Neurobehavioral Responses and Toxic Brain Reactions of Juvenile Rats Exposed to Iprodione and Chlorpyrifos, Alone and in a Mixture
Abstract
1. Introduction
2. Materials and Methods
2.1. Tested Compounds
2.2. Animals and Experimental Design
2.3. Neurobehavioral Assessments
2.3.1. Open Field Test
2.3.2. Elevated plus Maze
2.3.3. Forced Swimming Test (FST)
2.4. Sampling
2.5. Biochemical Assessment of Brain Tissue Homogenate
2.5.1. Assessment of Brain AChE and Nor-Epinephrine (NE) Levels
2.5.2. Measurement of Oxidative Stress and Apoptosis Biomarkers in Brain Homogenates
2.6. Histopathological Evaluation
2.7. Data Analysis
3. Results
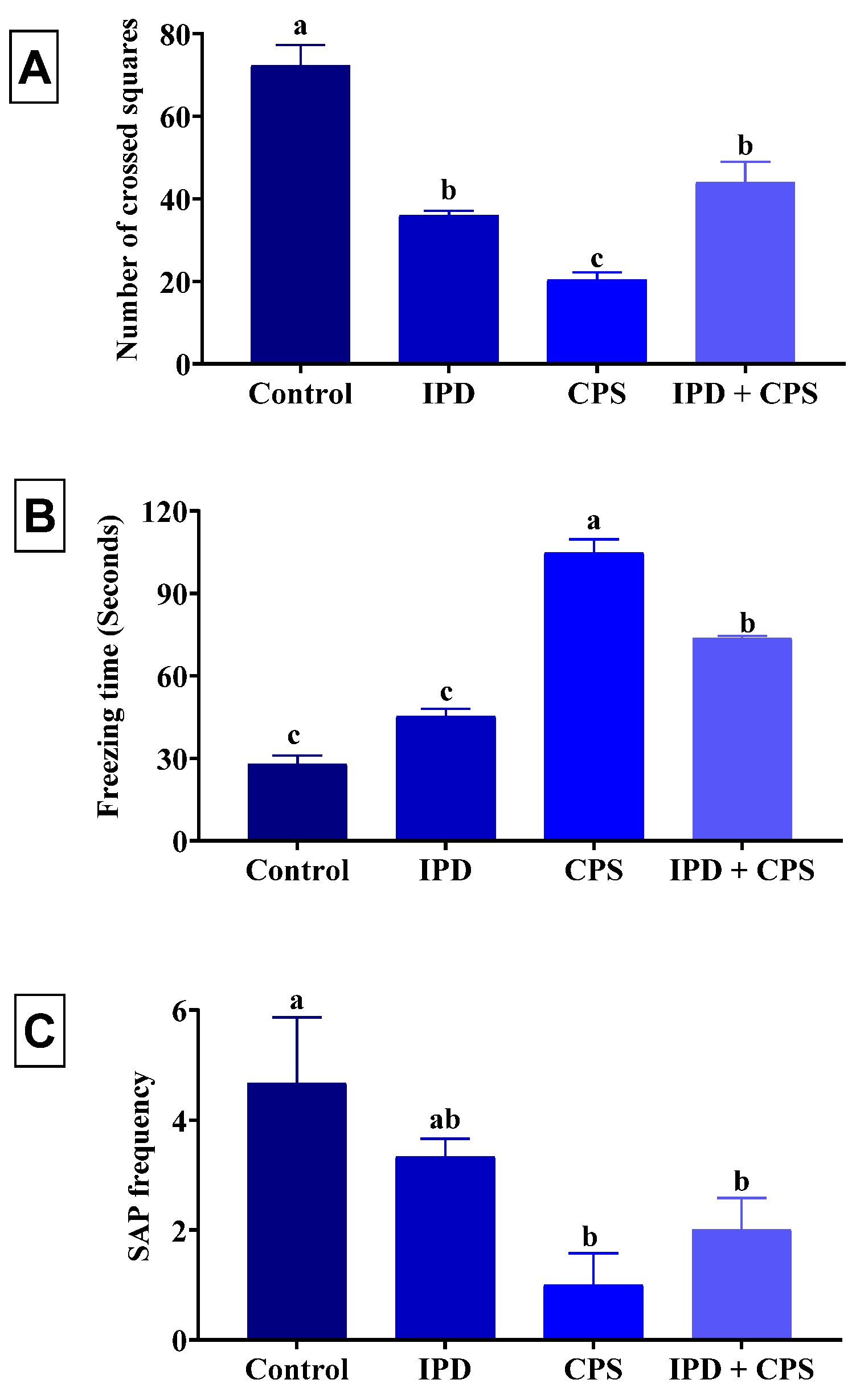
3.1. Effects on Locomotors Activity and Anxiety in OFT
3.2. Effects on Depression in the FST
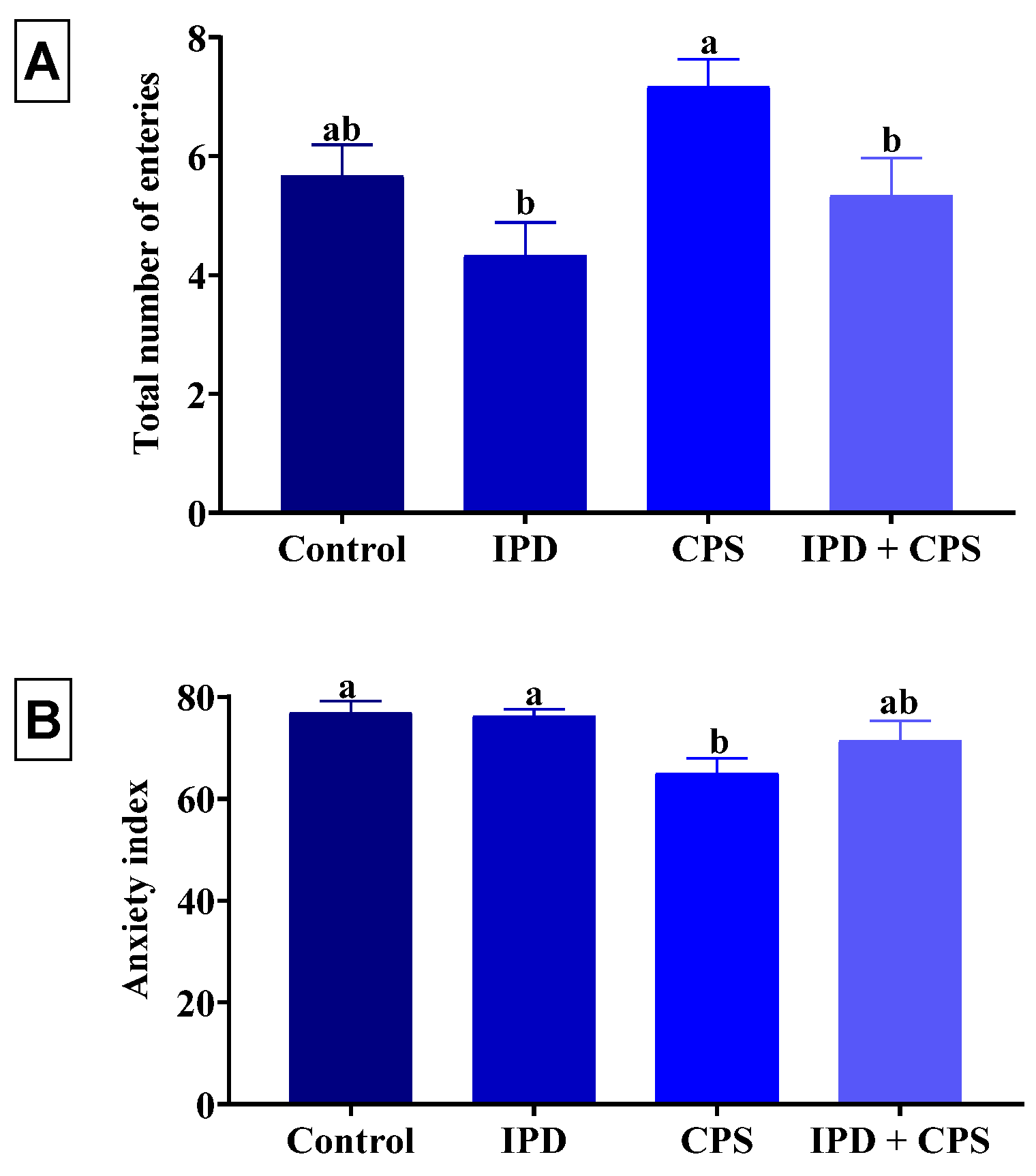
3.3. Effects on Anxiety in EPM
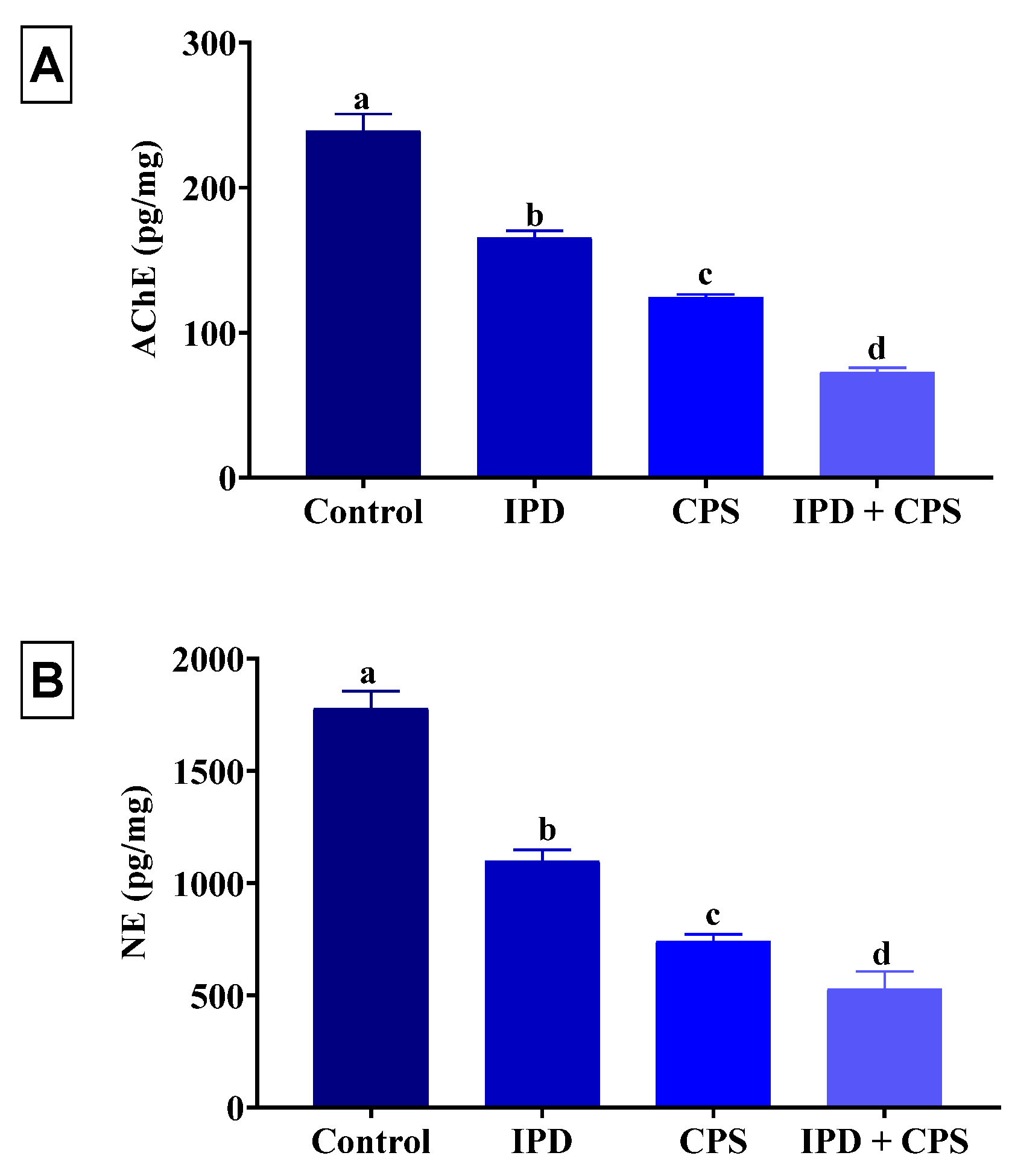
3.4. Effects of Brain AChE and NE Neurotransmitter
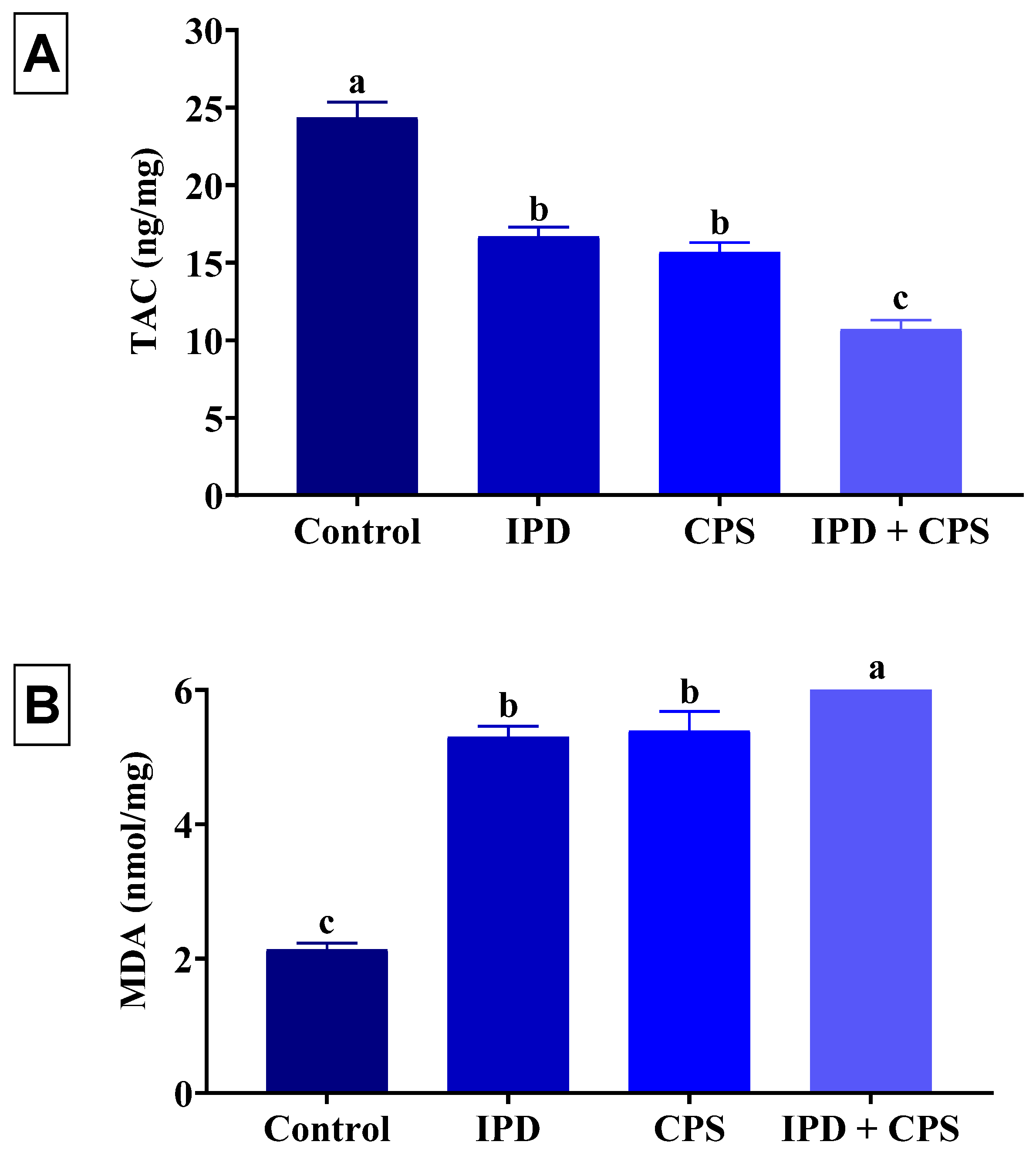
3.5. Effects on Brain Oxidative Status
3.6. Histopathological Findings
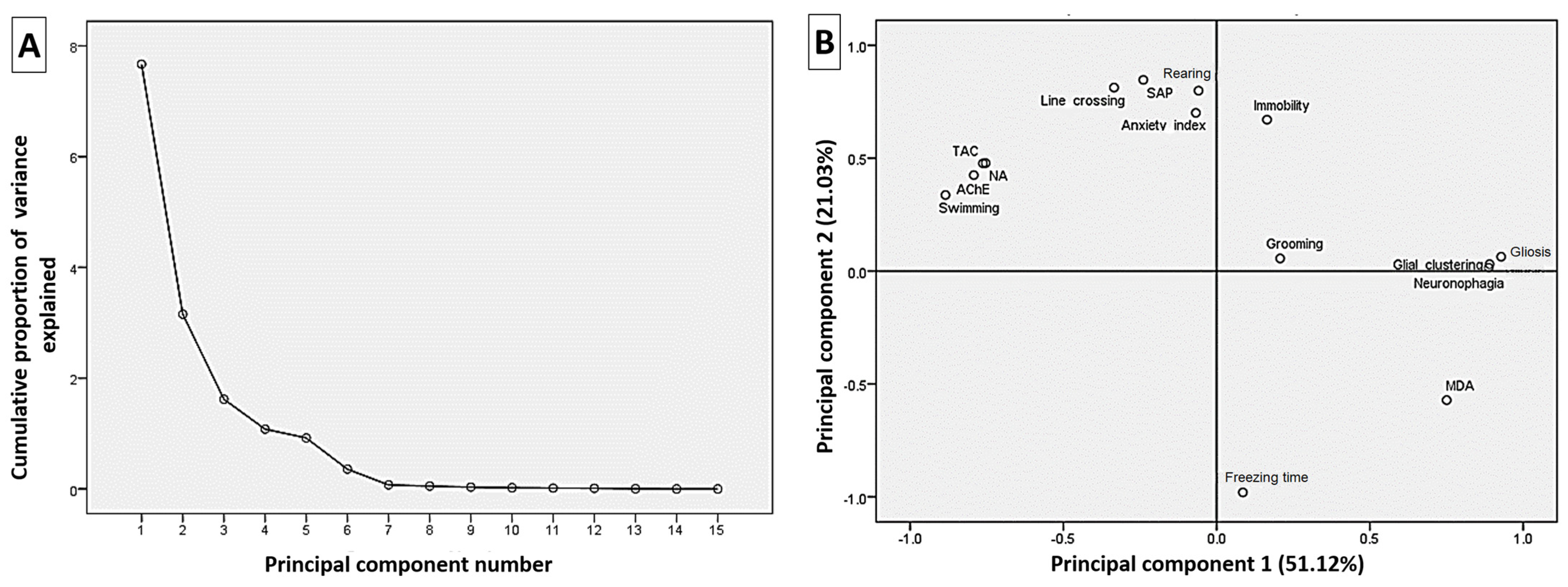
3.7. Correlation Analysis of the Estimated Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Park, W.; An, G.; Lim, W.; Song, G. Exposure to iprodione induces ROS production and mitochondrial dysfunction in porcine trophectoderm and uterine luminal epithelial cells, leading to implantation defects during early pregnancy. Chemosphere 2022, 307, 135894. [Google Scholar] [CrossRef]
- Sharma, A.; Shukla, A.; Attri, K.; Kumar, M.; Kumar, P.; Suttee, A.; Singh, G.; Barnwal, R.P.; Singla, N. Global trends in pesticides: A looming threat and viable alternatives. Ecotoxicol. Environ. Saf. 2020, 201, 110812. [Google Scholar] [CrossRef]
- Abd-Elhakim, Y.M.; El-Sharkawy, N.I.; Mohammed, H.H.; Ebraheim, L.L.; Shalaby, M.A. Camel milk rescues neurotoxic impairments induced by fenpropathrin via regulating oxidative stress, apoptotic, and inflammatory events in the brain of rats. Food Chem. Toxicol. 2020, 135, 111055. [Google Scholar] [CrossRef]
- Ma, M.; Chen, C.; Yang, G.; Wang, Y.; Wang, T.; Li, Y.; Qian, Y. Combined anti-androgenic effects of mixtures of agricultural pesticides using in vitro and in silico methods. Ecotoxicol. Environ. Saf. 2019, 186, 109652. [Google Scholar] [CrossRef]
- Abd El-Hakim, Y.M.; Mohamed, W.A.; El-Metwally, A.E. Spirulina platensis attenuates furan reprotoxicity by regulating oxidative stress, inflammation, and apoptosis in testis of rats. Ecotoxicol. Environ. Saf. 2018, 161, 25–33. [Google Scholar] [CrossRef]
