The Migration Rules of Malathion during Indoor Simulated Lake Freezing
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. Instruments and Reagents
2.3. Experimental Design and Procedure
2.4. Operating Parameters of Ultra Performance Liquid Chromatography
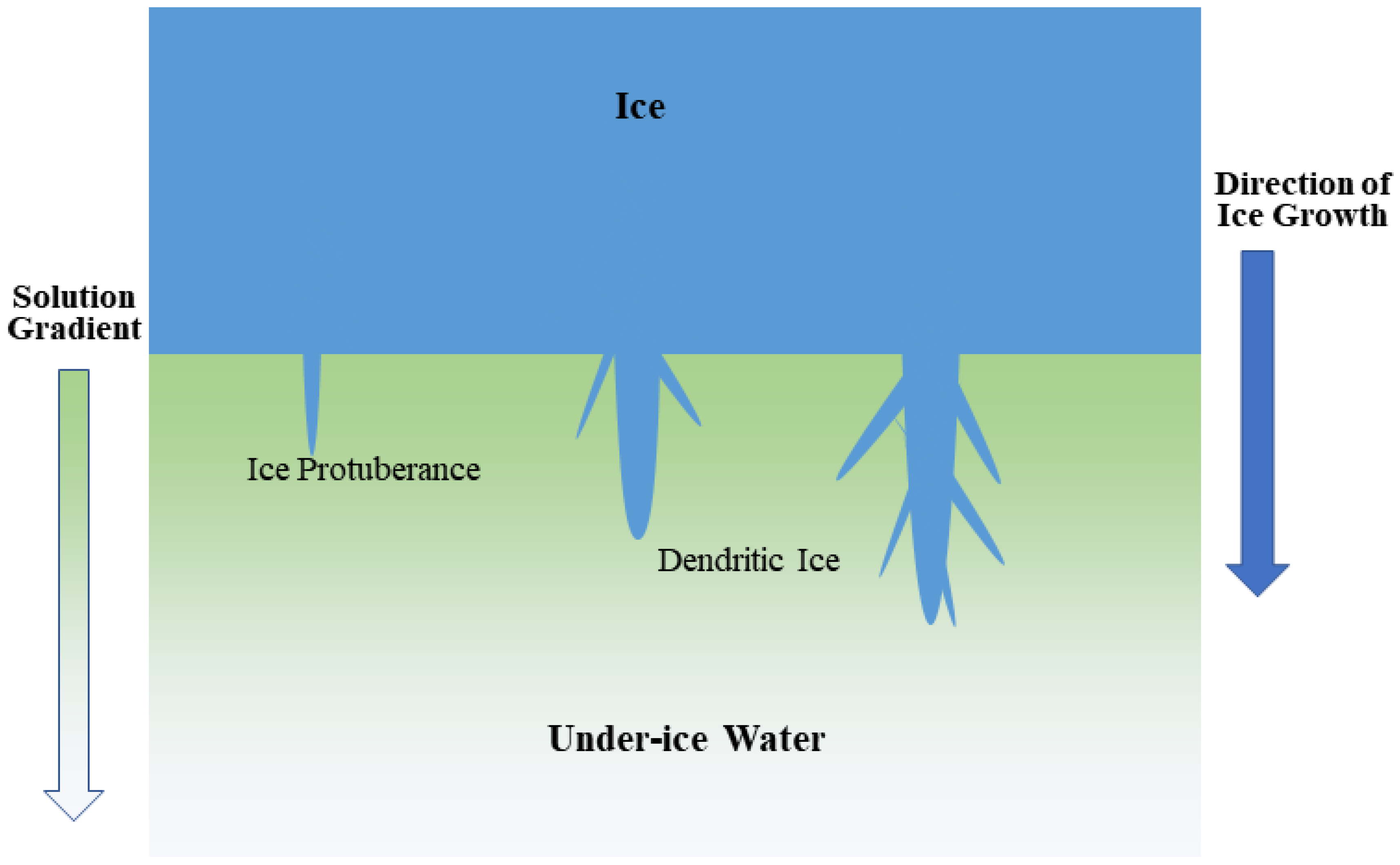
2.5. Observation of Malathion Distribution in Ice
2.6. Data Analysis Methods
3. Results
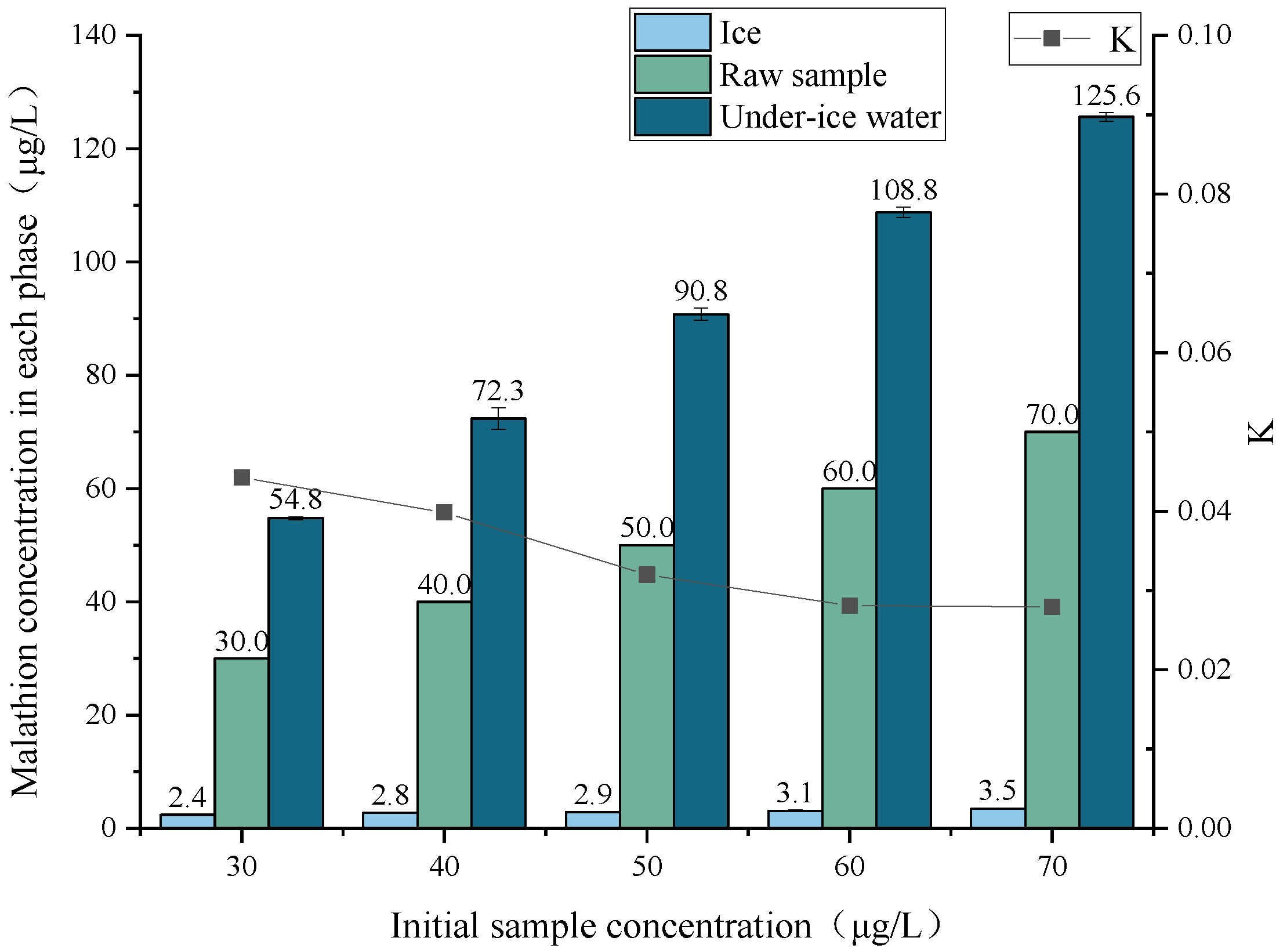
3.1. Effect of Initial Sample Concentration on the Migration Rule of Malathion
3.2. Effect of Freezing Ratio on the Migration Rule of Malathion
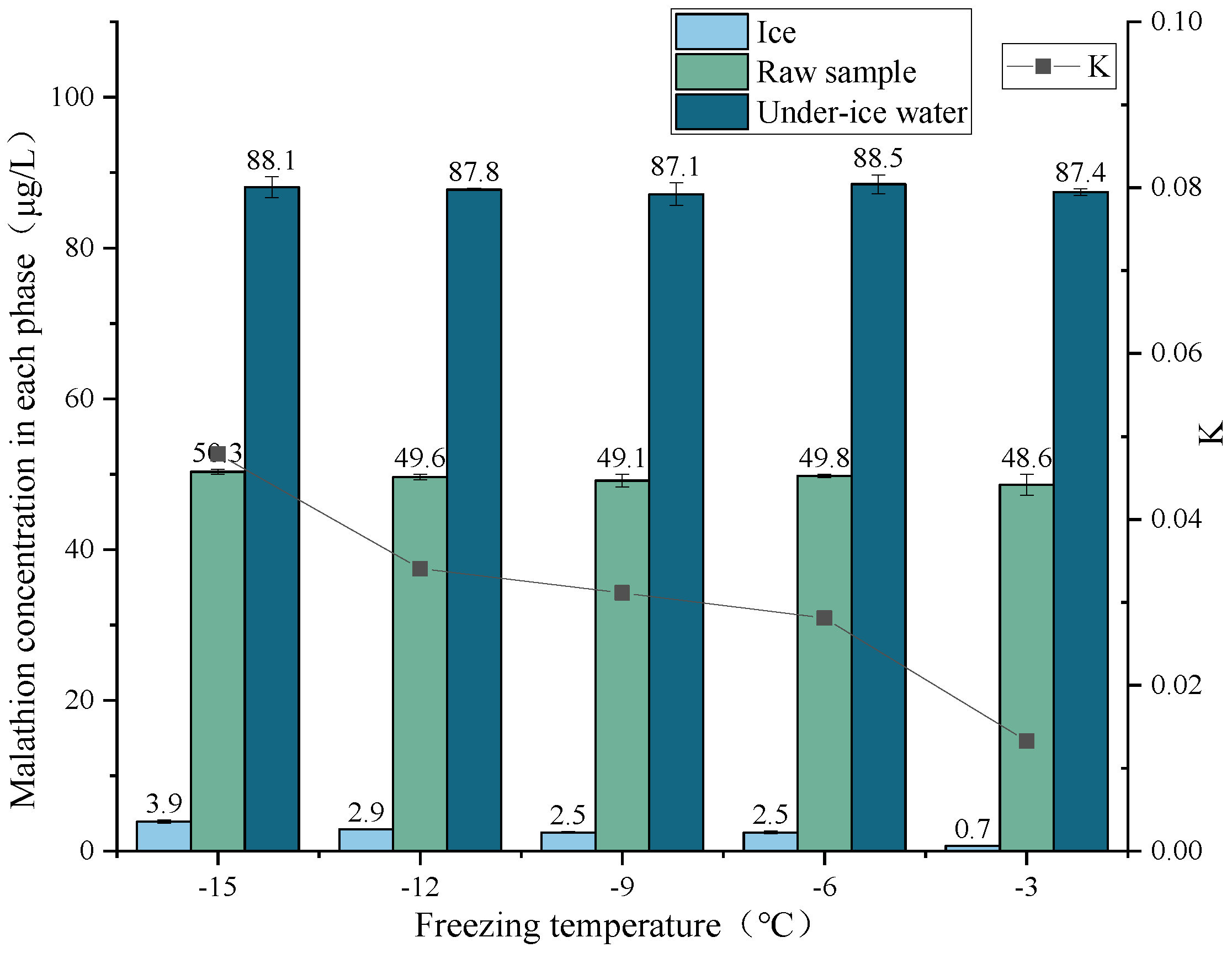
3.3. Effect of Freezing Temperature on the Migration Rule of Malathion
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A. Mass Conservation Verification
| Freezing Ratio (%) | Raw Sample | Ice | Under-Ice Water | CHECK IN/OUT (%) | |||
|---|---|---|---|---|---|---|---|
| Concentration (μg/L) | Volume (L) | Concentration (μg/L) | Volume (L) | Concentration (μg/L) | Volume (L) | ||
| 15 | 48.8 | 1 | 3.3 | 0.15 | 56.8 | 0.85 | 99.95 |
| 30 | 52.2 | 1 | 2.9 | 0.3 | 73.3 | 0.7 | 99.96 |
| 45 | 51 | 1 | 3.2 | 0.45 | 90.1 | 0.55 | 99.99 |
| 60 | 49.1 | 1 | 3.9 | 0.6 | 117 | 0.4 | 100.08 |
| Initial Concentration (μg/L) | Raw Sample | Ice | Under-Ice Water | CHECK IN/OUT (%) | |||
|---|---|---|---|---|---|---|---|
| Concentration (μg/L) | Volume (L) | Concentration (μg/L) | Volume (L) | Concentration (μg/L) | Volume (L) | ||
| 30 | 30 | 1 | 2.4 | 0.45 | 54.8 | 0.55 | 104.07 |
| 40 | 40 | 1 | 2.8 | 0.45 | 72.3 | 0.55 | 102.56 |
| 50 | 50 | 1 | 2.9 | 0.45 | 90.8 | 0.55 | 102.49 |
