Biological and Physiological Responses of Root-knot Disease Development on Five Cucurbits Exposed to Different Concentrations of Sulfur Dioxide
Abstract
1. Introduction
2. Materials and Methods
2.1. Exposure System
2.2. Isolation, Identification and Mass Culturing of Root-knot Nematode, Meloidogyne incognita
2.3. Inoculation with Root-Knot Nematode
2.4. Germplasm Collection and Plant Culture
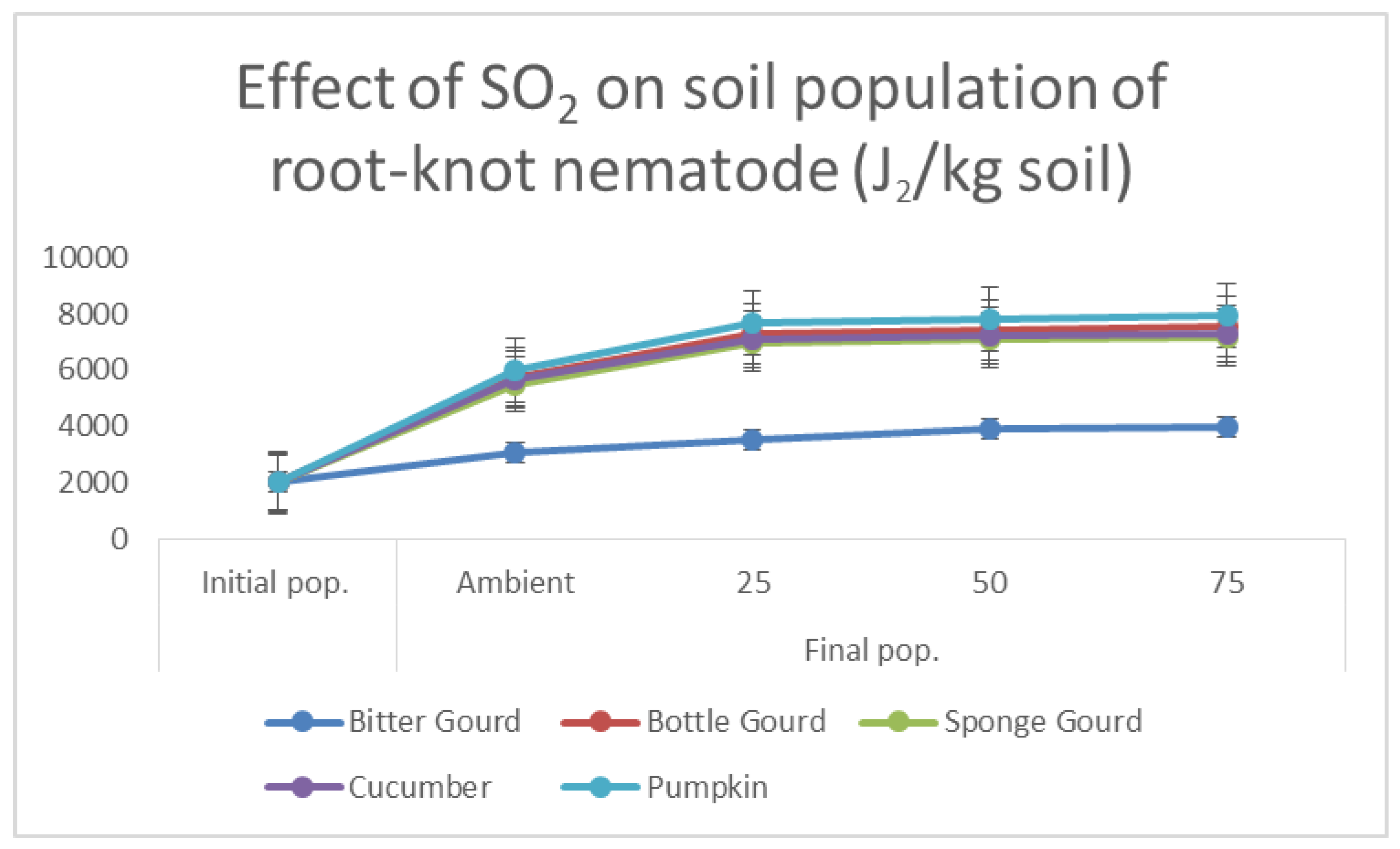
2.5. Soil Population, Number of Galls and Egg Masses of M. incognita
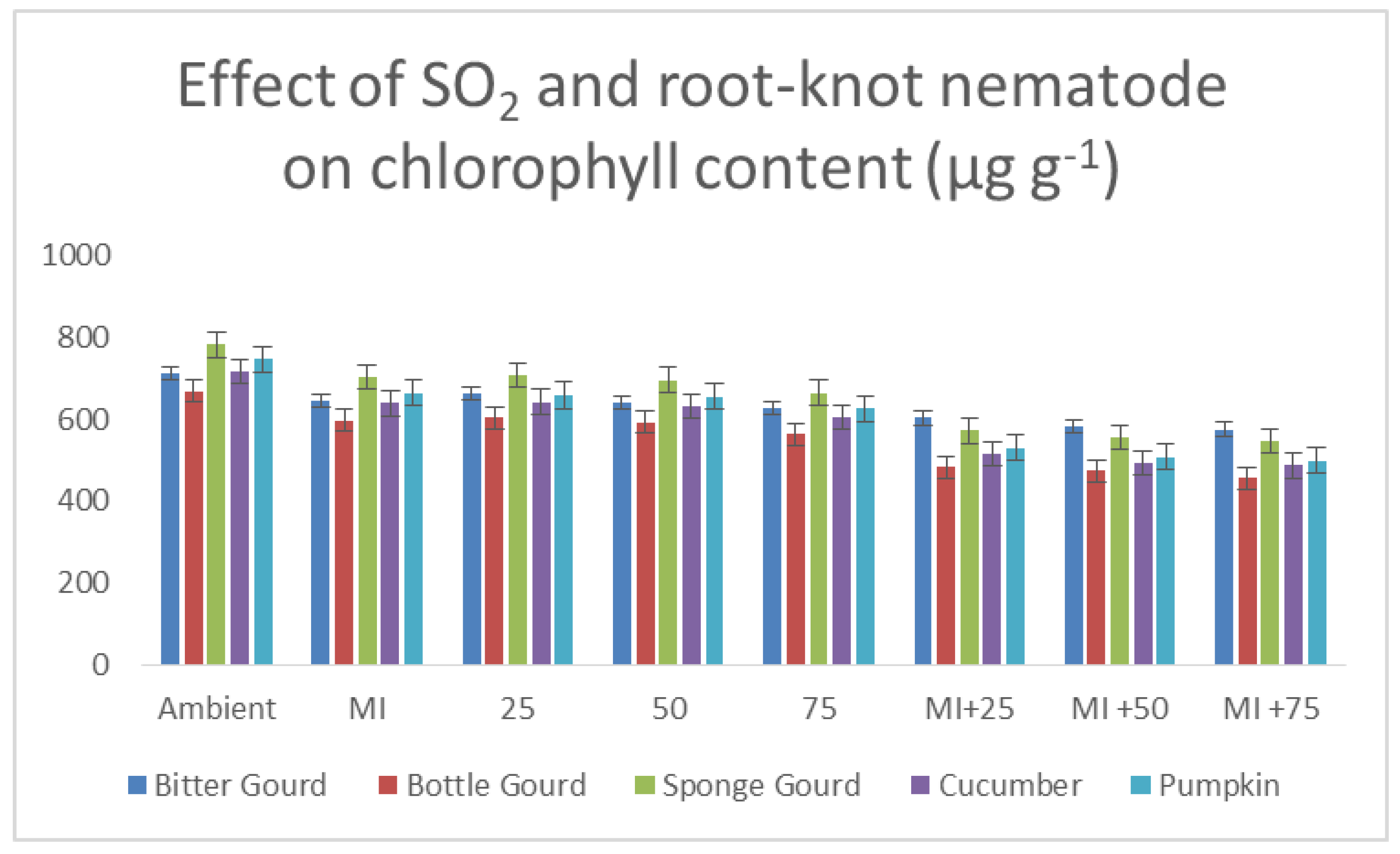
2.6. Estimation of Chlorophyll and Carotenoid Contents
2.7. Determination of Number of Trichomes and Stomata
2.8. Estimation of Foliar Phenolic Compounds and Salicylic Acid Contents
2.9. Physiological Parameters
2.10. Statistical Analysis
3. Results
3.1. Symptoms
3.2. Gas Injury
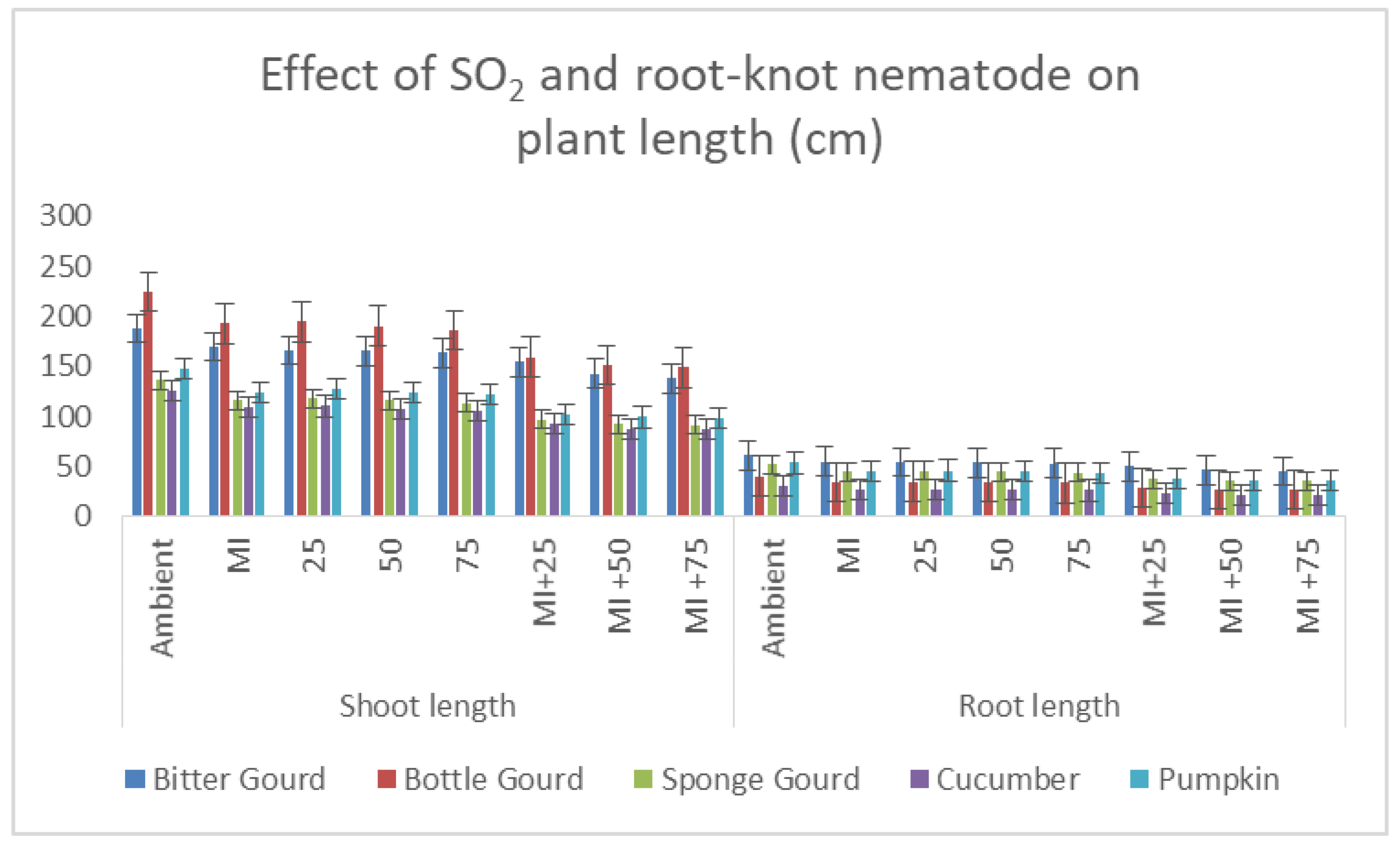
3.3. Plant Growth Parameters
3.4. Number of Trichome and Stomata
3.5. Foliar Pigments, Salicylic Acid Contents and Phenolic Compounds
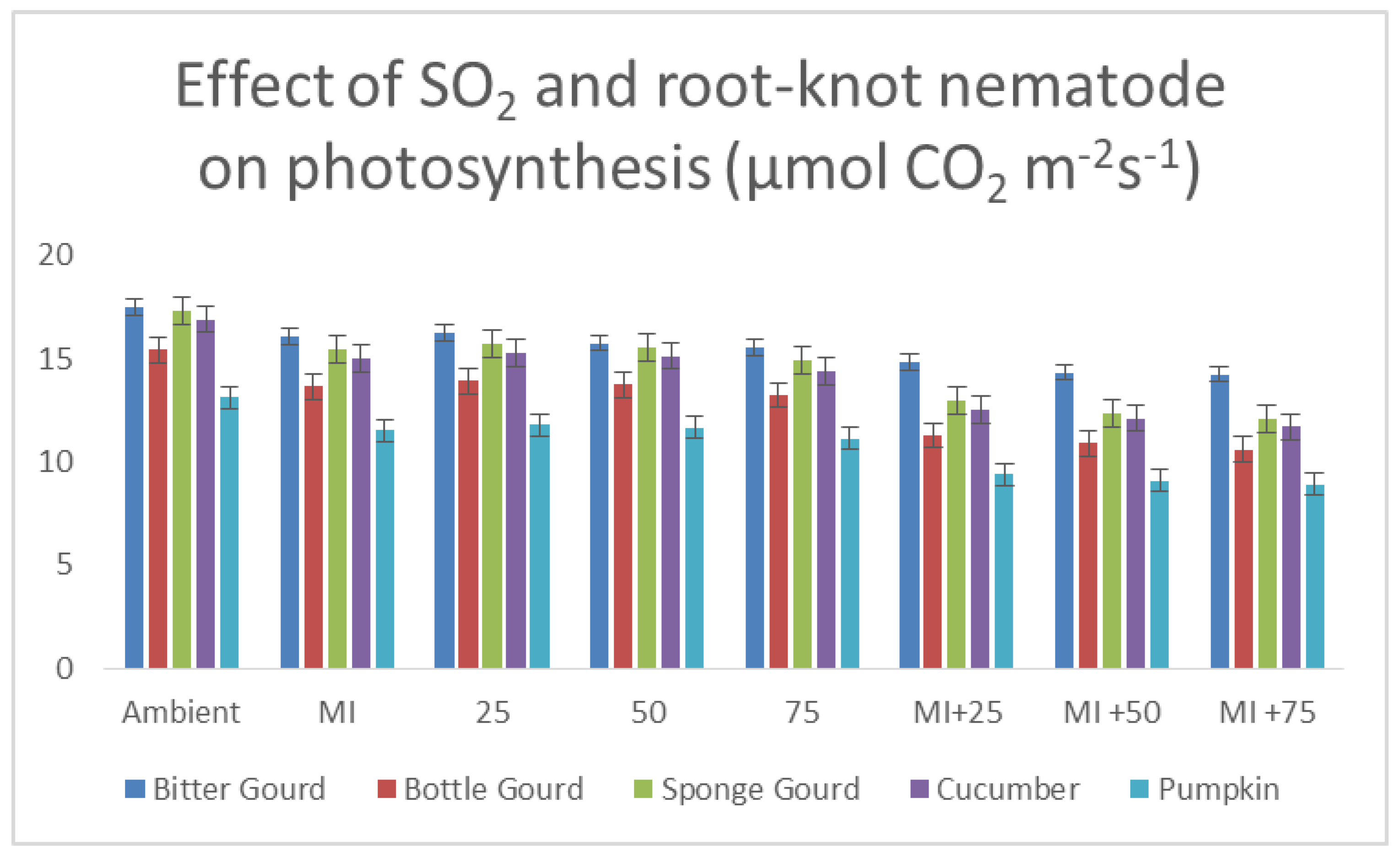
3.6. Rate of Photosynthesis and Transpiration and Stomatal Conductance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agrawal, M.; Deepak, S.S. Physiological and Biochemical Responses of Two Cultivars of Wheat to Elevated Levels of CO2 and SO2, Singly and in Combination. Environ. Pollut. 2003, 121, 189–197. [Google Scholar] [CrossRef]