- Visalakshmi, V.; Raju, M.; Rao, A.U.; Kumar, K.M.; Satyanarayana, N.H. Compatibility and efficacy of insecticide and fungicide combinations on major pests and sheath blight of paddy. Nat. Environ. Pollut. Technol. 2016, 15, 233. [Google Scholar]
- Ranjan, A.; Jindal, T. Overview of organophosphate compounds. In Toxicology of Organophosphate Poisoning: New Insights; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1–25. [Google Scholar]
- Perez-Fernandez, C.; Morales-Navas, M.; Aguilera-Sáez, L.M.; Abreu, A.C.; Guardia-Escote, L.; Fernández, I.; Garrido-Cárdenas, J.A.; Colomina, M.T.; Giménez, E.; Sánchez-Santed, F. Medium and long-term effects of low doses of Chlorpyrifos during the postnatal, preweaning developmental stage on sociability, dominance, gut microbiota and plasma metabolites. Environ. Res. 2020, 184, 109341. [Google Scholar] [CrossRef]
- Foong, S.Y.; Ma, N.L.; Lam, S.S.; Peng, W.; Low, F.; Lee, B.H.; Alstrup, A.K.; Sonne, C. A recent global review of hazardous chlorpyrifos pesticide in fruit and vegetables: Prevalence, remediation and actions needed. J. Hazard. Mater. 2020, 400, 123006. [Google Scholar] [CrossRef]
- European Food Safety Authority. The 2016 European Union report on pesticide residues in food. EFSA J. 2018, 16, e05348. [Google Scholar]
- Angioni, A.; Dedola, F.; Garau, A.; Sarais, G.; Cabras, P.; Caboni, P. Chlorpyrifos residues levels in fruits and vegetables after field treatment. J. Environ. Sci. Health Part B 2011, 46, 544–549. [Google Scholar]
- Burke, R.D.; Todd, S.W.; Lumsden, E.; Mullins, R.J.; Mamczarz, J.; Fawcett, W.P.; Gullapalli, R.P.; Randall, W.R.; Pereira, E.F.; Albuquerque, E.X. Developmental neurotoxicity of the organophosphorus insecticide chlorpyrifos: From clinical findings to preclinical models and potential mechanisms. J. Neurochem. 2017, 142, 162–177. [Google Scholar] [CrossRef]
- Xu, M.-Y.; Wang, P.; Sun, Y.-J.; Wu, Y.-J. Disruption of Kidney Metabolism in Rats after Subchronic Combined Exposure to Low-Dose Cadmium and Chlorpyrifos. Chem. Res. Toxicol. 2018, 32, 122–129. [Google Scholar] [CrossRef]
- Laporte, B.; Gay-Quéheillard, J.; Bach, V.; Villégier, A.-S. Developmental neurotoxicity in the progeny after maternal gavage with chlorpyrifos. Food Chem. Toxicol. 2018, 113, 66–72. [Google Scholar] [CrossRef]
