Abstract
Cadmium has been well recognized as a critical toxic agent in acute and chronic poisoning cases in occupational and nonoccupational settings and environmental exposure situations. Cadmium is released into the environment after natural and anthropogenic activities, particularly in contaminated and industrial areas, causing food pollution. In the body, cadmium has no biological activity, but it accumulates primarily in the liver and kidney, which are considered the main targets of its toxicity, through oxidative stress and inflammation. However, in the last few years, this metal has been linked to metabolic diseases. The pancreas–liver–adipose axis is largely affected by cadmium accumulation. Therefore, this review aims to collect bibliographic information that establishes the basis for understanding the molecular and cellular mechanisms linked to cadmium with carbohydrate, lipids, and endocrine impairments that contribute to developing insulin resistance, metabolic syndrome, prediabetes, and diabetes.
1. Introduction
Heavy metals are an environmental threat; every year, there are acute and chronic poisoning cases in occupational and nonoccupational exposure conditions. Epidemiological studies have demonstrated that environmental exposure to low levels of toxic metals develops diverse pathologies. Thus, there is a growing need to understand the molecular events, physiological adaptations, and physiopathological modifications generated by heavy metal exposure. Cadmium (Cd) is a nonessential metal, considered one of the top five most hazardous environmental contaminants by the Agency for Toxic Substances and Disease Registry (ATSDR) [1].
Around 45,000 tons of Cd per year from different sources, such as volcanic emissions, burning fossil fuels, and abrasion of sedimentary rocks, contaminate farmland, water tables, air, and oceans [2]. The United States Geological Survey Mineral Yearbook provides helpful information on global Cd production, where it is explained that the most important sources between 2017–2020 came from Australia, 29%; China, 20%; Germany, 19%; Peru, 11%; and others, 29%. Meanwhile, the refinery production in 2020 and 2021 was led by China, the Republic of Korea, Japan, Canada, Kazakhstan, Russia, and Mexico. Industrial globalization and anthropogenic sources such as phosphate fertilizers (54–58%), atmospheric deposition (39–41%), and sewage sludge (2–5%) contribute to Cd contamination in vegetables, fruits, tubercules, cereals, and legumes [3]. In addition, cigarette smoking may represent an additional Cd source that may equal or exceed that of food.
Cadmium poses a significant health risk due to a meager elimination rate (20–40 years); even very low Cd concentrations can accumulate in multiple tissues. The metal cannot degrade to less toxic species and is poorly excreted [4]. The liver, kidney, lungs, testes, prostate, heart, skeletal system, nervous system, and immune system are target organs for Cd toxicity. Furthermore, the principal reservoirs for cadmium are the liver, lungs, bones, and kidneys [5]. Hence, Itai-Itai disease, osteomalacia, osteoporosis, bone fractures, severely impaired renal function, emphysema, anosmia, chronic rhinitis, liver and cardiovascular diseases, testicular dysfunction, and cancer are associated with Cd toxicity. However, adipocyte damage or Langerhans islet dysfunction is rarely mentioned; thus, diabetes and metabolic alterations are not commonly associated with Cd toxicity. The pancreas–liver–adipose axis is central to managing and storing carbohydrates and lipids (Figure 1). However, environmental acute, intermediate, and chronic Cd exposure produces biochemical and hormonal alterations that augment the risk of developing metabolic diseases. This review summarizes studies examining Cd environmental exposure and exposing biological and cellular mechanisms associated with the homeostasis impairment of carbohydrates and lipids focused on related metabolic disorders.

Figure 1.
Pancreas–liver–adipose axis in response to cadmium exposure. Cellular response to cadmium toxicity of pancreas, liver, and adipose on metabolism, cell cycle, oxidative stress, and inflammation.
2. Minimal Risk Levels of Environmental Cadmium Exposure
According to ATSDR, minimal risk level to humans (MRL) for Cd exposure is defined as an estimate of daily exposure to the metal without an appreciable risk (noncarcinogenic) over a specified duration of exposure. Studies on Cd toxicity in humans and animals via inhalation have established that MRL for acute Cd exposure corresponds to 0.03 µg Cd/m3, and chronic duration corresponds to 0.01 µg Cd/m3. Meanwhile, acute, intermediate, and chronic oral MRL ranges from 1.12 to 65.6 mg Cd/kg/day, 0.5 µg Cd/kg/day, and 0.1 µg Cd/kg/day, respectively. Adverse effects have been established from dose-response data as the point of departure for MRL. Two MRLs have been identified, the lowest observed adverse effect level (LOAEL) and no observed adverse effect level (NOAEL). Both MRLs are time-depend, concentration (dose), and Cd toxicokinetic [1].
3. Cadmium Intake, Absorption, and Distribution
Extensive ranges of Cd concentrations have been reported in foodstuffs from various countries. For example, in Beijing, China, most foodstuffs have Cd concentrations of 0.005–0.100 mg/kg [6]. Estimations of daily Cd intake have been made in several noncontaminated areas. The average daily intake reported for European countries and North America is usually 15–25 μg/day for a 70 kg person [7]. However, the FAO/WHO established a provisional tolerable weekly intake for safe Cd intake. In 1993, Cd intake was set at 400–500 µg per person per week or 140–260 µg/day for over 50 years or 2000 mg of Cd over a lifetime. Presently, tolerable intake is 25 µg per kg body weight per month or 0.83 µg/kg/day or 58 µg/day for a 70 kg person. Exceeding these values breaks the renal threshold, and urinary Cd values increase to above 5.24 µg/g of creatinine [8].
The gastrointestinal tract absorbs 5–10% of the Cd it is exposed to [9]. Meanwhile, the absorption from the respiratory tract is 10–40%. The enterocytes carry Cd through several channels, carriers, and receptors, such as Ca2+ channels, transient receptor potential (TRP) channels (e.g., TRPA1, TRPV5/6, TRPML1), solute carriers (e.g., divalent metal transporter 1(DMT1), zinc transporter proteins (ZnT), and zinc-iron permease (ZIP), organic cation transporter 2 (SLC22A2), amino acid/cystine transporter (SLC7A9/SLC3A1), Ferroportin-1 (FPN1/SLC40A1)), and receptors (e.g., Lipocalin-2 Receptor (Lip2-R/SLC22A17) [10]. Then, Cd2+ bound to metallothionein (MT) forms the complex Cd-MT and gets into the bloodstream. The Cd-MT complex formation in tissue prevents some toxic effects of metal, but on the other side, the Cd-MT complex can increase cadmium transport to the kidney and liver [11]. Cd may also be bound to low-molecular-weight proteins, such as microglobulins, and only a low proportion to high-molecular-weight proteins, e.g., albumin and transferrin [12]. Consequently, Cd is distributed to different organs and tissues, mainly in the liver (acute exposure) and kidney (chronic exposure).
4. Cadmium Toxicity
(a) Interaction with essential biometals. According to the Irving–Williams series, Cd accumulation in organs and tissues can displace zinc, iron, magnesium, manganese, calcium, and selenium from structural domains and active centers of proteins and enzymes. Cd may cause a secondary deficit of oligo-elements and metabolism disruption, which results in functional impairment in many organs. The interaction of Cd with iron and copper is relatively well understood and described because Fenton and Haber–Weiss reactions produce oxidative stress, increasing hydroxyl (•OH) and superoxide (O2•−) radicals, respectively [13].
(b) Cell signaling impairment. Toxicity could result from Cd interacting with cellular components, such as surface receptors [14]. Several studies have shown cellular signaling pathways impairment after Cd exposure, such as interference with receptors, second messengers, transcription factors, and cell cycle, which cause defects on arrest, differentiation, immortalization, or apoptosis; there have even been reports of post-translational modifications associated with Cd toxicity and cell organelles damage [4]. In addition, Cd can switch on the mitogen-activated protein kinase (MAPK) pathway after inducing phosphorylation on protein kinases for MAPK. Other proteins activated after Cd exposure are transcription factors such as activator protein 1 (AP-1), nuclear factor kappa B (NF-κB), and metal transcription factor -1 (MTF-1), which in turn increase the activity of genes and their expression [4,15].
(c) DNA damage by cadmium. Direct interaction of Cd with DNA causes cancer by epigenetic or indirect genotoxic mechanisms [16]. Cadmium inhibits DNA methylation and impairs gene expression [17]. DNA hypomethylation produces an excessive synthesis of proteins responsible for cell proliferation, which is associated with the development of malignancies. This was found when intracell reactive oxygen species (ROS) increase, which alters the expression of many genes and transcription factors [4,18]. The main overexpression reported in cells exposed to Cd is on c-fos and c-jun, suggesting a possible mechanism responsible for Cd-carcinogenesis.
5. Cadmium and Inflammation
It was shown that Cd could modulate an innate immune response [19]. The exact mechanism by which Cd activates an inflammatory response has yet to be fully understood. However, Cd, in a time and concentration-dependent manner, leads to the upregulation of critical pro-inflammatory cytokines and chemokines (TNF-α, IL-1β, IL-8, IL-6, IL-18) and transcription factor genes (Nk-fb1, Nkfb-p65, MYD88) [20]. In vitro studies have demonstrated that nontoxic concentrations of Cd can stimulate IL-6 and IL-8 production through MAPK phosphorylation and NF-kB activation without affecting cell morphology and viability. Likewise, through the MAPK pathway or phosphatidylinositol 3-kinase (PI3K)/Akt signaling, Cd induces cyclooxygenase-2, prostaglandin E2, and macrophage inflammatory protein-2 [21,22]. In addition, it has been reported that Cd activates the ERK1/2 signaling pathway-dependent and NF-κB independent pathways, leading to elevated expression of pro-inflammatory mediators, including IL-8 [23].
6. Cadmium and Oxidative Stress
Cadmium cannot generate free radicals directly. Hence, ROS increase after exposure to metal is due to Fenton and Haber–Weiss reactions, as mentioned before. Oxidative stress disturbs the cellular redox balance in favor of the pro-oxidant molecules that disrupt normal cell function [24]. ROS can modulate signal transduction, but the uncontrolled increase carries fatal cell events. Since mitochondria are the major site of ROS production, they are a target of Cd toxicity [25]. ROS production substantially increases in chronic Cd exposure leading to lipid peroxidation, mtDNA cleavage, and impaired ATP generation by mitochondrial damage [26,27]. Cd can unstabilize the semiubiquinones of complex III mitochondrial, which makes them more prone to transfer electrons to molecular oxygen to form superoxide radicals [28,29,30].
Multiprotein complexes of the NOX family are another source of ROS generation. ROS derived from NADPH oxidase plays an essential role in normal cell functioning. However, NADPH oxidase overactivity has been implicated in pathology development and cell injury. ROS overproduction NADPH-dependent reactions after Cd exposure has been found in the liver, kidney, neuronal cells, and multiple cancer cell lines [31,32,33,34]. Since Cd can induce NADPH oxidase overactivity, the production of high free radical levels results in damage or cell death.
7. Antioxidative Defense
The cell possesses an antioxidant defense consisting of enzymes, peptides, proteins, and metabolites in each subcellular compartment that minimizes oxidative damage [35]. Cd-induced disturbances in redox balance impair signal transduction cascades, leading to a genic response. The stress-response genes encode MT, heat shock proteins (HSP), glutathione (GSH, the most important cell antioxidant), enzymes (e.g., superoxide dismutase (SOD), catalase (CAT), γ-glutamyl cysteine synthetase, GSH peroxidase (GPx), GSH reductase (GR), GSH transferase (GST)), vitamins (e.g., vitamin C and E), and other antioxidant molecules involved in oxidative stress response [36,37,38]. The most studied transcriptional factors activated by Cd and Cd-induced oxidative stress are metal regulatory transcription factor 1 (MTF1) and NFE2-related factor 2 (NRF2) [39,40,41,42,43,44,45].
Increases and decreases in SOD activity have been described during Cd exposure [46,47]. Discrepancies are attributable to exposure or administration conditions, the organ studied, and specific SOD isoforms localized in cell compartments, Cu/Zn-SOD (cytosolic isoform) and Mn-SOD (mitochondrial isoform) [48,49,50,51]. Cd inhibits Cu/Zn-SOD isoform activity because the Cd-enzyme interaction causes perturbations in the catalytic site [52,53]. After Cd exposure, a decrease in Mn-SOD isoform activity has also been reported [54].
CAT and GPx maintain physiologic cell hydrogen peroxide levels. Whereas CAT activity is in the peroxisomes, cytoplasm, nucleus, and mitochondria [55,56]; the detoxification of H2O2 by the five isoforms of GPx occurs via GSH oxidation in its active site through selenocysteine [57,58]. According to the literature, CAT activity is better for severe stress situations such as cancer [59,60], while peroxidases act better against low levels of H2O2, indicating a role in the fine-tuning of signal transduction [61].
Thiol groups of cysteine residues in antioxidative proteins and peptides are vital players in redox sensing and regulation [58,62]. During Cd-induced stress, thiols form Cd–thiol and ROS-thiol complexes that protect, mobilizes, and detoxify cell. Thiols are found in GSH, MT, other small peptides, and proteins that bind Cd in covalent attachments. The GSH system protects cells against xenobiotic compounds and oxidants through GSH as a substrate for GST [63,64,65]. GSH transfers reducing equivalents to GST, and also to GPx, glutaredoxins (Grx), and ascorbate [66]. The GSH levels are maintained by GR activity. Therefore, the GSH, at the same time, neutralizes ROS and eliminates Cd accumulated in the cell, diminishing damage risks [67]. Likewise, MTs that possess numerous thiol groups bind to Cd and other divalent metals and free radicals (such as •OH and O2•−) [68,69].
8. Cadmium and Pancreatic β-Cells
The impaired function of insulin-producing pancreatic β cells in the setting of insulin resistance is the leading underlying cause of type 2 diabetes (T2D). β-cell dysfunction is multifactorial; nevertheless, environmental factors lead to progressive β-cell failure. Studies have shown that acute Cd exposure decreases serum insulin in animals and humans [70,71]. Cadmium is transported into β-cells by DMT, ZIP, and calcium channels. At the same time, zinc exporters (ZnT family widely distributed into β-cell) avoid Cd leak, which explains its accumulation in subcellular compartments, such as insulin granules, endoplasmic reticulum, mitochondria, etc. [72]. In the insulin granules, the hormone is stored as a crystalline hexamer containing two zinc ions and one calcium ion, which prevents amyloidogenesis and degradation [73,74]. Meanwhile, Cd accumulation per se could cause insulin impairment because it may compete with and displace Zn in insulin, promoting hormone degradation. Additionally, amyloid fiber accumulation can be another consequence of Cd toxicity and cell death, thereby diminishing insulin.
Evidence suggests that chronic low-level exposure to Cd in the environment (LOAEL and NOAEL dose) has also been associated with an increased risk of developing dysglycemia and T2D. However, it is unrelated to the death of β-cells [75]. Chronic-Cd exposure in drinking water elicits changes in insulin secretion and insulin resistance development [76,77]. Progressive hyperinsulinemia is an adaptive response by β-cells. However, sustained hyperfunction of β-cells can alter their capacity to downregulate insulin secretion rate over a long-time period [78,79,80]. Most risk variants for T2D in healthy populations act through impairing insulin secretion and, consequently, β-cell dysfunction rather than insulin action that results in insulin resistance. According to this, abnormalities of β-cell function or mass are critical to T2D development [81,82]. The β-cell integrity is essential to an appropriate response to fluctuating metabolic demand for insulin. Glucose is the main regulator of transcription and translation in β-cells, which is necessary for the long-term maintenance of the highly differentiated cell state and the secretory requirements imposed by prolonged elevations of glucose concentration [83,84,85]. The glucose metabolism into β-cells couples the glycolysis and oxidative phosphorylation to increase ATP; in turn, ATP induces K+-channel close, which hyperpolarizes β-cells, activating voltage-dependent Ca2+ channels that cause mobilization and fusion of insulin granules and, finally, hormone secretion [86]. In each step, Cd accumulation and hyperinsulinemia can interfere. Therefore, β-cells must be prepared to protect against Cd effects. The β-cell adapts in response to fluctuations in demand for insulin. Hyperglycemia increases insulin demands; therefore, β-cells must increase hormone biosynthesis. Insulin synthesis occurs by augmenting transcriptional factors activity, faster insulin translation, and maturation, or via hypertrophy and hyperplasia β-cells, which restore glucose homeostasis [77]. β-cell hypertrophy is associated with p-Ras, p-Raf-1, p-MEK-1, and p-ERK1 pathways [87]. Meanwhile, hyperplasia is linked with c-fos protooncogene, activated by Ras cascade. Ras and c-fos are upregulated by Cd exposure, while the p53 tumor suppression mechanism is inhibited [88,89]. Cadmium may recruit transcription factors usually recruited by zinc, increasing mitogenesis and propagating the replication of damaged DNA. To avoid carcinogenic effects, β-cells increase the pancreatic and duodenal homeobox-1 (PDX-1), a master transcriptional factor induced by the MAPK pathway that plays a critical role in pancreatic development, β-cell differentiation, maintenance of normal function, and regulates the β-cell size and tumorigenesis [90,91]. Moreover, PDX-1 is the principal transcriptional factor in insulin production, and, therefore, it is possible to think that a β-cell adaptation to Cd could be the insulin over-synthesis [91,92].
The regulation and β-cell maintenance concerning mass cell preservation (the integrity of architecture, structure, number, and cell size) and function (physiology) are necessary for T2D not to develop. Serum response factor (SRF), a regulator of myogenic and neurogenic genes, is highly enriched in β cells [93]. SRF is a glucose concentration-sensitive regulator of insulin gene expression [94], and together with the constitutive expression of active peroxisome proliferator-activated receptor (PPARγ), confers glucose homeostasis and the preservation of β-cell mass, preventing apoptosis due to Cd accumulation [95]. The forkhead box class O family (FOXOs) are another transcription factor that participate in β-cell differentiation, proliferation, and survival [96]. FoxO1 is predominantly expressed in the β-cell [97]. FoxO1 accompanies the nuclear exclusion of Pdx-1, suppressing β-cell proliferation [98]. In addition, FoxO1 seems to exert a protective effect against oxidative stress-induced damage, preventing a state of premature β-cell senescence [97,99].
