir, D.C.G. Biological monitoring of polyfluoroalkyl substances: A review. Environ. Sci. Technol. 2006, 40, 3463–3473. [Google Scholar] [CrossRef] [PubMed]
- Houde, M.; De Silva, A.O.; Muir, D.C.; Letcher, R.J. Monitoring of perfluorinated compounds in aquatic biota: An updated review: PFCs in aquatic biota. Environ. Sci. Technol. 2011, 45, 7962–7973. [Google Scholar] [CrossRef]
- Giesy, J.P.; Kannan, K. Global distribution of perfluorooctane sulfonate in wildlife. Environ. Sci. Technol. 2001, 35, 1339–1342. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.J.; Clemen, L.A.; Ellefson, M.E.; Johnson, H.O. Compound–specific, quantitative characterization of organic fluorochemicals in biological matrices. Environ. Sci. Technol. 2001, 35, 766–770. [Google Scholar] [CrossRef]
- DeWitt, J.C.; Peden–Adams, M.M.; Keller, J.M.; Germolec, D.R. Immunotoxicity of perfluorinated compounds: Recent developments. Toxicol. Pathol. 2012, 40, 300–311. [Google Scholar] [CrossRef]
- Gomis, M.I.; Vestergren, R.; Borg, D.; Cousins, I.T. Comparing the toxic potency in vivo of long–chain perfluoroalkyl acids and fluorinated alternatives. Environ. Int. 2018, 113, 1–9. [Google Scholar] [CrossRef]
- UNEP. Stockholm Convention on Persistent Organic Pollutants (POPs). 2009. Available online: http://chm.pops.int/Portals/0/Repository/convention_text/UNEP–POPS–COP–CONVTEXTFULL.English.PDF (accessed on 26 April 2023).
- UNEP. Stockholm Convention on Persistent Organic Pollutants (POPs). 2021. Available online: http://chm.pops.int/TheConvention/POPsReviewCommittee/Meetings/POPRC16/Overview/tabid/8472/ctl/Download/mid/25103/Default.aspx?id=53&ObjID=29737 (accessed on 26 April 2023).
- UNEP. Stockholm Convention on Persistent Organic Pollutants (POPs). 2022. Available online: http://chm.pops.int/TheConvention/POPsReviewCommittee/Meetings/POPRC17/Overview/tabid/8900/Default.aspx (accessed on 26 April 2023).
- Birnbaum, L.S.; Grandjean, P. Alternatives to PFASs: Perspectives on the science. Environ. Health. Perspect. 2015, 123, A104–A105. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; DeWitt, J.C.; Higgins, C.P.; Cousins, I.T. A never–ending story of per–and polyfluoroalkyl substances (PFASs)? Environ. Sci. Technol. 2017, 54, 3325. [Google Scholar] [CrossRef]
- Bao, J.; Li, C.L.; Liu, Y.; Wang, X.; Yu, W.J.; Liu, Z.Q.; Shao, L.X.; Jin, Y.H. Bioaccumulation of perfluoroalkyl substances in greenhouse vegetables with long–term groundwater irrigation near fluorochemical plants in Fuxin, China. Environ. Res. 2020, 188, 109751. [Google Scholar] [CrossRef]
- Bao, J.; Yu, W.J.; Liu, Y.; Wang, X.; Jin, Y.H.; Dong, G.H. Perfluoroalkyl substances in groundwater and home–produced vegetables and eggs around a fluorochemical industrial park in China. Ecotoxicol. Environ. Saf. 2019, 171, 199–205. [Google Scholar] [CrossRef]
- Gebbink, W.A.; Bossi, R.; Rigét, F.F.; Rosing–Asvid, A.; Sonne, C.; Dietz, R. Observation of emerging per–and polyfluoroalkyl substances (PFASs) in Greenland marine mammals. Chemosphere 2016, 144, 2384–2391. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Qin, H.; Li, J.; Zhang, Q.; Zhang, H.; Wang, Z.; He, X. Atmospheric chlorinated polyfluorinated ether sulfonate and ionic perfluoroalkyl acids in 2006 to 2014 in Dalian, China. Environ. Toxicol. Chem. 2017, 36, 2581–2586. [Google Scholar] [CrossRef]
- Shi, Y.; Vestergren, R.; Zhou, Z.; Song, X.; Xu, L.; Liang, Y.; Cai, Y. Tissue distribution and whole body burden of the chlorinated polyfluoroalkyl ether sulfonic acid F–53B in crucian carp (Carassius carassius): Evidence for a highly bioaccumulative contaminant of emerging concern. Environ. Sci. Technol. 2015, 49, 14156–14165. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Liang, Y.; Shi, Y.; Xu, L.; Cai, Y. Occurrence and transport of perfluoroalkyl acids (PFAAs), including short–chain PFAAs in Tangxun Lake, China. Environ. Sci. Technol. 2013, 47, 9249–9257. [Google Scholar] [CrossRef]