| 60 | 60 | 1 | 3.1 | 0.45 | 108.8 | 0.55 | 102.06 |
| 70 | 70 | 1 | 3.5 | 0.45 | 125.6 | 0.55 | 100.94 |
| Freezing Temperature (°C) | Raw Sample | Ice | Under-Ice Water | CHECK IN/OUT (%) | |||
|---|---|---|---|---|---|---|---|
| Concentration (μg/L) | Volume (L) | Concentration (μg/L) | Volume (L) | Concentration (μg/L) | Volume (L) | ||
| −15 | 50.3 | 1 | 3.9 | 0.45 | 88.1 | 0.55 | 99.82 |
| −12 | 49.6 | 1 | 2.9 | 0.45 | 87.8 | 0.55 | 99.99 |
| −9 | 49.1 | 1 | 2.5 | 0.45 | 87.1 | 0.55 | 99.86 |
| −6 | 49.8 | 1 | 2.5 | 0.45 | 88.5 | 0.55 | 100.00 |
| −3 | 48.6 | 1 | 0.7 | 0.45 | 87.4 | 0.55 | 99.56 |
References
- Verpoorter, C.; Kutser, T.; Seekell, D.A.; Tranvik, L.J. A global inventory of lakes based on high-resolution satellite imagery. Geophys. Res. Lett. 2014, 41, 6396–6402. [Google Scholar] [CrossRef]
- Li, Z.J.; Wang, X.; Li, Q.S.; Xu, S.G.; Xu, X.Z.; Bai, Y. Study on the partitioning of nitrobenzene in water-ice system under different conditions. Sci. Sin. 2008, 38, 1131–1138. [Google Scholar] [CrossRef]
- Lan, C.J.; Shen, Y.; Wang, B.X.; Wu, J.L.; Zeng, H.A.; Ma, L. A survey of higher aquatic plants and macrobenthic invertebrates in lakes of the Mengxin Plateau. J. Lake Sci. 2010, 22, 888–893. [Google Scholar]
- Fang, X.; Stefan, H.G. Simulations of climate effects on water temperature, dissolved oxygen, and ice and snow covers in lakes of the contiguous US under past and future climate scenarios. Limnol. Oceanogr. 2009, 54, 2359–2370. [Google Scholar] [CrossRef]
