Pesticides and Parabens Contaminating Aquatic Environment: Acute and Sub-Chronic Toxicity towards Early-Life Stages of Freshwater Fish and Amphibians
Abstract
1. Introduction
2. Materials and Methods
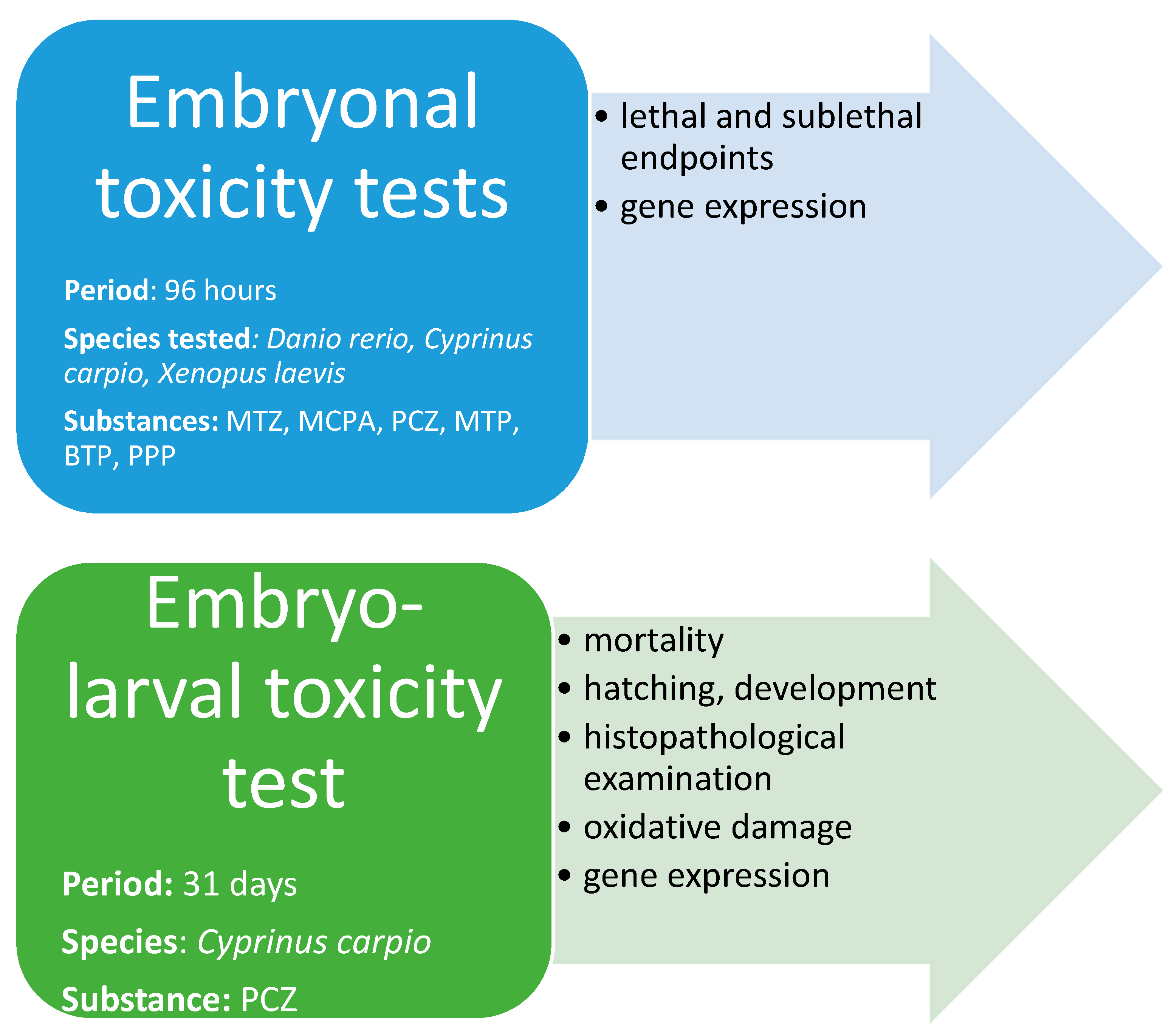
2.1. Experimental Design
2.1.1. Embryonal Toxicity Tests—Lethal and Sublethal Endpoints
2.1.2. Embryonal Toxicity Tests—Study on Gene Expression
2.1.3. Embryo-Larval Toxicity Test
2.2. Assays on Gene Expression
2.3. Control of the Tested Substances
2.3.1. Verification of Real Concentrations of Pesticides in Testing Solutions
2.3.2. Verification of Real Concentrations of Parabens in Testing Solutions
2.4. Statistical Analysis
3. Results and Discussion
3.1. Embryonal Toxicity Tests—Lethal and Sublethal Endpoints
3.1.1. Effects of Pesticides