- Miller, M.J. Retrofit SO2 and NOx Control Technologies for Coal-Fired Power Plants. Environ. Prog. 1986, 5, 171–177. [Google Scholar] [CrossRef]
- EPA. Office of Air Quality Planning and Standards; EPA: Washington, DC, USA, 2021. [Google Scholar]
- Chen, J.; Li, C.; Ristovski, Z.; Milic, A.; Gu, Y.; Islam, M.S.; Wang, S.; Hao, J.; Zhang, H.; He, C.; et al. A Review of Biomass Burning: Emissions and Impacts on Air Quality, Health and Climate in China. Sci. Total Environ. 2017, 579, 1000–1034. [Google Scholar] [CrossRef] [PubMed]
- Nawawi, N.H.; Jaafar, M.N. Assessment of Air Pollution Control Technologies to Reduce SOx Emission from Thermal Oxidizer for Oil and Gas Industry. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Kuching, Malaysia, 15–16 July 2021; IOP Publishing: Bristol, UK, 2021; Volume 1195, p. 012046. [Google Scholar]
- Matić, B.I.; Dejanović, S.M. Scope of Air Quality Monitoring Within the Network of Public Health Institutes in Republic of Serbia. Tehnika 2020, 75, 261–265. [Google Scholar] [CrossRef]