- Belzile, C.; Gibson, J.A.; Vincent, W.F. Colored dissolved organic matter and dissolved organic carbon exclusion from lake ice: Implications for irradiance transmission and carbon cycling. Limnol. Oceanogr. 2002, 47, 1283–1293. [Google Scholar] [CrossRef]
- Powers, S.M.; Labou, S.G.; Baulch, H.M. Ice duration drives winter nitrate accumulation in north temperate lakes. Limnol. Oceanogr. Lett. 2017, 2, 177–186. [Google Scholar] [CrossRef]
- Golosov, S.; Maher, O.A.; Schipunova, E.; Terzhevik, A.; Zdorovennova, G.; Kirillin, G. Physical background of the development of oxygen depletion in ice-covered lakes. Oecologia 2007, 151, 331–340. [Google Scholar] [CrossRef]
- Terry, J.A.; Sadeghian, A.; Lindenschmidt, K.-E. Modelling dissolved oxygen/sediment oxygen demand under ice in a shallow eutrophic prairie reservoir. Water 2017, 9, 131. [Google Scholar] [CrossRef]
- Hampton, S.E.; Galloway, A.W.; Powers, S.M. Ecology under lake ice. Ecol. Lett. 2017, 20, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y. Study on the Migration Mechanism of Pollutants in the Icing Process of the WuLiangsuhai Lake and Its Application. Ph.D. Thesis, Inner Mongolia Agricultural University, Hohhot, China, 2012. [Google Scholar]
- Bertilsson, S.; Burgin, A.; Carey, C.C. The under-ice microbiome of seasonally frozen lakes. Limnol. Oceanogr. 2013, 58, 1998–2012. [Google Scholar] [CrossRef]
- Huang, W.F.; Han, H.W.; Niu, F.J.; Li, Z.J. In situ observation of water temperature and its stratification characteristics in a seasonal ice-covered hot-melt shallow lake. Adv. Water Sci. 2016, 27, 280–289. [Google Scholar] [CrossRef]