9. Protective and Antioxidant β-Cell Capacity
Inflammation and oxidative stress are linked to insulin resistance and Cd accumulation. Exacerbated pro-inflammatory cytokines levels secreted by immune cells infiltrated in Langerhans islet could cause damage or death of β-cell. However, low Cd concentrations in no resolutive conditions, such as chronic exposure at NOAEL or LOAEL dose and sustained hyperglycemia, lead to oxidative stress, causing adaptation and preservation of cell programs [100,101,102]. In vitro, MIN6 cells enlightened Cd toxicity through sub-micromolar concentrations of ceramide that induce IL-6 and IL-1 expression and enhance PGE2 production by COX upregulation [103,104]. Ceramides activate protein kinase C (PKC) and NF-κB pathways, initiating inflammation via NLRP3 inflammasome [105]. Due to β-cells being susceptible to uncontrolled ROS and RNS that generate oxidative distress (nonphysiologic stress), endoplasmic reticulum (ER) stress, DNA damage, and the formation of advanced glycation end products (AGEs), the protective and antioxidant β-cell defense must respond by first up-regulating MT and SOD, and secondarily CAT, GSH, and GPx at the correct levels by the stress or damage stimuli [106].
9.1. Nonenzymatic Antioxidant Defense in β-Cell
In mouse and human Langerhans islets, MT expression (constitutive and/or inducible) against oxidative stress and heavy metals has been studied for decades [107]. β-cells strongly expressed Mt1 and Mt2. The mRNA level of Mt1 is relatively higher than Mt2. In human β-cells, MT1E, MT1F, MT1X, and MT2A participate in differentiation, maturation, and other significant biological roles [107,108,109]. The MTs are the first line of defense in β-cell, when their thiols are oxidized, releasing Zn. Cytoplasmic free Zn increase activates MTF1, binding to metal response elements (MREs) and ensuing upregulation of MT gene expression [110]. MT gene expression in β-cells is also regulated by glucose [108,111,112,113,114]. Langerhans islets exposure to H2O2, IL-1β, TNF-α, and IFNγ markedly increases Mt1a mRNA levels [109,115]. Interestingly, Mt1a upregulation parallels oxidative stress-responsive genes, such as Cat, Gst, and Sod2 [116,117]. Evidence suggests that MT can transfer Zn to SOD1 [118]. In addition to MT in β-cells, GSH has been proposed as the primary cell defense; however, the antioxidant activity of MT is about 50 times higher against oxidative DNA damage and about 10 times higher against lipid peroxidation [119].
GSH can react directly or with enzymatic antioxidants against heavy metals, ROS, RNS, lipoperoxides, and oxidized DNA [120,121]. GSH is present in an adequate concentration to protect and detoxify β-cells. GSH and GPx activity avoids mitochondria damage in Cd presence or high glucose fluxes [122]. GST and GR activity are indispensable to maintaining GSH levels over oxidized form (GSSG). Hyperglycemia and hyperlipidemia aggravate oxidative stress in β-cells by reducing antioxidant capability, evidenced in reduced oxidized glutathione (GSH/GSSG ratio) [123]. In addition, GSH synergically acts with thioredoxins, peroxiredoxins, and other thiol-peroxidases to protect membranes and DNA from lipid peroxidation, scavenging free radicals, and xenobiotics.
9.2. Antioxidant Enzymes
The harmful effects of oxidative stress on β-cell death and dysfunction have been extensively discussed. β-cells have been shown to express low enzymatic concentrations of CAT, SOD, and GPx compared to other tissues such as the liver, brain, kidney, and heart. Regarding the liver, β-cells are only equipped with about 50% of the SOD and 5% of H2O2-scavenging enzymes GPx and CAT [124]. CuZn-SOD and Mn-SOD gene expression levels are 30–40% of those in the liver, whereas GPx gene expression is 15%, and CAT gene expression is barely 5% in β-cells [125]. Therefore, β-cells are highly susceptible to oxidative stress and cytotoxicity. Cadmium exposure, hyperglycemia, and hyperlipidemia (mainly FFA) provoke an overexpression of the GPx4 isoform as the stress response. A mechanism that contributes to lipid-induced oxidative stress in β-cells is the modulation of the respiratory chain by Cd (complex III), glucose, and FFA (both on complex I) in mitochondria, which seem to be the radical source [126,127,128]. Hyperlipidemia and an over-FFA uptake significantly increase superoxide anion levels and reduce antioxidative defense, suggesting that hyperlipidemia may be a driving force for ROS production. This idea is strengthened because, in the presence of high glucose levels, SOD isoforms and CAT maintain their activity. However, exposure to high concentrations of long-chain FFA results in toxic levels of H2O2 and the inability to protect β-cells [129]. Nevertheless, environmental conditions of Cd exposure have not been well-studied.
Redox balance is regulated at the transcriptional level in response to the harmful effects of excess nutrients or xenobiotics in β-cell. The Nrf2/Keap1 pathway regulates the expression of over 100 genes and functions related to oxidative stress and cell survival, including the enzymatic and nonenzymatic antioxidants, growth factors, and transcription factors related to inflammation [39,130]. The role of the Nrf2/Keap1 pathway in β-cells has been extensively explored in metabolic and diabetic pathologies. Nrf2 suppression increases intracellular ROS and aggravates damage in Langerhans islets and β-cell lines [131,132]. Conversely, overexpression of Nrf2 reduces DNA adduct formation and reestablishes insulin secretion [132]. However, the β-cell Nrf2 pathway has not been studied in environmental conditions of Cd exposure (Figure 2).
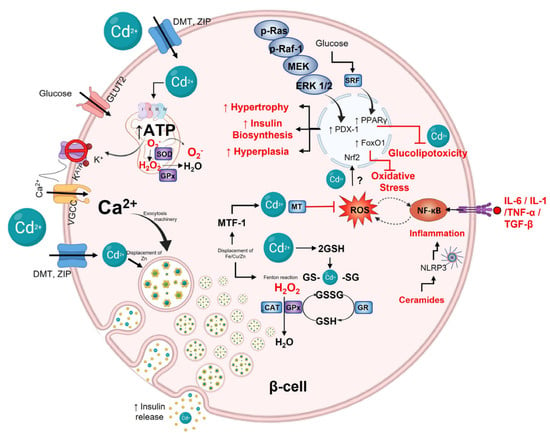
Figure 2.
Cadmium effect on pancreatic β-cell. Glucose is the primary stimulus for insulin release. Glucose enters β-cells through GLUT2; after oxidative metabolism in mitochondria, ATP increases and then closes K+ATP channels, depolarizing the plasmatic membrane and, consequently, opening VGCC channels, which causes calcium influx entry to the cytoplasm and activating exocytotic machinery for mobilizing and fusion insulin granules. Cadmium can make entry into the cells via DMT and ZIP transporters. Inside, Cd2+ can displace Zn2+ on insulin hexamers affecting the hormone stability and action. p-Ras, p-Raf−1m MEK, and ERK 1/2 pathway can be activated by Cd2+ modulating PDX−1 expression to increase insulin biosynthesis, hyperplasia, and hypertrophy to maintain hyperinsulinemia. Furthermore, glucose through SRF can activate PPARγ to protect against Cd2+-induced glucolipotoxicity. Cd2+ can interfere with redox balance in cells, generating ROS via Fenton reactions or interfering with mitochondrial complex III. When Cd2+ displaces Zn2+, it activates MTF, increasing MT and GSH expression. MT and GSH can form complexes with Cd2+ by sulfhydryl groups of cysteine. Finally, cytokines such as IL−6, IL−1, TNF-α, and TGF−β, as well as ceramides by NLRP3 inflammasome, activate NF-kB, which regulates and is regulated by ROS.
10. Cadmium and Liver
In acute Cd exposure, the liver takes up and stores a significant quantity of metal. Cd-hepatotoxicity during the initial hours after exposure produces ischemia, Kupffer cell activation, and neutrophil infiltration [133]. Liver histopathological hallmarks after acute Cd exposure are cytoplasmic vacuolization, necrosis, congested blood vessels, mitophagy, fatty droplets, glycogen depletion, and portal fibrosis [134]. However, oral subacute Cd exposure to LOAEL dose causes sinusoid obstruction of the central vein, leukocyte infiltration in portal zones, and hepatocyte hyperplasia but mild apoptotic signs [135]. Cadmium hepatotoxicity is closely related to inflammation. Previous studies have demonstrated that Cd-activated Kupffer cells release a number of inflammatory mediators, such as TNF-α, IL-1β, IL-6, and IL-8, which initiate a cascade of cellular responses, leading to liver damage [133,135]. Hepatotoxicity is reduced when Kupffer cells are selectively inhibited or suppressed [134,136]. At the same time, inflammation is accompanied by ROS, and vice versa, since ROS induces inflammation. Both cytokine and ROS signaling activate transcription factors, such as NF-κB and AP-1, which enhance the expression of pro-inflammatory and adhesion molecule genes [33,137,138].
Oxidative stress and inflammation disrupt the insulin metabolic pathway in the liver. Inflammation per se can induce insulin resistance via the MAPK pathway. Low-level Cd exposure indirectly stimulates MAPK in mice and cell cultures through oxidative stress [27,33,87]. Mechanisms include MT depletion and GSH reduction [69,139], ROS overproduction through NADPH oxidase activity [34,140], and complex III mitochondrial by accumulating unstable semiubiquinones. Increased superoxide radicals [141,142] and free iron, zinc, and selenium replacement with lost enzymatic activity (e.g., CAT, SOD, and GPx) have also been found [143,144]. Notably, oxidative stress, TNF-α, and IL-1β activate the c-Jun NH2-terminal kinase (JNK), generating insulin signaling interruption by modifying the phosphorylation of insulin receptor and insulin receptor substrate-1, provoking insulin resistance [145,146,147]. Changes in mitogen signaling consequently alter the metabolic pathway. This redundancy causes loss of glucose homeostasis and a high lipogenic rate observed in Cd-exposed animal models.
11. Cadmium Impairs Hepatic Glucose Homeostasis
Studies investigating the impact of environmentally relevant doses of Cd showed sex specificity in glucose metabolism disruption, with females being more sensitive [148,149]. Low-level maternal Cd exposure influences glucose homeostasis in offspring and increases the risk of developing T2D [128]. Chapatwala et al. demonstrated that Cd administration in environmental and toxic dosages for 30 days increased pyruvate carboxylase, phosphoenol pyruvate carboxykinase, fructose-1, 6-diphosphatase, and glucose-6-phosphatase in the liver, which indicates that Cd may induce gluconeogenesis from noncarbohydrate sources [148,149]. Additionally, Cd can alter PI3K/Akt/mTOR signaling, interfering in metabolic actions such as glycolysis and glycogenesis, promoting gluconeogenesis and glycogenolysis via dysregulation of FOXO1 and glycogen synthase kinase-3β (GSK3β) [150,151,152,153]. Hepatic glycogen synthesis results from postprandial insulin signaling [154,155]. Phosphorylation-based glycogen synthase (GS) regulation by insulin occurs through Akt phosphorylation and GSK3β. Thus inactivated, GSK3β kinase activity toward GS is diminished, and dephosphorylated GS is, in turn, more active. In hyperglycemic states associated with insulin resistance, GSK3β is overactivated, which impedes glycogen synthesis. Likewise, Cd exposure seems to be an inductor of the activity of GSK3β or an inhibitor of GS because animals exposed to a chronic environmental LOAEL dose show insulin resistance and continuous reduction in hepatic glycogen levels [77,135,156,157].
12. Cadmium, De Novo Lipogenesis, and Dyslipidemia
De novo lipogenesis (DNL) is a natural pathway to control glucose levels and energy storage, encouraging hepatic triglyceride biosynthesis, which is dependent on proper insulin signaling. This process is an extension of the complex metabolic networks and is provided with substrate primarily through glycolysis and the metabolism of carbohydrates. However, when carbohydrate pathways are impaired, hypertriglyceridemia and breaking the balance between lipogenesis and lipolysis have been observed as common factors in physiopathological processes such as insulin resistance, obesity, and T2D [158]. Regulation of the lipogenic pathway is in two levels, first by transcriptional regulation of enzymes integral to fatty acids (FA) synthesis, and second by allosteric regulation of acetyl-CoA carboxylase (ACC). The transcriptional regulation of DNL by sterol regulatory element-binding protein 1c (SREBP1c) is activated via PI3K/Akt/mTOR (insulin signaling), whereas increased glucose concentrations activate the carbohydrate response element-binding protein (ChREBP); both are induced by feeding [158,159,160]. The acetyl-CoA produced during glycolysis through ACC activity generates malonyl-CoA that is incorporated into the synthesis FA and then, through the initial acylation of glycerol-3-phosphate, rendered in consecutive cycles, mono, di, and triacylglycerol. Triglycerides interact with microsomal triglyceride transfer protein (MTP), which aids the partial lipidation of the nascent apolipoprotein B 100, a hallmark of very low-density lipoproteins (VLDL). MTP and apoB100 are modulated via FoxO1 through insulin signaling. Therefore, both hyperinsulinemia and insulin resistance overfeed the lipogenic conditions. It is named “the lipid paradox,” characterized by hypertriglyceridemia in fasting and postprandial states [158].
The oral cadmium LOAEL dose exposure results in a GSK3β decrease, providing excess lipogenic substrates. In addition, SREBP-1c is strongly expressed in the liver of environmental Cd-exposure animal models [135]. The evidence suggests that hepatic Cd accumulation induces insulin resistance, favors DNL, and modifies the lipidome serum and hepatic system [76,77,157]. The lipidome is the content, distribution, and type of lipids in tissue or lipoproteins. The overproduction of triglyceride-rich lipoproteins (TRLs) would be a straightforward consequence of the metabolic toxicity caused by Cd in the liver. In particular, rats exposed to Cd LOAEL dose overproduce large VLDL (VLDL1) [76]. VLDL1 is a TRL, and its formation is highly dependent on hepatic triglyceride accumulation (cytosolic lipid droplets) and FFA fluxes from serum to the liver [161,162]. Previous works have shown that chronic cadmium LOAEL dose exposure develops hepatic steatosis and small low-density lipoproteins (sLDL) [76]. VLDL1 presence is a prerequisite for forming sLDL [163,164]. The combination of sLDL and insulin resistance is a factor risk to atherosclerosis development [76,160]. Experimental evidence suggests that Cd could contribute to the initiation of atherosclerosis and promote the progression of cardiovascular disease through damage, stress, and inflammation. In addition, environmental Cd exposure generates small HDL sub-fractions (HDL-3c) [76]. These sub-fractions present low content of free and esterified cholesterol and phospholipids but are rich in triglycerides. Together, hypertriglyceridemia, low cholesterol level in HDL, and augmented FFA concentration are a consensus of metabolic dyslipidemia. Therefore, Cd exposure can be a risk factor for multi-tissular steatosis, atherosclerosis, and metabolic dyslipidemia associated with defects in the enzymatic activity of cholesterol ester transfer protein, lecithin-cholesterol acyltransferase, and phospholipid transfer protein (PLTP) [165]. Nevertheless, only a lack of PLTP activity has been observed after Cd administration [166]. Likewise, triglyceride-rich HDL3c could be a promoter of oxidative stress and inflammation in the liver and other tissues [167].
13. Hepatic Antioxidant Defense
The liver has a robust defense system to prevent injury associated with Cd exposure or other attacking molecules. The studies of Cd exposure in experimental animals and in vitro cellular models have yielded substantial information regarding metal toxicity and cellular defense mechanisms. Concerning mechanisms postulated to prevent hepatotoxicity, the literature mentioned the activity of sulfhydryl group binding, implicating membrane proteins, cytoplasmic proteins, and enzymes [168]. In addition, the interaction with an excessive quantity of essential metals such as zinc, selenium, copper, and calcium maintains low Cd toxicity [169]. Metal homeostasis is critical for many enzymes, transcription factors, and other subcellular proteins [75]. The substitution of native metals by Cd is often proposed as the leading molecular mechanism of Cd hepatotoxicity. In hepatocytes, Cd uptake and accumulation are mediated by transporters on essential metals [169]. Calcium channels and carriers actively participate in Cd uptake because they interact with sulfhydryl groups [170,171]. Furthermore, membrane transporters such as DMT1, ZIP8, and ZIP14 play a role in Cd uptake [11,172]. It has been shown that Cd-ferritin and Cd-MT complexes may enter hepatocytes by receptor-mediated endocytosis [173]. Numerous experiments have demonstrated that the isoform presence of MT-1/-2 shields cells from Cd toxicity [69,171,174,175].
Metallothionein and GSH are primary line defenses that maintain the hepatocytes’ redox state and, consequently, prevent inflammation, xenobiotic injury, metal toxicity, and many harmful effects. Complex formation between Cd and GSH has been implicated as the initial step in hepatic detoxification before transferring the metal to MT and other cysteine-rich peptides [176]. In chronic Cd exposure, the redox status is compromised. In cells and primary rat hepatocyte cultures, Cd-induced GSH loss and cell death indicate that GSH is critical in cell function and survival [142]. GSH administration not only reduces the generation of free radicals but also lowers the accumulation of Cd in the liver [177]. Hepatic Cd accumulation, metabolic impairment, dyslipidemia, and steatosis caused by metal establish an oxidative status and ROS generation in hepatocytes that activate hepatic stellate cells and Kupffer cells that favor a profibrotic status. Therefore, various cellular defense mechanisms could be started. Enzymes and other antioxidant components constitute the system to defend against Cd-induced oxidative damage [38]. In normal conditions, GSSG is regenerated to GSH by the action of GR; however, Cd inhibits GR activity, which induces mortality and hepatotoxicity. Furthermore, the electrophilic sites of oxidized molecules are neutralized by GST and the thiol group of GSH, thereby rendering the products more water-soluble and less toxic. It has been reported that Cd decreases hepatic GST activity [178,179].
