A New Advanced and Validated Method for the Determination of Potentially Toxic Metals and Trace and Ultra-Trace Elements in Peritoneal Fluid to Assess the Health Risks of Women with Gynecological Pathologies
Abstract
1. Introduction
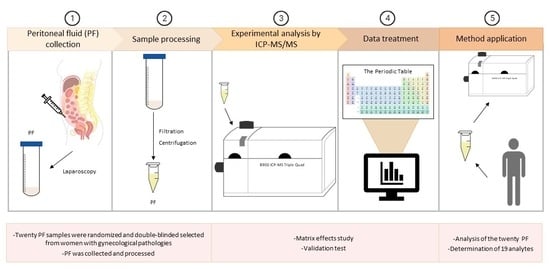
2. Materials and Methods
2.1. Instrumentation and Equipment
2.2. Study Group and Sample Collection
2.3. Reagents and Solutions
2.4. Calculation of the Limit of Detection (LOD) and Quantification (LOQ)
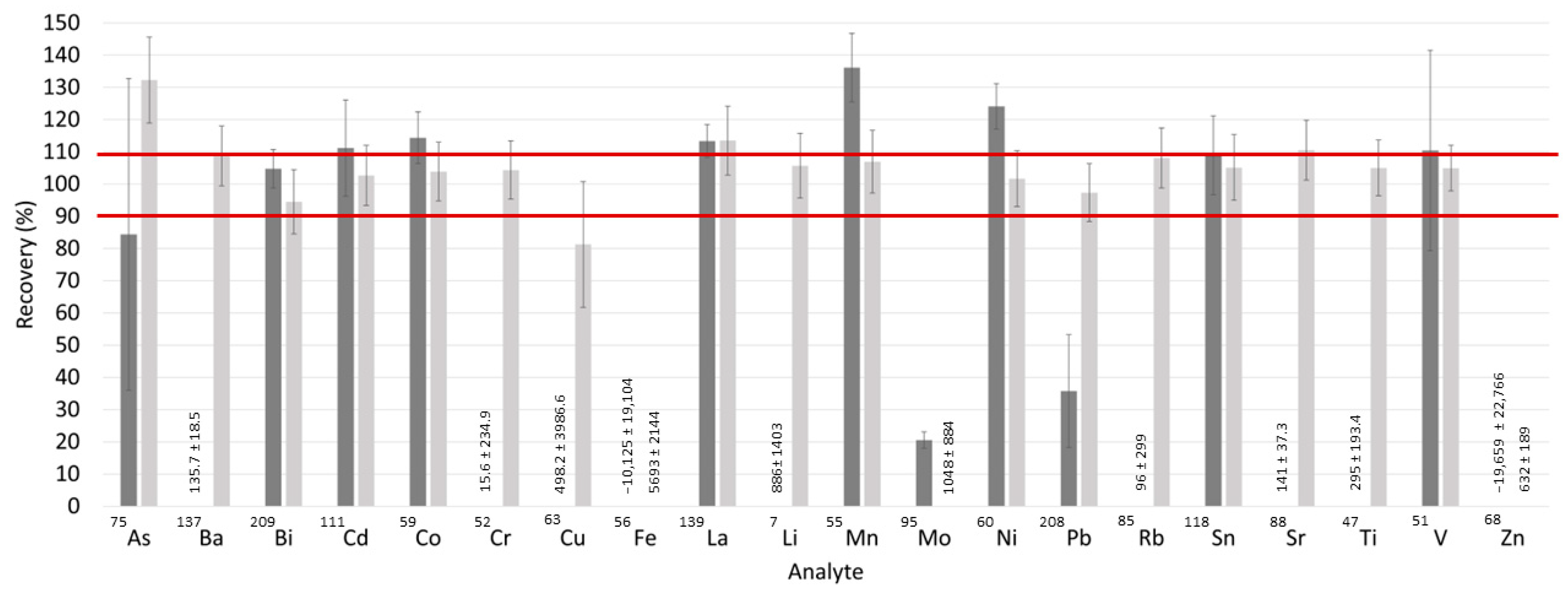
2.5. Matrix Effects Study
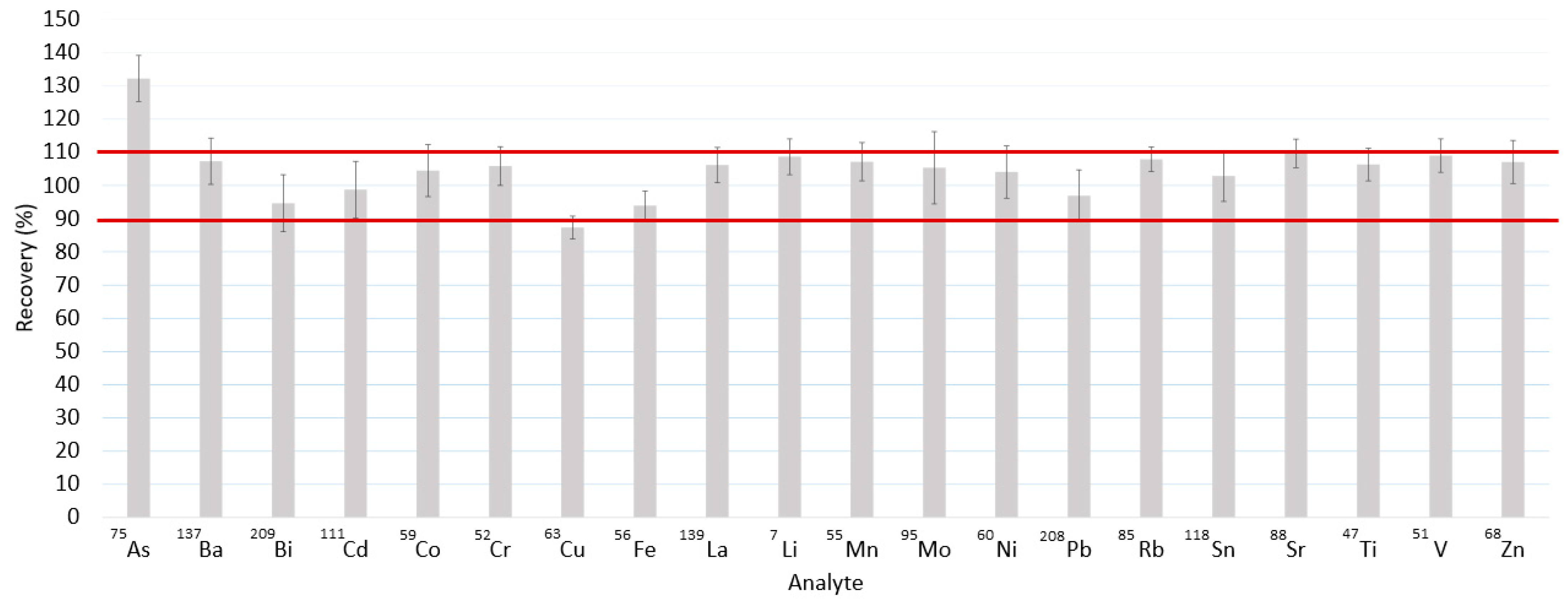
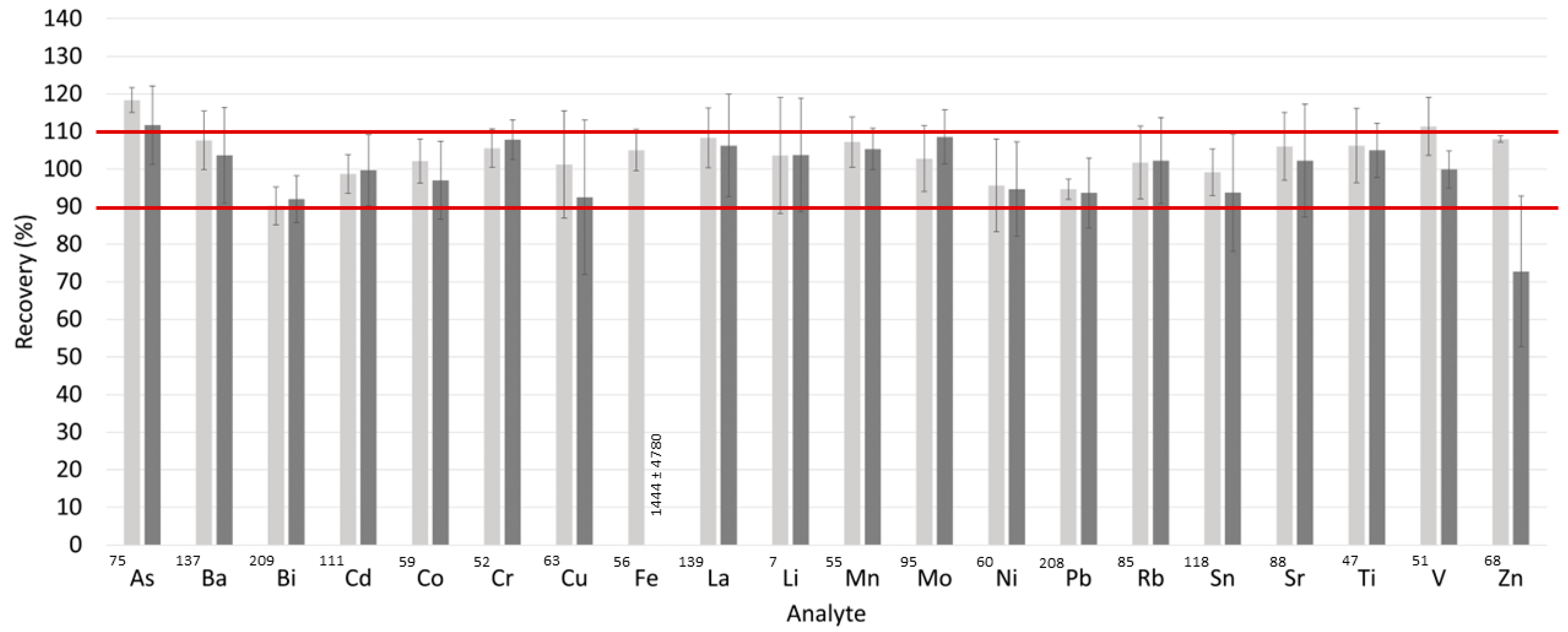
2.6. Method Validation
3. Results and Discussion
3.1. Method Optimization
3.1.1. Matrix Effects
3.1.2. Spectral Interferences
3.2. Analytical Figures of Merit
3.3. Complete Method Validation
3.4. Sample Analysis under Optimal Conditions
3.5. Essential and Non-Essential Elements
3.5.1. Elements Found at Major Concentrations in PF Samples
3.5.2. Elements Found at Intermediate Concentrations in PF Samples
3.5.3. Elements Found at Minor Concentrations in PF Samples
3.5.4. Non-Essential Hhuman Elements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- World Health Organization; Food and Agriculture Organization of the United Nations; International Atomic Energy Agency. Trace Elements in Human Nutrition and Health; World Health Organization: Geneva, Switzerland, 1996. [Google Scholar]
- Ali, H.; Khan, E. What Are Heavy Metals? Long-Standing Controversy over the Scientific Use of the Term ‘Heavy Metals’—Proposal of a Comprehensive Definition. Toxicol. Environ. Chem. 2018, 100, 6–19. [Google Scholar] [CrossRef]
- Pourret, O.; Hursthouse, A. It’s Time to Replace the Term “Heavy Metals” with “Potentially Toxic Elements” When Reporting Environmental Research. Int. J. Environ. Res. Public Health 2019, 16, 4446. [Google Scholar] [CrossRef] [PubMed]
- Pourret, O.; Bollinger, J.C. “Heavy Metal”—What to Do Now: To Use or Not to Use? Sci. Total Environ. 2017, 610–611, 419–420. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy Metal Toxicity and the Environment. In Molecular, Clinical and Environmental Toxicology; Springer: Berlin/Heidelberg, Germany, 2012; pp. 133–164. [Google Scholar] [CrossRef]
