Lack of Known Target-Site Mutations in Field Populations of Ostrinia furnacalis in China from 2019 to 2021
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Field Populations from 2019 to 2021
2.2. Individual Crude DNA Extraction
2.3. Examination of Insecticide Resistance Mutations
2.4. PCR Amplification and Sequencing
3. Results
3.1. PCR Product Evaluation and Chromatograms of the Insecticide Resistance Mutations
3.2. Individual Genotype Sequencing Results from 2019 to 2021
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nafus, D.; Schreiner, I. Review of the biology and control of the Asian corn borer, Ostrinia furnacalis (Lep: Pyralidae). Trop. Pest. Manag. 1991, 37, 41–56. [Google Scholar] [CrossRef]
- Chang, X.; Liu, G.; He, K.; Shen, Z.; Peng, Y.; Ye, G. Efficacy evaluation of two transgenic maize events expressing fused proteins to Cry1Ab-susceptible and-resistant Ostrinia furnacalis (Lepidoptera: Crambidae). J. Econ. Entomol. 2013, 106, 2548–2556. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Ding, J.; Zhao, Y.; Luo, J.; Mu, W.; Zhang, Z. Cyantraniliprole at sublethal dosages negatively affects the development, reproduction, and nutrient utilization of Ostrinia furnacalis (Lepidoptera: Crambidae). J. Econ. Entomol. 2017, 110, 230–238. [Google Scholar]
- Huang, J.M.; Rao, C.; Wang, S.; He, L.F.; Zhao, S.Q.; Zhou, L.Q.; Zhao, Y.X.; Yang, F.X.; Gao, C.F.; Wu, S.F. Multiple target-site mutations occurring in lepidopterans confer resistance to diamide insecticides. Insect Biochem. Molec. 2020, 121, 103367. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.C.; Du, W.M.; Zang, L.S.; Ruan, C.C.; Zhang, J.J.; Zou, Z.; Monticelli, L.S.; Harwood, J.D.; Desneux, N. Multiparasitism: A promising approach to simultaneously produce Trichogramma chilonis and T. dendrolimi on eggs of Antheraea pernyi. Entomol. Gen. 2021, 41, 627–636. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Lu, X.; He, K.L.; Zhou, D.R. Review of history, present situation and prospect of the Asian Borer research in China. J. Shenyang Agric. Univ. 2000, 31, 404–412. [Google Scholar]
- Zhao, Y.X.; Huang, J.M.; Ni, H.; Guo, D.; Yang, F.X.; Wang, X.; Wu, S.F.; Gao, C.F. Susceptibility of fall armyworm, Spodoptera frugiperda (JE Smith), to eight insecticides in China, with special reference to lambda-cyhalothrin. Pestic. Biochem. Physiol. 2020, 168, 104623. [Google Scholar] [CrossRef]
- Desneux, N.; Decourtye, A.; Delpuech, J.M. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef]