3.1.2. Effects of Selected Parabens
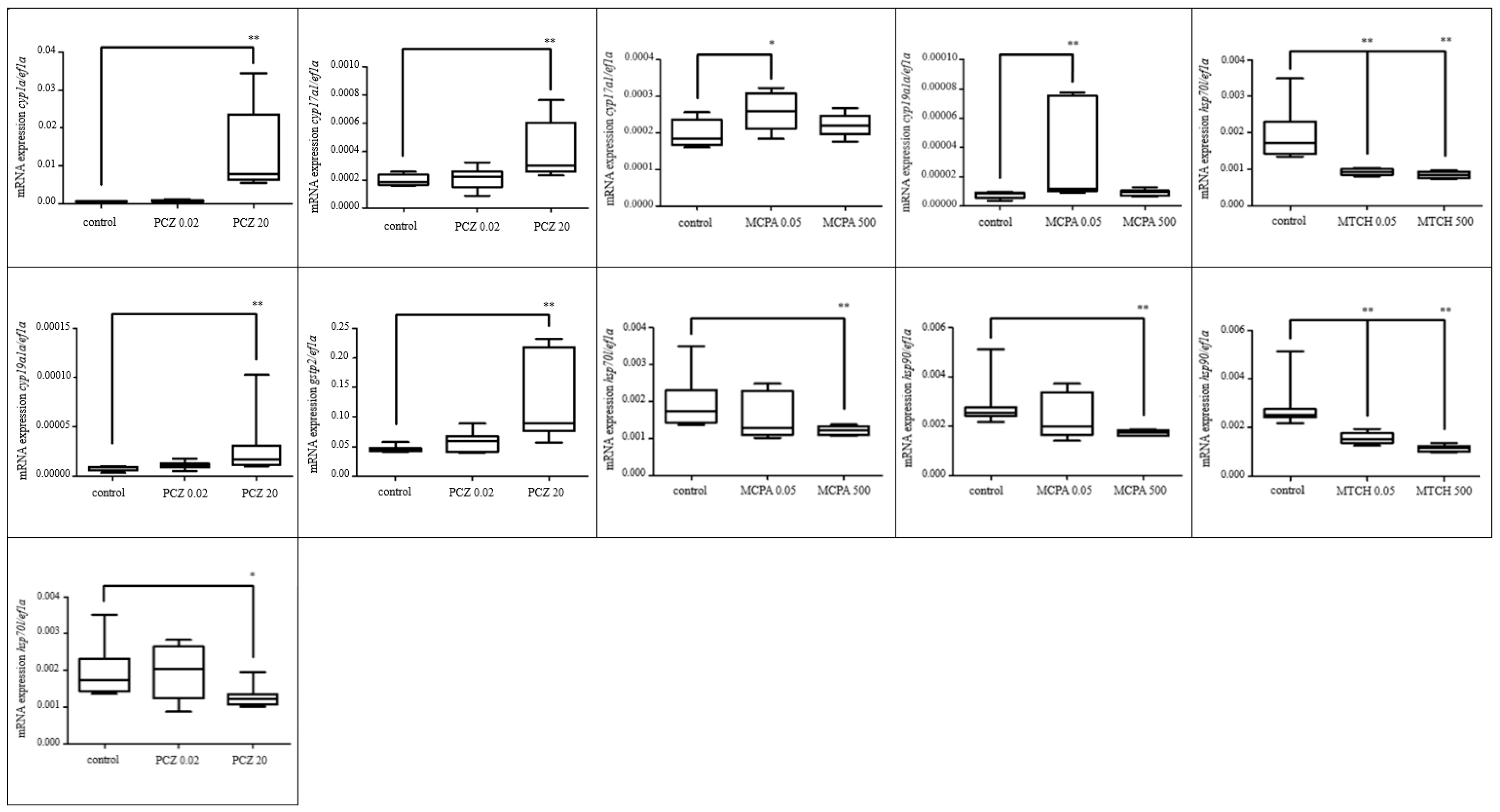
3.2. Embryonal Toxicity Tests—Study on Gene Expression
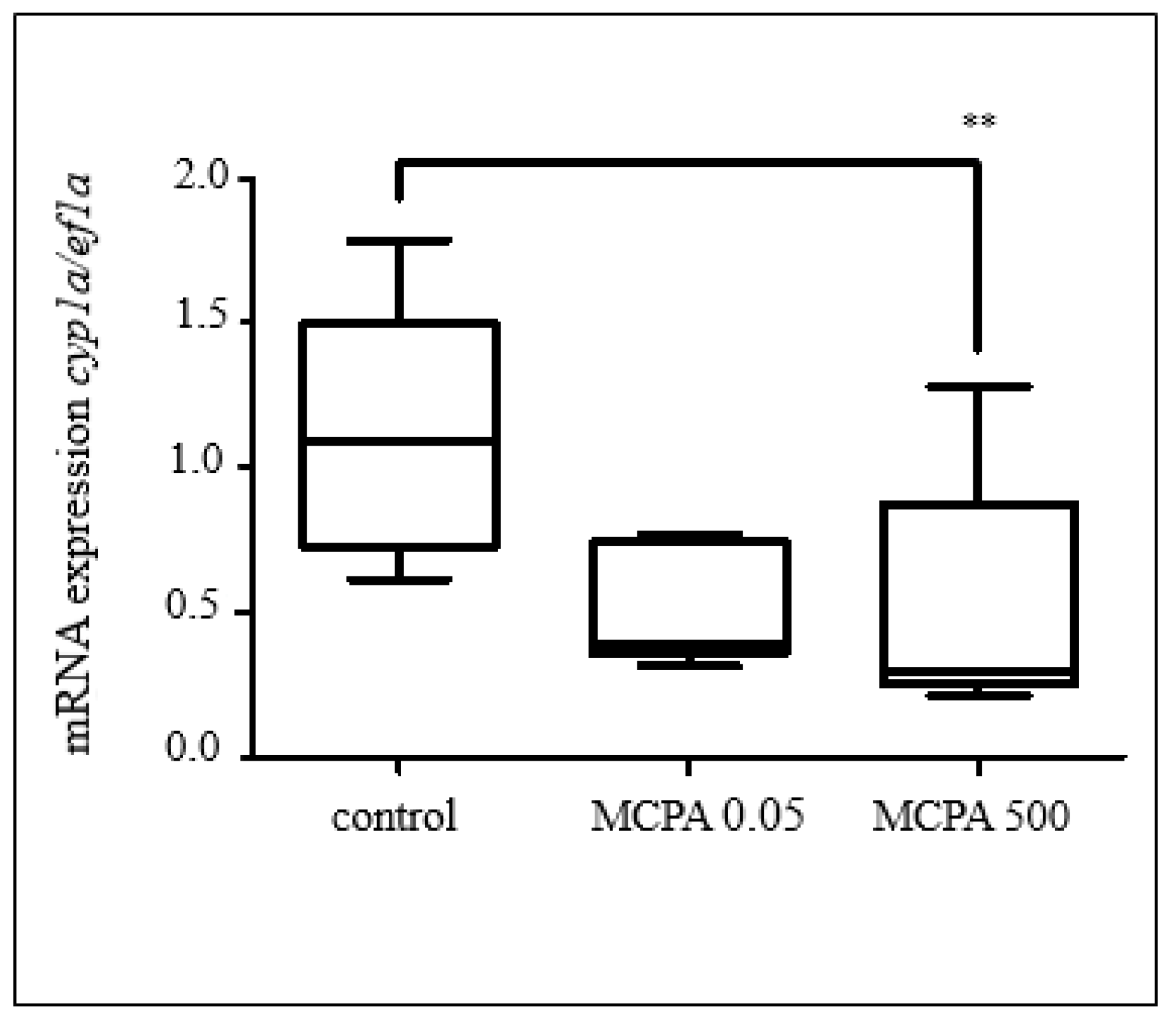
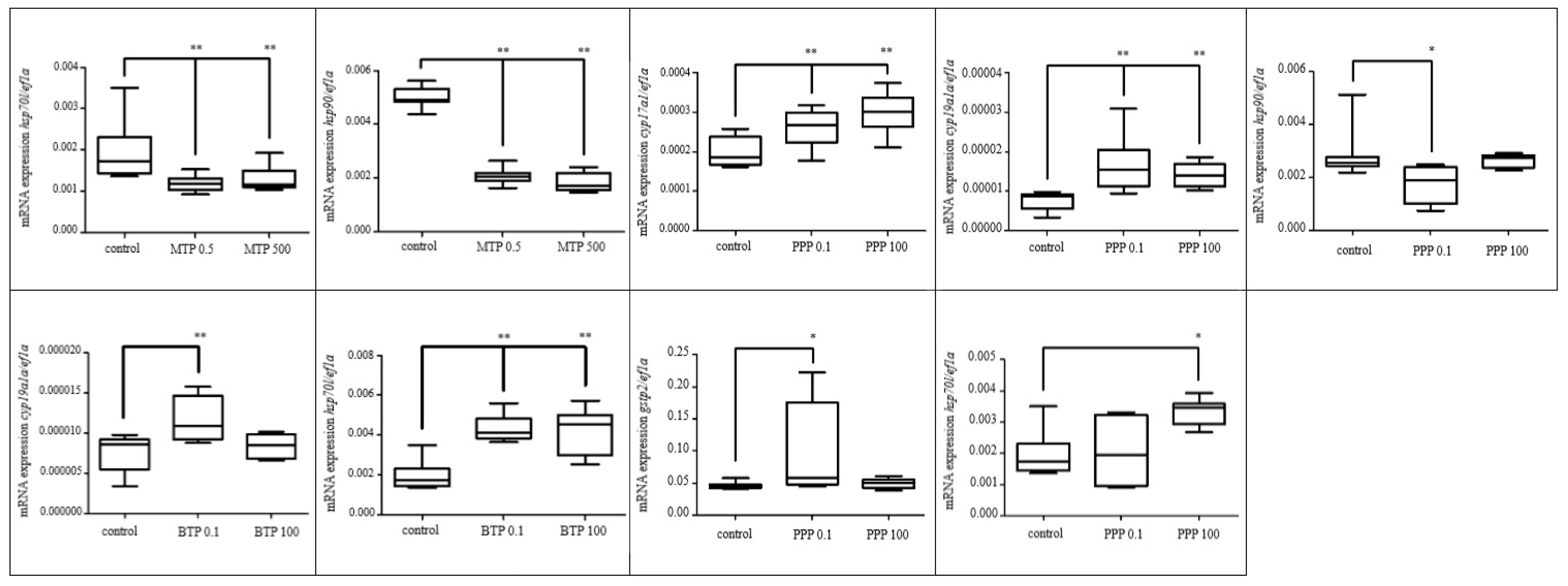
3.2.1. Effects of Selected Pesticides
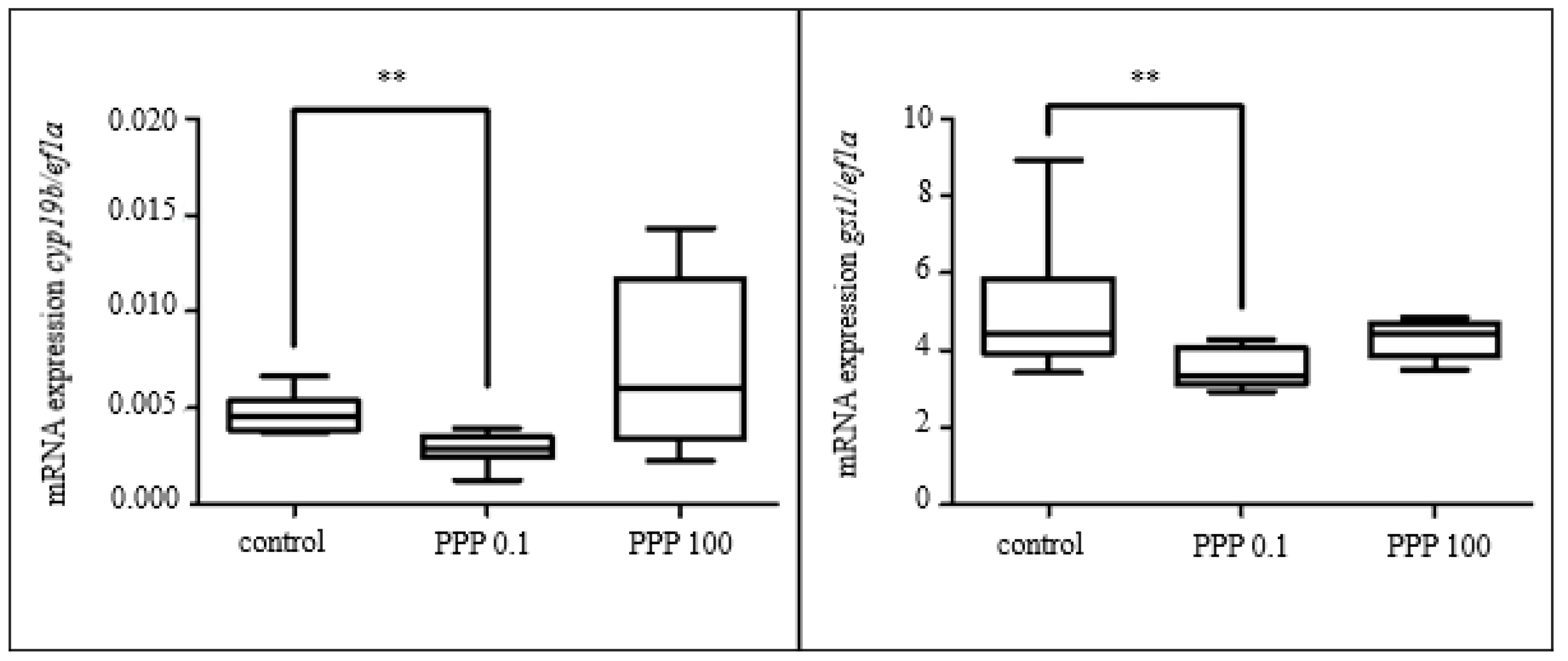
3.2.2. Effect of Selected Parabens
3.3. Embryo-Larval Toxicity Test
3.3.1. Mortality
3.3.2. Hatching, Development, and Morphometric and Condition Characteristics
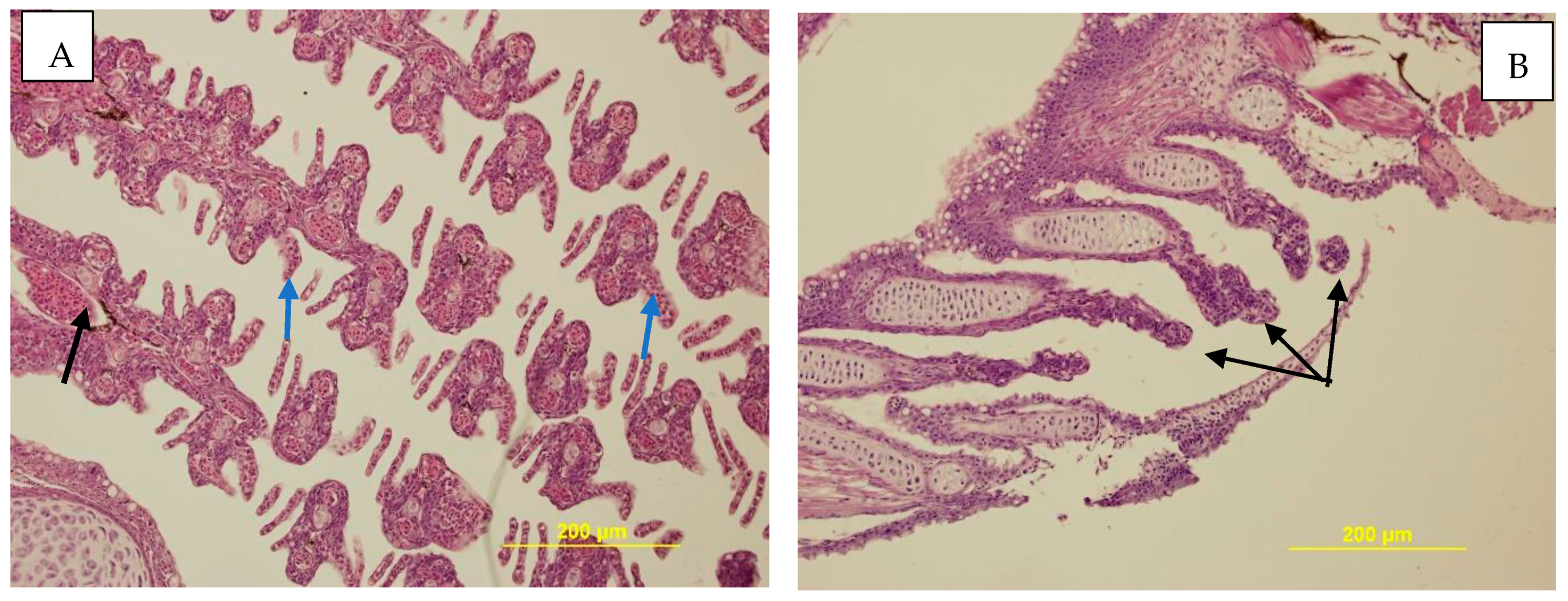
3.3.3. Histopathological Examination
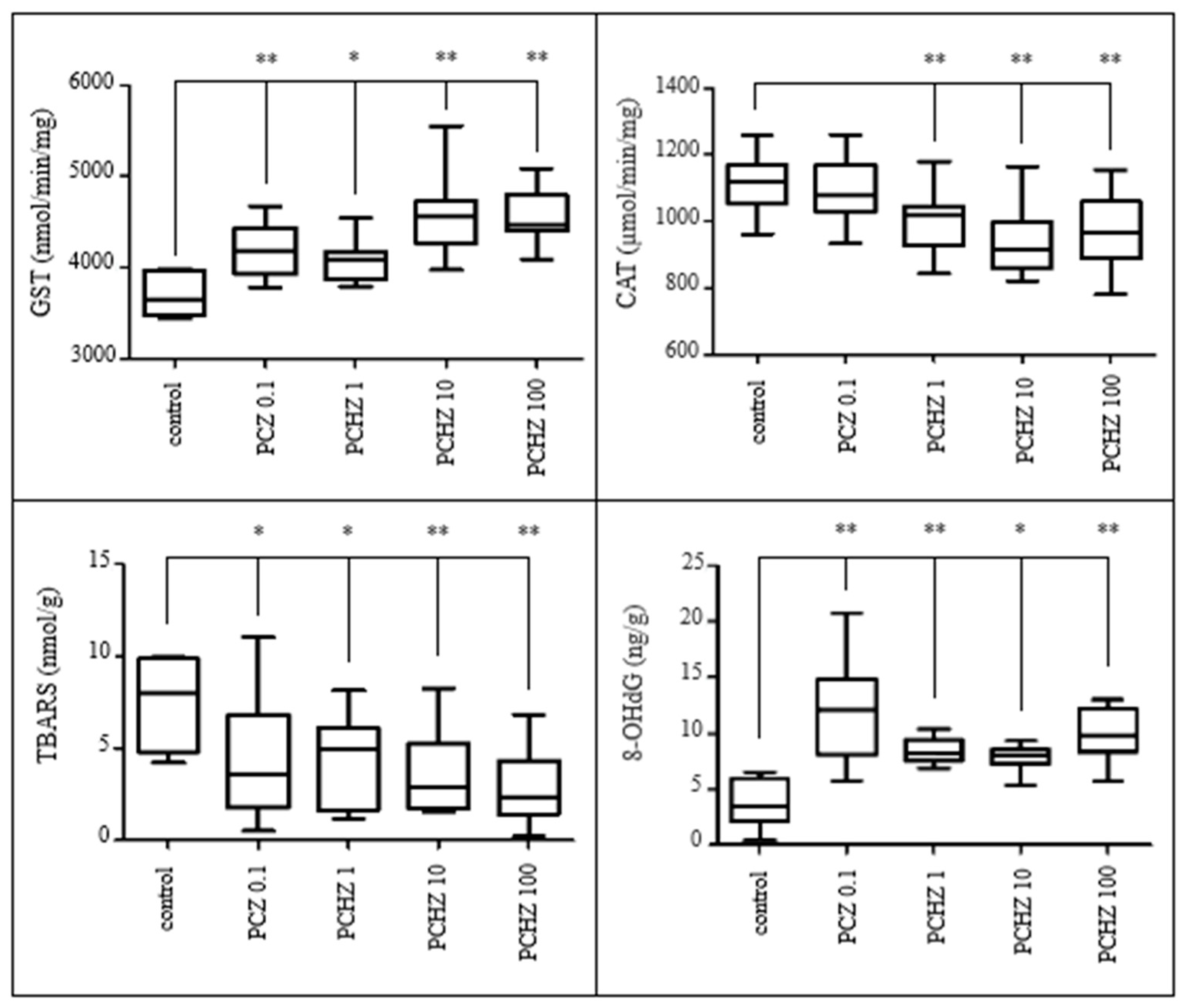
3.3.4. Indices on Oxidative Damage
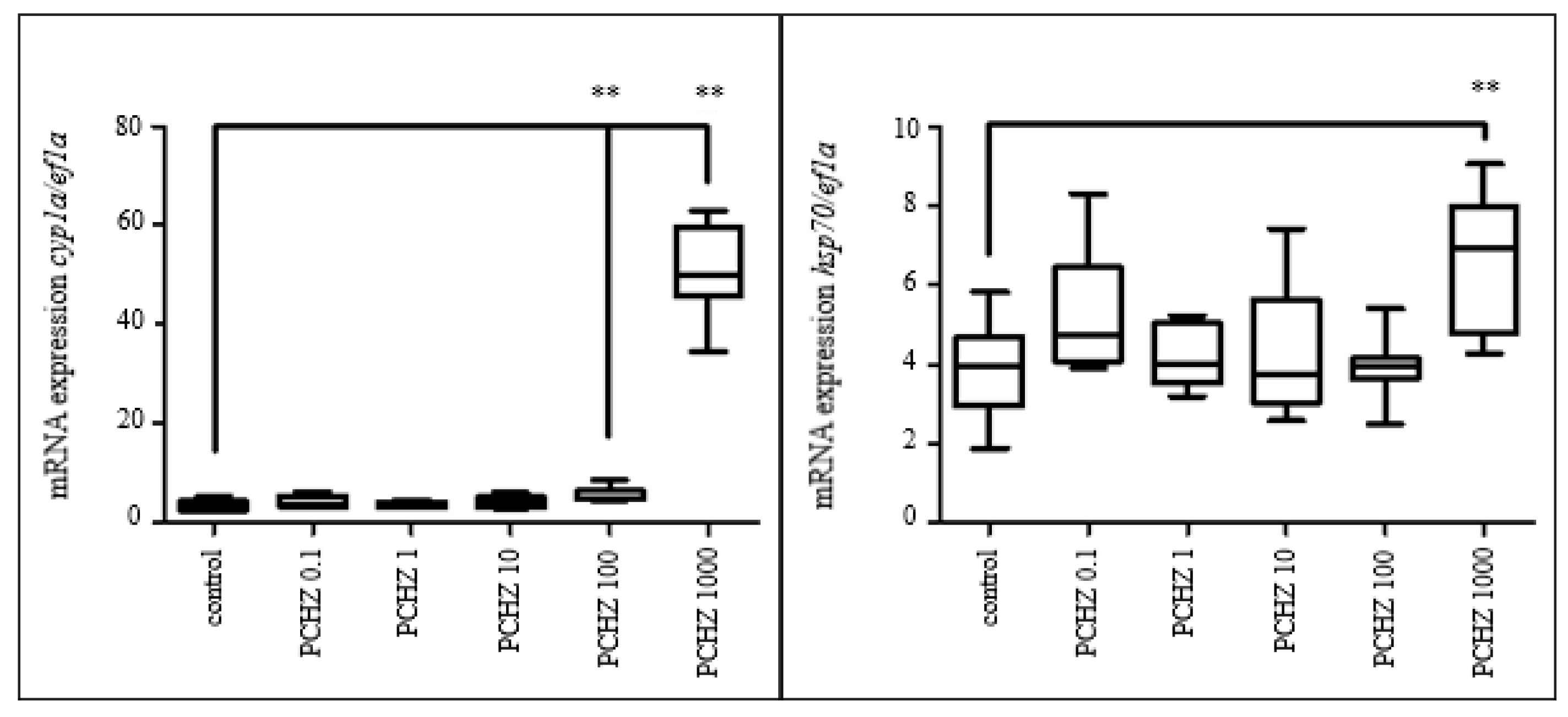
3.3.5. Gene Expression