- Wang, Y.W.; Ma, A.G.; Zhong, G.S. Effects of simulated warming on microbial populations in glacial foreland. Environ. Sci. 2020, 41, 2918–2923. [Google Scholar] [CrossRef]
- Ma, X.L. Study on the Growth Process of Reservoir Ice Cover and Its Water Quality Impact. Master’s Thesis, Dalian University of Technology, Dalian, China, 2021. [Google Scholar] [CrossRef]
- Yang, T.; Hei, P.; Song, J. Nitrogen variations during the ice-on season in the eutrophic lakes. Environ. Pollut. 2019, 247, 1089–1099. [Google Scholar] [CrossRef] [PubMed]
- Hampton, S.E.; Moore, M.V.; Ozersky, T.; Stanley, E.H.; Polashenski, C.M.; Galloway, A.W. Heating up a cold subject: Prospects for under-ice plankton research in lakes. J. Plankton Res. 2015, 37, 277–284. [Google Scholar] [CrossRef]
- Pieters, R.; Lawrence, G.A. Effect of salt exclusion from lake ice on seasonal circulation. Limnol. Oceanogr. 2009, 54, 401–412. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, S.; Li, C. A modified QWASI model for fate and transport modeling of Zn and Pb in a shallow lake during the ice-free period. Sci. Total Environ. 2019, 657, 577–584. [Google Scholar] [CrossRef]
- Melak, F.; Du Laing, G.; Ambelu, A.; Alemayehu, E. Application of freeze desalination for chromium (VI) removal from water. Desalination 2016, 377, 23–27. [Google Scholar] [CrossRef]
- Sun, C.; Li, C.; Liu, J.; Shi, X.; Zhao, S.; Wu, Y.; Tian, W. First-Principles Study on the Migrationof Heavy Metal Ions in Ice-Water Medium from Ulansuhai Lake. Water 2018, 10, 1149. [Google Scholar] [CrossRef]
- Li, W.P.; Xu, J.; Yu, L.H. Distribution characteristics of nutrient salts and phytoplankton during the ice-out period in the Wuliangsuhai. Ecol. Environ. Sci. 2014, 23, 1007–1013. [Google Scholar] [CrossRef]