- Rzymski, P.; Tomczyk, K.; Rzymski, P.; Poniedziałek, B.; Opala, T.; Wilczak, M. Impact of Heavy Metals on the Female Reproductive System. Ann. Agric. Environ. Med. 2015, 22, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Hruska, K.S.; Furth, P.A.; Seifer, D.B.; Sharara, F.I.; Flaws, J.A. Environmental Factors in Infertility. Clin. Obstet. Gynecol. 2000, 43, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; He, X.; Qi, K.; Wang, T.; Qi, Y.; Cui, L.; Wang, F.; Song, M. Effects of Environmental Contaminants on Fertility and Reproductive Health. J. Environ. Sci. 2019, 77, 210–217. [Google Scholar] [CrossRef]
- López-Botella, A.; Velasco, I.; Acién, M.; Sáez-Espinosa, P.; Todolí-Torró, J.-L.; Sánchez-Romero, R.; Gómez-Torres, M.J. Impact of Heavy Metals on Human Male Fertility—An Overview. Antioxidants 2021, 10, 1473. [Google Scholar] [CrossRef]
- Kopp, R.; Martínez, I.O.; Legradi, J.; Legler, J. Exposure to Endocrine Disrupting Chemicals Perturbs Lipid Metabolism and Circadian Rhythms. J. Environ. Sci. 2017, 62, 133–137. [Google Scholar] [CrossRef]
- Stoica, A.; Katzenellenbogen, B.S.; Martin, M.B. Activation of Estrogen Receptor-α by the Heavy Metal Cadmium. Mol. Endocrinol. 2000, 14, 545–553. [Google Scholar] [CrossRef]
- Pollack, A.Z.; Ranasinghe, S.; Sjaarda, L.A.; Mumford, S.L. Cadmium and Reproductive Health in Women: A Systematic Review of the Epidemiologic Evidence. In Current Environmental Health Reports; Springer: Berlin/Heidelberg, Germany, 2014; pp. 172–184. [Google Scholar] [CrossRef]
- Rzymski, P.; Niedzielski, P.; Rzymski, P.; Tomczyk, K.; Kozak, L.; Poniedziałek, B. Metal Accumulation in the Human Uterus Varies by Pathology and Smoking Status. Fertil Steril. 2016, 105, 1511–1518. [Google Scholar] [CrossRef]
- Yılmaz, B.K.; Evliyaoğlu, Ö.; Yorgancı, A.; Özyer, Ş.; Üstün, Y.E. Serum Concentrations of Heavy Metals in Women with Endometrial Polyps. J. Obstet. Gynaecol. 2020, 40, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Lai, G.; Yeh, C.; Yeh, C.; Chen, R.; Fu, C.; Chen, C.; Tzeng, C. Decreased zinc and increased lead blood levels are associated with endometriosis in Asian women. Reprod. Toxicol. 2017, 74, 77–84. [Google Scholar] [CrossRef]
- Dutta, S.; Gorain, B.; Choudhury, H.; Roychoudhury, S.; Sengupta, P. Environmental and Occupational Exposure of Metals and Female Reproductive Health. Environ. Sci. Pollut. Res. 2022, 29, 62067–62092. [Google Scholar] [CrossRef]
- Bhardwaj, J.K.; Paliwal, A.; Saraf, P. Effects of Heavy Metals on Reproduction Owing to Infertility. J. Biochem. Mol. Toxicol. 2021, 35, e22823. [Google Scholar] [CrossRef]