- Chu, C.; Zhou, Y.; Li, Q.Q.; Bloom, M.S.; Lin, S.; Yu, Y.J.; Chen, D.; Yu, H.Y.; Hu, L.W.; Yang, B.Y.; et al. Are perfluorooctane sulfonate alternatives safer? New insights from a birth cohort study. Environ. Int. 2020, 135, 105365. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Choi, K.; Ji, K.; Seo, J.; Kho, Y.; Park, J.; Kim, S.; Park, S.; Hwang, I.; Jeon, J.; et al. Trans–placental transfer of thirteen perfluorinated compounds and relations with fetal thyroid hormones. Environ. Sci. Technol. 2011, 45, 7465–7472. [Google Scholar] [CrossRef] [PubMed]
- Colles, A.; Bruckers, L.; Den Hond, E.; Govarts, E.; Morrens, B.; Schettgen, T.; Buekers, J.; Coertjens, D.; Nawrot, T.; Loots, I.; et al. Perfluorinated substances in the Flemish population (Belgium): Levels and determinants of variability in exposure. Chemosphere 2020, 242, 125250. [Google Scholar] [CrossRef] [PubMed]
- Wolf, C.J.; Fenton, S.E.; Schmid, J.E.; Calafat, A.M.; Kuklenyik, Z.; Bryant, X.A.; Thibodeaux, J.; Das, K.P.; White, S.S.; Lau, C.S.; et al. Developmental toxicity of perfluorooctanoic acid in the CD–1 mouse after cross–foster and restricted gestational exposures. Toxicol. Sci. 2007, 95, 462–473. [Google Scholar] [CrossRef]
- Prouillac, C.; Lecoeur, S. The role of the placenta in fetal exposure to xenobiotics: Importance of membrane transporters and human models for transfer studies. Drug Metab. Dispos. 2010, 38, 1623–1635. [Google Scholar] [CrossRef]
- Morello–Frosch, R.; Cushing, L.J.; Jesdale, B.M.; Schwartz, J.M.; Guo, W.; Guo, T.; Wang, M.; Harwani, S.; Petropoulou, S.E.; Duong, W.; et al. Environmental chemicals in an urban population of pregnant women and their newborns from San Francisco. Environ. Sci. Technol. 2016, 50, 12464–12472. [Google Scholar] [CrossRef]
- Zhang, T.; Sun, H.; Lin, Y.; Qin, X.; Zhang, Y.; Geng, X.; Kannan, K. Distribution of poly–and perfluoroalkyl substances in matched samples from pregnant women and carbon chain length related maternal transfer. Environ. Sci. Technol. 2013, 47, 7974–7981. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm–Benartzi, C.S.; Houseman, E.A.; Maccani, M.A.; Poage, G.M.; Koestler, D.C.; Langevin, S.M.; Gagne, L.A.; Banister, C.E.; Padbury, J.F.; Marsit, C.J. In utero exposures, infant growth, and DNA methylation of repetitive elements and developmentally related genes in human placenta. Environ. Health. Perspect. 2012, 120, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Bjerregaard–Olesen, C.; Bach, C.C.; Long, M.; Wielsøe, M.; Bech, B.H.; Henriksen, T.B.; Olsen, J.; Bonefeld–Jørgensen, E.C. Associations of fetal growth outcomes with measures of the combined xenoestrogenic activity of maternal serum perfluorinated alkyl acids in Danish pregnant women. Environ. Health. Perspect. 2019, 127, 017006. [Google Scholar] [CrossRef] [PubMed]
- Hjermitslev, M.H.; Long, M.; Wielsøe, M.; Bonefeld–Jørgensen, E.C. Persistent organic pollutants in Greenlandic pregnant women and indices of foetal growth: The ACCEPT study. Sci. Total. Environ. 2020, 698, 134118. [Google Scholar] [CrossRef]
- Liew, Z.; Goudarzi, H.; Oulhote, Y. Developmental exposures to perfluoroalkyl substances (PFASs): An update of associated health outcomes. Curr. Environ. Health. Rep. 2018, 5, 1–19. [Google Scholar] [CrossRef]
- Fisher, M.; Arbuckle, T.E.; Liang, C.L.; LeBlanc, A.; Gaudreau, E.; Foster, W.G.; Haines, D.; Davis, K.; Fraser, W.D. Concentrations of persistent organic pollutants in maternal and cord blood from the maternal–infant research on environmental chemicals (MIREC) cohort study. Environ. Health. 2016, 15, 59. [Google Scholar] [CrossRef]