- Xue, S.; Wen, Y.; Hui, X.; Zhang, L.; Zhang, Z.; Wang, J.; Zhang, Y. The migration and transformation of dissolved organic matter during the freezing processes of water. J. Environ. Sci. 2015, 27, 168–178. [Google Scholar] [CrossRef]
- Gruchlik, Y.; Linge, K.; Joll, C. Removal of organic micropollutants in waste stabilisation ponds: A review. J. Environ. Manag. 2018, 206, 202–214. [Google Scholar] [CrossRef] [PubMed]
- Stibal, M.; Bælum, J.; Holben, W.; Jacobsen, C. Exploring the functional diversity of the supraglacial environment: Microbial degradation of the pesticide 2, 4-D on the Greenland Ice Sheet. Appl. Environ. Microb. 2012, 78, 5070–5076. [Google Scholar] [CrossRef]
- Choi, Y.; Yoon, H.-I.; Lee, C. Activation of periodate by freezing for the degradation of aqueous organic pollutants. Environ. Sci. Technol. 2018, 52, 5378–5385. [Google Scholar] [CrossRef] [PubMed]
- Pućko, M.; Stern, G.A.; Burt, A.E. Current use pesticide and legacy organochlorine pesticide dynamics at the ocean-sea ice-atmosphere interface in resolute passage, Canadian Arctic, during winter-summer transition. Sci. Total Environ. 2017, 580, 1460–1469. [Google Scholar] [CrossRef] [PubMed]
- Bigot, M.; Hawker, D.W.; Cropp, R. Spring Melt and the Redistribution of Organochlorine Pesticides in the Sea-Ice Environment: A Comparative Study between Arctic and Antarctic Regions. Environ. Sci. Technol. 2017, 51, 8944–8952. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, X.; Li, Y.; Ya, M.; Hui, L. Polybrominated diphenyl ethers, organochlorine pesticides, and polycyclic aromatic hydrocarbons in water from the Jiulong River Estuary, China: Levels, distributions, influencing factors, and risk assessment. Environ. Sci. Pollut. Res. 2017, 24, 8933–8945. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, S.; Ren, C. Residues of organophosphorus insecticides in sediment around a highly eutrophic lake, Eastern China. J. Soil Sediment. 2015, 15, 436–444. [Google Scholar] [CrossRef]
- Wolfe, N.L.; Zepp, R.G.; Gordon, J.A.; Baughman, G.L.; Cline, D.M. Kinetics of chemical degradation of malathion in water. Environ. Sci. Technol. 1977, 11, 88–93. [Google Scholar] [CrossRef]
- Wang, T. Assimilation of malathion in the Indian River estuary, Florida. Bull. Environ. Contam. Toxicol. 1991, 47, 238–243. [Google Scholar] [CrossRef]
- Tankiewicz, M.; Fenik, J.; Biziuk, M. Determination of organophosphorus and organonitrogen pesticides in water samples. Trac-Trend. Anal. Chem. 2010, 29, 1050–1063. [Google Scholar] [CrossRef]
- Schiff, K.; Sutula, M. Organophosphorus pesticides in storm-water runoff from southern California (USA). Environ. Toxicol. Chem. 2004, 23, 1815–1821. [Google Scholar] [CrossRef]
- Quan, D.; Zhang, S.; Shi, X.H.; Sun, B.; Song, S.; Guo, Z.Y. Influence of environmental factors on the trophic state of water bodies in the Wulangsu Sea: An analysis based on monitoring data in 2013–2018. J. Lake Sci. 2020, 32, 10. [Google Scholar] [CrossRef]
- Williams, W.M.; Holden, P.W.; Parsons, D.W.; Lorber, M.N. Pesticides in Ground Water Data Base; 1988 Interim Report; US Deparment of Energy: Washington, DC, USA, 1988.