- Berger, N.; Rock, J. Peritoneal Fluid Environment in Endometriosis. Semin. Reprod. Med. 1985, 3, 313–318. [Google Scholar] [CrossRef]
- Koninckx, P. Endometriotic Disease: The Role of Peritoneal Fluid. Hum. Reprod. Update 1998, 4, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Su, M.H.; Cho, S.W.; Kung, Y.S.; Lin, J.H.; Lee, W.L.; Wang, P.H. Update on the Differential Diagnosis of Gynecologic Organ-Related Diseases in Women Presenting with Ascites. Taiwan. J. Obstet. Gynecol. 2019, 58, 587–591. [Google Scholar] [CrossRef]
- Bedaiwy, M.A.; Falcone, T. Peritoneal Fluid Environment in Endometriosis. Clinicopathological Implications. Minerva Ginecol. 2003, 55, 333–345. [Google Scholar] [PubMed]
- Dos Santos, L.R.S.S.R.; de Freitas Santos, A.; das Graças Andrade Korn, M. Effects of Furosemide Administration on the Concentration of Essential and Toxic Elements in Wistar Rats by Inductively Coupled Plasma Optical Emission Spectrometry. J. Trace Elem. Med. Biol. 2018, 48, 25–29. [Google Scholar] [CrossRef]
- Hou, X.; Amais, R.S.; Jones, B.T.; Donati, G.L. Inductively Coupled Plasma Optical Emission Spectrometry. In Encyclopedia of Analytical Chemistry; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2006. [Google Scholar] [CrossRef]
- Markiewicz, B.; Sajnóg, A.; Lorenc, W.; Hanć, A.; Komorowicz, I.; Suliburska, J.; Kocyłowski, R.; Barałkiewicz, D. Multielemental Analysis of 18 Essential and Toxic Elements in Amniotic Fluid Samples by ICP-MS: Full Procedure Validation and Estimation of Measurement Uncertainty. Talanta 2017, 174, 122–130. [Google Scholar] [CrossRef]
- López-Botella, A.; Sánchez, R.; Paul, R.; Aizpurua, J.; Gómez-Torres, M.J.; Todolí-Torró, J.-L. Analytical Determination of Heavy Metals in Human Seminal Plasma—A Systematic Review. Life 2023, 13, 925. [Google Scholar] [CrossRef]
- Cavey, T.; Ropert, M.; Loréal, O.; Bendavid, C.; Peoc’h, K. Metals: Common Clinical Applications in Inductively Coupled Plasma Mass Spectrometry. Ann. Biol. Clin. 2019, 77, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Bolea-Fernandez, E.; Balcaen, L.; Resano, M.; Vanhaecke, F. Overcoming Spectral Overlap via Inductively Coupled Plasma-Tandem Mass Spectrometry (ICP-MS/MS). A Tutorial Review. J. Anal. Spectrom. 2017, 32, 1660–1679. [Google Scholar] [CrossRef]
- Eurachem. The Fitness for Purpose of Analytical Methods: A Laboratory Guide to Method Validation and Related Topics; Eurachem Guides: Gembloux, Belgium, 1998. [Google Scholar]