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Désert, M.; Ravier, S.; Gille, G.; Quinapallo, A.; Armengaud, A.; Pochet, G.; Jean-Luc Savelli Wortham, H.; Quivet, E. Spatial and temporal distribution of current-use pesticides in ambient air of Provence-Alpes-Côte-d’Azur Region and Corsica, France. Atmos. Environ. 2018, 192, 241–256. [Google Scholar] [CrossRef]
- Rahman, M.; Hoque, M.d.S.; Bhowmik, S.; Ferdousi, S.; Kabiraz, M.P.; van Brakel, M.L. Monitoring of pesticides residues from fish feed, fish and vegetables in Bangladesh by GC-MS using the QuEChERS method. Heliyon 2021, 7, e06390. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, Z.F.; Liu, L.Y.; Zhu, F.J.; Ma, W.L. National-scale monitoring of historic used organochlorine pesticides (OCPs) and currently used pesticides (CUPs) in Chinese surface soil: Old topic and a new story. J. Hazard. Mater. 2022, 443, 130285. [Google Scholar] [CrossRef] [PubMed]
- Raby, M.; Lissemore, L.; Kaltenecker, G.; Beaton, D.; Prosser, R.S. Characterizing the exposure of streams in southern Ontario to agricultural pesticides. Chemosphere 2022, 294, 133769. [Google Scholar] [CrossRef] [PubMed]
- Dao, D.Q.; Taamalli, S.; Louis, F.; Kdouh, D.; Srour, Z.; Ngo, T.C.; Truong, D.H.; Fèvre-Nollet, V.; Ribaucour, M.; El Bakali, A.; et al. Hydroxyl radical-initiated decomposition of metazachlor herbicide in the gaseous and aqueous phases: Mechanism, kinetics, and toxicity evaluation. Chemosphere 2023, 312, 137234. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Available online: https://food.ec.europa.eu/plants/pesticides/eu-pesticides-database_en (accessed on 10 March 2023).