- Hernández, M.; Margalida, A. Pesticide abuse in Europe: Effects on the Cinereous vulture (Aegypius monachus) population in Spain. Ecotoxicology 2008, 17, 264–272. [Google Scholar] [CrossRef]
- Aktar, W.; Sengupta, D.; Chowdhury, A. Impact of pesticides use in agriculture: Their benefits and hazards. Interdiscip. Toxicol. 2009, 2, 1–12. [Google Scholar] [CrossRef]
- Liu, S.; Xu, Y.; Gao, Y.; Zhao, Y.; Zhang, A.; Zang, L.; Wu, C.; Zhang, L. Panaxadiol saponins treatment caused the subtle variations in the global transcriptional state of Asiatic corn borer, Ostrinia furnacalis. J. Ginseng Res. 2020, 44, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Liu, N. Insecticide resistance in mosquitoes: Impact, mechanisms, and research directions. Annu. Rev. Entomol. 2015, 60, 537–559. [Google Scholar] [CrossRef]
- Chen, C.; Shi, X.; Desneux, N.; Han, P.; Gao, X. Detection of insecticide resistance in Bradysia odoriphaga Yang et Zhang (Diptera: Sciaridae) in China. Ecotoxicology 2017, 26, 868–875. [Google Scholar] [CrossRef]
- Jiang, D.; Qian, C.; Wang, D.; Wang, F.; Zhao, S.; Yang, Y.; Baxter, S.W.; Wang, X.; Wu, Y. Varying contributions of three ryanodine receptor point mutations to diamide insecticide resistance in Plutella xylostella. Pest. Manag. Sci. 2021, 77, 4874–4883. [Google Scholar] [CrossRef] [PubMed]
- Paula, D.P.; Lozano, R.E.; Menger, J.P.; Andow, D.A.; Koch, R.L. Identification of point mutations related to pyrethroid resistance in voltage-gated sodium channel genes in Aphis glycines. Entomol. Gen. 2021, 41, 243–255. [Google Scholar] [CrossRef]
- Wang, X.; Xu, X.; Ullah, F.; Ding, Q.; Gao, X.; Desneux, N.; Song, D. Comparison of full-length transcriptomes of different imidacloprid-resistant strains of Rhopalosiphum padi (L.). Entomol. Gen. 2021, 41, 289–304. [Google Scholar] [CrossRef]
- Qian, W.; Liu, N.; Yang, Y.; Liu, J.; He, J.; Chen, Z.; Li, M.; Qiu, X. A survey of insecticide resistance-conferring mutations in multiple targets in Anopheles sinensis populations across Sichuan, China. Parasites Vectors 2021, 14, 169. [Google Scholar] [CrossRef]
- Jiang, X.; Qu, M.; Denholm, I.; Fang, J.; Jiang, W.; Han, Z. Mutation in acetylcholinesterase1 associated with triazophos resistance in rice stem borer, Chilo suppressalis (Lepidoptera: Pyralidae). Biochem. Biophys. Res. Commun. 2009, 378, 269–272. [Google Scholar] [CrossRef]
- Chang, C.; Cheng, X.; Huang, X.Y.; Dai, S.M. Amino acid substitutions of acetylcholinesterase associated with carbofuran resistance in Chilo suppressalis. Pest. Manag. Sci. 2014, 70, 1930–1935. [Google Scholar] [CrossRef]