- Abd-Elhakim, Y.M.; El Sharkawy, N.I.; El Bohy, K.M.; Hassan, M.A.; Gharib, H.S.; El-Metwally, A.E.; Arisha, A.H.; Imam, T.S. Iprodione and/or chlorpyrifos exposure induced testicular toxicity in adult rats by suppression of steroidogenic genes and SIRT1/TERT/PGC-1α pathway. Environ. Sci. Pollut. Res. 2021, 28, 56491–56506. [Google Scholar] [CrossRef]
- Hassan, M.A.; El Bohy, K.M.; El Sharkawy, N.I.; Imam, T.S.; El-Metwally, A.E.; Hamed Arisha, A.; Mohammed, H.A.; Abd-Elhakim, Y.M. Iprodione and chlorpyrifos induce testicular damage, oxidative stress, apoptosis and suppression of steroidogenic- and spermatogenic-related genes in immature male albino rats. Andrologia 2021, 53, e13978. [Google Scholar] [CrossRef]
- Lasagna, M.; Ventura, C.; Hielpos, M.S.; Mardirosian, M.N.; Martín, G.; Miret, N.; Randi, A.; Núñez, M.; Cocca, C. Endocrine disruptor chlorpyrifos promotes migration, invasion, and stemness phenotype in 3D cultures of breast cancer cells and induces a wide range of pathways involved in cancer progression. Environ. Res. 2022, 204, 111989. [Google Scholar] [CrossRef]
- Milošević, M.D.; Mašković, P.Z.; Stanković, V.D.; Paunović, M.G.; Mitić, M.N.; Matić, M.M.; Ognjanović, B.I. Protective effects of Viscum album L. leaf extract on chlorpyrifos-induced hepatotoxicity in Wistar rats. J. King Saud Univ. Sci. 2022, 34, 101957. [Google Scholar] [CrossRef]
- Taha, M.A.I.; Badawy, M.E.I.; Abdel-Razik, R.K.; Younis, H.M.; Abo-El-Saad, M.M. Mitochondrial dysfunction and oxidative stress in liver of male albino rats after exposing to sub-chronic intoxication of chlorpyrifos, cypermethrin, and imidacloprid. Pestic. Biochem. Physiol. 2021, 178, 104938. [Google Scholar] [CrossRef]
- Chen, W.-Q.; Zhang, Y.-Z.; Yuan, L.; Li, Y.-F.; Li, J. Neurobehavioral evaluation of adolescent male rats following repeated exposure to chlorpyrifos. Neurosci. Lett. 2014, 570, 76–80. [Google Scholar] [CrossRef]
- Garcia, S.J.; Seidler, F.J.; Slotkin, T.A. Developmental neurotoxicity of chlorpyrifos: Targeting glial cells. Environ. Toxicol. Pharmacol. 2005, 19, 455–461. [Google Scholar] [CrossRef]
- Vidair, C.A. Age dependence of organophosphate and carbamate neurotoxicity in the postnatal rat: Extrapolation to the human. Toxicol. Appl. Pharmacol. 2004, 196, 287–302. [Google Scholar] [CrossRef]
- Eronat, K.; Sağır, D. Protective effects of curcumin and Ganoderma lucidum on hippocampal damage caused by the organophosphate insecticide chlorpyrifos in the developing rat brain: Stereological, histopathological and immunohistochemical study. Acta Histochem. 2020, 122, 621. [Google Scholar] [CrossRef]