- Mohr, S.; Feibicke, M.; Berghahn, R.; Schmiediche, R.; Schmidt, R. Response of plankton communities in the freshwater pond and stream mesocosms to the herbicide metazachlor. Environ. Pollut. 2008, 152, 530–542. [Google Scholar] [CrossRef]
- Slaby, S.; Le Cor, F.; Dufour, V.; Auger, L.; Pasquini, L.; Cardoso, O.; Curtet, L.; Baudoin, J.M.; Wiest, L.; Vulliet, E.; et al. Distribution of pesticides and some of their transformation products in a small lentic waterbody: Fish, water, and sediment contamination in an agricultural watershed. Environ. Pollut. 2022, 292, 118403. [Google Scholar] [CrossRef]
- Quintana, J.; de la Cal, A.; Boleda, M.R. Monitoring the complex occurrence of pesticides in the Llobregat basin, natural and drinking waters in Barcelona metropolitan area (Catalonia, NE Spain) by a validated multi-residue online analytical method. Sci. Total Environ. 2019, 692, 952–965. [Google Scholar] [CrossRef]
- Lazartigues, A.; Fratta, C.; Baudot, R.; Wiest, L.; Feidt, C.; Thomas, M.; Cren-Olivé, C. Multiresidue method for the determination of 13 pesticides in three environmental matrices: Water, sediments, and fish muscle. Talanta 2011, 85, 1500–1507. [Google Scholar] [CrossRef]
- Velisek, J.V.; Stara, A.; Kubec, J.; Zuskova, E.; Buric, M.; Kouba, A. Effects of metazachlor and its major metabolite metazachlor OA on early life stages of marbled crayfish. Sci. Rep. 2020, 10, 875. [Google Scholar] [CrossRef]
- Polard, T.; Jean, S.; Gauthier, L.; Laplanche, C.; Merlina, G.; Sánchez-Pérez, J.M.; Pinelli, E. Mutagenic impact on fish of runoff events in agricultural areas in south-west France. Aquat. Toxicol. 2011, 101, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Haselman, J.T.; Kosian, P.A.; Korte, J.J.; Olmastead, A.W.; Degitz, S.J. Effect of multiple life stage exposure to the fungicide prochloraz in Xenopus laevis: Manifestations of antiandrogenic and other modes of toxicity. Aquat. Toxicol. 2018, 199, 240–251. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Wang, Y.; Li, J.; Wang, D.; Bao, Z.; Wang, Q.; Jin, Y. Health risks of chlorothalonil, carbendazim, prochloraz, their binary and ternary mixtures on embryonic and larval zebrafish based on metabolomics analysis. J. Hazard. Mater. 2021, 404, 124240. [Google Scholar] [CrossRef]
- Campos-Mañas, M.C.; Plaza-Bolaños, P.; Martínez-Piernas, A.B.; Sánchez-Pérez, J.A.; Agüera, A. Determination of pesticide levels in wastewater from an agro-food industry: Target, suspect and transformation product analysis. Chemosphere 2019, 232, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Campo, J.; Masiá, A.; Blasco, C.; Picó, Y. Occurrence and removal efficiency of pesticides in sewage treatment plants of four Mediterranean river basins. J. Hazard. Mater. 2013, 263, 146–157. [Google Scholar] [CrossRef]
- Belenguer, V.; Martinez-Capel, F.; Masiá, A.; Picó, Y. Patterns of presence and concentration of pesticides in fish and waters of the Júcar River (Eastern Spain). J. Hazard. Mater. 2014, 265, 271–279. [Google Scholar] [CrossRef]
- Petrarca, M.H.; Menezes-Sousa, D.; Ferreira, R.; Fernandes, J.O.; Vieira, L.R.; Guilhermino, L.; Cunha, S.C. Occurrence and risk assessment of endocrine-disrupting compounds in fish muscle: The case study of the Douro River estuary (North East Atlantic Ocean). Environ. Res. 2022, 215, 114236. [Google Scholar] [CrossRef]
- Dalhoff, K.; Gottardi, M.; Kretschmann, A.; Cedergreen, N. What causes the difference in synergistic potentials of propiconazole and prochloraz toward pyrethroids in Daphnia magna? Aquat. Toxicol. 2016, 172, 95–102. [Google Scholar] [CrossRef]
- Spaltro, A.; Pila, M.; Simonetti, S.; Álvarez-Torrellas, S.; García Rodríguez, J.; Ruiz, D.; Díaz Compañy, A.; Juan, A.; Allegretti, P. Adsorption and removal of phenoxy acetic herbicides from water by using commercial activated carbons: Experimental and computational studies. J. Contam. Hydrol. 2018, 218, 84–93. [Google Scholar] [CrossRef]
- Gaillard, J.; Thomas, M.; Lazartigues, A.; Bonnefille, B.; Pallez, C.; Dauchy, X.; Feidt, C.; Banas, D. Potential of barrage fish ponds for the mitigation of pesticide pollution in stream. Environ. Sci. Pollut. Res. 2016, 23, 23–35. [Google Scholar] [CrossRef]
- Morton, P.A.; Cassidy, R.; Floyd, S.; Doody, D.G.; McRoberts, W.C.; Jordan, P. Approaches to herbicide (MCPA) pollution mitigation in drinking water source catchments using enhanced space and time monitoring. Sci. Total Environ. 2021, 755, 142827. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, M.V.; Peris, A.; Postigo, C.; Moya-Garcés, A.; Monllor-Alcaraz, L.S.; Rambla-Alegre, M.; Eljarrat, E.; López de Alda, M. Evaluation of the occurrence and fate of pesticides in a typical Mediterranean delta ecosystem (Ebro River Delta) and risk assessment for aquatic organisms. Environ. Pollut. 2020, 274, 115813. [Google Scholar] [CrossRef]
- Tyohemba, R.L.; Pillay, L.; Humphries, M.S. Bioaccumulation of current-use herbicides in fish from a global biodiversity hotspot: Lake St Lucia, South Africa. Chemosphere 2021, 284, 131407. [Google Scholar] [CrossRef]
- Bermúdez-Saldaña, J.M.; Escuder-Gilabert, L.; Medina-Hernández, M.J.; Villanueva-Camañas, R.M.; Sagrado, S. Modelling bioconcentration of pesticides in fish using biopartitioning micellar chromatography. J. Chromatogr. A 2005, 1063, 153–160. [Google Scholar] [CrossRef]
- Wu, D.; Yun, Y.; Jiang, L.; Wu, C. Influence of dissolved organic matter on sorption and desorption of MCPA in ferralsol. Sci. Total Environ. 2018, 616–617, 1449–1456. [Google Scholar] [CrossRef] [PubMed]