- Starling, A.P.; Liu, C.; Shen, G.; Yang, I.V.; Kechris, K.; Borengasser, S.J.; Boyle, K.E.; Zhang, W.; Smith, H.A.; Calafat, A.M.; et al. Prenatal exposure to per–and polyfluoroalkyl substances, umbilical cord blood DNA methylation, and cardio–metabolic indicators in newborns: The Healthy Start Study. Environ. Health. Rep. 2020, 128, 127014. [Google Scholar] [CrossRef]
- Stratakis, N.V.; Conti, D.; Jin, R.; Margetaki, K.; Valvi, D.; Siskos, A.P.; Maitre, L.; Garcia, E.; Varo, N.; Zhao, Y.; et al. Prenatal exposure to perfluoroalkyl substances associated with increased susceptibility to liver injury in children. Hepatology 2020, 72, 1758–1770. [Google Scholar] [CrossRef]
- Fromme, H.; Mosch, C.; Morovitz, M.; Alba–Alejandre, I.; Boehmer, S.; Kiranoglu, M.; Faber, F.; Hannibal, I.; Genzel–Boroviczeny, O.; Koletzko, B.; et al. Pre–and postnatal exposure to perfluorinated compounds (PFCs). Environ. Sci. Technol. 2010, 44, 7123–7129. [Google Scholar] [CrossRef]
- Syme, M.R.; Paxton, J.W.; Keelan, J.A. Drug transfer and metabolism by the human placenta. Clin. Pharmacokinet. 2004, 43, 487–514. [Google Scholar] [CrossRef]
- Pan, Y.; Zhu, Y.; Zheng, T.; Cui, Q.; Buka, S.L.; Zhang, B.; Guo, Y.; Xia, W.; Yeung, L.W.Y.; Li, Y.; et al. Novel chlorinated polyfluorinated ether sulfonates and legacy per–/polyfluoroalkyl substances: Placental transfer and relationship with serum albumin and glomerular filtration rate. Environ. Sci. Technol. 2017, 51, 634–644. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.A.; Hungerbuehler, K. Exploring the use of molecular docking to identify bioaccumulative perfluorinated alkyl acids (PFAAs). Environ. Sci. Technol. 2015, 49, 12306–12314. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Meng, L.; Ma, D.; Cao, H.; Liang, Y.; Liu, H.; Wang, Y.; Jiang, G. The occurrence of PFAS in human placenta and their binding abilities to human serum albumin and organic anion transporter 4. Environ. Pollut. 2021, 273, 116460. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Shi, X.; Hu, Q.; Zhao, B.; Huang, M. Structural evidence of perfluorooctane sulfonate transport by human serum albumin. Chem. Res. Toxicol. 2012, 25, 990–992. [Google Scholar] [CrossRef]
- Chen, F.; Yin, S.; Kelly, B.C.; Liu, W. Isomer-specific transplacental transfer of perfluoroalkyl acids: Results from a survey of paired maternal, cord sera, and placentas. Environ. Sci. Technol. 2017, 51, 5756–5763. [Google Scholar] [CrossRef]
- Li, M.; Zeng, X.W.; Qian, Z.M.; Vaughn, M.G.; Sauvé, S.; Paul, G.; Lin, S.; Lu, L.; Hu, L.-W.; Yang, B.-Y.; et al. Isomers of perfluorooctanesulfonate (PFOS) in cord serum and birth outcomes in China: Guangzhou Birth Cohort Study. Environ. Int. 2017, 102, 1–8. [Google Scholar] [CrossRef]
- Carter, D.C.; Ho, J.X. Structure of serum albumin. Adv. Protein Chem. 1994, 45, 153–203. [Google Scholar]
- Alesio, J.L.; Slitt, A.; Bothun, G.D. Critical new insights into the binding of poly–and perfluoroalkyl substances (PFAS) to albumin protein. Chemosphere 2022, 287, 131979. [Google Scholar] [CrossRef]
- Goodfellow, I.; Bengio, Y.; Courville, A. Deep Learning; MIT Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Miller, T.H.; Baz–Lomba, J.A.; Harman, C.; Reid, M.J.; Owen, S.F.; Bury, N.R.; Thomas, K.V.; Barron, L.P. The first attempt at non–linear in silico prediction of sampling rates for polar organic chemical integrative samplers (POCIS). Environ. Sci. Technol. 2016, 50, 7973–7981. [Google Scholar] [CrossRef]
- Wu, C.; Li, B.; Xiong, N. An Effective Machine Learning Scheme to Analyze and Predict the Concentration of Persistent Pollutants in the Great Lakes. IEEE Access 2021, 9, 52252–52265. [Google Scholar] [CrossRef]
- Siami–Namini, S.; Tavakoli, N.; Namin, A.S. A comparison of ARIMA and LSTM in forecasting time series. In Proceedings of the 2018 17th IEEE International Conference on Machine Learning and Applications (ICMLA), Orlando, FL, USA, 17–20 December 2018; IEEE: Piscataway, NJ, USA, 2018; pp. 1394–1401. [Google Scholar]