- Khan, M.R.; Khan, M.M.; Mohiddin, F.A. Development of Alternaria Leaf Spot of Indian Mustard Caused by Alternaria brassicae Under the Stress of Low Levels of Sulfur Dioxide. Agric. Ecosyst. Environ. 2015, 199, 154–163. [Google Scholar] [CrossRef]
- Lee, H.K.; Khaine, I.; Kwak, M.J.; Jang, J.H.; Lee, T.Y.; Lee, J.K.; Kim, I.R.; Kim, W.I.; Oh, K.S.; Woo, S.Y. The Relationship Between SO2 Exposure and Plant Physiology: A Mini Review. Hortic. Environ. Biotechnol. 2017, 58, 523–529. [Google Scholar] [CrossRef]
- Khan, M.R.; Khan, M.M. Plants Response to Diseases in Sulphur Dioxide Stressed Environment. Plant Pathol. J. 2011, 10, 1–12. [Google Scholar] [CrossRef]
- Khan, M.R.; Khan, M.W. Sulphur Dioxide Effects on Plants and Pathogens. In Environmental Hazards, Plant and People; Iqbal, M., Srivastava, P.S., Siddiqi, T.O., Eds.; CBS Publishers and Distributors: Old Delhi, India, 2000; pp. 118–136. [Google Scholar]
- Rai, R.; Rajput, M.; Agrawal, M.; Agrawal, S.B. Gaseous Air Pollutants: A Review on Current and Future Trends of Emissions and Impact on Agriculture. J. Sci. Res. 2011, 55, 77–102. [Google Scholar]
- Wang, Z.; Cheng, J.; Zhang, X.; Yang, W.; Park, J.Y.; Kim, H.T.; Xu, L.H. Spermidine Protects Chlorella sp. from Oxidative Damage Caused by SO2 in Flue Gas from Coal-Fired Power Plants. ACS Sustain. Chem. Eng. 2020, 8, 15179–15188. [Google Scholar] [CrossRef]