- Osaili, T.M.; Al Sallagi, M.S.; Dhanasekaran, D.K.; Odeh, W.A.M.B.; Al Ali, H.J.; Al Ali, A.A.S.A.; Ismail, L.C.; Al Mehri, K.O.; Pisharath, V.A.; Holley, R.; et al. Pesticide residues in fresh fruits imported into the United Arab Emirates. Heliyon 2022, 8, e11946. [Google Scholar] [CrossRef]
- Silva, D.C.; Serrano, L.; Oliveira, T.M.A.; Mansano, A.S.; Almeida, E.A.; Vieira, E.M. Effects of parabens on antioxidant system and oxidative damages in Nile tilapia (Oreochromis niloticus). Ecotoxicol. Environ. Saf. 2018, 162, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Cheng, H.; Sang, N. Comprehensive assessment of estrogenic activities of parabens by in silico approach and in vitro assays. Sci. Total Environ. 2022, 845, 157194. [Google Scholar] [CrossRef]
- Cetinić, K.A.; Grgić, I.; Previšić, A.; Rožman, M. The curious case of methylparaben: Anthropogenic contaminant or natural origin? Chemosphere 2022, 294, 133781. [Google Scholar] [CrossRef]
- Maia, C.; Sousa, C.A.; Sousa, H.; Vale, F.; Simões, M. Parabens removal from wastewaters by microalgae—Ecotoxicity, metabolism and pathways. J. Chem. Eng. 2023, 453, 139631. [Google Scholar] [CrossRef]
- Emmanouil, C.; Bekyrou, M.; Psomopoulos, C.; Kungolos, A. An insight into ingredients of toxicological interest in personal care products and a small-scale sampling survey of the Greek market: Delineating a potential contamination source for water resources. Water 2019, 11, 2501. [Google Scholar] [CrossRef]
- Dambal, V.Y.; Selvan, K.P.; Lite, C.; Barathi, S.; Santosh, W. Developmental toxicity and induction of vitellogenin in embryo-larval stages of zebrafish (Danio rerio) exposed to methylparaben. Ecotoxicol. Environ. Saf. 2017, 141, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Bai, Y.; Sun, B.; Zhou, X.; Chen, L. Exposure to methylparaben at environmentally realistic concentrations significantly impairs neuronal health in adult zebrafish. J. Environ. Sci. 2023, 132, 134–144. [Google Scholar] [CrossRef]
- Bolujoko, N.B.; Ogunlaja, O.O.; Alfred, M.O.; Okewole, D.M.; Ogunlaja, A.; Olukanni, O.D.; Msagati, T.A.M.; Unuabonah Emmanuel, I. Occurrence and human exposure assessment of parabens in water sources in Osun State, Nigeria. Sci. Total Environ. 2022, 814, 152448. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Sun, B.; Tang, L.; Liu, M.; Huang, Z.; Zhou, X.; Chen, L. Hepatotoxicity caused by methylparaben in adult zebrafish. Aquat. Toxicol. 2022, 250, 106255. [Google Scholar] [CrossRef] [PubMed]
- Serra-Compte, A.; Maulvault, A.L.; Camacho, C.; Álvarez-Muñoz, D.; Barceló, D.; Rodríguez-Mozaz, S.; Marques, A. Effects of water warming and acidification on bioconcentration, metabolization and depuration of pharmaceuticals and endocrine disrupting compounds in marine mussels (Mytilus galloprovincialis). Environ. Pollut. 2018, 236, 824–834. [Google Scholar] [CrossRef]
- Raja, G.L.; Subhashree, D.K.; Lite, C.; Santosh, W.; Barathi, S. Transient exposure of methylparaben to zebrafish (Danio rerio) embryos altered cortisol level, acetylcholinesterase activity and induced anxiety-like behaviour. Gen. Comp. Endocrinol. 2019, 279, 53–59. [Google Scholar] [CrossRef]
- Sivaraman, L.; Pouliot, L.; Wang, B.; Brodie, T.; Graziano, M.; McNerney, M.E. Safety assessment of propylparaben in juvenile rats. Regul. Toxicol. Pharmacol. 2018, 92, 370–381. [Google Scholar] [CrossRef]
- Atli, E. The effects of ethylparaben and propylparaben on the development and fecundity of Drosophila melanogaster. Environ. Toxicol. Pharmacol. 2022, 92, 103856. [Google Scholar] [CrossRef]
- Calma, M.L.; Medina, P.M.B. Acute and chronic exposure of the holometabolous life cycle of Aedes aegypti L. to emerging contaminants naproxen and propylparaben. Environ. Pollut. 2020, 266, 115275. [Google Scholar] [CrossRef]
- Wang, N.; Hu, X.; Lu, S.; Ma, S.; Kang, L.; Liao, S.; Yu, Y. Interrelationship of anthropogenic activity and parabens in fish from Taihu Lake during 2009–2017. Environ. Pollut. 2019, 252, 1002–1009. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Lv, Y.-Z.; Zhang, L.-J.; Liu, W.-R.; Zhao, J.-L.; Yang, Y.-Y.; Jia, Y.-W.; Liu, Y.-S.; He, L.-Y.; Ying, G.-G. Bioaccumulation and risks of 24 personal care products in plasma of wild fish from the Yangtze River, China. Sci. Total Environ. 2019, 665, 810–819. [Google Scholar] [CrossRef]
- Moreno-Marenco, A.R.; Giraldo, L.; Moreno-Piraján, J.C. Adsorption of n-butylparaben from aqueous solution on surface of modified granular activated carbons prepared from African palm shell. Thermodynamic study of interactions. J. Environ. Chem. Eng. 2020, 8, 103969. [Google Scholar] [CrossRef]
- Okoye, C.O.; Okeke, E.S.; Okoye, K.C.; Echude, D.; Andong, F.A.; Chukwudozie, K.I.; Okoye, H.U.; Ezeonyejiaku, C.D. Occurrence and fate of pharmaceuticals, personal care products (PPCPs) and pesticides in African water systems: A need for timely intervention. Heliyon 2022, 8, 9143. [Google Scholar] [CrossRef] [PubMed]
- Bolujoko, N.B.; Unuabonah, E.I.; Alfred, M.O.; Ogunlaja, A.; Ogunlaja, O.O.; Omorogie, M.O.; Olukanni, O.D. Toxicity and removal of parabens from water: A critical review. Sci. Total Environ. 2021, 792, 148092. [Google Scholar] [CrossRef]
- Becerra-Herrera, M.; Miranda, V.; Richter, P. Rapid determination of parabens in water samples by ultra-high performance liquid chromatography coupled to time of flight mass spektrometry. Anal. Sci. 2019, 36, 675–679. [Google Scholar] [CrossRef]
- OECD. Test No. 236: Fish Embryo Acute Toxicity (FET) Test. OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, French, 2013. [Google Scholar]
- ISO 7346; Waterquality—Determination of the Acutelethal Toxicity of Substances to a Freshwaterfish [Brachydanio Rerio Hamilton-Buchanan (Teleostei, Cyprinidae)]—Part 1: Static Metod. European Committee for Standardization (CEN): Brussels, Belgium, 1996.