- Kibbey, T.C.; Jabrzemski, R.; O’Carroll, D.M. Predicting the relationship between PFAS component signatures in water and non–water phases through mathematical transformation: Application to machine learning classification. Chemosphere 2021, 282, 131097. [Google Scholar] [CrossRef] [PubMed]
- Young, W.; Wiggins, S.; Limm, W.; Fisher, C.M.; DeJager, L.; Genualdi, S. Analysis of Per–and Poly (fluoroalkyl) Substances (PFASs) in Highly Consumed Seafood Products from US Markets. J. Agric. Food Chem. 2022, 70, 13545–13553. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.B. Contribution of diet and other factors to the levels of selected polyfluorinated compounds: Data from NHANES 2003–2008. Int. J. Hyg. Environ. Health. 2014, 217, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Vestergren, R.; Berger, U.; Glynn, A.; Cousins, I.T. Dietary exposure to perfluoroalkyl acids for the Swedish population in 1999, 2005 and 2010. Environ. Int. 2012, 49, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Modaresi, S.M.S.; Wei, W.; Emily, M.; DaSilva, N.A.; Slitt, A.L. Per–and polyfluoroalkyl substances (PFAS) augment adipogenesis and shift the proteome in murine 3T3–L1 adipocytes. Toxicology 2022, 465, 153044. [Google Scholar] [CrossRef]
- Gützkow, K.B.; Haug, L.S.; Thomsen, C.; Sabaredzovic, A.; Becher, G.; Brunborg, G. Placental transfer of perfluorinated compounds is selective–a Norwegian Mother and Child sub–cohort study. Int. J. Hyg. Environ. Health. 2012, 215, 216–219. [Google Scholar] [CrossRef]
- Beesoon, S.; Webster, G.M.; Shoeib, M.; Harner, T.; Benskin, J.P.; Martin, J.W. Isomer profiles of perfluorochemicals in matched maternal, cord, and house dust samples: Manufacturing sources and transplacental transfer. Environ. Health. Perspect. 2011, 119, 1659–1664. [Google Scholar] [CrossRef]
- Kim, S.K.; Lee, K.T.; Kang, C.S.; Tao, L.; Kannan, K.; Kim, K.R.; Kim, C.K.; Lee, J.S.; Park, P.S.; Yoo, Y.W.; et al. Distribution of perfluorochemicals between sera and milk from the same mothers and implications for prenatal and postnatal exposures. Environ. Pollut. 2011, 159, 169–174. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kim, M.K.; Bae, J.; Yang, J.H. Concentrations of perfluoroalkyl compounds in maternal and umbilical cord sera and birth outcomes in Korea. Chemosphere 2013, 90, 1603–1609. [Google Scholar] [CrossRef]
- Olsen, G.W.; Burris, J.M.; Ehresman, D.J.; Froehlich, J.W.; Seacat, A.M.; Butenhoff, J.L.; Zobel, L.R. Half–life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ. Health. Perspect. 2007, 115, 1298–1305. [Google Scholar] [CrossRef]
- Kärrman, A.; Ericson, I.; van Bavel, B.; Darnerud, P.O.; Aune, M.; Glynn, A.; Lignell, S.; Lindström, G. Exposure of perfluorinated chemicals through lactation: Levels of matched human milk and serum and a temporal trend, 1996–2004, in Sweden. Environ. Health. Perspect. 2007, 115, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, C.; Haug, L.S.; Stigum, H.; Frøshaug, M.; Broadwell, S.L.; Becher, G. Changes in concentrations of perfluorinated compounds, polybrominated diphenyl ethers, and polychlorinated biphenyls in Norwegian breast–milk during twelve months of lactation. Environ. Sci. Technol. 2010, 44, 9550–9556. [Google Scholar] [CrossRef] [PubMed]
- Haug, L.S.; Huber, S.; Becher, G.; Thomsen, C. Characterisation of human exposure pathways to perfluorinated compounds—Comparing exposure estimates with biomarkers of exposure. Environ. Int. 2011, 37, 687–693. [Google Scholar] [CrossRef] [PubMed]
- LaKind, J.S.; Naiman, J.; Verner, M.A.; Lévêque, L.; Fenton, S. Exposure of perfluorinated chemicals through lactation: Levels of matched human milk and serum and a temporal trend, 1996–2004, in Sweden. Environ. Res. 2022, 115, 226–230. [Google Scholar]