- El-Eslamboly, A.A.; El-Wanis, A.; Mona, M.; Amin, A.W. Algal Application as a Biological Control Method of Root-knot Nematode Meloidogyne Incognita on Cucumber Under Protected Culture Conditions and Its Impact on Yield and Fruit Quality. Egypt. J. Biol. Pest Control 2019, 29, 1–9. [Google Scholar] [CrossRef]
- Asif, M.; Saleem, S.; Tariq, A.; Usman, M.; Haq, R.A.U. Pollutant Emissions from Brick Kilns and Their Effects on Climate Change and Agriculture. Asean J. Sci. Eng. 2021, 1, 135–140. [Google Scholar] [CrossRef]
- Khan, M.R.; Rizvi, T.F. Effect of Elevated Levels of CO2 on Powdery Mildew Development in Five Cucurbit Species. Sci. Rep. 2020, 10, 4986. [Google Scholar] [CrossRef] [PubMed]
- Eisenback, J.D.; Sasser, J.; Carter, C. Diagnostic Characters Useful in the Identification of the Four Most Common Species of Root-knot Nematodes (Meloidogyne spp.). In An Advance Treatise on Meloidogyne; North Carolina State University Graphics: Raleigh, NC, USA, 1985; Volume 1, pp. 95–112. [Google Scholar]