- Ubbels, G.A.; Hara, K.; Koster, C.H.; Kirschner, M.W. Evidence for a functional role of the cytoskeleton in determination of the dorsoventral axis in Xenopus laevis eggs. J. Embryol. Exp. Morphol. 1983, 77, 15–37. [Google Scholar] [CrossRef]
- Máchová, J.; Prokeš, M.; Kroupová, H.; Svobodová, Z.; Mácová, S.; Doleželová, P.; Velíšek, J. Early Ontogeny, Growth and Mortality of Common Carp (Cyprinus carpio) at Low Concentrations of Dimethyl Sulfoxide. Acta Vet. Brno. 2009, 78, 505–512. [Google Scholar] [CrossRef]
- Sehonova, P.; Plhalova, L.; Blahova, J.; Berankova, P.; Doubkova, V.; Prokes, M.; Tichy, F.; Vecerek, V.; Svobodova, Z. The effect of tramadol hydrochloride on early life stages of fish. Environ. Toxicol. Pharmacol. 2016, 44, 151–157. [Google Scholar] [CrossRef]
- OECD. Test No. 210: Fish, Early-Life Stage Toxicity Test. OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, French, 2013. [Google Scholar]
- Penaz, M.; Prokes, M.; Kouril, J.; Hamackova, J. Early development of the carp, Cyprinus carpio. Acta Sci. Nat. Brno. 1983, 17, 1–39. [Google Scholar]
- Vaclavik, J.; Sehonova, P.; Blahova, J.; Medkova, D.; Postulkova, E.; Maly, O.; Charvatova, M.; Stastny, K.; Lenz, J.; Mares, J.; et al. Foodborne fluoxetine impacts the immune response in rainbow trout (Oncorhynchus mykkis). Environ. Toxicol. Pharmacol. 2022, 90, 103818. [Google Scholar] [CrossRef] [PubMed]
- Zivna, D.; Sehonova, P.; Plhalova, L.; Marsalek, P.; Blahova, J.; Prokes, M.; Divisova, L.; Stancova, V.; Dobsikova, R.; Tichy, F.; et al. Effect of salicylic acid on early life stages of common carp (Cyprinus carpio). Environ. Toxicol. Pharmacol. 2015, 40, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Hodkovicova, N.; Hollerova, A.; Caloudova, H.; Blahova, J.; Franc, A.; Garajova, M.; Lenz, J.; Tichy, F.; Faldyna, M.; Kulich, P.; et al. Do foodborne polyethylene microparticles affect the health of rainbow trout (Oncorhynchus mykiss)? Sci. Total Environ. 2021, 793, 148490. [Google Scholar] [CrossRef] [PubMed]
- FAO (Food and Agriculture Organization). FAO Specifications and Evaluations for Plant Protection Products: Metazachlor; FAO: Rome, Italy, 1999. [Google Scholar]
- Mohr, S.; Berghahn, R.; Feibicke, M.; Meinecke, S.; Ottenströer, T.; Schmiedling, I.; Schmiediche, R.; Schmidt, R. Effects of the herbicide metazachlor on macrophytes and ecosystem function in freshwater pond and stream mesocosms. Aquat. Toxicol. 2007, 82, 73–84. [Google Scholar] [CrossRef]
- Guo, D.; Wang, Y.; Qian, Y.; Chen, C.; Jiao, B.; Cai, L.; Wang, Q. Joint acute and endocrine disruptive toxicities of malathion, cypermethrin and prochloraz to embryo-larval zebrafish, Danio rerio. Chemosphere 2017, 166, 63–71. [Google Scholar] [CrossRef]
- Domingues, I.; Oliveira, R.; Musso, C.; Cardoso, M.; Soares, A.M.; Loureiro, S. Prochloraz effects on biomarkers activity in zebrafish early life stages and adults. Environ. Toxicol. 2011, 28, 155–163. [Google Scholar] [CrossRef]
- Johansson, M.; Paha, H.; Kylin, H.; Merila, J. Toxicity of six pesticides to common frog (Rana temporaria) tadpoles. Environ. Toxicol. Chem. 2006, 25, 3164–3170. [Google Scholar] [CrossRef]
- Morton, P.A.; Fennell, C.; Cassidy, R.; Doody, D.; Fenton, O.; Mellander, P.-E.; Jordan, P. A review of the pesticide MCPA in the land-water environment and emerging research needs. Wiley Interdiscip. Rev. Water 2019, 7, 1–16. [Google Scholar] [CrossRef]
- Sun, Q.; Guo, W.; Wang, P.; Chang, Z.; Xia, X.; Du, Q. Toxicity of 2-methyl-4-chlorophenoxy acetic acid alone and in combination with cyhalofop-butyl to Cyprinus carpio embryos. Environ. Toxicol. Pharmacol. 2021, 87, 103697. [Google Scholar] [CrossRef]
- Merola, C.; Perugini, M.; Conte, A.; Angelozzi, G.; Bozzeli, M.; Amorena, M. Embryotocicity of methylparaben to zebrafish (Danio rerio) early-life stages. Comp. Biochem. Physiol. Part C Toxicol. Appl. Pharmacol. 2020, 236, 108792. [Google Scholar] [CrossRef]
- Bereketoglu, C.; Pradhan, A. Comparative transcriptional analysis of methylparaben and propylparaben in zebrafish. Sci. Total Environ. 2019, 671, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Merola, C.; Lai, O.; Conte, A.; Crescenzo, G.; Torelli, T.; Alloro, M.; Perugini, M. Toxicological assessment and developmental abnormalities induced by butylparaben and ethylparaben exposure in zebrafish early-life stages. Environ. Toxicol. Pharmacol. 2020, 80, 103504. [Google Scholar] [CrossRef] [PubMed]
- González-Doncel, M.; García-Mauriño, J.E.; San Segundo, L.; Beltrán, E.M.; Sastre, S.; Torija, C.F. Embryonic exposure of medaka (Oryzias latipes) to propylparaben: Effects on early development and post-hatching growth. Environ. Pollut. 2014, 184, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.M.; Kim, M.S.; Hwang, U.K.; Jeong, C.B.; Lee, J.S. Effects of methylparaben, ethylparaben, and propylparaben on life parameters and sex ratio in the marine copepod Tigriopus japonicus. Chemosphere 2019, 226, 388–394. [Google Scholar] [CrossRef]
- Yang, G.; Weng, Y.; Zhao, Y.; Wang, D.; Luo, T.; Jin, Y. Transcriptomic and targeted metabolomic analysis revealed the toxic effects of prochloraz on larval zebrafish. Sci. Total Environ. 2022, 822, 153625. [Google Scholar] [CrossRef]
- Guengerich, F.P. Cytochrome P450 and chemical toxikology. Chem. Res.Toxicol. 2008, 21, 70–83. [Google Scholar] [CrossRef]
- Zanger, U.M.; Schwab, M. Cytochrome P450 enzymes in drug metabolism: Regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol. Ther. 2013, 138, 103–141. [Google Scholar] [CrossRef]
- Garner, L.V.; Di Giulio, R.T. Glutathione transferase pi class 2 (GSTp2) protects against the cardiac deformities caused by exposure to PAHs but not PCB-126 in zebrafish embryos. Comp. Biochem. Physiol. Part-C Toxicol. Pharmacol. 2012, 155, 573–579. [Google Scholar] [CrossRef]
- Sanchez, W.; Piccini, B.; Porcher, J.M. Effect of prochloraz fungicide on biotransformation enzymes and oxidative stress parameters in three-spined stickleback (Gasterosteus aculeatus L.). J. Environ. Sci. Health B 2008, 43, 65–70. [Google Scholar] [CrossRef]
- Vinggaard, A.M.; Hass, U.; Dalgaard, M.; Andersen, H.R.; Bonefeld-Jørgensen, E.; Christiansen, S.; Laier, P.; Poulsen, M.E. Prochloraz: An imidazole fungicide with multiple mechanisms of action. Int. J. Androl. 2006, 29, 186–192. [Google Scholar] [CrossRef]
- Orton, F.; Lutz, I.; Kloas, W.; Routledge, E.J. Endocrine Disrupting Effects of Herbicides and Pentachlorophenol: In Vitro and in Vivo Evidence. Environ. Sci. Technol. 2009, 43, 2144–2150. [Google Scholar] [CrossRef] [PubMed]