- Lee, D.W.; Choi, J.Y.; Kim, W.T.; Je, Y.H.; Song, J.T.; Chung, B.K.; Boo, K.S.; Koh, Y.H. Mutations of acetylcholinesterase1 contribute to prothiofos-resistance in Plutella xylostella (L.). Biochem. Biophys. Res. Commun. 2007, 353, 591–597. [Google Scholar] [CrossRef]
- Cassanelli, S.; Reyes, M.; Rault, M.; Manicardi, G.C.; Sauphanor, B. Acetylcholinesterase mutation in an insecticide-resistant population of the codling moth Cydia pomonella (L.). Insect Biochem. Mol. Biol. 2006, 36, 642–653. [Google Scholar] [CrossRef] [PubMed]
- Boaventura, D.; Bolzan, A.; Padovez, F.E.; Okuma, D.M.; Omoto, C.; Nauen, R. Detection of a ryanodine receptor target-site mutation in diamide insecticide resistant fall armyworm, Spodoptera frugiperda. Pest. Manag. Sci. 2020, 76, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Troczka, B.; Zimmer, C.T.; Elias, J.; Schorn, C.; Bass, C.; Davies, T.E.; Field, L.M.; Williamson, M.S.; Slater, R.; Nauen, R. Resistance to diamide insecticides in diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae) is associated with a mutation in the membrane-spanning domain of the ryanodine receptor. Insect Biochem. Mol. Biol. 2012, 42, 873–880. [Google Scholar] [CrossRef]
- Guo, L.; Liang, P.; Zhou, X.; Gao, X. Novel mutations and mutation combinations of ryanodine receptor in a chlorantraniliprole resistant population of Plutella xylostella (L.). Sci. Rep. 2014, 4, 6924. [Google Scholar] [CrossRef]
- Roditakis, E.; Steinbach, D.; Moritz, G.; Vasakis, E.; Stavrakaki, M.; Ilias, A.; García-Vidal, L.; del Rosario Martinez-Aguirre, M.; Bielza, P.; Morou, E. Ryanodine receptor point mutations confer diamide insecticide resistance in tomato leafminer, Tuta absoluta (Lepidoptera: Gelechiidae). Insect Biochem. Mol. Biol. 2017, 80, 11–20. [Google Scholar] [CrossRef]
- Yao, R.; Zhao, D.D.; Zhang, S.; Zhou, L.Q.; Wang, X.; Gao, C.F.; Wu, S.F. Monitoring and mechanisms of insecticide resistance in Chilo suppressalis (Lepidoptera: Crambidae), with special reference to diamides. Pest. Manag. Sci. 2017, 73, 1169–1178. [Google Scholar] [CrossRef]
- Huang, N.-X.; Jaworski, C.; Desneux, N.; Zhang, F.; Yang, P.-Y.; Wang, S. Long-term, large-scale releases of Trichogramma promote pesticide decrease in maize in northeastern China. Entomol. Gen. 2020, 40, 1. [Google Scholar] [CrossRef]
- Smith, T.J.; Lee, S.H.; Ingles, P.J.; Knipple, D.C.; Soderlund, D.M. The L1014F point mutation in the house fly Vssc1 sodium channel confers knockdown resistance to pyrethroids. Insect Biochem. Mol. Biol. 1997, 27, 807–812. [Google Scholar] [CrossRef]
- Gholizadeh, S.; Nouroozi, B.; Ladonni, H. Molecular detection of knockdown resistance (kdr) in Blattella germanica (Blattodea: Blattellidae) from northwestern Iran. J. Med. Entomol. 2014, 51, 976–979. [Google Scholar] [CrossRef]