Although the liver is one tissue that expresses a high concentration and activity of detoxifying and antioxidant enzymes, Cd accumulation induces high oxidative stress and lipid peroxidation levels, thereby gradually decreasing their activity [180,181,182]. The most affected enzymatic activity observed after chronic environmental Cd exposure occurs in SOD, CAT, and GPx [183]. It has been proposed that the inactivation of the SOD isoforms results from a displacement of Cd by Zn or Mg from the catalytic domine through competitive inhibition or substitution [184]. However, the results depend on Cd concentration and exposure time.
An acute administration of 5 mg/kg decreases SOD activity [185]. In contrast, by 12 weeks, oral Cd exposure (50 ppm) increases hepatic SOD activity [51]. Likewise, the inhibition or reduction in GPx activity has been associated with Se depletion through Cd–Se–cys complex formation at the enzyme’s active site [186]. It has been suggested that competition between GPx and MTs for S-amino acids is a potential cause of GPx activity decrease during Cd stress [54,178]. Additionally, CAT activity increases after six days of daily intraperitoneal Cd administration or five days via gastric gavage in environmental concentrations but decreases its activity during acute intraperitoneal administration in toxicological levels [184,187,188]. Possible underlying mechanisms for decreased CAT activity have been postulated, such as the interaction between Cd and the catalytic subunit of CAT, Fe deficiency, since CAT has Fe as an essential element in its active center, and displacement of Fe from the catalytic domine [51].
Enzymatic and nonenzymatic antioxidant systems are essential to hepatic function in physiological or pathological conditions [189,190]. The hepatocytes sense redox balance via Nrf2. The Nrf2 activity increases antioxidant systems in different organelles. Notably, in mitochondria, Nrf2 activity protects against permeability, maintains the redox equilibrium, and enhances mitochondrial biogenesis by promoting the transcription of nuclear respiratory factor 1 (Nrf1). Therefore, Nrf2 protects mitochondria from oxidative stress. Correct mitochondrial function avoids hepatic steatosis, facilitating fatty acid metabolism by directly regulating related genes [190,191]. In hepatocytes, Cd stabilizes Nrf2, prevents degradation, and promotes nuclear translocation as a protective mechanism [192]. Cd exposure modulates NQO1 and HO-1 hepatic expression via Nrf2 [193,194]. Additionally, it has been speculated that through a specific G protein-coupled metal-binding receptor, Cd induces phospholipase C activity, releases intracellular Ca2+ and diacylglycerol, and provokes nuclear Nrf2 activation [195]. Furthermore, Zn displacement from MT by Cd induces the Nrf2 signaling pathway, which has two effects: suppresses inflammatory responses and enhances the antioxidant defense system by MTs upregulation. Consequently, it protects Keap1–Nrf2 oxidization upon Cd exposure [196,197]. Acute administration of a single CdCl2 dose in toxic concentration inhibits Nrf2 and HO-1 and activates inflammatory signaling pathways, NF-κB, NLRP3, and MAPK [194]. The absence or inhibition of Nrf2 causes a dramatic ROS elevation and, consequently, cell death [198,199] (Figure 3).
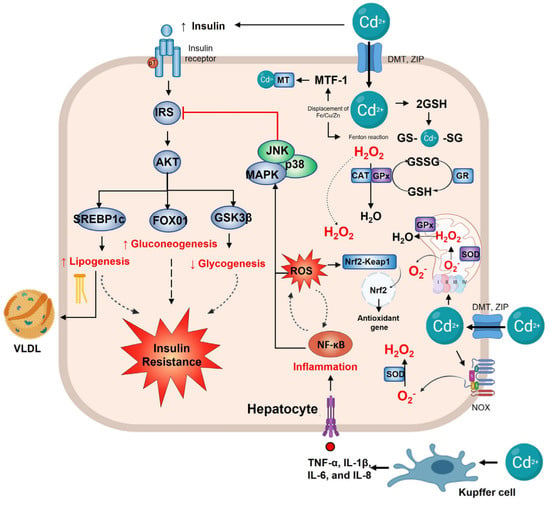
Figure 3.
Hepatic alterations in cadmium exposure. In the hepatocyte, the main effect of the insulin pathway is to regulate metabolism; when Cd2+ enters the cell, it disrupts the insulin signaling, increasing SREBP1c and FOX01 expression, thus, lipogenesis and gluconeogenesis. At the same time, it decreases GSK3β and glycogenesis, which leads to insulin resistance. Cadmium exposure activates the Kupffer cell, increasing TNF−α, IL-1γ/β, IL−6, and IL−8. Upregulated inflammation and NF−κB activate p38 and the JNK pathway to inhibit IRS and insulin signaling. Cd2+ also induces oxidative stress by the generation of •O2- via NOX or mitochondrial complex III. Mn−SOD and Cu/Zn-SOD play a vital role in detoxifying it, forming H2O2 that can be converted to H2O by GPx or CAT. The increased ROS by Cd2+ induces the Nrf2 gene to activate antioxidant gene transcription. On the other hand, Cd2+ can displace Fe, Cu, and Zn, leading to the Fenton reaction and producing more ROS. Zn2+ displacement activates MTF−1, which leads to GSH formation for Cd2+ detoxification.
14. Cadmium and Adipose Tissue
Adipose tissue is the main body energy reservoir and can store Cd [200]. In addition, adipose possesses endocrine functions, secreting adipokines, growth factors, cytokines, and chemokines [201]. Adipokines are mediators of various metabolic processes such as fatty acid oxidation, DNL, gluconeogenesis, glucose uptake, insulin signaling, and energy expenditure in metabolically active tissues such as the liver, skeletal muscle, and brain [201,202,203]. Clinically, adiponectin and leptin are the most important adipokines [204]. The endocrine function of adipose tissue is regulated significantly by the nutritional status linked to energy storage [205]. White adipocytes are tasked with storing excess energy as triglycerides. These undergo hyperplasia to increase the number of adipocytes and hypertrophy to increase the size of each adipocyte, allowing fatty tissue to expand when there are excessive nutrients [206]. As needed, during fasting and exercise, triglycerides stored in adipocytes are mobilized to provide fatty acids for energy utilization by the rest of the body. Stored triglycerides are in a constant state of flux, whereby energy storage and mobilization are determined mainly by the energetic necessity and hormonal fluctuations [207]. Therefore, through adipokines, adipose tissue cross-talks and regulates other body organs, metabolism, immunity, reproduction, and cardiovascular functions [206,208].
In white adipose tissue (WAT), Cd accumulation is also dose- and time-dependent [135,156]. Different concentrations of subcutaneous Cd administrations (3.5 times/week) for two weeks showed Cd accumulation in epididymal adipose tissue and abnormal adipocyte differentiation, expansion, and function [209,210]. In toxicological doses, adipocyte size is reduced, even in chronic exposure [211]. However, in chronic environmental exposure conditions, Cd was accumulated in retroperitoneal adipose, and histological analysis revealed cellular hypertrophy with a reduced adipocyte number per field. The hypertrophy was associated with increased triglyceride concentration in adipose tissue, fasting, and after insulin administration; however, a sustained lipolysis rate was observed, as was evidenced by serum FFA concentration [135]. The toxicological studies showed that WAT exposed to Cd reduces the expression level of mRNA and protein of peroxisome proliferator-activated receptor-gamma (PPAR-γ) and the protein of CCAAT/enhancer-binding protein (C/EBP), which suggests an adipogenesis impairment, but functional capacity and plasticity features [209,210,212]. Hypertrophy is a plasticity feature of adipocytes to store triglyceride. It is also an obesity-independent marker of insulin resistance and indicates a future risk of developing metabolic diseases driven by limited hyperplastic expansion and storage capacity [213,214]. Adipose tissue insulin resistance is an early hallmark of metabolic alteration, evidenced in chronic and subacute Cd exposure. A tyrosine phosphorylation reduction accompanies adipose insulin resistance on the insulin receptor [77,135]. Reduced signaling in the adipocyte metabolic pathway caused by Cd exposure has multiple consequences. For instance, insulin-stimulated glucose uptake in adipocytes depends on PI3K-Akt proper signaling [215,216], supporting GLUT4 trafficking to the plasma membrane [217], and glycogen synthesis. Additionally, the correct activation of PI3K-Akt has antiapoptotic and anti-inflammatory effects [218,219]. Chronic Cd exposure has been associated with diminished insulin receptors in rat adipocytes, which is related to the diabetogenic effect [220].
Adipocyte hypertrophy inevitably reaches a limit at which additional anabolic pressure is excessive due to cell expansion limitations. Adipocytes reach this threshold and initiate an inflammatory response [221]. Pro-inflammatory cytokines, such as TNF-α, inhibit adipocyte differentiation, increase lipolysis, interfere with insulin signaling, and induce a macrophage-like phenotype in preadipocytes [214,222]. TNF-α also induces increased IL-1β and IL-6, which polarizes adipose tissue macrophages, increasing subclass M1 recruitment that releases and produces more TNF-α, IL-6, IL-12, IL-23, and IL-1β. Meanwhile, M2 macrophages diminish, as well as IL-10, IL-4, and TGF-β cytokine levels [223,224]. Several findings showed that Cd activates the NF-κB pathway in adipocytes, leading to the upregulation of pro-inflammatory cytokines [20,225]. Studies have also demonstrated reliable data identifying chronic low-grade inflammation as an essential link between systemic insulin resistance and metabolic diseases. These effects include the activation of NF-κB and inhibition of AMPK signaling, leading to impaired insulin homeostasis [226]. Serum concentrations of several pro-inflammatory cytokines inhibit insulin signaling, which results in signal redundancy since insulin resistance induces inflammation and vice versa [227]. In addition, Cd exposure and inflammation negatively affect adipokine secretion. Subcutaneous Cd administrations have shown adipokine diminishing dose dependently [209,210]. However, chronic environmental exposure conditions increase leptin release, even associated with insulin concentration, while serum adiponectin concentration did not show changes [135]. Adiponectin plays an anti-inflammatory role and has insulin-sensitizing effects [228,229]. Moreover, adiponectin is a biomarker of healthy adipocyte expansion and prevents steatosis risk [230]. Meanwhile, leptin regulates food intake, lipid metabolism, energy expenditure, and glucose homeostasis [231]. Leptin releasing is proportional to adipocyte size; adipocyte hypertrophy carries hyperleptinemia, whereas lipodystrophy observes hypoleptinemia. The disturbance of insulin, adiponectin, and leptin contribute to metabolic diseases [135]. However, how or what Cd does in adipocytes impairment adipokine production and release have not been fully understood.
15. Adipose Tissue Antioxidant Defense
It has been shown that obesity contributes to the development of systemic oxidative stress in both human and animal studies [232,233]. Oxidative stress markers, such as protein carbonyls, lipid peroxidation products, and ROS production, increase in adipose tissues of obese subjects [234]. In the db/db mice model, ROS production and lipid peroxidation accumulation were also upregulated in adipocytes [235]. Possible triggers of obesity-induced oxidative stress include altered nutritional status, hyperglycemia, insulin resistance, hyperlipidemia, and low-grade chronic inflammation [236]. TNF-α, IL-6, and IL-1β signaling increase ROS production in the adipose tissue of obese individuals [37,237]. In cell cultures, 3T3-L1 adipocytes treated with TNF-α decreased the expression of antioxidants, such as GST, peroxiredoxin, and GPx, resulting in protein carbonylation, ROS generation, and mitochondrial dysfunction [238,239]. In adipocytes, ROS can be produced by NADPH oxidase (Nox), xanthine oxidases, and mitochondria. In particular, isoform Nox4 activity has been found in obese mice [240]. ROS production is induced by glucose and palmitate via Nox4 rather than mitochondrial oxidation. Likewise, xanthine oxidase increases activity in obese subjects, generating H2O2 that potentially links adipocyte DNL with ROS production [237]. Oxidative stress in adipose tissue activates NF-κB and MAPK, downregulating anti-inflammatory adipokines and adiponectin production while upregulating pro-inflammatory cytokines [232,240,241].
The antioxidant defense in white adipose tissue is limited; thereby, ROS accumulation contributes directly to the metabolic imbalance (impairs glucose uptake, over-release of free fatty acids, and jeopardizes mitochondrial biogenesis and functions) [242,243], linking excessive nutrient stress, Cd exposure, and insulin resistance [244,245]. Studies have shown that ROS production increases parallel with adipocyte fat accumulation; consequently, adipose tissue is a significant source of plasma ROS [246]. In the adipocytes, the activity and expression of SOD1, CAT, peroxiredoxins, and GPx have been reported. However, the antioxidant defense of adipose tissue concerning Cd exposure has yet to be well-studied. It has been found that toxic concentration of Cd exposure by oral gavage showed an activity decrease in SOD, CAT, and GPx [247]. MTs play a critical role in adipogenesis and maintaining adipocyte size beyond their chelating capacity [210]. On the other hand, in the adipocyte insulin resistance of obese mice and humans, antioxidant peroxiredoxin 3 (Prdx3) decreases significantly. Mitochondria are severely affected when Prx3 falls because H2O2 induces mitophagy [248]. Obesity-induced inflammation also decreases the expression of Prdx3 and GPx in epididymal adipose tissue [244]. Prdx3-deficient adipocytes exhibit high superoxide production, decreased mitochondrial potential, and altered adipokine expression. Prdx3 knockout mice observed adipocyte hypertrophy and fat overstorage [248]. Meanwhile, the Prdx2 isoform seems to participate in adipocyte differentiation because its silencing inhibits adipogenesis and increases ROS production in the 3T3-L1 cell line [249]. Obese models that augment ROS production present a low rate of GSH synthesis and reduce CAT and SOD1 activity and insulin sensibility [250]. Likewise, adipocyte-specific HO-1 knockout causes hyperglycemia and hyperinsulinemia in female mice but not male mice, indicating a sex-depended protective role [251]. The adipocyte-specific overexpression of HO-1 attenuated high-fat diet-induced adiposity and inflammation, improving insulin sensitivity and adiponectin levels [252] (Figure 4).
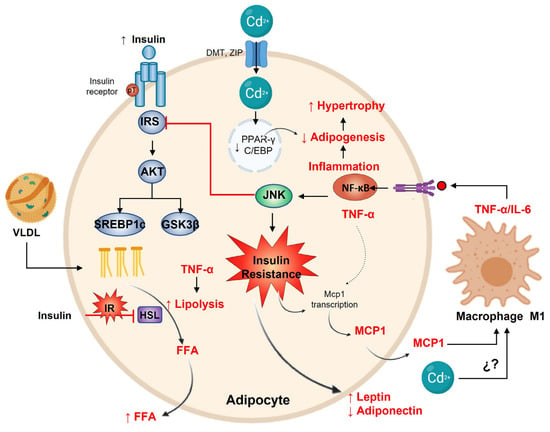
Figure 4.
Adipocyte response to cadmium. The prime function of adipocytes is to store lipids in response to SREBP1c with the insulin pathway. Contrarily, Cd2+ decreases adipogenesis by reducing PPARγ and C/EBP expression. Insulin resistance promotes MCP-1 and macrophage M1 differentiation with the increase in TNF-α and IL-6 secretion, leading to the adipocyte inflamed. The inflammation activates NF-kB, which in turn regulates adipogenesis and hypertrophy. On the other hand, TNF-α promotes lipolysis with FFA liberation (the original opposite effect of insulin signaling) and MCP-1 transcription. Finally, on insulin resistance, adipocytes increase leptin and decrease adiponectin release.
16. Cadmium and Obesity
Based on the relationship between heavy metals such as lead and obesity [253,254], the role of cadmium in developing this pathology has also been studied [255]. Despite multiple studies showing an association between environmental exposure to Cd and obesity, data are inconsistent. Cadmium exposure during the prenatal period has long been associated with lower birth weight and gestational age [256,257,258]. Low birth weight, often followed by rapid adiposity gain, is a consistent risk factor for cardiovascular and metabolic impairment later in life [259]. In the juvenile stage, an association exists between higher body weight, waist, and hip circumference in young females (8–15 years old) with high blood Cd levels [260]. In adulthood, Haswell-Elkins et al., 2007, showed a positive correlation between Cd levels and waist circumference but not with body weight [261]. Skalnaya et al., 2014, reported in women aged 22–35 years a weak but significant correlation between hair Cd concentration and high body weight values [262], whereas Filippini et al., 2016, demonstrated this association in Italian females but not in men [263]. The report on associations of cumulative exposure to heavy metal mixtures with obesity and its comorbidities among US adults in NHANES 2003–2014 showed a correlation between Cd serum and urinary concentration with higher body mass index, concluding that cumulative exposure to heavy metals as mixtures is associated with obesity and its related chronic conditions such as hypertension and T2D [264]. A study that included 5544 Chinese adults evaluated blood Cd levels related to diabetes or obesity. Results showed a positive relationship between blood Cd levels, fasting hyperglycemia, and prediabetes prevalence, but not with obesity [265].
In support of these human observations, animal studies have shown that early-life Cd exposure also increases fat mass, adiposity, and body weight in male mice [266,267]. Similar results have been found in chronic Cd exposure at LOAEL dose for five months. Male Wistar rats show a higher body weight, abdominal perimeter, BMI, and fat percentage, when exposed to an LOAEL dose from early-life stage [156]. However, young adult female and male rats exposed to a subacute and acute oral environmental amount of Cd resulted in low body weight, the delta of weight gain, and abdominal perimeter [131,268]. Regardless of the time, route of administration, concentration, or gender of experimental Cd exposed animals, adipose tissue shows significantly impaired physiology and function.
17. Cadmium and Diabetes
The evidence indicates that Cd exposure by direct and indirect mechanisms disrupts the pancreas–liver–adipose axis and produces a metabolic impairment, clinically exhibiting dysglycemia, hyperinsulinemia, insulin resistance, and dyslipidemia. These parameters are associated with metabolic syndrome development and its complications, such as atherosclerosis, hypertension, cerebrovascular and cardiovascular diseases, β-cell exhaustion, nonalcoholic fatty liver and its progression to fibrosis or cirrhosis, and prediabetes and diabetes. Therefore, Cd accumulation shows a diabetogenic effect in short-term acute exposure and long-term chronic expositions [77,269,270]. The mechanism(s) by which Cd may alter glucose homeostasis involves changes in glucose transporter expression, gluconeogenesis, and metabolism mediators impairment (leptin, glucose-dependent insulinotropic polypeptide, and pancreas polypeptide) [148,219]. However, impaired insulin levels after glucose stimulation and pancreatic β-cell dysfunction are likely Cd toxicity factors. In vitro and in vivo, Cd accumulates within human pancreatic β-cells and alters glucose-stimulated insulin release [269,271,272].