- Lee, J.E.; Park, J.H.; Shin, I.C.; Koh, H.C. Reactive oxygen species regulated mitochondria-mediated apoptosis in PC12 cells exposed to chlorpyrifos. Toxicol. Appl. Pharmacol. 2012, 263, 148–162. [Google Scholar] [CrossRef]
- Saulsbury, M.D.; Heyliger, S.O.; Wang, K.; Johnson, D.J. Chlorpyrifos induces oxidative stress in oligodendrocyte progenitor cells. Toxicology 2009, 259, 1–9. [Google Scholar] [CrossRef]
- Aboubakr, M.; Elshafae, S.M.; Abdelhiee, E.Y.; Fadl, S.E.; Soliman, A.; Abdelkader, A.; Abdel-Daim, M.M.; Bayoumi, K.A.; Baty, R.S.; Elgendy, E. Antioxidant and anti-inflammatory potential of thymoquinone and lycopene mitigate the chlorpyrifos-induced toxic neuropathy. Pharmaceuticals 2021, 14, 940. [Google Scholar] [CrossRef]
- Gupta, P.K. Chapter 35—Herbicides and fungicides. In Reproductive and Developmental Toxicology, 3rd ed.; Gupta, R.C., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 665–689. [Google Scholar]
- Bian, Y.; Wang, J.; Liu, F.; Mao, B.; Huang, H.; Xu, J.; Li, X.; Guo, Y. Residue behavior and removal of iprodione in garlic, green garlic, and garlic shoot. J. Sci. Food Agric. 2020, 100, 4705–4713. [Google Scholar] [CrossRef]
- Stensvand, A.; Christiansen, A. Investigation on fungicide residues in greenhouse-grown strawberries. J. Agric. Food Chem. 2000, 48, 917–920. [Google Scholar] [CrossRef]
- González-Rodríguez, R.M.; Rial-Otero, R.; Cancho-Grande, B.; Simal-Gándara, J. Occurrence of fungicide and insecticide residues in trade samples of leafy vegetables. Food Chem. 2008, 107, 1342–1347. [Google Scholar] [CrossRef]
- Omirou, M.; Vryzas, Z.; Papadopoulou-Mourkidou, E.; Economou, A. Dissipation rates of iprodione and thiacloprid during tomato production in greenhouse. Food Chem. 2009, 116, 499–504. [Google Scholar] [CrossRef]
- Wang, X.; Xu, G.; Wang, F.; Sun, H.; Li, Y. Iprodione residues and dissipation rates in tobacco leaves and soil. Bull. Environ. Contam. Toxicol. 2012, 89, 877–881. [Google Scholar] [CrossRef]
- Bletsou, A.A.; Hanafi, A.H.; Dasenaki, M.E.; Thomaidis, N.S. Development of specific LC-ESI-MS/MS methods to determine bifenthrin, lufenuron, and iprodione residue levels in green beans, peas, and chili peppers under Egyptian field conditions. Food Anal. Methods 2013, 6, 1099–1112. [Google Scholar] [CrossRef]
- Wang, Z.; Cang, T.; Qi, P.; Zhao, X.; Xu, H.; Wang, X.; Zhang, H.; Wang, X. Dissipation of four fungicides on greenhouse strawberries and an assessment of their risks. Food Control 2015, 55, 215–220. [Google Scholar] [CrossRef]