- Hinfray, N.; Nóbrega, R.H.; Caulier, M.; Baudiffier, D.; Maillot-Maréchal, E.; Chadili, E.; Palluel, O.; Porcher, J.M.; Schulz, R.; Brion, F. Cyp17a1 and Cyp19a1 in the zebrafish testis are differentially affected by oestradiol. J. Endocrinol. 2013, 216, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Glanemann, C.; Loos, A.; Gorret, N.; Willis, L.B.; O’Brien, X.M.; Lessard, P.A.; Sinskey, A.J. Disparity between changes in mRNA abundance and enzyme activity in Corynebacterium glutaminicum: Implications for DNA microarray analysis. Appl. Microbiol. Biotechnol. 2003, 61, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Feder, M.E.; Hofmann, G.E. Heat-shock proteins, molecular chaperones, and the stress response: Evolutionary and ecological physiology. Annu. Rev. Physiol. 1999, 61, 243–282. [Google Scholar] [CrossRef]
- Evans, T.G.; Yamamoto, Y.; Jeffery, W.R.; Krone, P.H. Zebrafish Hsp70 is required for embryonic lens formation. Cell Stress Chaperones. 2005, 10, 66–78. [Google Scholar] [CrossRef]
- Lele, Z.; Hartson, S.D.; Martin, C.C.; Whiteshell, L.; Matts, R.L.; Krone, P.H. Disruption of zebrafish somite development by pharmacologic inhibition of Hsp90. Dev. Biol. 1999, 210, 56–70. [Google Scholar] [CrossRef]
- Yang, C.; Lim, W.; Song, G. Mediation of oxidative stress toxicity induced by pyrethroid pesticides in fish. Comp. Biochem. Physiol. Part-C Toxicol. Pharmacol. 2020, 234, 108758. [Google Scholar] [CrossRef]
- Sepici-Diçel, A.; Benli, A.C.K.; Selvi, M.; Sarikaya, R.; Şahin, D.; Özkul, I.A.; Erkoç, F. Sublethal cyfluthrin toxicity to carp (Cyprinus carpio L.) fingerlings: Biochemical, hematological, histopathological alterations. Ecotoxicol. Environ. Saf. 2009, 72, 1433–1439. [Google Scholar] [CrossRef]
- Ullah, S.; Li, Z.; Arifeen, M.Z.U.; Khan, S.U.; Fahad, S. Multiple biomarkers based appraisal of deltamethrin induced toxicity in silver carp (Hypophthalmichthys molitrix). Chemosphere 2019, 214, 519–533. [Google Scholar] [CrossRef]
- D’Souza, U.J.A. Pesticide toxicity and oxidative stress: A review. Borneo Rev. Med. Sci. 2017, 11, 9–19. [Google Scholar]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alexandria J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Modrá, H.; Haluzová, I.; Blahová, J.; Maršálek, P.; Svobodová, Z. Effects of subchronic exposure to Spartakus (prochloraz 450gl−1) on haematological, biochemical and biometric indices of common carp Cyprinus carpio. Toxicol. Lett. 2010, 196, S319. [Google Scholar] [CrossRef]
- Lundqvist, J.; Hellman, B.; Oskarsson, A. Fungicide prochloraz induces oxidative stress and DNA damage in vitro. Food Chem. Toxicol. 2016, 91, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Lidova, J.; Stara, A.; Kouba, A.; Velisek, J. The effects of cypermethrin on oxidative stress and antioxidant biomarkers in marbled crayfish (Procambarus fallax f. virginalis). Neuroendocrinol. Lett. 2016, 37, 53–59. [Google Scholar]
- Stara, A.; Zuskova, E.; Velisek, J. Acute toxicity effect of cypermethrin on common carp (Cyprinus carpio). Neuroendocrinol. Lett. 2016, 37, 60–66. [Google Scholar]
- Toni, C.; Loro, V.L.; Santi, A.; de Menezes, C.C.; Cattaneo, R.; Clasen, B.E.; Zanella, R. Exposure to tebuconazol in rice field and laboratory conditions induces oxidative stress in carp (Cyprinus carpio). Comp. Biochem. Physiol. 2011, 153, 128–132. [Google Scholar] [CrossRef]
- Sehonova, P.; Hodkovicova, N.; Urbanova, M.; Orn, S.; Blahova, J.; Svobodova, Z.; Faldyna, M.; Chloupek, P.; Briedikova, K.; Carlsson, G. Effects of antidepressants with different modes of action on early life stages of fish and amphibians. Environ. Pollut. 2019, 254, 112999. [Google Scholar] [CrossRef]
- Hodkovicova, N.; Sehonova, P.; Blahova, J.; Faldyna, M.; Marsalek, P.; Mikula, P.; Chloupek, P.; Dobsikova, R.; Vecerek, V.; Vicenova, M.; et al. The effect of the antidepressant venlafaxine on gene expression of biotransformation enzymes in zebrafish (Danio rerio) embryos. Environ. Sci. Pollut. Res. 2020, 27, 1686–1696. [Google Scholar] [CrossRef]
- Karaca, M.; Varisli, L.; Korkmaz, K.; Ozaydin, O.; Parcin, F.; Orhan, H. Organochlorine pesticides and antioxidant enzymes are inversely correlated with liver enzyme gene expression in Cyprinus carpio. Toxicol. Lett. 2014, 230, 198–207. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, Z.; Chen, D.; Wang, L.; Yao, H.; Zhao, F.; Xing, H.; Xu, S. Effect of atrazine and chlorpyrifos exposure on heat shock protein response in the brain of common carp (Cyprinus carpio L.). Pestic. Biochem. Physiol. 2013, 107, 277–283. [Google Scholar] [CrossRef]



| Group Tested | Concentrations Tested (µg/L) | The Concentration of Solvent Used in Testing Solutions 3 |
|---|---|---|
| Control | X | |
| Control with ethanol | Ethanol 0.01% | |
| Control with DMSO | DMSO 0.01% | |
| MTCH | 0.05; 50; 500; 5000;100,000 | DMSO 0.01% |
| PCZ | 0.02; 0.2; 20; 200; 20,000 | DMSO 0.01% |
| MCPA | 0.05; 50; 500; 5000; 100,000 | Ethanol 0.01% |
| MTP | 0.5; 50; 500; 5000; 100,000 | Ethanol 0.01% |
| PPP | 0.1; 10; 100; 1000; 100,000 | Ethanol 0.01% |
| BTP | 0.1; 10; 100; 1000; 100,000 | Ethanol 0.01% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medkova, D.; Hollerova, A.; Riesova, B.; Blahova, J.; Hodkovicova, N.; Marsalek, P.; Doubkova, V.; Weiserova, Z.; Mares, J.; Faldyna, M.; et al. Pesticides and Parabens Contaminating Aquatic Environment: Acute and Sub-Chronic Toxicity towards Early-Life Stages of Freshwater Fish and Amphibians. Toxics 2023, 11, 333. https://doi.org/10.3390/toxics11040333
Medkova D, Hollerova A, Riesova B, Blahova J, Hodkovicova N, Marsalek P, Doubkova V, Weiserova Z, Mares J, Faldyna M, et al. Pesticides and Parabens Contaminating Aquatic Environment: Acute and Sub-Chronic Toxicity towards Early-Life Stages of Freshwater Fish and Amphibians. Toxics. 2023; 11(4):333. https://doi.org/10.3390/toxics11040333
Chicago/Turabian StyleMedkova, Denisa, Aneta Hollerova, Barbora Riesova, Jana Blahova, Nikola Hodkovicova, Petr Marsalek, Veronika Doubkova, Zuzana Weiserova, Jan Mares, Martin Faldyna, and et al. 2023. "Pesticides and Parabens Contaminating Aquatic Environment: Acute and Sub-Chronic Toxicity towards Early-Life Stages of Freshwater Fish and Amphibians" Toxics 11, no. 4: 333. https://doi.org/10.3390/toxics11040333
APA StyleMedkova, D., Hollerova, A., Riesova, B., Blahova, J., Hodkovicova, N., Marsalek, P., Doubkova, V., Weiserova, Z., Mares, J., Faldyna, M., Tichy, F., Svobodova, Z., & Lakdawala, P. (2023). Pesticides and Parabens Contaminating Aquatic Environment: Acute and Sub-Chronic Toxicity towards Early-Life Stages of Freshwater Fish and Amphibians. Toxics, 11(4), 333. https://doi.org/10.3390/toxics11040333