Epidemiological studies have also shown that subjects with occupational exposure to Cd have signs of prediabetes [273,274]. A positive association between Cd concentrations in urine (2–4.0 µg/g creatinine) and blood (1.2–2.5 µg/L) with prediabetes or diabetes [275,276]. Different studies suggest that risk increases linearly up to approximately two µg/g of creatinine. Swaddiwudhipong et al., 2012, found that persons occupationally or environmentally exposed to high Cd levels in Northwestern Thailand had a significant prevalence of diabetes [277]. Likewise, Zhang et al. 2014, showed hyperglycemia in residents in a polluted area in China [278]. Another study by Tangvarasittichai et al., 2015, demonstrated that Cd exposure was associated with a higher prevalence of diabetes [279]. In the prospective study realized by Xun et al., Cd exposure was related to the development of diabetes [280]. Using National Health and Nutrition Examination Survey (NHANES) data, urine Cd was positively associated with diabetes by Wallia et al. [281]. In the same way, the most compelling evidence came from a cross-sectional study by Menke et al. (2016). They used data from NHANES (1999–2010) to assess whether increased urinary Cd was associated with impaired T2D in the United States [282]. Despite evidence about Cd and diabetes development, some studies have revealed no relationship [283,284,285].
18. Final Remarks
Cadmium at environmental or toxic levels is accumulated in multiple tissues, depending on the time and route of administration or exposure, dose, interaction with biometals, previous diseases or comorbidities, age, and gender of population analyses. The pancreas–liver–adipose axis is susceptible to Cd accumulation and produces clinical disruptions such as metabolic syndrome. The molecular mechanisms are not yet fully understood; however, oxidative stress and inflammation are common features. Both are described in metabolic disorders and thereby link closely with diabetes progression. Each tissue of the axis possesses a different threshold for intracellular Cd management. Of course, the liver has a robust defense; contrarily the pancreas is very susceptible to Cd effects. It can be hypothesized that both pancreas and fatty tissue in the environmental conditions of Cd exposure generate hyperplasia or hypertrophy as adaptation mechanisms, but only a few works approach this topic. Hyperplasia and hypertrophy in both tissues also cause endocrine impairment, such as hyperinsulinemia that progresses to insulin resistance and long-term β-cell exhaustion. At the same time, adipocytes observe differential secretion of leptin and adiponectin, which alters the axis’ and other tissues’ metabolic functionality. The population risk analyses must consider these clinical data to clarify the link to diabetes. Meanwhile, evidence strongly suggests that Cd should be considered a risk factor for developing metabolic diseases. Future research must focus on molecular mechanisms that affect the pancreas–liver–adipose axis to find therapeutical strategies that minimize metabolic alterations.
Author Contributions
Funding
Vicerrectoria de Investigación y Posgrado [VIEP; TRMS-NAT22-1], CONACyT, and the “Sistema Nacional de Investigadores” of Mexico for the financial support of this research project [DMG, 758984].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The authors thank CONACyT, and the “Sistema Nacional de Investigadores” of Mexico, Vicerrectoria de Investigación y Posgrado [VIEP; TRMS-NAT22-1] through Ygnacio Martínez Laguna, and Robert Simpson, for editing the English language text.
Conflicts of Interest
The authors declare no conflict of interest.
References
- ATSDR, Agency for Toxic Substance and Disease Registry. Toxicological Profile for Cadmium; Department of Health and Humans Services, Public Health Service, Centers for Disease Control: Atlanta, GA, USA, 2012.
- Nordberg, G.F.; Fowler, B.A.; Nordberg, M. Handbook on the Toxicology of Metals; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Smolders, E.; Mertens, J. Cadmium. In Heavy Metals in Soils; Springer: Dordrecht, The Netherlands, 2013; pp. 283–311. [Google Scholar] [CrossRef]
- Thévenod, F.; Lee, W.-K. Cadmium and Cellular Signaling Cascades: Interactions between Cell Death and Survival Pathways. Arch. Toxicol. 2013, 87, 1743–1786. [Google Scholar] [CrossRef] [PubMed]
- Egger, A.E.; Grabmann, G.; Pechriggl, E.J.; Artner, C.; Bernhard, D. Chemical Imaging and Assessment of Cadmium Distribution in the Human Body. Metallomics 2019, 11, 2010–2019. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Gong, W.; Li, B.; Zuo, J.; Pan, L.; Liu, X. Analysis of Heavy Metals in Foodstuffs and an Assessment of the Health Risks to the General Public via Consumption in Beijing, China. Int. J. Environ. Res. Public Health 2019, 16, 909. [Google Scholar] [CrossRef] [PubMed]
- WHO. Chapter 6.3 Cadmium General Description; WHO: Copenhagen, Denmark, 2000. [Google Scholar]
- FAO Joint; WHO Expert Committee on Food Additives. Ninety-First Meeting (Safety Evaluation of Certain Food Additives and Contaminants); World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Asagba, S.O. Cadmium Absorption. In Encyclopedia of Metalloproteins; Springer Science + Business Media: New York, NY, USA, 2013; pp. 332–337. [Google Scholar] [CrossRef]
- Thévenod, F.; Fels, J.; Lee, W.-K.; Zarbock, R. Channels, Transporters and Receptors for Cadmium and Cadmium Complexes in Eukaryotic Cells: Myths and Facts. BioMetals 2019, 32, 469–489. [Google Scholar] [CrossRef] [PubMed]
- Nordberg, M.; Nordberg, G.F. Metallothionein and Cadmium Toxicology-Historical Review and Commentary. Biomolecules 2022, 12, 360. [Google Scholar] [CrossRef] [PubMed]
- Freisinger, E.; Vašák, M. Cadmium in Metallothioneins. Met. Ions Life Sci. 2013, 11, 339–371. [Google Scholar] [CrossRef]
- Valko, M.; Jomova, K.; Rhodes, C.J.; Kuča, K.; Musílek, K. Redox- and Non-Redox-Metal-Induced Formation of Free Radicals and Their Role in Human Disease. Arch. Toxicol. 2016, 3, 1–37. [Google Scholar] [CrossRef]
- Đukić-Ćosić, D.; Baralić, K.; Javorac, D.; Djordjevic, A.B.; Bulat, Z. An Overview of Molecular Mechanisms in Cadmium Toxicity. Curr. Opin. Toxicol. 2020, 19, 56–62. [Google Scholar] [CrossRef]
- Thévenod, F. Cadmium and Cellular Signaling Cascades: To Be or Not to Be? Toxicol. Appl. Pharmacol. 2009, 238, 221–239. [Google Scholar] [CrossRef]
- Hartwig, A. Cadmium and Cancer. Met. Ions Life Sci. 2013, 11, 491–507. [Google Scholar] [CrossRef]
- Hartwig, A. Mechanisms in Cadmium-Induced Carcinogenicity: Recent Insights. BioMetals 2010, 23, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Rhodes, C.; Moncol, J.; Izakovic, M.; Mazur, M. Free Radicals, Metals and Antioxidants in Oxidative Stress-Induced Cancer. Chem. Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Razzuoli, E.; Mignone, G.; Lazzara, F.; Vencia, W.; Ferraris, M.; Masiello, L.; Vivaldi, B.; Ferrari, A.; Bozzetta, E.; Amadori, M. Impact of Cadmium Exposure on Swine Enterocytes. Toxicol. Lett. 2018, 287, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Hossein-Khannazer, N.; Azizi, G.; Eslami, S.; Alhassan Mohammed, H.; Fayyaz, F.; Hosseinzadeh, R.; Usman, A.B.; Kamali, A.N.; Mohammadi, H.; Jadidi-Niaragh, F.; et al. The Effects of Cadmium Exposure in the Induction of Inflammation. Immunopharmacol. Immunotoxicol. 2019, 42, 1–8. [Google Scholar] [CrossRef]
- Miyahara, T.; Katoh, T.; Watanabe, M.; Mikami, Y.; Uchida, S.; Hosoe, M.; Sakuma, T.; Nemoto, N.; Takayama, K.; Komurasaki, T. Involvement of Mitogen-Activated Protein Kinases and Protein Kinase C in Cadmium-Induced Prostaglandin E2 Production in Primary Mouse Osteoblastic Cells. Toxicology 2004, 200, 159–167. [Google Scholar] [CrossRef]
- Rockwell, P.; Martinez, J.; Papa, L.; Gomes, E. Redox Regulates COX-2 Upregulation and Cell Death in the Neuronal Response to Cadmium. Cell. Signal. 2004, 16, 343–353. [Google Scholar] [CrossRef]
- Cormet-boyaka, E.; Jolivette, K.; Bonnegarde-bernard, A.; Rennolds, J.; Hassan, F.; Mehta, P.; Tridandapani, S.; Webster-marketon, J.; Boyaka, P.N. An NF-ΚB-Independent and Erk1/2-Dependent Mechanism Controls CXCL8/IL-8 Responses of Airway Epithelial Cells to Cadmium. Toxicol. Sci. 2012, 125, 418–429. [Google Scholar] [CrossRef]
- Nair, A.R.; DeGheselle, O.; Smeets, K.; Van Kerkhove, E.; Cuypers, A. Cadmium-Induced Pathologies: Where Is the Oxidative Balance Lost (or Not)? Int. J. Mol. Sci. 2013, 14, 6116. [Google Scholar] [CrossRef]
- Cannino, G.; Ferruggia, E.; Luparello, C.; Rinaldi, A.M. Cadmium and Mitochondria. Mitochondrion 2009, 9, 377–384. [Google Scholar] [CrossRef]
- Dorta, D.J.; Leite, S.; DeMarco, K.C.; Prado, I.M.R.; Rodrigues, T.; Mingatto, F.E.; Uyemura, S.A.; Santos, A.C.; Curti, C. A Proposed Sequence of Events for Cadmium-Induced Mitochondrial Impairment. J. Inorg. Biochem. 2003, 97, 251–257. [Google Scholar] [CrossRef]
- Souza, V.; Flores, K.; Ortiz, L.; Gómez-Quiroz, L.; Gutierrez-Ruiz, M. Liver and Cadmium Toxicity. J. Drug Metab. Toxicol. 2012, S5, 5. [Google Scholar] [CrossRef]
- Wang, H.; Wang, A.; Wang, X.; Zeng, X.; Xing, H. AMPK/PPAR-γ/NF-ΚB Axis Participates in ROS-Mediated Apoptosis and Autophagy Caused by Cadmium in Pig Liver. Environ. Pollut. 2022, 294, 118659. [Google Scholar] [CrossRef] [PubMed]
- Poliandri, A.H.B.; Esquifino, A.I.; Cano, P.; Jiménez, V.; Lafuente, A.; Cardinali, D.P.; Duvilanski, B.H. In Vivo Protective Effect of Melatonin on Cadmium-Induced Changes in Redox Balance and Gene Expression in Rat Hypothalamus and Anterior Pituitary. J. Pineal Res. 2006, 41, 238–246. [Google Scholar] [CrossRef]
- Chatterjee, S.; Kundu, S.; Bhattacharyya, A. Mechanism of Cadmium Induced Apoptosis in the Immunocyte. Toxicol. Lett. 2008, 177, 83–89. [Google Scholar] [CrossRef]
- El-Kott, A.F.; Alshehri, A.S.; Khalifa, H.S.; Abd-Lateif, A.E.; Karim, M.; Alshehri, M.A.; El-Maksoud, M.M.A.; Eid, R.A.; Bin-Meferij, M.M. Cadmium Chloride Induces Memory Deficits and Hippocampal Damage by Activating the JNK/P66Shc/NADPH Oxidase Axis. Int. J. Toxicol. 2020, 39, 477–490. [Google Scholar] [CrossRef]
- Mohammadi-Bardbori, A.; Rannug, A. Arsenic, Cadmium, Mercury and Nickel Stimulate Cell Growth via NADPH Oxidase Activation. Chem. Biol. Interact. 2014, 224, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Souza, V.; Escobar, M.d.C.; Bucio, L.; Hernández, E.; Gómez-Quiroz, L.E.; Gutiérrez Ruiz, M.C. NADPH Oxidase and ERK1/2 Are Involved in Cadmium Induced-STAT3 Activation in HepG2 Cells. Toxicol. Lett. 2009, 187, 180–186. [Google Scholar] [CrossRef]
- Gupta, D.K.; Pena, L.B.; Romero-Puertas, M.C.; Hernández, A.; Inouhe, M.; Sandalio, L.M. NADPH Oxidases Differentially Regulate ROS Metabolism and Nutrient Uptake under Cadmium Toxicity. Plant Cell Environ. 2016, 40, 509–526. [Google Scholar] [CrossRef]
- Halliwell, B. Reactive Species and Antioxidants. Redox Biology Is a Fundamental Theme of Aerobic Life. Plant Physiol. 2006, 141, 312. [Google Scholar] [CrossRef]
- Andrews, G.K. Regulation of Metallothionein Gene Expression by Oxidative Stress and Metal Ions. Biochem. Pharmacol. 2000, 59, 95–104. [Google Scholar] [CrossRef]
- Hauck, A.K.; Bernlohr, D.A. Oxidative Stress and Lipotoxicity. J. Lipid Res. 2016, 57, 1976–1986. [Google Scholar] [CrossRef] [PubMed]
- Cuypers, A.; Plusquin, M.; Remans, T.; Jozefczak, M.; Keunen, E.; Gielen, H.; Opdenakker, K.; Nair, A.R.; Munters, E.; Artois, T.J.; et al. Cadmium Stress: An Oxidative Challenge. BioMetals 2010, 23, 927–940. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Qu, W.; Kadiiska, M.B. Role of Oxidative Stress in Cadmium Toxicity and Carcinogenesis. Toxicol. Appl. Pharmacol. 2009, 238, 209–214. [Google Scholar] [CrossRef]
- Liu, L.; Tao, R.; Huang, J.; He, X.; Qu, L.; Jin, Y.; Zhang, S.; Fu, Z. Hepatic Oxidative Stress and Inflammatory Responses with Cadmium Exposure in Male Mice. Environ. Toxicol. Pharmacol. 2015, 39, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Ma, H.; Liu, G.; Fan, S.; Guo, Z. Mechanism of Cadmium Exposure Induced Hepatotoxicity in the Mud Crab (Scylla Paramamosain): Activation of Oxidative Stress and Nrf2 Signaling Pathway. Antioxidants 2022, 11, 978. [Google Scholar] [CrossRef]
- Wimmer, U.; Wang, Y.; Georgiev, O.; Schaffner, W. Two Major Branches of Anti-Cadmium Defense in the Mouse: MTF-1/Metallothioneins and Glutathione. Nucleic Acids Res. 2005, 33, 5715–5727. [Google Scholar] [CrossRef]
- Wang, Y.; Mandal, A.K.; Son, Y.O.K.; Pratheeshkumar, P.; Wise, J.T.F.; Wang, L.; Zhang, Z.; Shi, X.; Chen, Z. Roles of ROS, Nrf2, and Autophagy in Cadmium-Carcinogenesis and Its Prevention by Sulforaphane. Toxicol. Appl. Pharmacol. 2018, 353, 23–30. [Google Scholar] [CrossRef]
- Montes, S.; Juárez-Rebollar, D.; Nava-Ruíz, C.; Sánchez-García, A.; Heras-Romero, Y.; Rios, C.; Méndez-Armenta, M. Immunohistochemical Study of Nrf2-Antioxidant Response Element as Indicator of Oxidative Stress Induced by Cadmium in Developing Rats. Oxidative Med. Cell. Longev. 2015, 2015, 1–9. [Google Scholar] [CrossRef]
- Son, Y.O.; Pratheeshkumar, P.; Roy, R.V.; Hitron, J.A.; Wang, L.; Zhang, Z.; Shi, X. Nrf2/P62 Signaling in Apoptosis Resistance and Its Role in Cadmium-Induced Carcinogenesis. J. Biol. Chem. 2014, 289, 28660–28675. [Google Scholar] [CrossRef]
- Saed-Moucheshi, A.; Sohrabi, F.; Fasihfar, E.; Baniasadi, F.; Riasat, M.; Mozafari, A.A. Superoxide Dismutase (SOD) as a Selection Criterion for Triticale Grain Yield under Drought Stress: A Comprehensive Study on Genomics and Expression Profiling, Bioinformatics, Heritability, and Phenotypic Variability. BMC Plant Biol. 2021, 21, 148. [Google Scholar] [CrossRef]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide Dismutases: Dual Roles in Controlling ROS Damage and Regulating ROS Signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef] [PubMed]