- Seinhorst, J.W. The Quantitative Extraction of Nematodes from Soil. Nematologica 1956, 1, 249–267. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids: Measurement and Characterization by UV-Vis Spectroscopy. In Current Protocols in Food Analytical Chemistry (CPFA); Wrolstad, R.E., Acree, T.E., An, H., Decker, E.A., Penner, M.H., Reid, D.S., Schwartz, S.J., Shoemaker, C.F., Sporns, P., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2001; pp. F4.3.1–F4.3.8. [Google Scholar]
- Ghouse, A.K.M.; Yunus, M.; Chowdhury, K.A. Research Trends in Plant Anatomy; Open Library: Washington, DC, USA, 1972. [Google Scholar]
- Torriero, A.A.; Luco, J.M.; Sereno, L.; Raba, J. Voltammetric Determination of Salicylic Acid in Pharmaceuticals Formulations of Acetylsalicylic Acid. Talanta 2004, 62, 247–254. [Google Scholar] [CrossRef]
- Roura, E.; Andrés-Lacueva, C.; Estruch, R.; Lamuela-Raventós, R.M. Total Polyphenol Intake Estimated by a Modified Folin–Ciocalteu Assay of Urine. Clin. Chem. 2006, 52, 749–752. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2014; Available online: http://www.R-project.org/ (accessed on 1 February 2023).
- De Mendiburu, F. Statistical Procedures for Agricultural Research; John Wiley & Sons: New York, NY, USA, 2014; Available online: http://www.tarwi.lemonila.au/-Femendiburu (accessed on 1 February 2023).