- Ponce, G.; Sanchez, I.P.; García, S.M.; Torrado, J.M.; Lozano-Fuentes, S.; Lopez-Monroy, B.; Flores, A.E. First report of L1014F kdr mutation in Culex quinquefasciatus in Mexico. Insect Sci. 2016, 23, 829–834. [Google Scholar] [CrossRef]
- Da Cruz, D.L.; Paiva, M.H.S.; Guedes, D.R.D.; de Souza Gomes, E.C.; Pires, S.G.; Gomez, L.F.; Ayres, C.F.J. First report of the L1014F kdr mutation in wild populations of Anopheles arabiensis in Cabo Verde, West Africa. Parasites Vectors 2021, 14, 582. [Google Scholar] [CrossRef] [PubMed]
- Endersby, N.; Viduka, K.; Baxter, S.; Saw, J.; Heckel, D.G.; McKechnie, S. Widespread pyrethroid resistance in Australian diamondback moth, Plutella xylostella (L.), is related to multiple mutations in the para sodium channel gene. Bull. Entomol. Res. 2011, 101, 393–405. [Google Scholar] [CrossRef]
- Liu, N.; Feng, X.; Li, M.; Qiu, X. First detection of the kdr mutation (L1014F) in the plague vector Xenopsylla cheopis (Siphonaptera: Pulicidae). Parasites Vectors 2019, 12, 526. [Google Scholar] [CrossRef]
- Mota-Sanchez, D.; Wise, J. Arthropod Pesticide Resistance Database (APRD). Michigan State University. 2021. Available online: https://www.pesticideresistance.org/ (accessed on 1 June 2021).
- Jing, D.P.; Guo, J.F.; Jiang, Y.Y.; Zhao, J.Z.; Sethi, A.; He, K.L.; Wang, Z.Y. Initial detections and spread of invasive Spodoptera frugiperda in China and comparisons with other noctuid larvae in cornfields using molecular techniques. Insect Sci. 2020, 27, 780–790. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Zhai, Y.; Yang, Y.; Wu, Y.; Wang, X. Cadherin protein is involved in the action of Bacillus thuringiensis Cry1Ac toxin in Ostrinia furnacalis. Toxins 2021, 13, 658. [Google Scholar] [CrossRef] [PubMed]
- Atsumi, S.; Miyamoto, K.; Yamamoto, K.; Narukawa, J.; Kawai, S.; Sezutsu, H.; Kobayashi, I.; Uchino, K.; Tamura, T.; Mita, K. Single amino acid mutation in an ATP-binding cassette transporter gene causes resistance to Bt toxin Cry1Ab in the silkworm, Bombyx mori. Proc. Natl. Acad. Sci. USA 2012, 109, E1591–E1598. [Google Scholar] [CrossRef]
- Liang, J.G.; Zhang, D.D.; Li, D.Y.; Zhao, S.Y.; Wang, C.Y.; Xiao, Y.T.; Dong, X.; Yang, Y.Z.; Li, G.P.; Wang, L.L. Expression profiles of Cry1Ab protein and its insecticidal efficacy against the invasive fall armyworm for Chinese domestic GM maize DBN9936. J. Integr. Agr. 2021, 20, 792–803. [Google Scholar] [CrossRef]