- Candas, D.; Li, J.J. MnSOD in Oxidative Stress Response-Potential Regulation via Mitochondrial Protein Influx. Antioxid. Redox Signal. 2014, 20, 1599–1617. [Google Scholar] [CrossRef]
- Bresciani, G.; da Cruz, I.; González-Gallego, J. Manganese Superoxide Dismutase and Oxidative Stress Modulation. Adv. Clin. Chem. 2015, 68, 87–130. [Google Scholar] [CrossRef] [PubMed]
- López, E.; Arce, C.; Oset-Gasque, M.; Cañadas, S.; González, M. Cadmium Induces Reactive Oxygen Species Generation and Lipid Peroxidation in Cortical Neurons in Culture. Free Radic. Biol. Med. 2006, 40, 940–951. [Google Scholar] [CrossRef] [PubMed]
- Jurczuk, M.; Brzóska, M.M.; Moniuszko-Jakoniuk, J.; Gałażyn-Sidorczuk, M.; Kulikowska-Karpińska, E. Antioxidant Enzymes Activity and Lipid Peroxidation in Liver and Kidney of Rats Exposed to Cadmium and Ethanol. Food Chem. Toxicol. 2004, 42, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Gungor, H.; Kara, H. Effects of Selenium, Zinc, Insulin and Metallothionein on Cadmium-Induced Oxidative Stress and Metallothionein Gene Expression Levels in Diabetic Rats. J. Basic Clin. Physiol. Pharmacol. 2020, 31, 2. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.H.; Shih, C.M.; Huang, C.J.; Lin, C.M.; Chou, C.M.; Tsai, M.L.; Liu, T.P.; Chiu, J.F.; Chen, C.T. Effects of Cadmium on Structure and Enzymatic Activity of Cu,Zn-SOD and Oxidative Status in Neural Cells. J. Cell. Biochem. 2006, 98, 577–589. [Google Scholar] [CrossRef]
- Jihen, E.H.; Sonia, S.; Fatima, H.; Mohamed Tahar, S.; Abdelhamid, K. Interrelationships between Cadmium, Zinc and Antioxidants in the Liver of the Rat Exposed Orally to Relatively High Doses of Cadmium and Zinc. Ecotoxicol. Environ. Saf. 2011, 74, 2099–2104. [Google Scholar] [CrossRef]
- Glorieux, C.; Zamocky, M.; Sandoval, J.; Verrax, J.; Calderon, P. Regulation of Catalase Expression in Healthy and Cancerous Cells. Free Radic. Biol. Med. 2015, 87, 84–97. [Google Scholar] [CrossRef]
- Schrader, M.; Fahimi, H. Mammalian Peroxisomes and Reactive Oxygen Species. Histochem. Cell Biol. 2004, 122, 383–393. [Google Scholar] [CrossRef]
- Kalyanaraman, B. Teaching the Basics of Redox Biology to Medical and Graduate Students: Oxidants, Antioxidants and Disease Mechanisms. Redox Biol. 2013, 1, 244–257. [Google Scholar] [CrossRef] [PubMed]
- Svensson, B.E. Abilities of Peroxidases to Catalyse Peroxidase-Oxidase Oxidation of Thiols. Biochem. J. 1988, 256, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Glorieux, C.; Calderon, P. Catalase, a Remarkable Enzyme: Targeting the Oldest Antioxidant Enzyme to Find a New Cancer Treatment Approach. Biol. Chem. 2017, 398, 1095–1108. [Google Scholar] [CrossRef] [PubMed]
- Matés, J.M. Effects of Antioxidant Enzymes in the Molecular Control of Reactive Oxygen Species Toxicology. Toxicology 2000, 153, 83–104. [Google Scholar] [CrossRef] [PubMed]
- Panday, S.; Talreja, R.; Kavdia, M. The Role of Glutathione and Glutathione Peroxidase in Regulating Cellular Level of Reactive Oxygen and Nitrogen Species. Microvasc. Res. 2020, 131, 104010. [Google Scholar] [CrossRef]
- Vairavamurthy, M.A.; Goldenberg, W.S.; Ouyang, S.; Khalid, S. The Interaction of Hydrophilic Thiols with Cadmium: Investigation with a Simple Model, 3-Mercaptopropionic Acid. Mar. Chem. 2000, 70, 181–189. [Google Scholar] [CrossRef]
- Mah, V.; Jalilehvand, F. Cadmium(II) Complex Formation with Glutathione. J. Biol. Inorg. Chem. 2010, 15, 441–458. [Google Scholar] [CrossRef]
- Leverrier, P.; Montigny, C.; Garrigos, M.; Champeil, P. Metal Binding to Ligands: Cadmium Complexes with Glutathione Revisited. Anal. Biochem. 2007, 371, 215–228. [Google Scholar] [CrossRef]
- Delalande, O.; Desvaux, H.; Godat, E.; Valleix, A.; Junot, C.; Labarre, J.; Boulard, Y. Cadmium—Glutathione Solution Structures Provide New Insights into Heavy Metal Detoxification. FEBS J. 2010, 277, 5086–5096. [Google Scholar] [CrossRef]
- Adamis, P.D.B.; Gomes, D.S.; Pinto, M.L.C.C.; Panek, A.D.; Eleutherio, E.C.A. The Role of Glutathione Transferases in Cadmium Stress. Toxicol. Lett. 2004, 154, 81–88. [Google Scholar] [CrossRef]
- Sandbichler, A.M.; Höckner, M. Cadmium Protection Strategies—A Hidden Trade-Off? Int. J. Mol. Sci. 2016, 17, 139. [Google Scholar] [CrossRef] [PubMed]
- Henkel, G.; Krebs, B. Metallothioneins: Zinc, Cadmium, Mercury, and Copper Thiolates and Selenolates Mimicking Protein Active Site Features—Structural Aspects and Biological Implications. Chem. Rev. 2004, 104, 801–824. [Google Scholar] [CrossRef] [PubMed]
- Sabolić, I.; Breljak, D.; Škarica, M.; Herak-Kramberger, C.M. Role of Metallothionein in Cadmium Traffic and Toxicity in Kidneys and Other Mammalian Organs. BioMetals 2010, 23, 897–926. [Google Scholar] [CrossRef]
- Lei, L.; Jin, T.; YF, Z. Insulin Expression in Rats Exposed to Cadmium—PubMed. Biomed. Environ. Sci. 2007, 20, 295–301. [Google Scholar] [PubMed]
- Lei, L.J.; Jin, T.Y.; Zhou, Y.F. Effects of Cadmium on Levels of Insulin in Rats. Wei Sheng Yan Jiu 2005, 34, 394–396. [Google Scholar] [PubMed]
- El Muayed, M.; Raja, M.R.; Zhang, X.; MacRenaris, K.W.; Bhatt, S.; Chen, X.; Urbanek, M.; O’Halloran, T.V.; Lowe, W.L. Accumulation of Cadmium in Insulin-Producing β Cells. Islets 2012, 4, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.V. Zinc and Insulin in Pancreatic Beta-Cells. Endocrine 2014, 45, 178–189. [Google Scholar] [CrossRef]
- Dodson, G.; Steiner, D. The Role of Assembly in Insulin’s Biosynthesis. Curr. Opin. Struct. Biol. 1998, 8, 189–194. [Google Scholar] [CrossRef]
- Moulis, J.-M. Cellular Mechanisms of Cadmium Toxicity Related to the Homeostasis of Essential Metals. BioMetals 2010, 23, 877–896. [Google Scholar] [CrossRef]
- Sarmiento-Ortega, V.E.; Treviño, S.; Flores-Hernández, J.Á.; Aguilar-Alonso, P.; Moroni-González, D.; Aburto-Luna, V.; Diaz, A.; Brambila, E. Changes on Serum and Hepatic Lipidome after a Chronic Cadmium Exposure in Wistar Rats. Arch. Biochem. Biophys. 2017, 635, 52–59. [Google Scholar] [CrossRef]
- Treviño, S.; Waalkes, M.P.; Flores Hernández, J.A.; León-Chavez, B.A.; Aguilar-Alonso, P.; Brambila, E. Chronic Cadmium Exposure in Rats Produces Pancreatic Impairment and Insulin Resistance in Multiple Peripheral Tissues. Arch. Biochem. Biophys. 2015, 583, 27–35. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A. Pathogenesis of Type 2 Diabetes Mellitus. Med. Clin. N. Am. 2004, 88, 787–835. [Google Scholar] [CrossRef] [PubMed]
- Paschen, M.; Moede, T.; Valladolid-Acebes, I.; Leibiger, B.; Moruzzi, N.; Jacob, S.; García-Prieto, C.F.; Brismar, K.; Leibiger, I.B.; Berggren, P.-O. Diet-Induced β-Cell Insulin Resistance Results in Reversible Loss of Functional β-Cell Mass. FASEB J. 2019, 33, 204–218. [Google Scholar] [CrossRef]
- Shanik, J.; Xu, Y.; Skrha, J.; Dankner, R.; Zick, Y.; Roth, J. Insulin Resistance and Hyperinsulinemia: Is Hyperinsulinemia the Cart or the Horse? Diabetes Care 2008, 31 (Suppl. S2), S262–S268. [Google Scholar] [CrossRef] [PubMed]
- Gastaldelli, A.; Ferrannini, E.; Miyazaki, Y.; Matsuda, M.; DeFronzo, R.A. San Antonio metabolism study Beta-Cell Dysfunction and Glucose Intolerance: Results from the San Antonio Metabolism (SAM) Study. Diabetologia 2004, 47, 31–39. [Google Scholar] [CrossRef]
- Yazıcı, D.; Sezer, H. Insulin Resistance, Obesity and Lipotoxicity. Adv. Exp. Med. Biol. 2017, 960, 277–304. [Google Scholar] [CrossRef]
- Swisa, A.; Glaser, B.; Dor, Y. Metabolic Stress and Compromised Identity of Pancreatic Beta Cells. Front. Genet. 2017, 08, 21. [Google Scholar] [CrossRef]
- Kahn, S.E. The Relative Contributions of Insulin Resistance and Beta-Cell Dysfunction to the Pathophysiology of Type 2 Diabetes. Diabetologia 2003, 46, 3–19. [Google Scholar] [CrossRef]
- Fu, Z.; Gilbert, E.R.; Liu, D. Regulation of Insulin Synthesis and Secretion and Pancreatic Beta-Cell Dysfunction in Diabetes. Curr. Diabetes Rev. 2013, 9, 25–53. [Google Scholar] [CrossRef]
- Corkey, B.E. Metabolic Regulation of Insulin Secretion. In Pancreatic Beta Cell in Health and Disease; Springer: Japan, Tokyo, 2007; pp. 53–74. [Google Scholar]
- Ali, I.; Damdimopoulou, P.; Stenius, U.; Halldin, K. Cadmium at Nanomolar Concentrations Activates Raf–MEK–ERK1/2 MAPKs Signaling via EGFR in Human Cancer Cell Lines. Chem. Biol. Interact. 2015, 231, 44–52. [Google Scholar] [CrossRef]
- Thévenod, F.; Friedmann, J.; Katsen, A.; Hauser, I. Up-Regulation of Multidrug Resistance P-Glycoprotein via Nuclear Factor-KappaB Activation Protects Kidney Proximal Tubule Cells from Cadmium- and Reactive Oxygen Species-Induced Apoptosis. J. Biol. Chem. 2000, 275, 1887–1896. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-K.; Thévenod, F. Cell Organelles as Targets of Mammalian Cadmium Toxicity. Arch. Toxicol. 2020, 94, 1017–1049. [Google Scholar] [CrossRef] [PubMed]
- Okada, T.; Chong, W.L.; Hu, J.; Hinault, C.; Michael, M.D.; Krützfeldt, J.; Yin, C.; Holzenberger, M.; Stoffel, M.; Kulkarni, R.N. Insulin Receptors in β-Cells Are Critical for Islet Compensatory Growth Response to Insulin Resistance. Proc. Natl. Acad. Sci. USA 2007, 104, 8977–8982. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, B.R.; Brun, T.; Sarret, E.J.; Ishihara, H.; Schaad, O.; Descombes, P.; Wollheim, C.B. Oligonucleotide Microarray Analysis Reveals PDX1 as an Essential Regulator of Mitochondrial Metabolism in Rat Islets. J. Biol. Chem. 2004, 279, 31121–31130. [Google Scholar] [CrossRef]
- Poitout, V.; Hagman, D.; Stein, R.; Artner, I.; Robertson, R.P.; Harmon, J.S. Regulation of the Insulin Gene by Glucose and Fatty Acids. J. Nutr. 2006, 136, 873–876. [Google Scholar] [CrossRef]
- Sarkar, A.; Zhang, M.; Liu, S.; Sarkar, S.; Brunicardi, F.C.; Berger, D.H.; Belaguli, N.S. Serum Response Factor Expression Is Enriched in Pancreatic β Cells and Regulates Insulin Gene Expression. FASEB J. 2011, 25, 2592–2603. [Google Scholar] [CrossRef]
- Treisman, R. The Serum Response Element. Trends Biochem. Sci. 1992, 17, 423–426. [Google Scholar] [CrossRef]
- Vivas, Y.; Martínez-García, C.; Izquierdo, A.; Garcia-Garcia, F.; Callejas, S.; Velasco, I.; Campbell, M.; Ros, M.; Dopazo, A.; Dopazo, J.; et al. Early Peroxisome Proliferator-Activated Receptor Gamma Regulated Genes Involved in Expansion of Pancreatic Beta Cell Mass. BMC Med. Genom. 2011, 4, 86. [Google Scholar] [CrossRef]
- Kitamura, T.; Ido Kitamura, Y. Role of FoxO Proteins in Pancreatic β Cells. Endocr. J. 2007, 54, 507–515. [Google Scholar] [CrossRef]
- Kitamura, T. The Role of FOXO1 in β-Cell Failure and Type 2 Diabetes Mellitus. Nat. Rev. Endocrinol. 2013, 9, 615–623. [Google Scholar] [CrossRef]
- Lv, L.; Chen, H.; Sun, J.; Lu, D.; Chen, C.; Liu, D. PRMT1 Promotes Glucose Toxicity-Induced β Cell Dysfunction by Regulating the Nucleo-Cytoplasmic Trafficking of PDX-1 in a FOXO1-Dependent Manner in INS-1 Cells. Endocrine 2015, 49, 669–682. [Google Scholar] [CrossRef]
- Kitamura, T.; Nakae, J.; Kitamura, Y.; Kido, Y.; Biggs, W.; Wright, C.; White, M.; Arden, K.; Accili, D. The Forkhead Transcription Factor Foxo1 Links Insulin Signaling to Pdx1 Regulation of Pancreatic Beta Cell Growth. J. Clin. Investig. 2002, 110, 1839–1847. [Google Scholar] [CrossRef] [PubMed]
- Ying, W.; Fu, W.; Lee, Y.S.; Olefsky, J.M. The Role of Macrophages in Obesity-Associated Islet Inflammation and β-Cell Abnormalities. Nat. Rev. Endocrinol. 2020, 16, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, K.; Nagai, R. Islet Inflammation in Type 2 Diabetes and Physiology. J. Clin. Investig. 2017, 127, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Ying, W.; Lee, Y.S.; Dong, Y.; Seidman, J.S.; Yang, M.; Isaac, R.; Seo, J.B.; Yang, B.H.; Wollam, J.; Riopel, M.; et al. Expansion of Islet-Resident Macrophages Leads to Inflammation Affecting β Cell Proliferation and Function in Obesity. Cell Metab. 2019, 29, 457–474.e5. [Google Scholar] [CrossRef] [PubMed]
- El Alwani, M.; Wu, B.X.; Obeid, L.M.; Hannun, Y.A. Bioactive Sphingolipids in the Modulation of the Inflammatory Response. Pharmacol. Ther. 2006, 112, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Xu, Y.; Xu, J.; Zhang, J.; Xi, Y.; Pi, H.; Yang, L.; Yu, Z.; Wu, Q.; Meng, Z.; et al. Cadmium Exposure Impairs Pancreatic β-Cell Function and Exaggerates Diabetes by Disrupting Lipid Metabolism. Environ. Int. 2021, 149, 106406. [Google Scholar] [CrossRef] [PubMed]
- Palomer, X.; Pizarro-Delgado, J.; Barroso, E.; Vázquez-Carrera, M. Palmitic and Oleic Acid: The Yin and Yang of Fatty Acids in Type 2 Diabetes Mellitus. Trends Endocrinol. Metab. 2018, 29, 178–190. [Google Scholar] [CrossRef]
- Ježek, P.; Holendová, B.; Jabůrek, M.; Tauber, J.; Dlasková, A.; Plecitá-Hlavatá, L. The Pancreatic β-Cell: The Perfect Redox System. Antioxidants 2021, 10, 197. [Google Scholar] [CrossRef]
- Chabosseau, P.; Rutter, G.A. Zinc and Diabetes. Arch. Biochem. Biophys. 2016, 611, 79–85. [Google Scholar] [CrossRef]
- Bellomo, E.A.; Meur, G.; Rutter, G.A. Glucose Regulates Free Cytosolic Zn2+ Concentration, Slc39 (ZiP), and Metallothionein Gene Expression in Primary Pancreatic Islet β-Cells. J. Biol. Chem. 2011, 286, 25778–25789. [Google Scholar] [CrossRef] [PubMed]
- Bensellam, M.; Laybutt, D.R.; Jonas, J.-C. Emerging Roles of Metallothioneins in Beta Cell Pathophysiology: Beyond and Above Metal Homeostasis and Antioxidant Response. Biology 2021, 10, 176. [Google Scholar] [CrossRef] [PubMed]
- Günther, V.; Lindert, U.; Schaffner, W. The Taste of Heavy Metals: Gene Regulation by MTF-1. Biochim. Biophys. Acta BBA Mol. Cell Res. 2012, 1823, 1416–1425. [Google Scholar] [CrossRef]
- Zimny, S.; Gogolin, F.; Abel, J.; Gleichmann, H. Metallothionein in Isolated Pancreatic Islets of Mice: Induction by Zinc and Streptozotocin, a Naturally Occurring Diabetogen. Arch. Toxicol. 1993, 67, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Ohly, P.; Gleichmann, H. Metallothionein: In Vitro Induction With Zinc and Streptozotocin in Pancreatic Islets of Mice. Exp. Clin. Endocrinol. Diabetes 1995, 103, 79–82. [Google Scholar] [CrossRef]