- Loutfy, N.; Malhat, F.; Kamel, E.; Saber, A. Residual pattern and dietary intake of iprodione on grapes under Egyptian field conditions: A prelude to risk assessment profile. Hum. Ecol. Risk Assess. Int. J. 2015, 21, 265–279. [Google Scholar] [CrossRef]
- Celeiro, M.; Llompart, M.; Lamas, J.P.; Lores, M.; Garcia-Jares, C.; Dagnac, T. Determination of fungicides in white grape bagasse by pressurized liquid extraction and gas chromatography tandem mass spectrometry. J. Chromatogr. A 2014, 1343, 18–25. [Google Scholar] [CrossRef]
- Rose, G.; Lane, S.; Jordan, R. The fate of fungicide and insecticide residues in Australian wine grape by-products following field application. Food Chem. 2009, 117, 634–640. [Google Scholar] [CrossRef]
- Yang, X.; Luo, J.; Li, S.; Liu, C. Evaluation of nine pesticide residues in three minor tropical fruits from southern China. Food Control 2016, 60, 677–682. [Google Scholar] [CrossRef]
- Sullivan, P.; Clark, J.; Agardy, F.; Rosenfeld, P. Synthetic chemicals in a balanced diet. In Toxic Legacy: Synthetic Toxins in the Food, Water and Air of American Cities; Elsevier: Amsterdam, The Netherlands, 2007; pp. 37–87. [Google Scholar]
- Blystone, C.R.; Lambright, C.S.; Furr, J.; Wilson, V.S.; Gray, L.E., Jr. Iprodione delays male rat pubertal development, reduces serum testosterone levels, and decreases ex vivo testicular testosterone production. Toxicol. Lett. 2007, 174, 74–81. [Google Scholar] [CrossRef]
- Pisani, C.; Voisin, S.; Arafah, K.; Durand, P.; Perrard, M.-H.; Guichaoua, M.-R.; Bulet, P.; Prat, O. Ex vivo assessment of testicular toxicity induced by carbendazim and iprodione, alone or in a mixture. ALTEX Altern. Anim. Exp. 2016, 33, 393–413. [Google Scholar] [CrossRef]
- Wei, Y.; Meng, Y.; Huang, Y.; Liu, Z.; Zhong, K.; Ma, J.; Zhang, W.; Li, Y.; Lu, H. Development toxicity and cardiotoxicity in zebrafish from exposure to iprodione. Chemosphere 2021, 263, 127860. [Google Scholar] [CrossRef]
- Chaufan, G.; Galvano, C.; Nieves, M.; Mudry, M.D.; Ríos de Molina, M.D.C.; Andrioli, N.B. Oxidative Response and Micronucleus Centromere Assay in HEp-2 Cells Exposed to Fungicide Iprodione. Chem. Res. Toxicol. 2019, 32, 745–752. [Google Scholar] [CrossRef]
- United State Environmental Protection Agency (USEPA) Registration Eligibility Decision (RED) Iprodione. Prevention, Pesticides and Toxic Substances. 1998. Available online: http://www.epa.gov/oppsrrd1/REDs/2335.pdf. (accessed on 28 April 2023).