- Papadopoulou, E.; Sabaredzovic, A.; Namork, E.; Nygaard, U.C.; Granum, B.; Haug, L.S. Exposure of Norwegian toddlers to perfluoroalkyl substances (PFAS): The association with breastfeeding and maternal PFAS concentrations. Environ. Int. 2016, 94, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Pan, Y.; Wei, X.; Dai, J. Temporal trends in prenatal exposure (1998–2018) to emerging and legacy per–and polyfluoroalkyl substances (PFASs) in cord plasma from the Beijing Cord Blood Bank, China. Environ. Sci. Technol. 2020, 54, 12850–12859. [Google Scholar] [CrossRef]
- Aimuzi, R.; Luo, K.; Chen, Q.; Wang, H.; Feng, L.; Ouyang, F.; Zhang, J. Perfluoroalkyl and polyfluoroalkyl substances and fetal thyroid hormone levels in umbilical cord blood among newborns by prelabor caesarean delivery. Environ. Int. 2019, 130, 104929. [Google Scholar] [CrossRef]
- Xie, S.; Wang, T.; Liu, S.; Jones, K.C.; Sweetman, A.J.; Lu, Y. Industrial source identification and emission estimation of perfluorooctane sulfonate in China. Environ. Int. 2013, 52, 1–8. [Google Scholar] [CrossRef]
- Sheng, N.; Wang, J.; Guo, Y.; Wang, J.; Dai, J. Interactions of perfluorooctanesulfonate and 6: 2 chlorinated polyfluorinated ether sulfonate with human serum albumin: A comparative study. Chem. Res. Toxicol. 2020, 33, 1478–1486. [Google Scholar] [CrossRef]
- Nyberg, E.; Awad, R.; Bignert, A.; Ek, C.; Sallsten, G.; Benskin, J.P. Inter-individual, inter-city, and temporal trends of per-and polyfluoroalkyl substances in human milk from Swedish mothers between 1972 and 2016. Environ. Sci. Process Impacts 2020, 20, 1136–1147. [Google Scholar] [CrossRef]
- Eriksson, U.; Mueller, J.F.; Toms, L.M.L.; Hobson, P.; Kärrman, A. Temporal trends of PFSAs, PFCAs and selected precursors in Australian serum from 2002 to 2013. Environ. Pollut. 2020, 220, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Sun, X.; Xu, J.; Tan, H.; Zeng, E.Y.; Chen, D. Transplacental transfer of environmental chemicals: Roles of molecular descriptors and placental transporters. Environ. Sci. Technol. 2020, 55, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Zhuang, T.; Liu, X.; Fu, J.; Zhang, J.; Fu, J.; Wang, L.; Zhang, A.; Liang, Y.; Song, M.; et al. Prenatal exposure to per–and polyfluoroalkyl substances (PFASs) and association between the placental transfer efficiencies and dissociation constant of serum proteins–PFAS complexes. Environ. Sci. Technol. 2019, 53, 6529–6538. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Shao, L.X.; Liu, Y.; Cui, S.W.; Wang, X.; Lu, G.L.; Wang, X.; Jin, Y.H. Target analysis and suspect screening of per–and polyfluoroalkyl substances in paired samples of maternal serum, umbilical cord serum, and placenta near fluorochemical plants in Fuxin, China. Chemosphere 2022, 307, 135731. [Google Scholar] [CrossRef] [PubMed]
- Eryasa, B.; Grandjean, P.; Nielsen, F.; Valvi, D.; Zmirou–Navier, D.; Sunderland, E.; Weihe, P.; Oulhote, Y. Physico–chemical properties and gestational diabetes predict transplacental transfer and partitioning of perfluoroalkyl substances. Environ. Int. 2019, 130, 104874. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, K.; Zheng, P.; Yin, S.; Jin, H.; Bai, X.; Li, Y.; Zheng, J.; Dai, Y.; Zhao, M.; et al. Prenatal exposure and transplacental transfer of perfluoroalkyl substance isomers in participants from the upper and lower reaches of the Yangtze River. Environ. Pollut. 2021, 270, 116202. [Google Scholar] [CrossRef]
- Li, Y.; Yu, N.; Du, L.; Shi, W.; Yu, H.; Song, M.; Wei, S. Transplacental transfer of per–and polyfluoroalkyl substances identified in paired maternal and cord sera using suspect and nontarget screening. Environ. Sci. Technol. 2020, 54, 3407–3416. [Google Scholar] [CrossRef]