- Ohly, P.; Dohle, C.; Abel, J.; Seissler, J.; Gleichmann, H. Zinc Sulphate Induces Metallothionein in Pancreatic Islets of Mice and Protects against Diabetes Induced by Multiple Low Doses of Streptozotocin. Diabetologia 2000, 43, 1020–1030. [Google Scholar] [CrossRef] [PubMed]
- Duprez, J.; Roma, L.; Close, A.; Jonas, J. Protective Antioxidant and Antiapoptotic Effects of ZnCl2 in Rat Pancreatic Islets Cultured in Low and High Glucose Concentrations. PLoS ONE 2012, 7, e46831. [Google Scholar] [CrossRef] [PubMed]
- Jonas, J.C.; Bensellam, M.; Duprez, J.; Elouil, H.; Guiot, Y.; Pascal, S.M.A. Glucose Regulation of Islet Stress Responses and β-Cell Failure in Type 2 Diabetes. Diabetes Obes. Metab. 2009, 11, 65–81. [Google Scholar] [CrossRef]
- Ortis, F.; Naamane, N.; Flamez, D.; Ladrière, L.; Moore, F.; Cunha, D.; Colli, M.; Thykjaer, T.; Thorsen, K.; Orntoft, T.; et al. Cytokines Interleukin-1beta and Tumor Necrosis Factor-Alpha Regulate Different Transcriptional and Alternative Splicing Networks in Primary Beta-Cells. Diabetes 2010, 59, 358–374. [Google Scholar] [CrossRef]
- Cardoso, S.; Seiça, R.; Moreira, P.I. Diabesity and Brain Energy Metabolism: The Case of Alzheimer’s Disease. In Advances in Neurobiology; Springer: New York, NY, USA, 2017; Volume 19, pp. 117–150. [Google Scholar]
- Boyd, S.D.; Ullrich, M.S.; Skopp, A.; Winkler, D.D. Copper Sources for Sod1 Activation. Antioxidants 2020, 9, 500. [Google Scholar] [CrossRef]
- Miura, T.; Muraoka, S.; Ogiso, T. Antioxidant Activity of Metallothionein Compared with Reduced Glutathione. Life Sci. 1997, 60, 301–309. [Google Scholar] [CrossRef]
- Langston, W.; Circu, M.L.; Aw, T.Y. Insulin Stimulation of γ-Glutamylcysteine Ligase Catalytic Subunit Expression Increases Endothelial GSH during Oxidative Stress: Influence of Low Glucose. Free Radic. Biol. Med. 2008, 45, 1591. [Google Scholar] [CrossRef] [PubMed]
- Franklin, C.C.; Backos, D.S.; Mohar, I.; White, C.C.; Forman, H.J.; Kavanagh, T.J. Structure, Function, and Post-Translational Regulation of the Catalytic and Modifier Subunits of Glutamate Cysteine Ligase. Mol. Asp. Med. 2009, 30, 86. [Google Scholar] [CrossRef] [PubMed]
- Merry, T.; Tran, M.; Stathopoulos, M.; Wiede, F.; Fam, B.; Dodd, G.; Clarke, I.; Watt, M.; Andrikopoulos, S.; Tiganis, T. High-Fat-Fed Obese Glutathione Peroxidase 1-Deficient Mice Exhibit Defective Insulin Secretion but Protection from Hepatic Steatosis and Liver Damage. Antioxid. Redox Signal. 2014, 20, 2114–2129. [Google Scholar] [CrossRef] [PubMed]
- Alnahdi, A.; John, A.; Raza, H. N-Acetyl Cysteine Attenuates Oxidative Stress and Glutathione-Dependent Redox Imbalance Caused by High Glucose/High Palmitic Acid Treatment in Pancreatic Rin-5F Cells. PLoS ONE 2019, 14, e0226696. [Google Scholar] [CrossRef] [PubMed]
- Lenzen, S. Oxidative Stress: The Vulnerable β-Cell. Biochem. Soc. Trans. 2008, 36, 343–347. [Google Scholar] [CrossRef]
- Grankvist, K.; Marklund, S.L.; Taljedal, I.B. CuZn-Superoxide Dismutase, Mn-Superoxide Dismutase, Catalase and Glutathione Peroxidase in Pancreatic Islets and Other Tissues in the Mouse. Biochem. J. 1981, 199, 393–398. [Google Scholar] [CrossRef]
- Aronis, A.; Madar, Z.; Tirosh, O. Mechanism Underlying Oxidative Stress-Mediated Lipotoxicity: Exposure of J774.2 Macrophages to Triacylglycerols Facilitates Mitochondrial Reactive Oxygen Species Production and Cellular Necrosis. Free Radic. Biol. Med. 2005, 38, 1221–1230. [Google Scholar] [CrossRef]
- Mulder, H.; Ling, C. Mitochondrial Dysfunction in Pancreatic β-Cells in Type 2 Diabetes. Mol. Cell. Endocrinol. 2009, 297, 34–40. [Google Scholar] [CrossRef]
- Jacquet, A.; Cottet-Rousselle, C.; Arnaud, J.; Julien Saint Amand, K.; Ben Messaoud, R.; Lénon, M.; Demeilliers, C.; Moulis, J.-M. Mitochondrial Morphology and Function of the Pancreatic β-Cells INS-1 Model upon Chronic Exposure to Sub-Lethal Cadmium Doses. Toxics 2018, 6, 20. [Google Scholar] [CrossRef]
- Oh, Y.S.; Bae, G.D.; Baek, D.J.; Park, E.Y.; Jun, H.S. Fatty Acid-Induced Lipotoxicity in Pancreatic Beta-Cells During Development of Type 2 Diabetes. Front. Endocrinol. 2018, 9, 384. [Google Scholar] [CrossRef] [PubMed]
- Pall, M.L.; Levine, S. Nrf2, a Master Regulator of Detoxification and Also Antioxidant, Anti-Inflammatory and Other Cytoprotective Mechanisms, Is Raised by Health Promoting Factors. Sheng Li Xue Bao 2015, 67, 1–18. [Google Scholar]
- Newsholme, P.; Keane, K.N.; Carlessi, R.; Cruzat, V. Oxidative Stress Pathways in Pancreatic β-Cells and Insulin-Sensitive Cells and Tissues: Importance to Cell Metabolism, Function, and Dysfunction. Am. J. Physiol. Physiol. 2019, 317, C420–C433. [Google Scholar] [CrossRef] [PubMed]
- Yagishita, Y.; Fukutomi, T.; Sugawara, A.; Kawamura, H.; Takahashi, T.; Pi, J.; Uruno, A.; Yamamoto, M. Nrf2 Protects Pancreatic β-Cells From Oxidative and Nitrosative Stress in Diabetic Model Mice. Diabetes 2014, 63, 605–618. [Google Scholar] [CrossRef] [PubMed]
- El-Mansy, A.A.; Mazroa, S.A.; Hamed, W.S.; Yaseen, A.H.; El-Mohandes, E.A. Histological and Immunohistochemical Effects of Curcuma Longa on Activation of Rat Hepatic Stellate Cells after Cadmium Induced Hepatotoxicity. Biotech. Histochem. 2016, 91, 170–181. [Google Scholar] [CrossRef] [PubMed]
- El-Sokkary, G.H.; Nafady, A.A.; Shabash, E.H. Melatonin Administration Ameliorates Cadmium-Induced Oxidative Stress and Morphological Changes in the Liver of Rat. Ecotoxicol. Environ. Saf. 2010, 73, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Sarmiento-Ortega, V.E.; Moroni-González, D.; Díaz, A.; Eduardo, B.; Samuel, T. Oral Subacute Exposure to Cadmium LOAEL Dose Induces Insulin Resistance and Impairment of the Hormonal and Metabolic Liver-Adipose Axis in Wistar Rats. Biol. Trace Elem. Res. 2021, 200, 4370–4384. [Google Scholar] [CrossRef]
- Harstad, E.B.; Klaassen, C.D. Gadolinium Chloride Pretreatment Prevents Cadmium Chloride-Induced Liver Damage in Both Wild-Type and MT-Null Mice. Toxicol. Appl. Pharmacol. 2002, 180, 178–185. [Google Scholar] [CrossRef]
- Souza, V.; Escobar, M.D.C.; Gómez-Quiroz, L.; Bucio, L.; Hernández, E.; Cossio, E.C.; Gutiérrez-Ruiz, M.C. Acute Cadmium Exposure Enhances AP-1 DNA Binding and Induces Cytokines Expression and Heat Shock Protein 70 in HepG2 Cells. Toxicology 2004, 197, 213–228. [Google Scholar] [CrossRef]
- Matović, V.; Buha, A.; Dukić-Ćosić, D.; Bulat, Z. Insight into the Oxidative Stress Induced by Lead and/or Cadmium in Blood, Liver and Kidneys. Food Chem. Toxicol. 2015, 78, 130–140. [Google Scholar] [CrossRef]
- Dai, S.; Yin, Z.; Yuan, G.; Lu, H.; Jia, R.; Xu, J.; Song, X.; Li, L.; Shu, Y.; Liang, X.; et al. Quantification of Metallothionein on the Liver and Kidney of Rats by Subchronic Lead and Cadmium in Combination. Environ. Toxicol. Pharmacol. 2013, 36, 1207–1216. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Y.; Xia, M.Z.; Wang, H.; Liu, X.J.; Hu, Y.F.; Chen, Y.H.; Zhang, C.; Xu, D.X. Cadmium Selectively Induces MIP-2 and COX-2 through PTEN-Mediated Akt Activation in RAW264.7 Cells. Toxicol. Sci. 2014, 138, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Okoye, C.N.; MacDonald-Jay, N.; Kamunde, C. Effects of Bioenergetics, Temperature and Cadmium on Liver Mitochondria Reactive Oxygen Species Production and Consumption. Aquat. Toxicol. 2019, 214, 105264. [Google Scholar] [CrossRef] [PubMed]
- Gebhardt, R. Prevention of Cadmium-Induced Toxicity in Liver-Derived Cells by the Combination Preparation Hepeel®. Environ. Toxicol. Pharmacol. 2009, 27, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Gao, J.; Zhang, K.; Liu, X.; Li, J. Effects of Chronic Cadmium Poisoning on Zn, Cu, Fe, Ca, and Metallothionein in Liver and Kidney of Rats. Biol. Trace Elem. Res. 2012, 149, 57–63. [Google Scholar] [CrossRef]
- Beyrami, M.; Karimi, E.; Oskoueian, E. Synthesized Chrysin-Loaded Nanoliposomes Improves Cadmium-Induced Toxicity in Mice. Environ. Sci. Pollut. Res. 2020, 27, 40643–40651. [Google Scholar] [CrossRef]
- Pal, M.; Febbraio, M.A.; Lancaster, G.I. The Roles of C-Jun NH2-Terminal Kinases (JNKs) in Obesity and Insulin Resistance. J. Physiol. 2016, 594, 267–279. [Google Scholar] [CrossRef]
- Solinas, G.; Becattini, B. JNK at the Crossroad of Obesity, Insulin Resistance, and Cell Stress Response. Mol. Metab. 2017, 6, 174–184. [Google Scholar] [CrossRef]
- Schmitz-Peiffer, C.; Laybutt, D.R.; Burchfield, J.G.; Gurisik, E.; Narasimhan, S.; Mitchell, C.J.; Pedersen, D.J.; Braun, U.; Cooney, G.J.; Leitges, M.; et al. Inhibition of PKCε Improves Glucose-Stimulated Insulin Secretion and Reduces Insulin Clearance. Cell Metab. 2007, 6, 320–328. [Google Scholar] [CrossRef]
- Chapatwala, K.D.; Rajanna, E.; Desaiah, D. Cadmium Induced Changes in Gluconeogenic Enzymes in Rat Kidney and Liver. Drug Chem. Toxicol. 1980, 3, 407–420. [Google Scholar] [CrossRef]
- Chapatwala, K.D.; Hobson, M.; Desaiah, D.; Rajanna, B. Effect of Cadmium on Hepatic and Renal Gluconeogenic Enzymes in Female Rats. Toxicol. Lett. 1982, 12, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Bashir, N.; Shagirtha, K.; Manoharan, V.; Miltonprabu, S. The Molecular and Biochemical Insight View of Grape Seed Proanthocyanidins in Ameliorating Cadmium-Induced Testes-Toxicity in Rat Model: Implication of PI3K/Akt/Nrf-2 Signaling. Biosci. Rep. 2019, 39, BSR20180515. [Google Scholar] [CrossRef] [PubMed]
- Shati, A.A.; Alfaifi, M.Y. Trans-Resveratrol Inhibits Tau Phosphorylation in the Brains of Control and Cadmium Chloride-Treated Rats by Activating PP2A and PI3K/Akt Induced-Inhibition of GSK3β. Neurochem. Res. 2019, 44, 357–373. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Zhang, Y.; Xing, H.; Xu, S. Ameliorative Effect of Selenomethionine on Cadmium-Induced Hepatocyte Apoptosis via Regulating PI3K/AKT Pathway in Chickens. Biol. Trace Elem. Res. 2019, 195, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Xin, C.; Guangliang, S.; Qing, Z.; Qingqing, L.; Hang, Y.; Yiming, Z.; Shu, L. Astilbin Protects Chicken Peripheral Blood Lymphocytes from Cadmium-Induced Necroptosis via Oxidative Stress and the PI3K/Akt Pathway. Ecotoxicol. Environ. Saf. 2020, 190, 110064. [Google Scholar] [CrossRef]
- Fang, X.; Yu, S.X.; Lu, Y.; Bast, R.C.; Woodgett, J.R.; Mills, G.B. Phosphorylation and Inactivation of Glycogen Synthase Kinase 3 by Protein Kinase A. Proc. Natl. Acad. Sci. USA 2000, 97, 11960–11965. [Google Scholar] [CrossRef]
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef]
- Sarmiento-Ortega, V.E.; Moroni-González, D.; Díaz, A.; Morán, C.; Brambila, E.; Treviño, S. Sodium Metavanadate Treatment Improves Glycogen Levels in Multiple Tissues in a Model of Metabolic Syndrome Caused by Chronic Cadmium Exposure in Wistar Rats. BioMetals 2021, 34, 245–258. [Google Scholar] [CrossRef]
- Sarmiento-Ortega, V.; Brambila, E.; Flores-Hernández, J.; Díaz, A.; Peña-Rosas, U.; Moroni-González, D.; Aburto-Luna, V.; Treviño, S. The NOAEL Metformin Dose Is Ineffective against Metabolic Disruption Induced by Chronic Cadmium Exposure in Wistar Rats. Toxics 2018, 6, 55. [Google Scholar] [CrossRef]
- Treviño, S.; Diaz, A. Vanadium and Insulin: Partners in Metabolic Regulation. J. Inorg. Biochem. 2020, 208, 111094. [Google Scholar] [CrossRef]
- Treviño, S.; Sánchez-Lara, E.; Sarmiento-Ortega, V.E.; Sánchez-Lombardo, I.; Flores-Hernández, J.Á.; Pérez-Benítez, A.; Brambila-Colombres, E.; González-Vergara, E. Hypoglycemic, Lipid-Lowering and Metabolic Regulation Activities of Metforminium Decavanadate (H2Metf)3 [V10O28]·8H2O Using Hypercaloric-Induced Carbohydrate and Lipid Deregulation in Wistar Rats as b. J. Inorg. Biochem. 2015, 147, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Santamaria-Juarez, C.; Atonal-Flores, F.; Diaz, A.; Sarmiento-Ortega, V.E.; Garcia-Gonzalez, M.; Aguilar-Alonso, P.; Lopez-Lopez, G.; Brambila, E.; Treviño, S. Aortic Dysfunction by Chronic Cadmium Exposure Is Linked to Multiple Metabolic Risk Factors That Converge in Anion Superoxide Production. Arch. Physiol. Biochem. 2020, 128, 748–756. [Google Scholar] [CrossRef]
- Hirano, T. Pathophysiology of Diabetic Dyslipidemia. J. Atheroscler. Thromb. 2018, 25, 771–782. [Google Scholar] [CrossRef]
- Adiels, M.; Olofsson, S.O.; Taskinen, M.R.; Borén, J. Diabetic Dyslipidaemia. Curr. Opin. Lipidol. 2006, 17, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Packard, C.J. Triacylglycerol-Rich Lipoproteins and the Generation of Small, Dense Low-Density Lipoprotein. Biochem. Soc. Trans. 2003, 31, 1066–1069. [Google Scholar] [CrossRef] [PubMed]
- Adiels, M.; Taskinen, M.R.; Packard, C.; Caslake, M.J.; Soro-Paavonen, A.; Westerbacka, J.; Vehkavaara, S.; Häkkinen, A.; Olofsson, S.O.; Yki-Järvinen, H.; et al. Overproduction of Large VLDL Particles Is Driven by Increased Liver Fat Content in Man. Diabetologia 2006, 49, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Ji, A.; Wroblewski, J.M.; Webb, N.R.; Van Der Westhuyzen, D.R. Impact of Phospholipid Transfer Protein on Nascent High-Density Lipoprotein Formation and Remodeling. Arter. Thromb. Vasc. Biol. 2014, 34, 1910–1916. [Google Scholar] [CrossRef]
- Ramakrishnan, R.; Elangovan, P.; Pari, L. Protective Role of Tetrahydrocurcumin: An Active Polyphenolic Curcuminoid on Cadmium-InducedOxidative Damage in Rats. Appl. Biochem. Biotechnol. 2017, 183, 51–69. [Google Scholar] [CrossRef]
- Camont, L.; Lhomme, M.; Rached, F.; Le Goff, W.; Nègre-Salvayre, A.; Salvayre, R.; Calzada, C.; Lagarde, M.; Chapman, M.J.; Kontush, A. Small, Dense High-Density Lipoprotein-3 Particles Are Enriched in Negatively Charged Phospholipids: Relevance to Cellular Cholesterol Efflux, Antioxidative, Antithrombotic, Anti-Inflammatory, and Antiapoptotic Functionalities. Arter. Thromb. Vasc. Biol. 2013, 33, 2715–2723. [Google Scholar] [CrossRef]
- Jaeschke, H.; Gores, G.J.; Cederbaum, A.I.; Hinson, J.A.; Pessayre, D.; Lemasters, J.J. Mechanisms of Hepatotoxicity. Toxicol. Sci. 2002, 65, 166–176. [Google Scholar] [CrossRef]