- Lai, Q.; Sun, X.; Li, L.; Li, D.; Wang, M.; Shi, H. Toxicity effects of procymidone, iprodione and their metabolite of 3,5-dichloroaniline to zebrafish. Chemosphere 2021, 272, 129577. [Google Scholar] [CrossRef]
- Morgan, A.M.; El-Aty, A.A. Reproductive toxicity evaluation of Pestban insecticide exposure in male and female rats. Toxicol. Res. 2008, 24, 137–150. [Google Scholar] [CrossRef]
- Fuochi, S.; Galasso, M.E.; Colombo, R.; Giaquinto, D.; De Girolamo, P.; D’Angelo, L. Puberty onset curve in CD (Sprague Dawley) and Long Evans outbred male rats. Lab. Anim. 2022, 56, 471–475. [Google Scholar] [CrossRef]
- Prut, L.; Belzung, C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: A review. European J. Pharmacol. 2003, 463, 3–33. [Google Scholar] [CrossRef]
- Walf, A.A.; Frye, C.A. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat. Protoc. 2007, 2, 322–328. [Google Scholar] [CrossRef]
- Sánchez-Amate, M.; Flores, P.; Sánchez-Santed, F. Effects of chlorpyrifos in the plus-maze model of anxiety. Behav. Pharmacol. 2001, 12, 285–292. [Google Scholar] [CrossRef]
- Porsolt, R.D.; Le Pichon, M.; Jalfre, M. Depression: A new animal model sensitive to antidepressant treatments. Nature 1977, 266, 730–732. [Google Scholar] [CrossRef]
- Koracevic, D.; Koracevic, G.; Djordjevic, V.; Andrejevic, S.; Cosic, V. Method for the measurement of antioxidant activity in human fluids. J. Clin. Pathol. 2001, 54, 356–361. [Google Scholar] [CrossRef]
- Esterbauer, H.; Cheeseman, K.H.; Dianzani, M.U.; Poli, G.; Slater, T.F. Separation and characterization of the aldehydic products of lipid peroxidation stimulated by ADP-Fe2+ in rat liver microsomes. Biochem. J. 1982, 208, 129–140. [Google Scholar] [CrossRef]
- Ruehl-Fehlert, C.; Kittel, B.; Morawietz, G.; Deslex, P.; Keenan, C.; Mahrt, C.R.; Nolte, T.; Robinson, M.; Stuart, B.P.; Deschl, U. Revised guides for organ sampling and trimming in rats and mice–part 1: A joint publication of the RITA and NACAD groups. Exp. Toxicol. Pathol. 2003, 55, 91–106. [Google Scholar] [CrossRef]
- Suvarna, K.S.; Layton, C.; Bancroft, J.D. Bancroft’s Theory and Practice of Histological Techniques E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Prism, G. Version 8; Prism 8 for Windows; GraphPad Software Inc.: San Diego, CA, USA, 2019. [Google Scholar]
- Granato, D.; Santos, J.S.; Escher, G.B.; Ferreira, B.L.; Maggio, R.M. Use of principal component analysis (PCA) and hierarchical cluster analysis (HCA) for multivariate association between bioactive compounds and functional properties in foods: A critical perspective. Trends Food Sci. Technol. 2018, 72, 83–90. [Google Scholar] [CrossRef]
- Van Waes, V.; Enache, M.; Berton, O.; Vinner, E.; Lhermitte, M.; Maccari, S.; Darnaudéry, M. Effect of prenatal stress on alcohol preference and sensitivity to chronic alcohol exposure in male rats. Psychopharmacology 2011, 214, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Mendola, P.; Selevan, S.G.; Gutter, S.; Rice, D. Environmental factors associated with a spectrum of neurodevelopmental deficits. Ment. Retard. Dev. Disabil. Res. Rev. 2002, 8, 188–197. [Google Scholar] [CrossRef]
- Zimcikova, E.; Simko, J.; Karesova, I.; Kremlacek, J.; Malakova, J. Behavioral effects of antiepileptic drugs in rats: Are the effects on mood and behavior detectable in open-field test? Seizure 2017, 52, 35–40. [Google Scholar] [CrossRef]
- Kudavidanage, E.P.; Dissanayake, D.; Keerthirathna, W.R.; Nishshanke, N.L.W.; Peiris, L.D.C. Commercial formulation of chlorpyrifos alters neurological behaviors and fertility. Biology 2020, 9, 49. [Google Scholar] [CrossRef]
- Moser, V.C. Comparisons of the acute effects of cholinesterase inhibitors using a neurobehavioral screening battery in rats. Neurotoxicology Teratol. 1995, 17, 617–625. [Google Scholar] [CrossRef]
- Serrano-Medina, A.; Ugalde-Lizárraga, A.; Bojorquez-Cuevas, M.S.; Garnica-Ruiz, J.; González-Corral, M.A.; García-Ledezma, A.; Pineda-García, G.; Cornejo-Bravo, J.M. Neuropsychiatric Disorders in Farmers Associated with Organophosphorus Pesticide Exposure in a Rural Village of Northwest México. Int. J. Environ. Res. Public Health 2019, 16, 689. [Google Scholar] [CrossRef]
- Harrison, V.; Mackenzie Ross, S. Anxiety and depression following cumulative low-level exposure to organophosphate pesticides. Environ. Res. 2016, 151, 528–536. [Google Scholar] [CrossRef]
- Smolinsky, A.N.; Bergner, C.L.; LaPorte, J.L.; Kalueff, A.V. Analysis of grooming behavior and its utility in studying animal stress, anxiety, and depression. In Mood and Anxiety Related Phenotypes in Mice: Characterization Using Behavioral Tests; Humana: Totowa, NJ, USA, 2009; pp. 21–36. [Google Scholar]