- Todolí, J.-L.; Mermet, J.-M. Acid Interferences in Atomic Spectrometry: Analyte Signal Effects and Subsequent Reduction. Spectrochim. Acta Part B Spectrosc. 1999, 54, 895–929. [Google Scholar] [CrossRef]
- Darrouzes, J.; Bueno, M.; Lespes, G.; Holeman, M.; Potingautier, M. Optimisation of ICPMS Collision/Reaction Cell Conditions for the Simultaneous Removal of Argon Based Interferences of Arsenic and Selenium in Water Samples. Talanta 2007, 71, 2080–2084. [Google Scholar] [CrossRef]
- Allain, P.; Jaunault, L.; Mauras, Y.; Mermet, J.M.; Delaporte, T. Signal Enhancement of Elements Due to the Presence of Carbon-Containing Compounds in Inductively Coupled Plasma Mass Spectrometry. Anal. Chem. 1991, 63, 1497–1498. [Google Scholar] [CrossRef]
- Trumbo, P.; Yates, A.A.; Schlicker, S.; Poos, M. Dietary Reference Intakes. J. Am. Diet. Assoc. 2001, 101, 294–301. [Google Scholar] [CrossRef]
- Kurdoglu, Z.; Kurdoglu, M.; Demir, H.; Sahin, H.G. Serum Trace Elements and Heavy Metals in Polycystic Ovary Syndrome. Hum. Exp. Toxicol. 2012, 31, 452–456. [Google Scholar] [CrossRef]
- Lousse, J.C.; Defrère, S.; Van Langendonckt, A.; Gras, J.; González-Ramos, R.; Colette, S.; Donnez, J. Iron Storage Is Significantly Increased in Peritoneal Macrophages of Endometriosis Patients and Correlates with Iron Overload in Peritoneal Fluid. Fertil Steril 2009, 91, 1668–1675. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, Y.; Zhao, A.; Cai, X.; Yu, A.; Xu, Q.; Wang, P.; Yao, J.; Wang, Q.; Wang, W. Arsenic Exposure Diminishes Ovarian Follicular Reserve and Induces Abnormal Steroidogenesis by DNA Methylation. Ecotoxicol. Environ. Saf. 2022, 241, 113816. [Google Scholar] [CrossRef]
- Kuo, H.W.; Chen, S.F.; Wu, C.C.; Chen, D.R.; Lee, J.H. Serum and Tissue Trace Elements in Patients with Breast Cancer in Taiwan. Biol. Trace Elem. Res. 2002, 89, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, Y.; Guan, Q.; Xu, L.; Zhao, S.; Duan, J.; Wang, Y.; Xia, Y.; Xu, Q. Exposure to Multiple Trace Elements and Miscarriage during Early Pregnancy: A Mixtures Approach. Environ. Int. 2022, 162, 107161. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, W.; Guo, Y.; Zheng, B.; Li, H.; Chen, J.; Zhang, W. High Copper Levels in Follicular Fluid Affect Follicle Development in Polycystic Ovary Syndrome Patients: Population-Based and in Vitro Studies. Toxicol. Appl. Pharmacol. 2019, 365, 101–111. [Google Scholar] [CrossRef]