- Martelli, A.; Rousselet, E.; Dycke, C.; Bouron, A.; Moulis, J.M. Cadmium Toxicity in Animal Cells by Interference with Essential Metals. Biochimie 2006, 88, 1807–1814. [Google Scholar] [CrossRef] [PubMed]
- Souza, V.; Bucio, L.; Gutiérrez-Ruiz, M.C. Cadmium Uptake by a Human Hepatic Cell Line (WRL-68 Cells). Toxicology 1997, 120, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Min, K.S.; Ueda, H.; Tanaka, K. Involvement of Intestinal Calcium Transporter 1 and Metallothionein in Cadmium Accumulation in the Liver and Kidney of Mice Fed a Low-Calcium Diet. Toxicol. Lett. 2008, 176, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Fujishiro, H.; Okugaki, S.; Kubota, K.; Fujiyama, T.; Miyataka, H.; Himeno, S. The Role of ZIP8 Down-Regulation in Cadmium-Resistant Metallothionein-Null Cells. J. Appl. Toxicol. 2009, 29, 367–373. [Google Scholar] [CrossRef]
- DelRaso, N.J.; Foy, B.D.; Gearhart, J.M.; Frazier, J.M. Cadmium Uptake Kinetics in Rat Hepatocytes: Correction for Albumin Binding. Toxicol. Sci. 2003, 72, 19–30. [Google Scholar] [CrossRef]
- Nordberg, G.F.; Piscator, M.; Lind, B. Distribution of Cadmium among Protein Fractions of Mouse Liver. Acta Pharmacol. Toxicol. 1971, 29, 456–470. [Google Scholar] [CrossRef] [PubMed]
- Goering, P.L.; Klaassen, C.D. Tolerance to Cadmium-Induced Toxicity Depends on Presynthesized Metallothionein in Liver. J. Toxicol. Environ. Health 1984, 14, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Qi, K.; Zhang, L.; Bai, Z.; Ren, C.; Xu, X.; Zhang, Z.; Li, X. Glutathione Might Attenuate Cadmium-Induced Liver Oxidative Stress and Hepatic Stellate Cell Activation. Biol. Trace Elem. Res. 2019, 191, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.V.S.; Verma, S. Protective Effects of GSH, Vitamin E, and Selenium on Lipid Peroxidation in Cadmium-Fed Rats. Biol. Trace Elem. Res. 1996, 51, 161–168. [Google Scholar] [CrossRef]
- Renugadevi, J.; Prabu, S.M. Cadmium-Induced Hepatotoxicity in Rats and the Protective Effect of Naringenin. Exp. Toxicol. Pathol. 2010, 62, 171–181. [Google Scholar] [CrossRef]
- Prabu, S.M.; Shagirtha, K.; Renugadevi, J. Amelioration of Cadmium-Induced Oxidative Stress, Impairment in Lipids and Plasma Lipoproteins by the Combined Treatment with Quercetin and α-Tocopherol in Rats. J. Food Sci. 2010, 75, T132–T140. [Google Scholar] [CrossRef]
- Haouem, S.; El Hani, A. Effect of Cadmium on Lipid Peroxidation and on Some Antioxidants in the Liver, Kidneys and Testes of Rats Given Diet Containing Cadmium-Polluted Radish Bulbs. J. Toxicol. Pathol. 2013, 26, 359. [Google Scholar] [CrossRef] [PubMed]
- Zwolak, I. The Role of Selenium in Arsenic and Cadmium Toxicity: An Updated Review of Scientific Literature. Biol. Trace Elem. Res. 2020, 193, 44. [Google Scholar] [CrossRef] [PubMed]
- Unsal, V.; Dalkiran, T.; Çiçek, M.; Kölükçü, E. The Role of Natural Antioxidants Against Reactive Oxygen Species Produced by Cadmium Toxicity: A Review. Adv. Pharm. Bull. 2020, 10, 184. [Google Scholar] [CrossRef] [PubMed]
- Newairy, A.A.; El-Sharaky, A.S.; Badreldeen, M.M.; Eweda, S.M.; Sheweita, S.A. The Hepatoprotective Effects of Selenium against Cadmium Toxicity in Rats. Toxicology 2007, 242, 23–30. [Google Scholar] [CrossRef]
- Casalino, E.; Calzaretti, G.; Sblano, C.; Landriscina, C. Molecular Inhibitory Mechanisms of Antioxidant Enzymes in Rat Liver and Kidney by Cadmium. Toxicology 2002, 179, 37–50. [Google Scholar] [CrossRef]
- Yalin, S.; Comelekoglu, U.; Bagis, S.; Sahin, N.O.; Ogenler, O.; Hatungil, R. Acute Effect of Single-Dose Cadmium Treatment on Lipid Peroxidation and Antioxidant Enzymes in Ovariectomized Rats. Ecotoxicol. Environ. Saf. 2006, 65, 140–144. [Google Scholar] [CrossRef]
- Lazarus, M.; Orct, T.; Aladrović, J.; Ljubić, B.B.; Jurasović, J.; Blanuša, M. Effect of Selenium Pre-Treatment on Antioxidative Enzymes and Lipid Peroxidation in Cd-Exposed Suckling Rats. Biol. Trace Elem. Res. 2011, 142, 611–622. [Google Scholar] [CrossRef]
- Gong, P.; Chen, F.X.; Ma, G.F.; Feng, Y.; Zhao, Q.Y.; Wang, R. Endomorphin 1 Effectively Protects Cadmium Chloride-Induced Hepatic Damage in Mice. Toxicology 2008, 251, 35–44. [Google Scholar] [CrossRef]
- Tandon, S.K.; Singh, S.; Prasad, S.; Khandekar, K.; Dwivedi, V.K.; Chatterjee, M.; Mathur, N. Reversal of Cadmium Induced Oxidative Stress by Chelating Agent, Antioxidant or Their Combination in Rat. Toxicol. Lett. 2003, 145, 211–217. [Google Scholar] [CrossRef]
- Dey, A.; Lakshmanan, J. The Role of Antioxidants and Other Agents in Alleviating Hyperglycemia Mediated Oxidative Stress and Injury in Liver. Food Funct. 2013, 4, 1148–1184. [Google Scholar] [CrossRef]
- Tang, W.; Jiang, Y.F.; Ponnusamy, M.; Diallo, M. Role of Nrf2 in Chronic Liver Disease. World J. Gastroenterol. 2014, 20, 13079–13087. [Google Scholar] [CrossRef] [PubMed]
- Klaassen, C.D.; Reisman, S.A. Nrf2 the Rescue: Effects of the Antioxidative/Electrophilic Response on the Liver. Toxicol. Appl. Pharmacol. 2010, 244, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Lin, J.; Ge, J.; Wang, L.L.; Li, N.; Sun, X.T.; Cao, H.B.; Li, J.L. Selenium Triggers Nrf2-Mediated Protection against Cadmium-Induced Chicken Hepatocyte Autophagy and Apoptosis. Toxicol. Vitr. 2017, 44, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.G.; Wang, X.Y.; Wang, J.H.; Fan, R.F.; Wang, L. Trehalose Prevents Cadmium-Induced Hepatotoxicity by Blocking Nrf2 Pathway, Restoring Autophagy and Inhibiting Apoptosis. J. Inorg. Biochem. 2019, 192, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhu, Y.; Lu, Z.; Guo, W.; Tumen, B.; He, Y.; Chen, C.; Hu, S.; Xu, K.; Wang, Y.; et al. Cadmium Induces Acute Liver Injury by Inhibiting Nrf2 and the Role of NF-ΚB, NLRP3, and MAPKS Signaling Pathway. Int. J. Environ. Res. Public Health 2020, 17, 138. [Google Scholar] [CrossRef]
- Lawal, A.O.; Ellis, E.M. Nrf2-Mediated Adaptive Response to Cadmium-Induced Toxicity Involves Protein Kinase C Delta in Human 1321N1 Astrocytoma Cells. Environ. Toxicol. Pharmacol. 2011, 32, 54–62. [Google Scholar] [CrossRef]
- Wang, C.C.; Si, L.F.; Guo, S.N.; Zheng, J.L. Negative Effects of Acute Cadmium on Stress Defense, Immunity, and Metal Homeostasis in Liver of Zebrafish: The Protective Role of Environmental Zinc Dpre-Exposure. Chemosphere 2019, 222, 91–97. [Google Scholar] [CrossRef]
- Shinkai, Y.; Kimura, T.; Itagaki, A.; Yamamoto, C.; Taguchi, K.; Yamamoto, M.; Kumagai, Y.; Kaji, T. Partial Contribution of the Keap1-Nrf2 System to Cadmium-Mediated Metallothionein Expression in Vascular Endothelial Cells. Toxicol. Appl. Pharmacol. 2016, 295, 37–46. [Google Scholar] [CrossRef]
- Buha, A.; Baralić, K.; Djukic-Cosic, D.; Bulat, Z.; Tinkov, A.; Panieri, E.; Saso, L. The Role of Toxic Metals and Metalloids in Nrf2 Signaling. Antioxidants 2021, 10, 630. [Google Scholar] [CrossRef]
- He, X.; Chen, M.G.; Ma, Q. Activation of Nrf2 in Defense against Cadmium-Induced Oxidative Stress. Chem. Res. Toxicol. 2008, 21, 1375–1383. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Liu, M. Adipose Tissue in Control of Metabolism. J. Endocrinol. 2016, 231, R77–R99. [Google Scholar] [CrossRef] [PubMed]
- Ahima, R.S.; Lazar, M.A. Physiology. The Health Risk of Obesity—Better Metrics Imperative. Science 2013, 341, 856–858. [Google Scholar] [CrossRef]
- Akingbemi, B.T. Adiponectin Receptors in Energy Homeostasis and Obesity Pathogenesis. Prog. Mol. Biol. Transl. Sci. 2013, 114, 317–342. [Google Scholar] [CrossRef] [PubMed]
- Martínez-García, M.Á.; Moncayo, S.; Insenser, M.; Álvarez-Blasco, F.; Luque-Ramírez, M.; Escobar-Morreale, H.F. Postprandial Responses of Circulating Energy Homeostasis Mediators to Single Macronutrient Challenges: Influence of Obesity and Sex Hormones. Food Funct. 2021, 12, 1051–1062. [Google Scholar] [CrossRef]
- Treviño, S.; Cortezano-Esteban, S.; Hernández-Fragoso, H.; Díaz, A.; Vázquez-Roque, R.; Enrique Sarmiento-Ortega, V.; Moroni-González, D.; Pelayo, R.; Brambila, E. Clinical Monitored in Subjects Metabolically Healthy and Unhealthy before and during a SARS-CoV-2 Infection- A Cross-Sectional Study in Mexican Population. Cytokine 2022, 153, 155868. [Google Scholar] [CrossRef]
- Jaganathan, R.; Ravindran, R.; Dhanasekaran, S. Emerging Role of Adipocytokines in Type 2 Diabetes as Mediators of Insulin Resistance and Cardiovascular Disease. Can. J. Diabetes 2018, 42, 446–456.e1. [Google Scholar] [CrossRef]
- Coelho, M.; Oliveira, T.; Fernandes, R. Biochemistry of Adipose Tissue: An Endocrine Organ. Arch. Med. Sci. 2013, 9, 191. [Google Scholar] [CrossRef]
- Qatanani, M.; Lazar, M.A. Mechanisms of Obesity-Associated Insulin Resistance: Many Choices on the Menu. Genes Dev. 2007, 21, 1443–1455. [Google Scholar] [CrossRef]
- Unamuno, X.; Gómez-Ambrosi, J.; Rodríguez, A.; Becerril, S.; Frühbeck, G.; Catalán, V. Adipokine Dysregulation and Adipose Tissue Inflammation in Human Obesity. Eur. J. Clin. Investig. 2018, 48, e12997. [Google Scholar] [CrossRef]
- Kawakami, T.; Sugimoto, H.; Furuichi, R.; Kadota, Y.; Inoue, M.; Setsu, K.; Suzuki, S.; Sato, M. Cadmium Reduces Adipocyte Size and Expression Levels of Adiponectin and Peg1/Mest in Adipose Tissue. Toxicology 2010, 267, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, T.; Nishiyama, K.; Kadota, Y.; Sato, M.; Inoue, M.; Suzuki, S. Cadmium Modulates Adipocyte Functions in Metallothionein-Null Mice. Toxicol. Appl. Pharmacol. 2013, 272, 625–636. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, R.; Ribeiro, M.; Kajdacsy-Balla, A. The Toxic Effect of Environmental Cadmium on Visceral Adipose Tissue. FASEB J. 2020, 34, 1. [Google Scholar] [CrossRef]
- Lee, E.J.; Moon, J.Y.; Yoo, B.S. Cadmium Inhibits the Differentiation of 3T3-L1 Preadipocyte through the C/EBPα and PPARγ Pathways. Drug Chem. Toxicol. 2012, 35, 225–231. [Google Scholar] [CrossRef]
- Hammarstedt, A.; Gogg, S.; Hedjazifar, S.; Nerstedt, A.; Smith, U. Impaired Adipogenesis and Dysfunctional Adipose Tissue in Human Hypertrophic Obesity. Physiol. Rev. 2018, 98, 1911–1941. [Google Scholar] [CrossRef]
- Gustafson, B.; Gogg, S.; Hedjazifar, S.; Jenndahl, L.; Hammarstedt, A.; Smith, U. Inflammation and Impaired Adipogenesis in Hypertrophic Obesity in Man. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E999–E1003. [Google Scholar] [CrossRef]
- Zhu, S.; Sun, F.; Li, W.; Cao, Y.; Wang, C.; Wang, Y.; Liang, D.; Zhang, R.; Zhang, S.; Wang, H.; et al. Apelin Stimulates Glucose Uptake through the PI3K/Akt Pathway and Improves Insulin Resistance in 3T3-L1 Adipocytes. Mol. Cell. Biochem. 2011, 353, 305–313. [Google Scholar] [CrossRef]
- Tsuchiya, A.; Kanno, T.; Nishizaki, T. PI3 Kinase Directly Phosphorylates Akt1/2 at Ser473/474 in the Insulin Signal Transduction Pathway. J. Endocrinol. 2014, 220, 49–59. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Simental-Mendía, L.E.; Barreto, G.E.; Sahebkar, A. Metabolic Effects of Antidiabetic Drugs on Adipocytes and Adipokine Expression. J. Cell. Physiol. 2019, 234, 16987–16997. [Google Scholar] [CrossRef]
- Summers, S.A.; Whiteman, E.L.; Birnbaum, M.J. Insulin Signaling in the Adipocyte. Int. J. Obes. 2000, 24, S67–S70. [Google Scholar] [CrossRef]
- Han, J.C.; Park, S.Y.; Hah, B.G.; Choi, G.H.; Kim, Y.K.; Kwon, T.H.; Kim, E.K.; Lachaal, M.; Jung, C.Y.; Lee, W. Cadmium Induces Impaired Glucose Tolerance in Rat by Down-Regulating GLUT4 Expression in Adipocytes. Arch. Biochem. Biophys. 2003, 413, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Ficková, M.; Eybl, V.; Kotyzová, D.; Mičková, V.; Möstbök, S.; Brtko, J. Long Lasting Cadmium Intake Is Associated with Reduction of Insulin Receptors in Rat Adipocytes. Biometals 2003, 16, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Reilly, S.M.; Saltiel, A.R. Adapting to Obesity with Adipose Tissue Inflammation. Nat. Rev. Endocrinol. 2017, 13, 633–643. [Google Scholar] [CrossRef]
- Laurencikiene, J.; Van Harmelen, V.; Nordström, E.A.; Dicker, A.; Blomqvist, L.; Näslund, E.; Langin, D.; Arner, P.; Rydén, M. NF-ΚB Is Important for TNF-α-Induced Lipolysis in Human Adipocytes. J. Lipid Res. 2007, 48, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.G.; Araujo, T.G.; Carvalho, B.M.; Guadagnini, D.; Rocha, G.Z.; Bagarolli, R.A.; Carvalheira, J.B.C.; Saad, M.J.A. Acute Exercise Induces a Phenotypic Switch in Adipose Tissue Macrophage Polarization in Diet-Induced Obese Rats. Obesity 2013, 21, 2545–2556. [Google Scholar] [CrossRef]
- Lumeng, C.N.; Saltiel, A.R. Inflammatory Links between Obesity and Metabolic Disease. J. Clin. Investig. 2011, 121, 2111–2117. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Zhang, J.; Qi, X.; Cui, Y.; Yin, K.; Lin, H. Cadmium Induced Inflammation and Apoptosis of Porcine Epididymis via Activating RAF1/MEK/ERK and NF-ΚB Pathways. Toxicol. Appl. Pharmacol. 2021, 415, 115449. [Google Scholar] [CrossRef]
- Farkhondeh, T.; Llorens, S.; Pourbagher-Shahri, A.M.; Ashrafizadeh, M.; Talebi, M.; Shakibaei, M.; Samarghandian, S. An Overview of the Role of Adipokines in Cardiometabolic Diseases. Molecules 2020, 25, 5218. [Google Scholar] [CrossRef]
- Park, S.E.; Park, C.Y.; Sweeney, G. Biomarkers of Insulin Sensitivity and Insulin Resistance: Past, Present and Future. Crit. Rev. Clin. Lab. Sci. 2015, 52, 180–190. [Google Scholar] [CrossRef]
- Fasshauer, M.; Blüher, M. Adipokines in Health and Disease. Trends Pharmacol. Sci. 2015, 36, 461–470. [Google Scholar] [CrossRef]
- Tumminia, A.; Vinciguerra, F.; Parisi, M.; Graziano, M.; Sciacca, L.; Baratta, R.; Frittitta, L. Adipose Tissue, Obesity and Adiponectin: Role in Endocrine Cancer Risk. Int. J. Mol. Sci. 2019, 20, 2863. [Google Scholar] [CrossRef] [PubMed]
- Stern, J.H.; Rutkowski, J.M.; Scherer, P.E. Adiponectin, Leptin, and Fatty Acids in the Maintenance of Metabolic Homeostasis through Adipose Tissue Crosstalk. Cell Metab. 2016, 23, 770–784. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Sánchez, N. There and Back Again: Leptin Actions in White Adipose Tissue. Int. J. Mol. Sci. 2020, 21, 6039. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased Oxidative Stress in Obesity and Its Impact on Metabolic Syndrome. J. Clin. Investig. 2004, 114, 1752–1761. [Google Scholar] [CrossRef]