- Carvalho, R.; Yang, Y.; Field, L.M.; Gorman, K.; Moores, G.; Williamson, M.S.; Bass, C. Chlorpyrifos resistance is associated with mutation and amplification of the acetylcholinesterase-1 gene in the tomato red spider mite, tetranychus evansi. Pestic. Biochem. Physiol. 2012, 104, 143–149. [Google Scholar] [CrossRef]
- Wu, S.; Zuo, K.; Kang, Z.; Yang, Y.; Oakeshott, J.G.; Wu, Y. A point mutation in the acetylcholinesterase-1 gene is associated with chlorpyrifos resistance in the plant bug Apolygus lucorum. Insect Biochem. Mol. Biol. 2015, 65, 75–82. [Google Scholar] [CrossRef]
- Alon, M.; Alon, F.; Nauen, R.; Morin, S. Organophosphates’ resistance in the B-biotype of Bemisia tabaci (Hemiptera: Aleyrodidae) is associated with a point mutation in an ace1-type acetylcholinesterase and overexpression of carboxylesterase. Insect Biochem. Mol. Biol. 2008, 38, 940–949. [Google Scholar] [CrossRef]
- Zhang, N.N.; Liu, C.F.; Yang, F.; Dong, S.I.; Han, Z.J.; Liu, N. Resistance mechanisms to chlorpyrifos and F392W mutation frequencies in the acetylcholine esterase ace1 allele of field populations of the tobacco whitefly, Bemisia tabaci in China. J. Insect Sci. 2012, 12, 41. [Google Scholar] [CrossRef] [PubMed]
- Vontas, J.G.; Hejazi, M.; Hawkes, N.J.; Cosmidis, N.; Loukas, M.; Hemingway, J. Resistance-associated point mutations of organophosphate insensitive acetylcholinesterase, in the olive fruit fly Bactrocera oleae. Insect Mol. Biol. 2002, 11, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wu, Y.; Chen, S.; Devine, G.; Denholm, I.; Jewess, P.; Moores, G. The involvement of microsomal oxidases in pyrethroid resistance in Helicoverpa armigera from Asia. Insect Biochem. Mol. Biol. 2004, 34, 763–773. [Google Scholar] [CrossRef]
- Wu, G.; Li, Q.; Liu, X.; Li-Byarlay, H.; He, B. Differential state-dependent effects of deltamethrin and tefluthrin on sodium channels in central neurons of Helicoverpa armigera. Pestic. Biochem. Physiol. 2021, 175, 104836. [Google Scholar] [CrossRef] [PubMed]
- Roditakis, E.; Vasakis, E.; Grispou, M.; Stavrakaki, M.; Nauen, R.; Gravouil, M.; Bassi, A. First report of Tuta absoluta resistance to diamide insecticides. J. Pest. Sci. 2015, 88, 9–16. [Google Scholar] [CrossRef]
- Cho, S.R.; Kyung, Y.; Shin, S.; Kang, W.J.; Jung, D.H.; Lee, S.J.; Park, G.H.; Kim, S.I.; Cho, S.W.; Kim, H.K. Susceptibility of field populations of Plutella xylostella and Spodoptera exigua to four diamide insecticides. Korean J. Appl. Entomol. 2018, 57, 43–50. [Google Scholar]
- Troczka, B.J.; Williamson, M.S.; Field, L.M.; Davies, T.E. Rapid selection for resistance to diamide insecticides in Plutella xylostella via specific amino acid polymorphisms in the ryanodine receptor. Neurotoxicology 2017, 60, 224–233. [Google Scholar] [CrossRef]