- Xie, X.; Ling, J.; Mao, Z.; Li, Y.; Zhao, J.; Yang, Y.; Li, Y.; Liu, M.; Gu, X.; Xie, B. Negative Regulation of Root-knot Nematode Parasitic Behavior by Root-Derived Volatiles of Wild Relatives of Cucumis metuliferus CM3. Hortic. Res. 2022, 9, uhac051. [Google Scholar] [CrossRef]
- Khan, M.R. Plant Nematodes-Methodology, Morphology, Systematics, Biology and Ecology; Science Publishers: Enfield, NH, USA, 2008; p. 360. ISBN 9781578085330. [Google Scholar]
- Khan, M.R. Plant nematodes-Global crop production. In Nematode Diseases of Crops and Their Sustainable Management; Khan, M.R., Quientinella, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 1–47. [Google Scholar]
- Abd-Elgawad, M.M.M. Understanding molecular plant–nematode interactions to develop alternative approaches for nematode control. Plants 2022, 11, 2141. [Google Scholar] [CrossRef]
- El-Sappah, A.H.; Islam, M.M.; El-Awady, H.H.; Yan, S.; Qi, S.; Liu, J.; Cheng, G.-T.; Liang, Y. Tomato natural resistance genes in controlling the root-knot nematode. Genes 2019, 10, 925. [Google Scholar] [CrossRef]
- Khan, M.R.; Khan, M.W.; Pasha, M.J. Effects of Sulfur Dioxide on the Development of Powdery Mildew of Cucumber. Environ. Exp. Bot. 1998, 40, 265–273. [Google Scholar] [CrossRef]
- Khan, M.R.; Khan, M.W. The Interaction of SO2 and Root-knot Nematode on Tomato. Environ. Pollut. 1993, 81, 91–102. [Google Scholar] [CrossRef]
- Khan, M.R.; Khan, M.W. Effects of Root-knot Nematode, Meloidogyne incognita, on the Sensitivity of Tomato to Sulfur Dioxide and Ozone. Environ. Exp. Bot. 1997, 38, 117–130. [Google Scholar] [CrossRef]
- Weber, D.E.; Reinert, R.A.; Barker, K.R. Ozone and sulfur dioxide effects on reproduction and host-parasite relationships of selected plant-parasitic nematodes. Phytopathology 1979, 69, 624–628. [Google Scholar] [CrossRef]
- Pratt, G.C.; Kromroy, K.W.; Krupa, S.V. Effects of ozone and sulphur dioxide on injury and foliar concentrations of sulphur and chlorophyll in soybean Glycine max. Environ. Pollut. Ser. A Ecol. Biol. 1983, 32, 91–99. [Google Scholar] [CrossRef]
- Jaubert-Possamai, S.; Noureddine, Y.; Favery, B. MicroRNAs, new players in the plant–nematode interaction. Front. Plant Sci. 2019, 10, 1180. [Google Scholar] [CrossRef] [PubMed]





| Cucurbits | Bitter Gourd | Bottle Gourd | Sponge Gourd | Cucumber | Pumpkin | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatments | No. of Galls/ Root | No. of Egg Masses/ Root | No. of Galls/ Root | No. of Egg Masses/ Root | No. of Galls/ Root | No. of Egg Masses/ Root | No. of Galls/ Root | No. of Egg Masses/ Root | No. of Galls/ Root | No. of Egg Masses/ Root |
| Ambient (Inoculated) | 11.19 c | 5.15 c | 171.44 b | 99.31 b | 185.33 b | 106.33 b | 165.51 b | 92.11 b | 199.33 b | 110.03 b |
| MI + 25 | 12.87 b | 5.94 b | 218.07 a | 126.12 a | 235.00 a | 134.83 a | 208.87 a | 116.06 a | 256.14 a | 141.06 a |
| MI + 50 | 14.16 a | 6.53 a | 221.50 a | 128.21 a | 238.52 a | 136.74 a | 211.03 a | 117.35 a | 260.72 a | 143.48 a |
| MI + 75 | 14.46 a | 6.66 a | 224.76 a | 130.39 a | 241.30 a | 138.87 a | 215.16 a | 119.47 a | 263.31 a | 145.24 a |
| LSD (p ≤ 0.05) | ||||||||||
| SO2 Level: Treatment | 0.72 | 0.33 | 10.96 | 6.35 | 11.79 | 6.77 | 10.93 | 6.07 | 12.85 | 7.08 |
| Cucurbits | Bitter Gourd | Bottle Gourd | Sponge Gourd | Cucumber | Pumpkin | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatments | Shoot | Root | Shoot | Root | Shoot | Root | Shoot | Root | Shoot | Root |