- Osman, K.; Åkesson, A.; Berglund, M.; Bremme, K.; Schu¨tz, A.; Schu¨tz, S.; Ask, K.; Vahter, M. Toxic and Essential Elements in Placentas of Swedish Women. Clin. Biochem. 2000, 33, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Liang, K.; Li, M.; Rong, C.; Zheng, J.; Li, J. Metal Elements Associate with in Vitro Fertilization (IVF) Outcomes in 195 Couples. J. Trace Elem. Med. Biol. 2021, 68, 126810. [Google Scholar] [CrossRef]
- Guo, Y.; Huo, X.; Li, Y.; Wu, K.; Liu, J.; Huang, J.; Zheng, G.; Xiao, Q.; Yang, H.; Wang, Y.; et al. Monitoring of Lead, Cadmium, Chromium and Nickel in Placenta from an e-Waste Recycling Town in China. Sci. Total Environ. 2010, 408, 3113–3117. [Google Scholar] [CrossRef]
- Guan, H.; Wang, M.; Li, X.; Piao, F.; Li, Q.; Xu, L.; Kitamura, F.; Yokoyama, K. Manganese Concentrations in Maternal and Umbilical Cord Blood: Related to Birth Size and Environmental Factors. Eur. J. Public Health 2014, 24, 150–157. [Google Scholar] [CrossRef]
- Baruthio, F.; Pierre, F. Cobalt Determination in Serum and Urine by Electrothermal Atomic Absorption Spectrometry. Biol. Trace Elem. Res. 1993, 39, 21–31. [Google Scholar] [CrossRef]
- Ćwiertnia, A.; Kozłowski, M.; Cymbaluk-Płoska, A. The Role of Iron and Cobalt in Gynecological Diseases. Cells 2023, 12, 117. [Google Scholar] [CrossRef]
- Weir, A.; Westerhoff, P.; Fabricius, L.; Hristovski, K.; Von Goetz, N. Titanium Dioxide Nanoparticles in Food and Personal Care Products. Environ. Sci. Technol. 2012, 46, 2242–2250. [Google Scholar] [CrossRef]
- Jin, Y.; Li, Z.; An, H.; Pang, Y.; Li, K.; Zhang, Y.; Zhang, L.; Yan, L.; Wang, B.; Ye, R.; et al. Environmental Titanium Exposure and Reproductive Health: Risk of Low Birth Weight Associated with Maternal Titanium Exposure from a Nested Case-Control Study in Northern China. Ecotoxicol. Environ. Saf. 2021, 208, 111632. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Barium in Drinking-Water; Background Document for Development of WHO Guidelines for Drinking-Water Quality; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Flannery, B.M.; Middleton, K.B. Updated Interim Reference Levels for Dietary Lead to Support FDA’s Closer to Zero Action Plan. Regul. Toxicol. Pharmacol. 2022, 133, 105202. [Google Scholar] [CrossRef]