- Wei, Y.; Yan, R.; Zhou, Q.; Qiao, L.; Zhu, G.; Chen, M. Monitoring and mechanisms of chlorantraniliprole resistance in Chilo suppressalis (Lepidoptera: Crambidae) in China. J. Econ. Entomol. 2019, 112, 1348–1353. [Google Scholar] [CrossRef]
- Lv, S.L.; Shi, Y.; Zhang, J.C.; Liang, P.; Zhang, L.; Gao, X.W. Detection of ryanodine receptor target-site mutations in diamide insecticide-resistant Spodoptera frugiperda in China. Insect Sci. 2021, 28, 639–648. [Google Scholar] [CrossRef]
- Li, G.; Huang, J.; Ji, T.; Tian, C.; Zhao, X.; Feng, H. Baseline susceptibility and resistance allele frequency in Ostrinia furnacalis related to Cry1 toxins in the Huanghuaihai summer corn region of China. Pest. Manag. Sci. 2020, 76, 4311–4317. [Google Scholar] [CrossRef]
- Liu, X.; Liu, S.; Long, Y.; Wang, Y.; Zhao, W.; Shwe, S.M.; Wang, Z.; He, K.; Bai, S. Baseline Susceptibility and Resistance Allele Frequency in Ostrinia furnacalis in Relation to Cry1Ab Toxins in China. Toxins 2022, 14, 255. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, Y.; Huang, J.; Jin, W.; Yang, Y.; Wu, Y. CRISPR-mediated knockout of the ABCC2 gene in Ostrinia furnacalis confers high-level resistance to the Bacillus thuringiensis Cry1Fa toxin. Toxins 2020, 12, 246. [Google Scholar] [CrossRef] [PubMed]
- Zang, L.S.; Wang, S.; Zhang, F.; Desneux, N. Biological control with Trichogramma in China: History, present status, and perspectives. Annu. Rev. Entomol. 2021, 66, 463–484. [Google Scholar] [CrossRef]
- Gong, Y.; Li, M.; Li, T.; Liu, N. Molecular and functional characterization of three novel carboxylesterases in the detoxification of permethrin in the mosquito, Culex quinquefasciatus. Insect Sci. 2022, 29, 199–214. [Google Scholar] [CrossRef]
- Gong, Y.H.; Ai, G.M.; Li, M.; Shi, X.Y.; Diao, Q.Y.; Gao, X.W. Functional characterization of carboxylesterase gene mutations involved in Aphis gossypii resistance to organophosphate insecticides. Insect Mol. Biol. 2017, 26, 702–714. [Google Scholar] [CrossRef]
- Li, R.; Zhu, B.; Liang, P.; Gao, X. Identification of carboxylesterase genes contributing to multi-insecticide resistance in Plutella xylostella (L.). Entomol. Gen. 2022, 42, 967–976. [Google Scholar] [CrossRef]
- Li, X.; Li, R.; Zhu, B.; Gao, X.; Liang, P. Overexpression of cytochrome P450 CYP6BG1 may contribute to chlorantraniliprole resistance in Plutella xylostella (L.). Pest. Manag. Sci. 2018, 74, 1386–1393. [Google Scholar] [CrossRef]