- Jones, P.D.; Hu, W.; De Coen, W.; Newsted, J.L.; Giesy, J.P. Binding of perfluorinated fatty acids to serum proteins. Environ. Toxicol. Chem. 2003, 22, 2639–2649. [Google Scholar] [CrossRef]
- Qin, P.; Liu, R.; Pan, X.; Fang, X.; Mou, Y. Impact of carbon chain length on binding of perfluoroalkyl acids to bovine serum albumin determined by spectroscopic methods. J. Agric. Food. Chem. 2010, 58, 5561–5567. [Google Scholar] [CrossRef]
- Bischel, H.N.; MacManus-Spencer, L.A.; Zhang, C.; Luthy, R.G. Strong associations of short-chain perfluoroalkyl acids with serum albumin and investigation of binding mechanisms. Environ. Toxicol. Chem. 2011, 30, 2423–2430. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Q.; Cai, Y.; Yuan, R.; Wang, F.; Zhou, B. Investigation of the interaction mechanism of perfluoroalkyl carboxylic acids with human serum albumin by spectroscopic methods. Int. J. Environ. Res. Public Health 2020, 17, 1319. [Google Scholar] [CrossRef] [PubMed]
- Salvalaglio, M.; Muscionico, I.; Cavallotti, C. Determination of energies and sites of binding of PFOA and PFOS to human serum albumin. J Phys Chem B. 2010, 114, 14860–14874. [Google Scholar] [CrossRef] [PubMed]
- Delva–Wiley, J.; Jahan, I.; Newman, R.H.; Zhang, L.; Dong, M. Computational analysis of the binding mechanism of GenX and HSA. ACS Omega 2021, 6, 29166–29170. [Google Scholar] [CrossRef]
- Li, W.; Hu, Y.; Bischel, H.N. In–vitro and in–silico assessment of per–and polyfluoroalkyl substances (PFAS) in aqueous film–forming foam (AFFF) binding to human serum albumin. Toxics 2021, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Chi, Q.; Li, Z.; Huang, J.; Ma, J.; Wang, X. Interactions of perfluorooctanoic acid and perfluorooctanesulfonic acid with serum albumins by native mass spectrometry, fluorescence and molecular docking. Chemosphere 2018, 198, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, H.; Kang, Y.; Cao, J. Effects of perfluorooctane sulfonate on the conformation and activity of bovine serum albumin. J. Photochem. Photobiol. B 2016, 159, 66–73. [Google Scholar] [CrossRef]
- Singam, E.R.A.; Tachachartvanich, P.; Fourches, D.; Soshilov, A.; Hsieh, J.C.; La Merrill, M.A.; Smith, M.T.; Durkin, K.A. Structure–based virtual screening of perfluoroalkyl and polyfluoroalkyl substances (PFASs) as endocrine disruptors of androgen receptor activity using molecular docking and machine learning. Environ. Res. 2020, 190, 109920. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H.; Kang, Y.; Fei, Z.; Cao, J. The interaction of perfluorooctane sulfonate with hemoglobin: Influence on protein stability. Chem. Biol. Interact. 2016, 254, 1–10. [Google Scholar] [CrossRef]
- Cheng, W.; Ng, C.A. Predicting relative protein affinity of novel per–and polyfluoroalkyl substances (PFASs) by an efficient molecular dynamics approach. Environ. Sci. Technol. 2018, 52, 7972–7980. [Google Scholar] [CrossRef]
- Zhang, L.; Ren, X.M.; Guo, L.H. Structure–based investigation on the interaction of perfluorinated compounds with human liver fatty acid binding protein. Environ. Sci. Technol. 2013, 47, 11293–11301. [Google Scholar] [CrossRef]
- Ren, X.M.; Qin, W.P.; Cao, L.Y.; Zhang, J.; Yang, Y.; Wan, B.; Guo, L.H. Binding interactions of perfluoroalkyl substances with thyroid hormone transport proteins and potential toxicological implications. Toxicology 2016, 366, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Le, S.T.; Kibbey, T.C.; Weber, K.P.; Glamore, W.C.; O’Carroll, D.M. A group–contribution model for predicting the physicochemical behavior of PFAS components for understanding environmental fate. Sci. Total. Environ. 2021, 764, 142882. [Google Scholar] [CrossRef] [PubMed]