- Mehri, A. Trace Elements in Human Nutrition (II)—An Update. Int. J. Prev. Med. 2020. [Google Scholar] [CrossRef]
- Popovic, M.; McNeill, F.E.; Chettle, D.R.; Webber, C.E.; Lee, C.V.; Kaye, W.E. Impact of Occupational Exposure on Lead Levels in Women. Environ. Health Perspect. 2005, 113, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Sun, H. Bismuth: Environmental Pollution and Health Effects. Encycl. Environ. Health 2011, 414–420. [Google Scholar] [CrossRef]
- Winship, K.A. Toxicity of Tin and Its Compounds. Advers. Drug React Acute Poisoning Rev. 1988, 7, 19–38. [Google Scholar]
- Satarug, S.; Haswell-Elkins, M.R.; Moore, M.R. Safe Levels of Cadmium Intake to Prevent Renal Toxicity in Human Subjects. Br. J. Nutr. 2000, 84, 791–802. [Google Scholar] [CrossRef]
- Jackson, L.W.; Zullo, M.D.; Goldberg, J.M. The Association between Heavy Metals, Endometriosis and Uterine Myomas among Premenopausal Women: National Health and Nutrition Examination Survey 1999–2002. Hum. Reprod. 2008, 23, 679–687. [Google Scholar] [CrossRef]
- Nasiadek, M.; Swiatkowska, E.; Nowinska, A.; Krawczyk, T.; Wilczynski, J.R.; Sapota, A. The Effect of Cadmium on Steroid Hormones and Their Receptors in Women with Uterine Myomas. Arch. Environ. Contam. Toxicol. 2011, 60, 734–741. [Google Scholar] [CrossRef]
| Isotope | Abundance (%) | Interference from the Matrix/Argon Plasma |
|---|---|---|
| 52Cr | 83.8 | 40Ar12C+, 35Cl17O+ |
| 56Fe | 91.7 | 40Ca16O+,40Ar16O+ |
| 63Cu | 69.2 | 36Ar12C14N1H+, 14N12C37Cl+, 16O12C35Cl+ |
| 68Zn | 18.6 | 40Ar14N2+, 35Cl16O17O+, 64S2+, 38Ar32S+ |
| 75As | 100 | 23Na12C40Ar, 12C31P16O2+, 40Ar35Cl+, 36Ar39K+ |
| LOD (µg L−1) | LOQ (µg L−1) | |||
|---|---|---|---|---|
| No Gas | He Gas | No Gas | He Gas | |
| 75As | 1.7 | 1.9 | 6 | 6 |
| 137Ba | 0.7 | 1.1 | 2 | 4 |
| 209Bi | 0.16 | 0.2 | 0.5 | 0.8 |
| 111Cd | 0.2 | 0.45 | 0.7 | 1.5 |
| 59Co | 0.19 | 0.2 | 0.6 | 0.9 |
| 52Cr | 0.3 | 0.5 | 1.1 | 1.6 |
| 63Cu | 3 | 2 | 9 | 7 |
| 56Fe | 60 | 4 | 200 | 12 |
| 139La | 0.05 | 0.14 | 0.18 | 0.5 |
| 7Li | 0.3 | 1.6 | 1.0 | 5 |
| 55Mn | 0.3 | 0.7 | 1.0 | 2 |
| 95Mo | 1.8 | 0.4 | 6 | 1.3 |
| 60Ni | 0.6 | 1.0 | 1.9 | 3 |
| 208Pb | 0.5 | 0.2 | 1.7 | 0.8 |
| 85Rb | 0.14 | 0.5 | 0.4 | 1.7 |
| 118Sn | 0.4 | 0.4 | 1.5 | 1.3 |
| 88Sr | 1.1 | 1.1 | 4 | 4 |
| 47Ti | 2 | 6 | 7 | 18 |
| 51V | 0.7 | 0.3 | 2 | 1.0 |
| 68Zn | 14 | 6 | 47 | 19 |
| Analyte | Peritoneal Fluid Sample | ||||
|---|---|---|---|---|---|
| Sample 1 | Sample 2 | Sample 3 | Sample 4 | Sample 5 | |