- Holly, K.S.; Orndorff, C.O.; Murray, T.A. MATSAP: An automated analysis of stretch-attend posture in rodent behavioral experiments. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef]
- Chen, W.-Q.; Yuan, L.; Xue, R.; Li, Y.-F.; Su, R.-B.; Zhang, Y.-Z.; Li, J. Repeated exposure to chlorpyrifos alters the performance of adolescent male rats in animal models of depression and anxiety. NeuroToxicology 2011, 32, 355–361. [Google Scholar] [CrossRef]
- Degroot, A.; Treit, D. Dorsal and ventral hippocampal cholinergic systems modulate anxiety in the plus-maze and shock-probe tests. Brain Res. 2002, 949, 60–70. [Google Scholar] [CrossRef]
- Schulz, H.; Desi, I.; Nagymajtenyi, L. Behavioral effects of subchronic intoxication with parathion-methyl in male Wistar rats. Neurotoxicology Teratol. 1990, 12, 125–127. [Google Scholar] [CrossRef]
- Eaton, D.L.; Daroff, R.B.; Autrup, H.; Bridges, J.; Buffler, P.; Costa, L.G.; Coyle, J.; McKhann, G.; Mobley, W.C.; Nadel, L. Review of the toxicology of chlorpyrifos with an emphasis on human exposure and neurodevelopment. Crit. Rev. Toxicol. 2008, 38, 1–125. [Google Scholar] [CrossRef]
- Slotkin, T.A.; Seidler, F.J. Developmental neurotoxicity of organophosphates targets cell cycle and apoptosis, revealed by transcriptional profiles in vivo and in vitro. Neurotoxicology Teratol. 2012, 34, 232–241. [Google Scholar] [CrossRef]
- Nuss, P. Anxiety disorders and GABA neurotransmission: A disturbance of modulation. Neuropsychiatr. Dis. Treat. 2015, 11, 165–175. [Google Scholar] [CrossRef]
- Martin, E.I.; Ressler, K.J.; Binder, E.; Nemeroff, C.B. The neurobiology of anxiety disorders: Brain imaging, genetics, and psychoneuroendocrinology. The Psychiatr. Clin. North Am. 2009, 32, 549–575. [Google Scholar] [CrossRef]
- Bouayed, J.; Rammal, H.; Soulimani, R. Oxidative Stress and Anxiety: Relationship and Cellular Pathways. Oxidative Med. Cell. Longev. 2009, 2, 623654. [Google Scholar] [CrossRef]
- Fedoce, A.D.G.; Ferreira, F.; Bota, R.G.; Bonet-Costa, V.; Sun, P.Y.; Davies, K.J.A. The role of oxidative stress in anxiety disorder: Cause or consequence? Free. Radic. Res. 2018, 52, 737–750. [Google Scholar] [CrossRef]
- Jaouad, B. Relationship between Oxidative Stress and Anxiety: Emerging Role of Antioxidants within Therapeutic or Preventive Approaches. In Anxiety Disorders; Vladimir, K., Ed.; IntechOpen: Rijeka, Croatia, 2011; Chapter 2. [Google Scholar]
- Higley, M.J.; Picciotto, M.R. Neuromodulation by acetylcholine: Examples from schizophrenia and depression. Curr. Opin. Neurobiol. 2014, 29, 88–95. [Google Scholar] [CrossRef]
- Nomani Lafmejani, R.; Zare, K.; Fazollahi, Z.; Roghani, M. Changes of serum level of acetylcholinesterase enzyme in lipopolysaccharide-induced model of depression in mice. J. Basic Clin. Pathophysiol. 2018, 6, 23–26. [Google Scholar]
- Nutt, D.J. Relationship of neurotransmitters to the symptoms of major depressive disorder. J. Clin. Psychiatry 2008, 69 (Suppl. E1), 4–7. [Google Scholar]
- Liu, Y.; Zhao, J.; Guo, W. Emotional Roles of Mono-Aminergic Neurotransmitters in Major Depressive Disorder and Anxiety Disorders. Front. Psychol. 2018, 9, 2201. [Google Scholar] [CrossRef]
- Garabrant, D.H.; Aylward, L.L.; Berent, S.; Chen, Q.; Timchalk, C.; Burns, C.J.; Hays, S.M.; Albers, J.W. Cholinesterase inhibition in chlorpyrifos workers: Characterization of biomarkers of exposure and response in relation to urinary TCPy. J. Expo. Sci. Environ. Epidemiol. 2009, 19, 634–642. [Google Scholar] [CrossRef]
- Moser, V.C.; Chanda, S.M.; Mortensen, S.R.; Padilla, S. Age-and gender-related differences in sensitivity to chlorpyrifos in the rat reflect developmental profiles of esterase activities. Toxicol. Sci. 1998, 46, 211–222. [Google Scholar] [CrossRef]
- Gallegos, C.E.; Bartos, M.; Gumilar, F.; Minetti, A.; Baier, C.J. Behavioral and neurochemical impairments after intranasal administration of chlorpyrifos formulation in mice. Pestic. Biochem. Physiol. 2023, 189, 105315. [Google Scholar] [CrossRef]
- Xu, F.; Chang, X.; Lou, D.; Wu, Q.; Zhou, Z. Chlorpyrifos exposure causes alternation in dopamine metabolism in PC12 cells. Toxicol. Mech. Methods 2012, 22, 309–314. [Google Scholar] [CrossRef]
- Li, J.-W.; Fang, B.; Pang, G.-F.; Zhang, M.; Ren, F.-Z. Age- and diet-specific effects of chronic exposure to chlorpyrifos on hormones, inflammation and gut microbiota in rats. Pestic. Biochem. Physiol. 2019, 159, 68–79. [Google Scholar] [CrossRef]