- Kibbey, T.C.; Jabrzemski, R.; O’Carroll, D.M. Supervised machine learning for source allocation of per–and polyfluoroalkyl substances (PFAS) in environmental samples. Chemosphere 2020, 252, 126593. [Google Scholar] [CrossRef] [PubMed]
- Tropsha, A.; Golbraikh, A. Predictive QSAR modeling workflow, model applicability domains, and virtual screening. Curr. Pharm. Des. 2007, 13, 3494–3504. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, M.; Madden, J.C.; Rowe, P.H.; Cronin, M.T.D. Structure–based modelling in reproductive toxicology:(Q) SARs for the placental barrier. SAR QSAR Environ. Res. 2007, 18, 57–76. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.; Ali, Z.A.; Wong, B.M. Harnessing Semi–Supervised Machine Learning to Automatically Predict Bioactivities of Per–and Polyfluoroalkyl Substances (PFASs). Environ. Sci. Technol. Lett. 2022. [Google Scholar] [CrossRef]
- Cheng, W.; Ng, C.A. Using machine learning to classify bioactivity for 3486 per–and polyfluoroalkyl substances (PFASs) from the OECD list. Environ. Sci. Technol. 2019, 53, 13970–13980. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.T.; Kuntz, D.; Wilson, A.K. Molecular Screening and Toxicity Estimation of 260,000 Perfluoroalkyl and Polyfluoroalkyl Substances (PFASs) through Machine Learning. J. Chem. Inf. Model. 2022, 62, 4569–4578. [Google Scholar] [CrossRef]
- Feinstein, J.; Sivaraman, G.; Picel, K.; Peters, B.; Vázquez–Mayagoitia, Á.; Ramanathan, A.; MacDonell, M.; Foster, I.; Yan, E. Uncertainty–informed deep transfer learning of perfluoroalkyl and polyfluoroalkyl substance toxicity. J. Chem. Inf. Model. 2021, 61, 5793–5803. [Google Scholar] [CrossRef]
- Eguchi, A.; Hanazato, M.; Suzuki, N.; Matsuno, Y.; Todaka, E.; Mori, C. Maternal–fetal transfer rates of PCBs, OCPs, PBDEs, and dioxin–like compounds predicted through quantitative structure–activity relationship modeling. Environ. Sci. Pollut. Res. 2018, 25, 7212–7222. [Google Scholar] [CrossRef]
- Abrahamsson, D.; Siddharth, A.; Robinson, J.F.; Soshilov, A.; Elmore, S.; Cogliano, V.; Ng, C.; Khan, E.; Ashton, R.; Chiu, W.A.; et al. Modeling the transplacental transfer of small molecules using machine learning: A case study on per-and polyfluorinated substances (PFAS). J. Exp. Sci. Environ. Epidemiol. 2022, 32, 808–819. [Google Scholar] [CrossRef] [PubMed]

| PFASs | Target Proteins | Research Content | Software | Ref. |
|---|---|---|---|---|
| PFOA PFOS | HSA | Structure and energies of the binding sites | AutoDock 3.0 package | [79] |
| PFOS | HSA | Binding sites, binding molar ratio | – | [39] |
| PFBA, PFHxA, PFOA, PFDA | HSA | Binding mechanism Binding affinity | AutoDock Vina, MGLTools, Discovery Studio 3.5 | [78] |
| PFOS, GenX | HSA | Binding sites | AutoDock 4 | [80] |
| PFBA, PFPeA, PFHxA, PFHpA, PFOA, PFNA, PFDA, PFUdA, PFTrA, PFTeA, PFPrS, PFBS, PFPeS, PFHxS, PFHpS, PFOS, PFNS, PFDS, FOSA, N–MeFOSAA, N–EtFOSAA, 4:2 FTS, 6:2 FTS, 8:2 FTS, HFPO–DA (GenX) | HSA | Binding affinity | AutoDock Vina (v 1.1.2) | [81] |
| PFOA, PFOS | SA | Binding sites | AutoDock | [82] |
| PFOS | BSA | Binding sites | AutoDock 4.2.3 | [83] |
| 29 PFASs | AR | Binding affinity | LigPrep, Glide | [84] |
| PFOS | Hb | Effects on the stability and conformation of Hb, binding sites | Autodock 4.2.3 | [85] |
| PFBA, PFPA, PFHxA, PFHpA, PFOA, PFNA, PFBS, PFHxS, PFOS, EEA, GenX, ADONA, 2m–PFOA, F–53, F–53B | hLFABP, rLFABP | Relative binding affinity | Autodock Vina (v1.1.2) | [86] |
| PFBA, PFPA, PFHxA, PFHpA, PFOA, PFNA, PFDA, PFUnA, PFDoA, PFTA, PFHxDA, PFOcDA, PFBS, PFHxS, PFOS, 6:2 FTOH, 8:2 FTOH | Liver fatty acid binding protein | Kd structure changes, binding strength | AutoDock 4.2 | [87] |
| PFBA, PFHxA, PFHpA, PFOA, PFNA, PFDA, PFUnA, PFDoA, PFOcDA, PFTA, PFBS, PFHxS, PFOS, 6:2 FTOH, 8:2 FTOH, 10:2 FTOH | Thyroid hormone transport proteins | Relative potency Kd | AutoDock 4.2 | [88] |
| Research Content | Dataset | Model | Validation | Significance | Ref. |