| 137Ba (No Gas) | 2.8 ± 0.3 | 2.7 ± 0.4 | 18.4 ±1.0 | 11.0 ±1.4 | 19.7 ± 0.8 |
| 137Ba (He) | 0.58 ± 0.15 | <LOQ (4) | 16.9 ± 1.4 | 13.8 ± 0.8 | 19.2 ± 0.7 |
| 209Bi (No Gas) | 0.34 ± 0.07 | 0.34 ± 0.12 | 0.77 ± 0.13 | 0.20 ± 0.02 | 0.17 ± 0.03 |
| 209Bi (He) | <LOQ (0.8) | <LOQ (0.8) | 0.85 ± 0.09 | 0.49 ± 0.03 | <LOQ (0.8) |
| 111Cd (No Gas) | <LOQ (0.5) | <LOQ (0.5) | <LOQ (0.5) | <LOQ (0.5) | 4.4 ± 0.4 |
| 111Cd (He) | <LOQ (1.5) | <LOQ (1.5) | <LOQ (1.5) | <LOQ (1.5) | 5.3 ± 1.7 |
| 59Co (No Gas) | <LOQ (0.3) | <LOQ (0.3) | <LOQ (0.3) | <LOQ (0.3) | 2.50 ± 0.06 |
| 59Co (He) | <LOQ (0.9) | <LOQ (0.9) | <LOQ (0.9) | <LOQ (0.9) | 1.9 ± 0.5 |
| 139La (No Gas) | <LOQ (0.14) | <LOQ (0.14) | 0.21 ± 0.03 | 0.24 ± 0.05 | 3.22 ± 0.16 |
| 139La (He) | <LOQ (0.5) | <LOQ (0.5) | <LOQ (0.5) | <LOQ (0.5) | 2.80 ± 0.09 |
| 7Li (No Gas) | 2.70 ± 0.07 | <LOQ (0.9) | 10.8 ± 0.2 | <LOQ (0.9) | <LOQ (0.9) |
| 7Li (He) | <LOQ (5) | <LOQ (5) | 9.8 ± 0.5 | <LOQ (5) | <LOQ (5) |
| 55Mn (No Gas) | 1.06 ± 0.04 | 2.18 ± 0.08 | <LOQ (0.7) | <LOQ (0.7) | 3.70 ± 0.11 |
| 55Mn (He) | <LOQ (2) | <LOQ (2) | <LOQ (2) | <LOQ (2) | <LOQ (2) |
| 60Ni (No Gas) | 3.0 ± 0.3 | 4.4 ± 0.6 | 5.10 ± 0.12 | 11.1 ± 0.4 | 6.8 ± 0.4 |
| 60Ni (He) | <LOQ (3) | <LOQ (3) | 5.5 ± 1.1 | 4.3 ± 0.9 | 6.2 ± 0.8 |
| 208Pb (No Gas) | <LOQ (1.7) | <LOQ (1.7) | 4.84 ± 0.08 | 2.77 ± 0.04 | <LOQ (1.7) |
| 208Pb (He) | 0.98 ± 0.07 | 1.57 ± 0.11 | 4.64 ± 0.18 | 2.49 ± 0.16 | 1.33 ± 0.12 |
| 137Ba | 209Bi | 111Cd | 59Co | 139La | 7Li | 55Mn | 59Mo | 60Ni | 208Pb | 85Rb | 118Sn | 88Sr | 47Ti | 51V | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| uip | 2.7 | 1.8 | 3.1 | 0.8 | 1.0 | 1.2 | 0.8 | 1.2 | 0.9 | 1.4 | 1.5 | 1.5 | 0.9 | 1.3 | 1.2 |
| urep | 1.1 | 1.1 | 0.9 | 1.4 | 1.4 | 2.3 | 1.3 | 1.7 | 1.7 | 1.4 | 1.7 | 2.2 | 1.9 | 1.8 | 1.4 |
| ut | 7.5 | 8.0 | 7.1 | 6.7 | 4.5 | 8.7 | 5.1 | 7.9 | 6.3 | 6.3 | 6.8 | 8.8 | 8.1 | 5.3 | 7.7 |
| Expanded relative uncertainty (U, k = 2) | 16.0 | 16.6 | 15.6 | 13.8 | 9.6 | 18.2 | 10.6 | 16.3 | 13.1 | 13.2 | 14.3 | 18.4 | 16.7 | 11.5 | 15.8 |
| 52Cr | 63Cu | 56Fe | 68Zn | |
|---|---|---|---|---|
| uip | 1.7 | 1.3 | 1.3 | 1.3 |
| urep | 2.5 | 1.8 | 1.6 | 1.9 |
| ut | 7.3 | 6.9 | 8.0 | 8.2 |
| Expanded relative uncertainty (U, k = 2) | 15.8 | 14.5 | 16.5 | 17.0 |
| Analyte | Mean ± SEM (μg L−1) | Min (μg L−1) | Max (μg L−1) | % > LOD |
|---|---|---|---|---|