| Ambient (Uninoculated) | 29.11 a | 10.06 a | 31.31 a | 9.31 c | 28.33 a | 9.83 a | 27.31 a | 9.09 c | 31.61 a | 10.71 ab |
| 25 | 25.68 bc | 8.85 bc | 27.24 b | 8.06 d | 24.68 b | 8.55 c | 23.81 b | 7.93 d | 27.12 b | 9.20 bc |
| 50 | 25.62 bc | 8.84 bc | 26.43 b | 7.87 d | 24.08 b | 8.38 c | 23.38 b | 7.79 d | 26.36 b | 8.92 c |
| 75 | 25.35 bc | 8.75 c | 26.02 b | 7.73 d | 23.66 b | 8.22 c | 22.89 b | 7.64 d | 25.89 b | 8.80 c |
| Ambient (Inoculated) | 26.20 b | 9.07 bc | 26.83 b | 7.97 d | 24.19 b | 8.39 c | 23.49 b | 7.84 d | 26.55 b | 8.97 c |
| MI + 25 | 23.81 cd | 10.13 bc | 22.14 c | 10.21 b | 20.28 c | 10.03 b | 20.07 c | 9.73 bc | 21.81 c | 11.24 ab |
| MI + 50 | 22.07 de | 10.55 bc | 21.13 c | 10.87 ab | 19.29 c | 10.71 b | 18.95 c | 10.32 ab | 21.12 c | 11.66 a |
| MI + 75 | 20.84 e | 10.94 ab | 20.82 c | 11.23 a | 19.07 c | 10.91 ab | 18.79 c | 10.97 a | 20.86 cd | 11.94 a |
| LSD (p ≤ 0.05) | ||||||||||
| Treatment | 0.59 | 0.22 | 0.61 | 0.06 | 0.55 | 0.19 | 0.53 | 0.18 | 0.6 | 0.2 |
| SO2 Level | 0.83 | 0.31 | 0.86 | 0.09 | 0.78 | 0.27 | 0.76 | 0.25 | 0.85 | 0.29 |
| SO2 Level: Treatment | 1.19 | 0.43 | 1.21 | 0.12 | 1.1 | 0.38 | 1.07 | 0.36 | 1.2 | 0.4 |
| Cucurbits | Bitter Gourd | Bottle Gourd | Sponge Gourd | Cucumber | Pumpkin | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatments | Trichomes (cm−2) | Stomata (cm−2) | Trichomes (cm−2) | Stomata (cm−2) | Trichomes (cm−2) | Stomata (cm−2) | Trichomes (cm−2) | Stomata (cm−2) | Trichomes (cm−2) | Stomata (cm−2) |
| Ambient (Uninoculated) | 120.31 a | 88.11 a | 129.31 a | 93.21 a | 126.11 a | 84.33 a | 127.61 a | 98.33 a | 121.11 a | 92.22 a |
| 25 | 112.01 b | 82.12 b | 115.34 b | 83.24 b | 112.87 b | 75.64 b | 115.10 b | 88.79 b | 107.79 b | 81.80 b |
| 50 | 108.28 bc | 79.39 bc | 114.31 b | 82.49 b | 111.86 b | 74.80 b | 113.57 b | 87.71 b | 106.70 b | 81.15 b |
| 75 | 106.11 bcd | 78.24 bc | 108.75 b | 78.48 b | 106.69 b | 71.17 b | 108.47 b | 83.68 b | 101.13 b | 77.19 bc |
| Ambient (Inoculated) | 108.52 bc | 79.56 bc | 115.09 b | 82.77 b | 112.36 b | 75.14 b | 114.34 b | 87.91 b | 107.06 b | 81.61 b |
| MI + 25 | 101.30 cde | 74.19 cd | 94.40 c | 68.23 c | 92.44 c | 62.07 c | 94.43 c | 72.37 c | 86.47 c | 66.03 c |
| MI + 50 | 98.65 de | 72.16 d | 90.52 c | 65.06 c | 89.92 c | 59.96 c | 93.16 c | 71.19 c | 83.69 c | 63.91 c |
| MI + 75 | 97.21 e | 71.02 d | 88.06 c | 63.38 c | 86.26 c | 58.02 c | 89.33 cd | 68.54 cd | 81.51 c | 61.97 cd |
| LSD (p ≤ 0.05) | ||||||||||
| Treatment | 2.52 | 1.86 | 2.56 | 1.85 | 2.5 | 1.68 | 2.55 | 1.97 | 2.38 | 1.81 |
| SO2 Level | 3.58 | 2.62 | 3.62 | 2.61 | 3.54 | 2.37 | 3.61 | 2.78 | 3.37 | 2.56 |
| SO2 Level: Treatment | 5.06 | 3.71 | 5.11 | 3.69 | 5.01 | 3.35 | 5.11 | 3.93 | 4.76 | 3.63 |
| Cucurbits | Bitter Gourd | Bottle Gourd | Sponge Gourd | Cucumber | Pumpkin | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatments | Salicylic Acid | Carotenoids | Salicylic Acid | Carotenoids | Salicylic Acid | Carotenoids | Salicylic Acid | Carotenoids | Salicylic Acid | Carotenoids |
| Ambient (Uninoculated) | 16.31 c | 34.51 a | 18.31 c | 35.21 a | 15.11 c | 41.51 a | 17.11 c | 35.12 a | 18.91 c | 38.12 a |
| 25 | 17.35 b | 32.20 b | 20.10 b | 31.79 b | 16.47 b | 37.61 b | 18.74 b | 31.50 b | 20.82 b | 33.62 b |
| 50 | 17.86 b | 30.99 bc | 20.29 b | 31.20 b | 16.64 b | 36.99 b | 18.92 b | 31.05 b | 20.99 b | 33.51 b |