- Aldridge, J.E.; Seidler, F.J.; Slotkin, T.A. Developmental exposure to chlorpyrifos elicits sex-selective alterations of serotonergic synaptic function in adulthood: Critical periods and regional selectivity for effects on the serotonin transporter, receptor subtypes, and cell signaling. Environ. Health Perspect. 2004, 112, 148–155. [Google Scholar] [CrossRef]
- Radice, S.; Marabini, L.; Gervasoni, M.; Ferraris, M.; Chiesara, E. Adaptation to oxidative stress: Effects of vinclozolin and iprodione on the HepG2 cell line. Toxicology 1998, 129, 183–191. [Google Scholar] [CrossRef]
- Hussein, R.M.; Mohamed, W.R.; Omar, H.A. A neuroprotective role of kaempferol against chlorpyrifos-induced oxidative stress and memory deficits in rats via GSK3β-Nrf2 signaling pathway. Pestic. Biochem. Physiol. 2018, 152, 29–37. [Google Scholar] [CrossRef]
- Yang, Y.Y.; Lai, C.T. Synergistic effect and field control efficacy of the binary mixture of permethrin and chlorpyrifos to brown planthopper (Nilaparvata lugens). J. Asia-Pac. Entomol. 2019, 22, 67–76. [Google Scholar] [CrossRef]
- Xu, L.; Luo, G.; Sun, Y.; Huang, S.; Xu, D.; Xu, G.; Han, Z.; Gu, Z.; Zhang, Y. Multiple down-regulated cytochrome P450 monooxygenase genes contributed to synergistic interaction between chlorpyrifos and imidacloprid against Nilaparvata lugens. J. Asia-Pac. Entomol. 2020, 23, 44–50. [Google Scholar] [CrossRef]


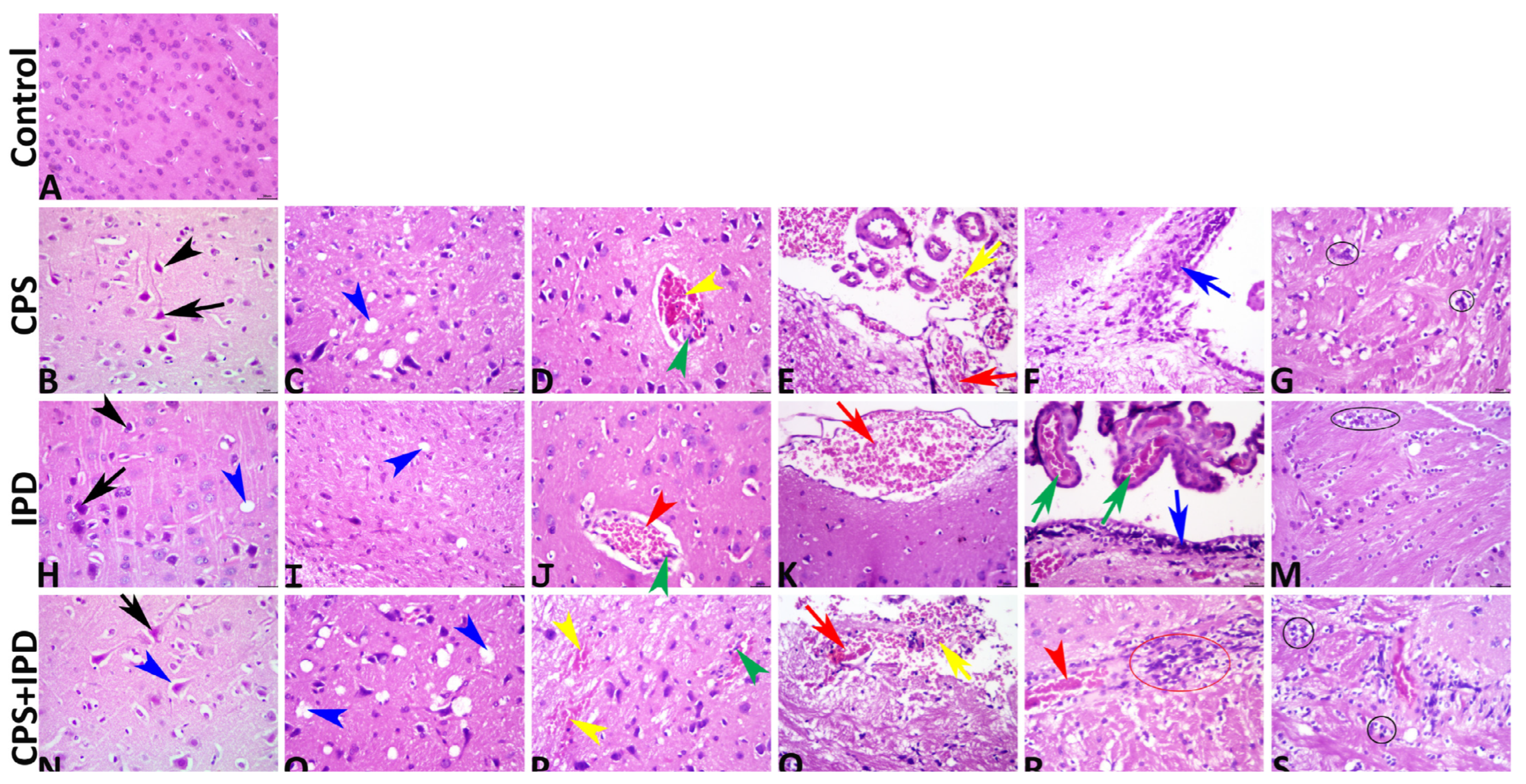
| Lesion | Control | IPD | CPS | IPD + CPS | |
|---|---|---|---|---|---|
| Percentage of neurons exhibited the lesion to the total number of neurons per images per group | Chromatolysis | 0.00 b ± 0.00 | 3.00 a ± 1.53 | 5.00 a ± 2.24 | 11.00 a ± 3.14 |
| Perineuronal vacuolation | 0.00 c ± 0.00 | 6.00 c ± 1.63 | 28.00 b ± 3.89 | 43.00 a ± 4.23 | |
| Neuronal necrosis | 0.00 c ± 0.00 | 4.00 c ± 1.63 | 10.00 b ± 2.11 | 23.00 a ± 3.00 | |
| Percentages of the area fractions of the lesion regarding the total areas of the images per group | Intracerebral blood vessels | 2.30 d ± 0.23 | 3.44 c ± 0.21 | 4.96 b ± 0.34 | 6.47 a ± 0.29 |
| Meningeal blood vessels | 0.70 d ± 0.09 | 1.19 c ± 0.11 | 1.79 b ± 0.15 | 2.82 a ± 0.12 | |
| Choroidal plexus blood vessels | 1.84 d ± 0.18 | 2.31 c ± 0.17 | 3.46 b ± 0.21 | 5.15 a ± 0.38 | |
| Intracerebral hemorrhages | 0.00 c ± 0.00 | 0.08 bc± 0.04 | 0.54 ab ± 0.15 | 0.95 a ± 0.28 | |
| Meningeal hemorrhages | 0.00 c ± 0.00 | 0.38 bc ± 0.27 | 1.42 ab ± 0.53 | 1.70 a ± 0.49 | |
| Neuropil microcavitations | 0.00 b ± 0.00 | 0.69 b ± 0.18 | 4.10 a ± 0.58 | 5.82 a ± 1.06 | |
| The frequencies of the lesion regarding the total number of images per group | Glial clustering | 0.00 b ± 0.00 | 9.00 a ± 3.14 | 4.00 ab ± 2.21 | 5.00 ab ± 2.24 |
| Gliosis | 0.00 b ± 0.00 | 2.00 b ± 1.33 | 9.00 a ± 2.33 | 9.00 a ± 2.77 | |
| Neuronophagia | 0.00 c ± 0.00 | 1.11 bc ± 0.61 | 2.00 ab ± 0.71 | 3.00 a ± 0.73 | |
| Endothelial hypertrophy | 0.00 b ± 0.00 | 4.00 ab ± 1.63 | 7.00 a ± 2.60 | 8.00 a ± 2.00 | |
| Cerebral inflammatory cell infiltration | 0.00 b ± 0.00 | 1.00 ab ± 0.54 | 3.00 ab ± 1.27 | 4.00 a ± 1.63 | |
| Meningeal inflammatory cell infiltration | 0.00 b ± 0.00 | 0.80 ab ± 0.53 | 1.40 a ± 0.60 | 2.00 a ± 0.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abd-Elhakim, Y.M.; El Sharkawy, N.I.; Gharib, H.S.A.; Hassan, M.A.; Metwally, M.M.M.; Elbohi, K.M.; Hassan, B.A.; Mohammed, A.T. Neurobehavioral Responses and Toxic Brain Reactions of Juvenile Rats Exposed to Iprodione and Chlorpyrifos, Alone and in a Mixture. Toxics 2023, 11, 431. https://doi.org/10.3390/toxics11050431
Abd-Elhakim YM, El Sharkawy NI, Gharib HSA, Hassan MA, Metwally MMM, Elbohi KM, Hassan BA, Mohammed AT. Neurobehavioral Responses and Toxic Brain Reactions of Juvenile Rats Exposed to Iprodione and Chlorpyrifos, Alone and in a Mixture. Toxics. 2023; 11(5):431. https://doi.org/10.3390/toxics11050431
Chicago/Turabian StyleAbd-Elhakim, Yasmina M., Nabela I. El Sharkawy, Heba S. A. Gharib, Mona A. Hassan, Mohamed M. M. Metwally, Khlood M. Elbohi, Bayan A. Hassan, and Amany Tharwat Mohammed. 2023. "Neurobehavioral Responses and Toxic Brain Reactions of Juvenile Rats Exposed to Iprodione and Chlorpyrifos, Alone and in a Mixture" Toxics 11, no. 5: 431. https://doi.org/10.3390/toxics11050431
APA StyleAbd-Elhakim, Y. M., El Sharkawy, N. I., Gharib, H. S. A., Hassan, M. A., Metwally, M. M. M., Elbohi, K. M., Hassan, B. A., & Mohammed, A. T. (2023). Neurobehavioral Responses and Toxic Brain Reactions of Juvenile Rats Exposed to Iprodione and Chlorpyrifos, Alone and in a Mixture. Toxics, 11(5), 431. https://doi.org/10.3390/toxics11050431