| 137Ba | 21.7 ± 31.8 | 2.7 | 110.7 | 85 |
| 209Bi | 4.7 ± 7.5 | 0.6 | 15.8 | 65 |
| 111Cd | 2.8 ± 1.5 | 0.9 | 4.4 | 55 |
| 59Co | 2.3 ± 0.3 | 2.1 | 2.5 | 40 |
| 139La | 0.6 ± 1.1 | 0.2 | 3.9 | 80 |
| 7Li | 12.7 ± 9.6 | 1.6 | 25.3 | 45 |
| 55Mn | 4.6 ± 4.0 | 1.1 | 14.6 | 75 |
| 95Mo | 17.9 | - | - | 40 |
| 60Ni | 5.4 ± 3.2 | 2.9 | 14.5 | 100 |
| 208Pb | 6.3 ± 5.9 | 1.8 | 17.9 | 80 |
| 85Rb | 150.7 ± 57.9 | 77.2 | 313.5 | 100 |
| 118Sn | 3.8 ± 2.1 | 2.2 | 7.4 | 75 |
| 88Sr | 33.5 ± 11.1 | 9.8 | 54.1 | 100 |
| 47Ti | 64.3 ± 68.9 | 12.2 | 219 | 100 |
| 51V | 7.4 ± 1.9 | 4.3 | 11.5 | 70 |
| Analyte | Mean ± SEM (μg L−1) | Min (μg L−1) | Max (μg L−1) | % > LOD |
|---|---|---|---|---|
| 52Cr | 6.6 ± 7.9 | 1.7 | 35.1 | 100 |
| 63Cu | 1115 ± 1466 | 304 | 7221 | 100 |
| 56Fe | 3118 ± 5123 | 332 | 17,832 | 100 |
| 68Zn | 6889 ± 13,385 | 169 | 47,040 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Botella, A.; Sánchez, R.; Todolí-Torró, J.-L.; Gómez-Torres, M.J.; Velasco, I.; Acién, M. A New Advanced and Validated Method for the Determination of Potentially Toxic Metals and Trace and Ultra-Trace Elements in Peritoneal Fluid to Assess the Health Risks of Women with Gynecological Pathologies. Toxics 2023, 11, 399. https://doi.org/10.3390/toxics11050399
López-Botella A, Sánchez R, Todolí-Torró J-L, Gómez-Torres MJ, Velasco I, Acién M. A New Advanced and Validated Method for the Determination of Potentially Toxic Metals and Trace and Ultra-Trace Elements in Peritoneal Fluid to Assess the Health Risks of Women with Gynecological Pathologies. Toxics. 2023; 11(5):399. https://doi.org/10.3390/toxics11050399
Chicago/Turabian StyleLópez-Botella, Andrea, Raquel Sánchez, José-Luis Todolí-Torró, María José Gómez-Torres, Irene Velasco, and Maribel Acién. 2023. "A New Advanced and Validated Method for the Determination of Potentially Toxic Metals and Trace and Ultra-Trace Elements in Peritoneal Fluid to Assess the Health Risks of Women with Gynecological Pathologies" Toxics 11, no. 5: 399. https://doi.org/10.3390/toxics11050399
APA StyleLópez-Botella, A., Sánchez, R., Todolí-Torró, J.-L., Gómez-Torres, M. J., Velasco, I., & Acién, M. (2023). A New Advanced and Validated Method for the Determination of Potentially Toxic Metals and Trace and Ultra-Trace Elements in Peritoneal Fluid to Assess the Health Risks of Women with Gynecological Pathologies. Toxics, 11(5), 399. https://doi.org/10.3390/toxics11050399