|---|---|---|---|---|---|
| To automatically predict the biological activity of PFASs in various human biological targets | The CF dataset, the C3F6 dataset | QSAR, unsupervised/semi-supervised machine learning models | Structural alerts were used to cross-check the validity of the predicted substructures | The first semi-supervised machine learning study of structure—activity relationships for predicting possible bioactivities in a variety of PFAS species | [93] |
| To predict the bioactivity of PFASs | The bioactivity information on 1012 PFASs for 26 bioassays | Logistic regression, random forests, multitask neural networks, graph convolutional models, and weave models | 30% of data were used to tune hyperparameters and evaluate models | To provide valuable insight into the behavior of those chemicals and thus facilitate high-throughput screening and prioritization | [94] |
| To find alternatives for the most commonly used PFAS molecules | The curated EPA dataset consists of 7751 PFAS molecules | Junction tree variational autoencoder (JTVAE) | No validation set but well processed | 22 promising new PFAS substitutes were identified | [95] |
| To classify the active and inactive PFASs for AR | The resulting dataset contained 568 active and 3934 inactive chemicals | Logistic regression, random forest, support-vector machine, k-nearest neighbors | A grid search cross-validation method was used to tune the parameters | 29 PFASs had strong potential for activity against the AR | [84] |
| To predict acute toxicity of PFAS compounds | LDToxDB of 13,329 unique compounds of any type with oral rat LD50 measurements | RF regressor, Gaussian process (GP) regression, deep neural network, graph convolutional neural network | Five-fold cross-validation | Predicting toxicity for PFASs with a defined chemical structure | [96] |
| To predict the maternal–fetal transfer rates of the POPs | The Chiba University Hospital’s Delivery Unit and various other obstetric units in Japan | Principal component analysis (PCA), multiple linear regression (MLR), partial least squares regression (PLS), random forest regression (RF) | Ten-fold cross-validation | Maternal transfer rate and molecular weight, and/or lipophilicity, might be important parameters for the maternal–fetal transport of organohalogen compounds | [97] |
| To develop a computational approach that can be used to evaluate the extent to which small molecules can cross the placenta and partition in the cord blood | From the literature | Support-vector machine (SVM), a random forest (RF), and an artificial neural network (ANN) | Shuffle-split cross-validation with an 80/20 split | These observations have important public health implications | [98] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Bao, J.; Liu, Y.; Wang, X.; Qu, W. A Review on Per- and Polyfluoroalkyl Substances in Pregnant Women: Maternal Exposure, Placental Transfer, and Relevant Model Simulation. Toxics 2023, 11, 430. https://doi.org/10.3390/toxics11050430
Wu Y, Bao J, Liu Y, Wang X, Qu W. A Review on Per- and Polyfluoroalkyl Substances in Pregnant Women: Maternal Exposure, Placental Transfer, and Relevant Model Simulation. Toxics. 2023; 11(5):430. https://doi.org/10.3390/toxics11050430
Chicago/Turabian StyleWu, Yuqing, Jia Bao, Yang Liu, Xin Wang, and Wene Qu. 2023. "A Review on Per- and Polyfluoroalkyl Substances in Pregnant Women: Maternal Exposure, Placental Transfer, and Relevant Model Simulation" Toxics 11, no. 5: 430. https://doi.org/10.3390/toxics11050430
APA StyleWu, Y., Bao, J., Liu, Y., Wang, X., & Qu, W. (2023). A Review on Per- and Polyfluoroalkyl Substances in Pregnant Women: Maternal Exposure, Placental Transfer, and Relevant Model Simulation. Toxics, 11(5), 430. https://doi.org/10.3390/toxics11050430