- Tian, K.; Feng, J.; Zhu, J.; Cheng, J.; Li, M.; Qiu, X. Pyrethrin-resembling pyrethroids are metabolized more readily than heavily modified ones by CYP9As from Helicoverpa armigera. Pestic. Biochem. Physiol. 2021, 176, 104871. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Gong, C.; Yao, X.; Jiang, C.; Yang, Q. Molecular identification of four novel cytochrome P450 genes related to the development of resistance of Spodoptera exigua (Lepidoptera: Noctuidae) to chlorantraniliprole. Pest. Manag. Sci. 2018, 74, 1938–1952. [Google Scholar] [CrossRef]
- Wei, Z.-H.; Wang, X.-Q.; Li, P.-R.; Tan, X.-L.; Yang, X.-Q. Diet-mediated effects of cadmium on the fitness-related traits and detoxification and antioxidative enzymes in the oriental armyworm, Mythimna separata. Entomol. Gen. 2020, 40, 407–419. [Google Scholar] [CrossRef]
- Xu, D.; Liao, H.; Li, L.; Wu, M.; Xie, W.; Wu, Q.; Zhang, Y.; Zhou, X.; Wang, S. The CYP392D8 gene is not directly associated with abamectin resistance, a case study in two highly resistant Tetranychus urticae strains. Entomol. Gen. 2022. [Google Scholar] [CrossRef]
- Zeng, X.; Pan, Y.; Song, J.; Li, J.; Lv, Y.; Gao, X.; Tian, F.; Peng, T.; Xu, H.; Shang, Q. Resistance risk assessment of the ryanoid anthranilic diamide insecticide cyantraniliprole in Aphis gossypii Glover. J. Agric. Food Chem. 2021, 69, 5849–5857. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wen, Z.; Li, K.; Xu, W.; Liang, N.; Yu, X.; Li, C.; Chu, D.; Guo, L. Cytochrome P450 Gene, CYP6CX3, Is Involved in the Resistance to Cyantraniliprole in Bemisia tabaci. J. Agric. Food Chem. 2022, 70, 12398–12407. [Google Scholar] [CrossRef] [PubMed]
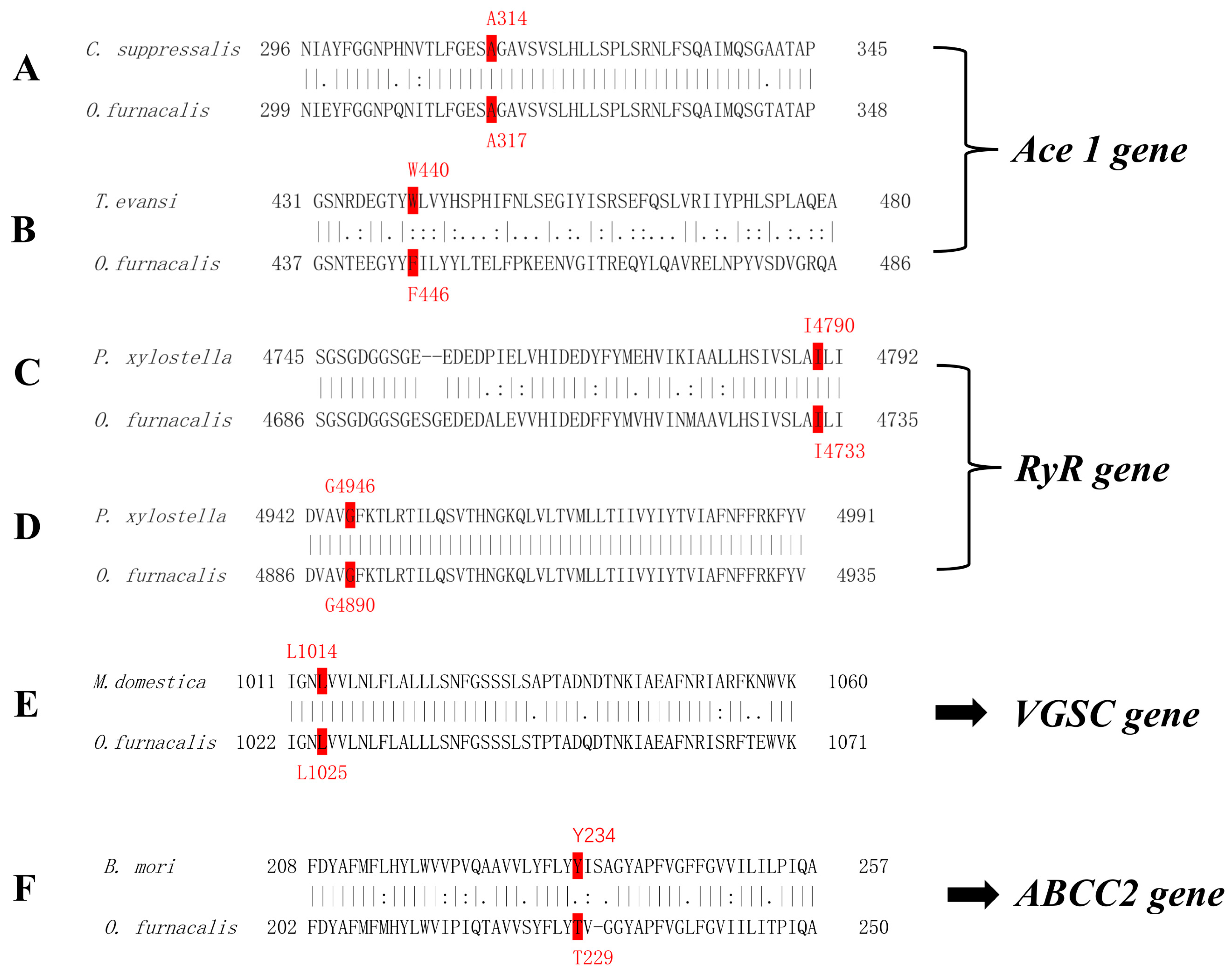
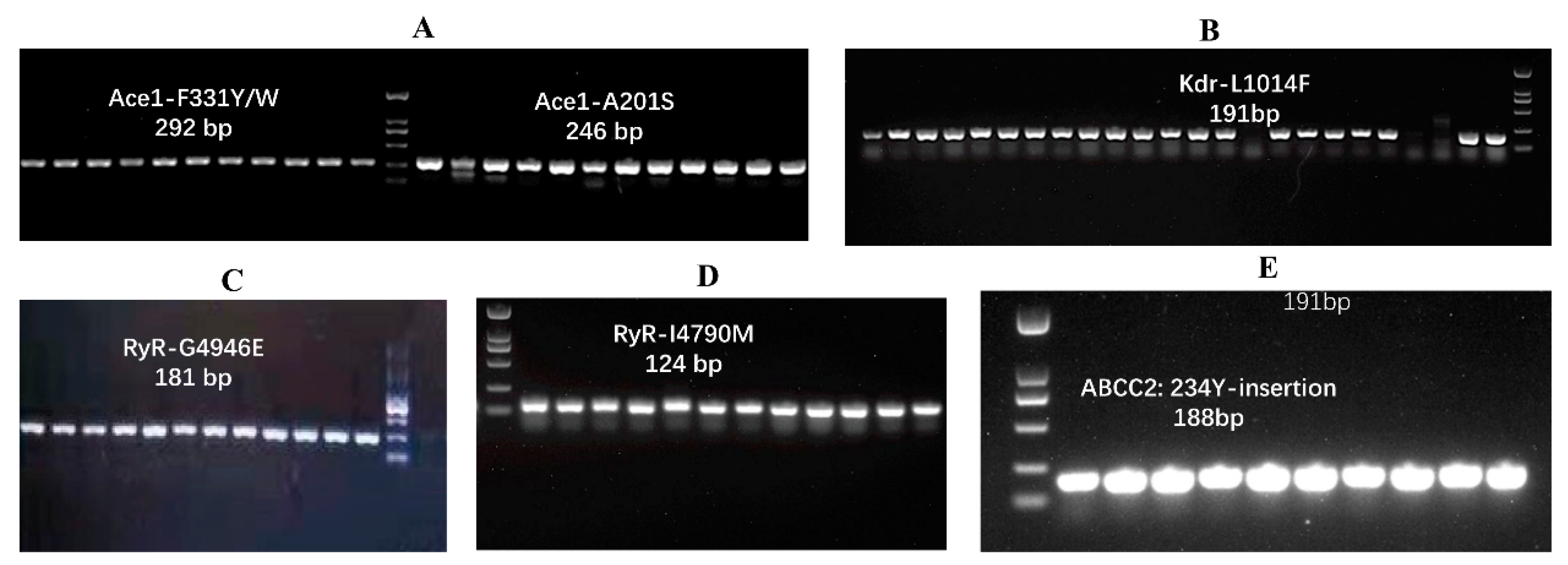
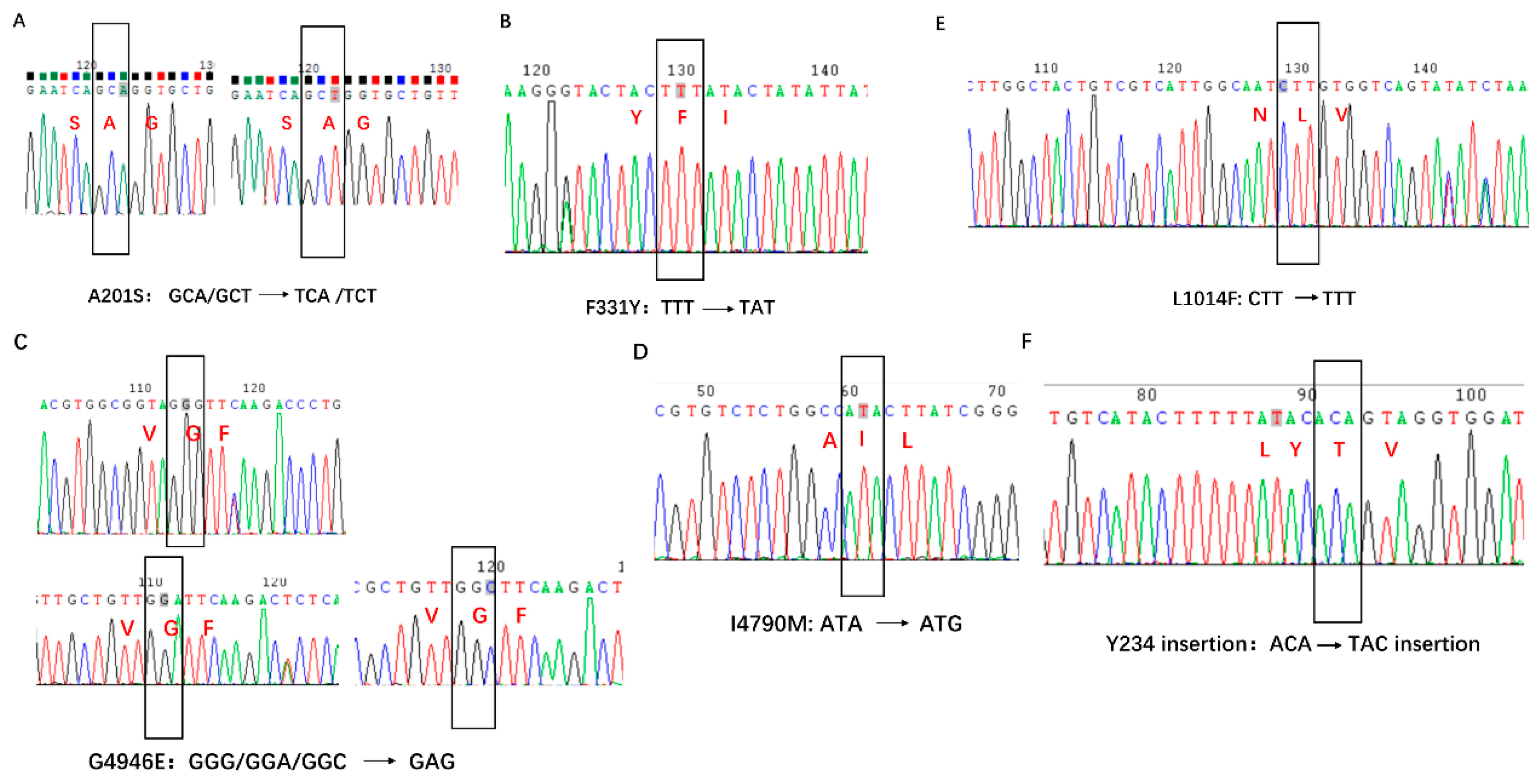
| Year | Province and Municipality | Location | Longitude, Latitude | Number Tested |
|---|---|---|---|---|
| 2019 | Guizhou | Bijie | 105.92° E, 26.84° N | 50 |
| Sichuan | Chongzhou | 103.67° E, 30.63° N | 50 | |
| Anhui | Bengbu | 116.8° E, 33.02° N | 60 | |
| Hebei | Hengshui | 115.28° E, 37.32° N | 45 | |
| Tianjing | Wuqing | 117.03° E, 39.22° N | 50 | |
| Liaoning | Shenyang | 123.56° E, 41.82° N | 30 | |
| 2020 | Yunnan | Puer | 101.38° E, 22.33° N | 25 |
| Guizhou | Bijie | 105.92° E, 26.84° N | 49 | |
| Chongqing | Wushan | 109.86° E, 31.1° N | 30 | |
| Sichuan | Chongzhou | 103.67° E, 30.63° N | 45 | |
| Jiangxi | Yongxiu | 115.81° E, 29.02° N | 50 | |
| Anhui | Bengbu | 116.8° E, 33.02° N | 50 | |
| Jiangsu | Binghai | 119.95° E, 34.1° N | 45 | |
| Henan | Nanyang | 112.8° E, 32.68° N | 30 | |
| Shandong | Changqing | 116.75° E, 36.55° N | 50 | |