- Wonisch, W.; Falk, A.; Sundl, I.; Winklhofer-Roob, B.M.; Lindschinger, M. Oxidative Stress Increases Continuously with BMI and Age with Unfavourable Profiles in Males. Aging Male 2012, 15, 159–165. [Google Scholar] [CrossRef]
- Chattopadhyay, M.; Khemka, V.K.; Chatterjee, G.; Ganguly, A.; Mukhopadhyay, S.; Chakrabarti, S. Enhanced ROS Production and Oxidative Damage in Subcutaneous White Adipose Tissue Mitochondria in Obese and Type 2 Diabetes Subjects. Mol. Cell. Biochem. 2015, 399, 95–103. [Google Scholar] [CrossRef]
- Boyer, F.; Diotel, N.; Girard, D.; Rondeau, P.; Essop, M.F.; Bourdon, E. Enhanced Oxidative Stress in Adipose Tissue from Diabetic Mice, Possible Contribution of Glycated Albumin. Biochem. Biophys. Res. Commun. 2016, 473, 154–160. [Google Scholar] [CrossRef]
- Manna, P.; Jain, S.K. Obesity, Oxidative Stress, Adipose Tissue Dysfunction, and the Associated Health Risks: Causes and Therapeutic Strategies. Metab. Syndr. Relat. Disord. 2015, 13, 423–444. [Google Scholar] [CrossRef]
- Hauck, A.K.; Huang, Y.; Hertzel, A.V.; Bernlohr, D.A. Adipose Oxidative Stress and Protein Carbonylation. J. Biol. Chem. 2019, 294, 1083–1088. [Google Scholar] [CrossRef]
- Curtis, J.M.; Grimsrud, P.A.; Wright, W.S.; Xu, X.; Foncea, R.E.; Graham, D.W.; Brestoff, J.R.; Wiczer, B.M.; Ilkayeva, O.; Cianflone, K.; et al. Downregulation of Adipose Glutathione S-Transferase A4 Leads to Increased Protein Carbonylation, Oxidative Stress, and Mitochondrial Dysfunction. Diabetes 2010, 59, 1132–1142. [Google Scholar] [CrossRef]
- Roškarić, P.; Šperanda, M.; Mašek, T.; Verbanac, D.; Starčević, K. Low Dietary N6/N3 Ratio Attenuates Changes in the NRF 2 Gene Expression, Lipid Peroxidation, and Inflammatory Markers Induced by Fructose Overconsumption in the Rat Abdominal Adipose Tissue. Antioxidants 2021, 10, 2005. [Google Scholar] [CrossRef]
- Den Hartigh, L.J.; Omer, M.; Goodspeed, L.; Wang, S.; Wietecha, T.; O’Brien, K.D.; Han, C.Y. Adipocyte-Specific Deficiency of NADPH Oxidase 4 Delays the Onset of Insulin Resistance and Attenuates Adipose Tissue Inflammation in Obesity. Arter. Thromb. Vasc. Biol. 2017, 37, 466–475. [Google Scholar] [CrossRef]
- Chait, A.; den Hartigh, L.J. Adipose Tissue Distribution, Inflammation and Its Metabolic Consequences, Including Diabetes and Cardiovascular Disease. Front. Cardiovasc. Med. 2020, 7, 22. [Google Scholar] [CrossRef]
- De Pauw, A.; Tejerina, S.; Raes, M.; Keijer, J.; Arnould, T. Mitochondrial (Dys)Function in Adipocyte (de)Differentiation and Systemic Metabolic Alterations. Am. J. Pathol. 2009, 175, 927–939. [Google Scholar] [CrossRef]
- Matsuzawa-Nagata, N.; Takamura, T.; Ando, H.; Nakamura, S.; Kurita, S.; Misu, H.; Ota, T.; Yokoyama, M.; Honda, M.; Miyamoto, K.-i.; et al. Increased Oxidative Stress Precedes the Onset of High-Fat Diet-Induced Insulin Resistance and Obesity. Metabolism 2008, 57, 1071–1077. [Google Scholar] [CrossRef]
- Paglialunga, S.; Ludzki, A.; Root-McCaig, J.; Holloway, G.P. In Adipose Tissue, Increased Mitochondrial Emission of Reactive Oxygen Species Is Important for Short-Term High-Fat Diet-Induced Insulin Resistance in Mice. Diabetologia 2015, 58, 1071–1080. [Google Scholar] [CrossRef]
- Fazakerley, D.J.; Minard, A.Y.; Krycer, J.R.; Thomas, K.C.; Stöckli, J.; Harney, D.J.; Burchfield, J.G.; Maghzal, G.J.; Caldwell, S.T.; Hartley, R.C.; et al. Mitochondrial Oxidative Stress Causes Insulin Resistance without Disrupting Oxidative Phosphorylation. J. Biol. Chem. 2018, 293, 7315–7328. [Google Scholar] [CrossRef]
- Greenstein, A.S.; Khavandi, K.; Withers, S.B.; Sonoyama, K.; Clancy, O.; Jeziorska, M.; Laing, I.; Yates, A.P.; Pemberton, P.W.; Malik, R.A.; et al. Local Inflammation and Hypoxia Abolish the Protective Anticontractile Properties of Perivascular Fat in Obese Patients. Circulation 2009, 119, 1661–1670. [Google Scholar] [CrossRef]
- Sun, J.; Jiao, Z.; Zhu, W.; Li, X.; Wang, P.; Wang, J.; Tai, T.; Wang, Y.; Wang, H.; Shi, G. Astilbin Attenuates Cadmium-Induced Adipose Tissue Damage by Inhibiting NF-ΚB Pathways and Regulating the Expression of HSPs in Chicken. Biol. Trace Elem. Res. 2022, 2022, 1–12. [Google Scholar] [CrossRef]
- Huh, J.Y.; Kim, Y.; Jeong, J.; Park, J.; Kim, I.; Huh, K.H.; Kim, Y.S.; Woo, H.A.; Rhee, S.G.; Lee, K.J.; et al. Peroxiredoxin 3 Is a Key Molecule Regulating Adipocyte Oxidative Stress, Mitochondrial Biogenesis, and Adipokine Expression. Antioxid. Redox Signal. 2012, 16, 229–243. [Google Scholar] [CrossRef]
- Kim, M.H.; Kim, J.Y.; Kim, J.H.; Lee, H.S.; Huh, J.W.; Lee, D.S. Peroxiredoxin 2 Deficiency Reduces White Adipogenesis Due to the Excessive ROS Generation. Cell Biol. Int. 2020, 44, 2086–2093. [Google Scholar] [CrossRef] [PubMed]
- Okuno, Y.; Fukuhara, A.; Hashimoto, E.; Kobayashi, H.; Kobayashi, S.; Otsuki, M.; Shimomura, I. Oxidative Stress Inhibits Healthy Adipose Expansion Through Suppression of SREBF1-Mediated Lipogenic Pathway. Diabetes 2018, 67, 1113–1127. [Google Scholar] [CrossRef] [PubMed]
- Hosick, P.A.; Weeks, M.F.; Hankins, M.W.; Moore, K.H.; Stec, D.E. Sex-Dependent Effects of HO-1 Deletion from Adipocytes in Mice. Int. J. Mol. Sci. 2017, 18, 611. [Google Scholar] [CrossRef]
- Cao, J.; Peterson, S.J.; Sodhi, K.; Vanella, L.; Barbagallo, I.; Rodella, L.F.; Schwartzman, M.L.; Abraham, N.G.; Kappas, A. Heme Oxygenase Gene Targeting to Adipocytes Attenuates Adiposity and Vascular Dysfunction in Mice Fed a High-Fat Diet. Hypertension 2012, 60, 467–475. [Google Scholar] [CrossRef]
- Kim, R.; Hu, H.; Rotnitzky, A.; Bellinger, D.; Needleman, H. A Longitudinal Study of Chronic Lead Exposure and Physical Growth in Boston Children. Environ. Health Perspect. 1995, 103, 952–957. [Google Scholar] [CrossRef]
- Wang, N.; Chen, C.; Nie, X.; Han, B.; Li, Q.; Chen, Y.; Zhu, C.; Chen, Y.; Xia, F.; Cang, Z.; et al. Blood Lead Level and Its Association with Body Mass Index and Obesity in China—Results from SPECT-China Study. Sci. Rep. 2015, 5, 18299. [Google Scholar] [CrossRef]
- Hildebrand, J.; Thakar, S.; Watts, T.L.; Banfield, L.; Thabane, L.; Macri, J.; Hill, S.; Samaan, M.C. The Impact of Environmental Cadmium Exposure on Type 2 Diabetes Risk: A Protocol for an Overview of Systematic Reviews. Syst. Rev. 2019, 8, 309. [Google Scholar] [CrossRef]
- Tian, L.L.; Zhao, Y.C.; Wang, X.C.; Gu, J.L.; Sun, Z.J.; Zhang, Y.L.; Wang, J.X. Effects of Gestational Cadmium Exposure on Pregnancy Outcome and Development in the Offspring at Age 4.5 Years. Biol. Trace Elem. Res. 2009, 132, 51–59. [Google Scholar] [CrossRef]
- Vidal, A.C.; Semenova, V.; Darrah, T.; Vengosh, A.; Huang, Z.; King, K.; Nye, M.D.; Fry, R.; Skaar, D.; Maguire, R.; et al. Maternal Cadmium, Iron and Zinc Levels, DNA Methylation and Birth Weight. BMC Pharmacol. Toxicol. 2015, 16, 20. [Google Scholar] [CrossRef]
- Lin, C.M.; Doyle, P.; Wang, D.; Hwang, Y.H.; Chen, P.C. Does Prenatal Cadmium Exposure Affect Fetal and Child Growth? Occup. Environ. Med. 2011, 68, 641–646. [Google Scholar] [CrossRef]
- Barker, D.J.P.; Eriksson, J.G.; Forsén, T.; Osmond, C. Fetal Origins of Adult Disease: Strength of Effects and Biological Basis. Int. J. Epidemiol. 2002, 31, 1235–1239. [Google Scholar] [CrossRef] [PubMed]
- Tinkov, A.A.; Filippini, T.; Ajsuvakova, O.P.; Skalnaya, M.G.; Aaseth, J.; Bjørklund, G.; Gatiatulina, E.R.; Popova, E.V.; Nemereshina, O.N.; Huang, P.-T.; et al. Cadmium and Atherosclerosis: A Review of Toxicological Mechanisms and a Meta-Analysis of Epidemiologic Studies. Environ. Res. 2018, 162, 240–260. [Google Scholar] [CrossRef] [PubMed]
- Haswell-Elkins, M.; McGrath, V.; Moore, M.; Satarug, S.; Walmby, M.; Ng, J. Exploring Potential Dietary Contributions Including Traditional Seafood and Other Determinants of Urinary Cadmium Levels among Indigenous Women of a Torres Strait Island (Australia). J. Expo. Sci. Environ. Epidemiol. 2007, 17, 298–306. [Google Scholar] [CrossRef]
- Skalnaya, M.G.; Tinkov, A.A.; Demidov, V.A.; Serebryansky, E.P.; Nikonorov, A.A.; Skalny, A.V. Hair Toxic Element Content in Adult Men and Women in Relation to Body Mass Index. Biol. Trace Elem. Res. 2014, 161, 13–19. [Google Scholar] [CrossRef]
- Filippini, T.; Michalke, B.; Malagoli, C.; Grill, P.; Bottecchi, I.; Malavolti, M.; Vescovi, L.; Sieri, S.; Krogh, V.; Cherubini, A.; et al. Determinants of Serum Cadmium Levels in a Northern Italy Community: A Cross-Sectional Study. Environ. Res. 2016, 150, 219–226. [Google Scholar] [CrossRef]
- Wang, X.; Mukherjee, B.; Park, S.K. Associations of Cumulative Exposure to Heavy Metal Mixtures with Obesity and Its Comorbidities among U.S. Adults in NHANES 2003–2014. Environ. Int. 2018, 121, 683–694. [Google Scholar] [CrossRef]
- Nie, X.; Wang, N.; Chen, Y.; Chen, C.; Han, B.; Zhu, C.; Chen, Y.; Xia, F.; Cang, Z.; Lu, M.; et al. Blood Cadmium in Chinese Adults and Its Relationships with Diabetes and Obesity. Environ. Sci. Pollut. Res. Int. 2016, 23, 18714–18723. [Google Scholar] [CrossRef]
- MERALI, Z.; SINGHAL, R.L. Diabetogenic Effects of Chronic Oral Cadmium Adminstration to Neonatal Rats. Br. J. Pharmacol. 1980, 69, 151–157. [Google Scholar] [CrossRef]
- Ba, Q.; Li, M.; Chen, P.; Huang, C.; Duan, X.; Lu, L.; Li, J.; Chu, R.; Xie, D.; Song, H.; et al. Sex-Dependent Effects of Cadmium Exposure in Early Life on Gut Microbiota and Fat Accumulation in Mice. Environ. Health Perspect. 2017, 125, 437–446. [Google Scholar] [CrossRef]
- Yu, Y.; Ma, R.; Yu, L.; Cai, Z.; Li, H.; Zuo, Y.; Wang, Z.; Li, H. Combined Effects of Cadmium and Tetrabromobisphenol a (TBBPA) on Development, Antioxidant Enzymes Activity and Thyroid Hormones in Female Rats. Chem. Biol. Interact. 2018, 289, 23–31. [Google Scholar] [CrossRef]
- Edwards, J.; Ackerman, C. A Review of Diabetes Mellitus and Exposure to the Environmental Toxicant Cadmium with an Emphasis on Likely Mechanisms of Action. Curr. Diabetes Rev. 2016, 12, 252–258. [Google Scholar] [CrossRef]
- Bimonte, V.M.; Besharat, Z.M.; Antonioni, A.; Cella, V.; Lenzi, A.; Ferretti, E.; Migliaccio, S. The Endocrine Disruptor Cadmium: A New Player in the Pathophysiology of Metabolic Diseases. J. Endocrinol. Investig. 2021, 44, 1363–1377. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, R.; Olsen, A.; Nguyen, J.; Wong, W.; Muayed, M.E.; Edwards, J. Pancreatic Islets Accumulate Cadmium in a Rodent Model of Cadmium-Induced Hyperglycemia. Int. J. Mol. Sci. 2021, 22, 360. [Google Scholar] [CrossRef] [PubMed]
- Fatima, G.; Raza, A.M.; Hadi, N.; Nigam, N.; Mahdi, A.A. Cadmium in Human Diseases: It’s More than Just a Mere Metal. Indian J. Clin. Biochem. 2019, 34, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Barregard, L.; Bergström, G.; Fagerberg, B. Cadmium Exposure in Relation to Insulin Production, Insulin Sensitivity and Type 2 Diabetes: A Cross-Sectional and Prospective Study in Women. Environ. Res. 2013, 121, 104–109. [Google Scholar] [CrossRef]
- Borné, Y.; Fagerberg, B.; Persson, M.; Sallsten, G.; Forsgard, N.; Hedblad, B.; Barregard, L.; Engström, G. Cadmium Exposure and Incidence of Diabetes Mellitus—Results from the Malmö Diet and Cancer Study. PLoS ONE 2014, 9, e112277. [Google Scholar] [CrossRef]
- Filippini, T.; Wise, L.A.; Vinceti, M. Cadmium Exposure and Risk of Diabetes and Prediabetes: A Systematic Review and Dose-Response Meta-Analysis. Environ. Int. 2022, 158, 106920. [Google Scholar] [CrossRef]
- Tinkov, A.A.; Filippini, T.; Ajsuvakova, O.P.; Aaseth, J.; Gluhcheva, Y.G.; Ivanova, J.M.; Bjørklund, G.; Skalnaya, M.G.; Gatiatulina, E.R.; Popova, E.V.; et al. The Role of Cadmium in Obesity and Diabetes. Sci. Total Environ. 2017, 601–602, 741–755. [Google Scholar] [CrossRef]
- Swaddiwudhipong, W.; Limpatanachote, P.; Mahasakpan, P.; Krintratun, S.; Punta, B.; Funkhiew, T. Progress in Cadmium-Related Health Effects in Persons with High Environmental Exposure in Northwestern Thailand: A Five-Year Follow-Up. Environ. Res. 2012, 112, 194–198. [Google Scholar] [CrossRef]
- Zhang, W.L.; Du, Y.; Zhai, M.M.; Shang, Q. Cadmium Exposure and Its Health Effects: A 19-Year Follow-up Study of a Polluted Area in China. Sci. Total Environ. 2014, 470–471, 224–228. [Google Scholar] [CrossRef]
- Madrigal, J.M.; Ricardo, A.C.; Persky, V.; Turyk, M. Associations between Blood Cadmium Concentration and Kidney Function in the U.S. Population: Impact of Sex, Diabetes and Hypertension. Environ. Res. 2019, 169, 180. [Google Scholar] [CrossRef]
- Wu, M.; Song, J.; Zhu, C.; Wang, Y.; Yin, X.; Huang, G.; Zhao, K.; Zhu, J.; Duan, Z.; Su, L. Association between Cadmium Exposure and Diabetes Mellitus Risk: A Prisma-Compliant Systematic Review and Meta-Analysis. Oncotarget 2017, 8, 113129. [Google Scholar] [CrossRef]
- Wallia, A.; Allen, N.B.; Badon, S.; El Muayed, M. Association between Urinary Cadmium Levels and Prediabetes in the NHANES 2005-2010 Population. Int. J. Hyg. Environ. Health 2014, 217, 854–860. [Google Scholar] [CrossRef]
- Menke, A.; Guallar, E.; Cowie, C.C. Metals in Urine and Diabetes in U.S. Adults. Diabetes 2016, 65, 164–171. [Google Scholar] [CrossRef]
- Swaddiwudhipong, W.; Mahasakpan, P.; Limpatanachote, P.; Krintratun, S. Correlations of Urinary Cadmium with Hypertension and Diabetes in Persons Living in Cadmium-Contaminated Villages in Northwestern Thailand: A Population Study. Environ. Res. 2010, 110, 612–616. [Google Scholar] [CrossRef]
- Kuo, C.C.; Moon, K.; Thayer, K.A.; Navas-Acien, A. Environmental Chemicals and Type 2 Diabetes: An Updated Systematic Review of the Epidemiologic Evidence. Curr. Diabetes Rep. 2013, 13, 831–849. [Google Scholar] [CrossRef]
- Moon, S.S. Association of Lead, Mercury and Cadmium with Diabetes in the Korean Population: The Korea National Health and Nutrition Examination Survey (KNHANES) 2009–2010. Diabet. Med. 2013, 30, e143–e148. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).