| 75 | 18.10 b | 30.23 bcd | 21.00 b | 29.65 b | 17.23 b | 35.20 b | 19.64 b | 29.71 bc | 21.75 b | 32.06 b |
| Ambient (Inoculated) | 17.66 b | 31.34 bc | 20.12 b | 31.58 b | 16.52 b | 37.40 b | 18.60 b | 31.47 b | 20.80 b | 33.85 b |
| MI + 25 | 18.81 a | 29.26 cd | 23.24 ab | 25.60 c | 19.13 a | 30.30 c | 21.71 a | 25.29 c | 24.24 a | 27.14 c |
| MI + 50 | 19.25 a | 28.30 d | 23.84 ab | 24.37 c | 19.52 a | 29.47 c | 22.23 a | 24.27 c | 24.77 a | 25.81 c |
| MI + 75 | 19.29 a | 27.99 d | 24.15 a | 24.08 c | 19.73 a | 29.22 c | 22.40 a | 23.99 c | 25.02 a | 25.58 c |
| LSD (p ≤ 0.05) | ||||||||||
| Treatment | 0.04 | 0.07 | 0.5 | 0.7 | 0.42 | 0.83 | 0.47 | 0.7 | 0.52 | 0.75 |
| SO2 Level | 0.06 | 1.02 | 0.71 | 0.99 | 0.59 | 1.17 | 0.67 | 0.98 | 0.74 | 1.06 |
| SO2 Level: Treatment | 0.09 | 1.45 | 1.01 | 1.4 | 0.83 | 1.66 | 0.94 | 1.39 | 0.05 | 1.5 |
| Cucurbits | Bitter Gourd | Bottle Gourd | Sponge Gourd | Cucumber | Pumpkin | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatments | Transpiration | Stomatal Conductance | Transpiration | Stomatal Conductance | Transpiration | Stomatal Conductance | Transpiration | Stomatal Conductance | Transpiration | Stomatal Conductance |
| Ambient (Uninoculated) | 1.91 d | 1.63 c | 2.31 c | 1.82 c | 2.81 c | 2.91 c | 2.82 c | 2.11 c | 2.11 c | 1.90 c |
| 25 | 2.04 cd | 1.75 bc | 2.54 b | 2.00 b | 3.07 b | 3.18 b | 3.09 b | 2.31 b | 2.33 b | 2.10 b |
| 50 | 2.09 bc | 1.78 b | 2.56 b | 2.01 b | 3.10 b | 3.22 b | 3.12 b | 2.33 b | 2.35 b | 2.12 b |
| 75 | 2.12 abc | 1.81 ab | 2.65 b | 2.09 b | 3.20 b | 3.31 b | 3.24 b | 2.41 b | 2.43 b | 2.19 b |
| Ambient (Inoculated) | 2.06 bcd | 1.77 b | 2.54 b | 2.00 b | 3.08 b | 3.18 b | 3.06 bc | 2.30 b | 2.33 bc | 2.09 bc |
| MI + 25 | 2.20 ab | 1.88 ab | 2.92 a | 2.29 a | 3.54 a | 3.67 a | 3.58 a | 2.68 a | 2.70 a | 2.43 a |
| MI + 50 | 2.25 a | 1.93 a | 3.00 a | 2.36 a | 3.60 a | 3.72 a | 3.62 a | 2.71 a | 2.77 a | 2.49 a |
| MI + 75 | 2.26 a | 1.94 a | 3.00 a | 2.36 a | 3.60 a | 3.73 a | 3.67 a | 2.75 a | 2.79 a | 2.51 a |
| LSD (p ≤ 0.05) | ||||||||||
| Treatment | 0.05 | 0.04 | 0.06 | 0.05 | 0.08 | 0.08 | 0.08 | 0.06 | 0.06 | 0.05 |
| SO2 Level | 0.07 | 0.06 | 0.09 | 0.07 | 0.1 | 0.11 | 0.1 | 0.08 | 0.08 | 0.08 |
| SO2 Level: Treatment | 0.1 | 0.08 | 0.13 | 0.10 | 0.15 | 0.16 | 0.16 | 0.11 | 0.12 | 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizvi, T.F.; Khan, M.R. Biological and Physiological Responses of Root-knot Disease Development on Five Cucurbits Exposed to Different Concentrations of Sulfur Dioxide. Toxics 2023, 11, 334. https://doi.org/10.3390/toxics11040334
Rizvi TF, Khan MR. Biological and Physiological Responses of Root-knot Disease Development on Five Cucurbits Exposed to Different Concentrations of Sulfur Dioxide. Toxics. 2023; 11(4):334. https://doi.org/10.3390/toxics11040334
Chicago/Turabian StyleRizvi, Tanveer Fatima, and Mujeebur Rahman Khan. 2023. "Biological and Physiological Responses of Root-knot Disease Development on Five Cucurbits Exposed to Different Concentrations of Sulfur Dioxide" Toxics 11, no. 4: 334. https://doi.org/10.3390/toxics11040334
APA StyleRizvi, T. F., & Khan, M. R. (2023). Biological and Physiological Responses of Root-knot Disease Development on Five Cucurbits Exposed to Different Concentrations of Sulfur Dioxide. Toxics, 11(4), 334. https://doi.org/10.3390/toxics11040334