| Hebei | Hengshui | 115.28° E, 37.32° N | 30 | |
| Tianjin | Wuqing | 117.03° E, 39.22° N | 50 | |
| Liaoning | Shenyang | 123.56° E, 41.82° N | 35 | |
| Liaoning a | Shenyang | 123.56° E, 41.82° N | 35 | |
| 2021 | Jiangxi | Yongxiu | 115.81° E, 29.02° N | 40 |
| Anhui | Bengbu | 116.8° E, 33.02° N | 50 | |
| Shandong | Changqing | 116.8° E, 33.02° N | 40 | |
| Hebei | Hengshui | 115.28° E, 37.32° N | 50 | |
| Tianjin | Wuqing | 117.03° E, 39.22° N | 35 |
| Insecticide Class | Target Gene | Mutations | References |
|---|---|---|---|
| Organophosphorus/Carbamate | Acetylcholinesterase (AchEs) | A201S, F331Y/W | [18,19,20] |
| diamide insecticides | ryanodine receptors (RyR) | G4946E, I4790M | [22,23,24] |
| Pyrethroids | voltage-gated sodium channel (VGSC) | Kdr L1014F | [28,30,31] |
| Bt toxin (Cry Ab) | ABC transporter (ABCC2) | 234 site Y insert | [37] |
| Mutations | Primer Pairs | Size of PCR Products (bp) | Sequencing Primer |
|---|---|---|---|
| Ace1-A201S | Forward: ATCGTGTTGCATCACTTGGA Reverse: CTGTTGCCGTTCCAGATTGC | 246 | Forward primer |
| Ace1-F331Y/W | Forward: CAACAACGAGTGGGGTACCTT Reverse: CTCGAACACTATCGCCTGCC | 292 | Reverse primer |
| RyR-G4946E | Forward: GACTGGCGCTACCAAGTGT Reverse: ATGCGTGACAGACTGCAAGA | 181 | Forward primer |
| RyR-I4790M | Forward: GAAGTGGTGCACATAGACGAAGA Reverse: GTGATCTCACCTTAAGATGGTAGTACC | 124 | Forward primer |
| Kdr-L1014F | Forward: GGAACTTTACAGATTTCATGCACA Reverse: TCTTAACGTTTTTGGTAATCAAG | 191 | Forward primer |
| 234Y-insertion | Forward: CGGCAAGCTCGTGAATCTTTTG Reverse: CGGCCTGTATTGGCGTTATCAA | 188 | Forward primer |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, Y.; Li, T.; Xiu, X.; Desneux, N.; Hou, M. Lack of Known Target-Site Mutations in Field Populations of Ostrinia furnacalis in China from 2019 to 2021. Toxics 2023, 11, 332. https://doi.org/10.3390/toxics11040332
Gong Y, Li T, Xiu X, Desneux N, Hou M. Lack of Known Target-Site Mutations in Field Populations of Ostrinia furnacalis in China from 2019 to 2021. Toxics. 2023; 11(4):332. https://doi.org/10.3390/toxics11040332
Chicago/Turabian StyleGong, Youhui, Ting Li, Xiaojian Xiu, Nicolas Desneux, and Maolin Hou. 2023. "Lack of Known Target-Site Mutations in Field Populations of Ostrinia furnacalis in China from 2019 to 2021" Toxics 11, no. 4: 332. https://doi.org/10.3390/toxics11040332
APA StyleGong, Y., Li, T., Xiu, X., Desneux, N., & Hou, M. (2023). Lack of Known Target-Site Mutations in Field Populations of Ostrinia furnacalis in China from 2019 to 2021. Toxics, 11(4), 332. https://doi.org/10.3390/toxics11040332