- Fadaei, A.; Dehghani, M.H.; Nasseri, S.; Mahvi, A.H.; Rastkari, N.; Shayeghi, M. Organophosphorous pesticides in surface water of Iran. Bull. Environ. Contam. Toxicol. 2012, 88, 867–869. [Google Scholar] [CrossRef] [PubMed]
- Wang, L. Removal Characteristics and Mechanism of Typical Endocrine Disruptors of Pesticides in Water. Ph.D. Thesis, Tongji University, Shanghai, China, 2006. [Google Scholar]
- Smith, A.; Gangolli, S. Organochlorine chemicals in seafood: Occurrence and health concerns. Food. Chem. Toxicol. 2002, 40, 767–779. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liang, Y.; Jiang, X. Analysis of eight organophosphorus pesticide residues in fresh vegetables retailed in agricultural product markets of Nanjing, China. Bull. Environ. Contam. Toxicol. 2008, 81, 377–382. [Google Scholar] [CrossRef]
- Sultatos, L. Mammalian toxicology of organophosphorus pesticides. J. Toxicol. Environ. Health 1994, 43, 271–289. [Google Scholar] [CrossRef]
- Ishihara, A.; Nishiyama, N.; Sugiyama, S.; Yamauchi, K. The effect of endocrine disrupting chemicals on thyroid hormone binding to Japanese quail transthyretin and thyroid hormone receptor. Gen. Comp. Endocrinol. 2003, 134, 36–43. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Sun, J. Overview of organophosphorus pesticides at home and abroad and views on the development of organophosphorus pesticides in China. Agrochemicals 1999, 3–5. [Google Scholar] [CrossRef]
- Wen, J.; Zhou, S.; Yu, J.J.; Zhong, Y. Degradation of Malathion in Different Water Environments. Pesticides 2013, 52, 210–212. [Google Scholar] [CrossRef]
- Oudejans, L.; Mysz, A.; Snyder, E.G. Remediating indoor pesticide contamination from improper pest control treatments: Persistence and decontamination studies. J. Hazard. Mater. 2020, 397, 122743. [Google Scholar] [CrossRef]
- Liu, L.; Miyawaki, O.; Nakamura, K. Progressive freeze-concentration of model liquid food. Food Sci. Technol. Int. Tokyo 1997, 3, 348–352. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, C.; Yu, A.; Zhao, W.; Ren, F.; Liu, Y. The Migration Pattern of Atrazine during the Processes of Water Freezing and Thawing. Toxics 2022, 10, 603. [Google Scholar] [CrossRef]
- Shum, E.; Papangelakis, V. Water recovery from inorganic solutions via natural freezing and melting. J. Water. Process. Eng. 2019, 31, 100787. [Google Scholar] [CrossRef]
- University of Cambridge. Constitutional Undercooling. Available online: https://www.doitpoms.ac.uk/tlplib/solidification_alloys/undercooling.php (accessed on 5 December 2020).
- Moharramzadeh, S.; Ong, S.K.; Alleman, J.; Cetin, K.S. Parametric study of the progressive freeze concentration for desalination. Desalination 2021, 510, 115077. [Google Scholar] [CrossRef]
- Abdelmoaty, H.M.; Mahgoub, A.U.; Abdeldayem, A.W. Performance analysis of salt reduction levels in indirect freeze desalination system with and without magnetic field exposure. Desalination 2021, 508, 115021. [Google Scholar] [CrossRef]




| Chemical Compound | Mass-To-Charge Ratio (m/z) | Collision Energy (eV) | |
|---|---|---|---|
| Parent Ion | Daughter Ion | ||
| Malathion | 330.96 | 98.98 | 25 |
| 126.99 | 13 | ||
| 285.01 | 7 | ||
| Time (min) | Flow Rate (mL/min) | Mobile Phase A (%) | Mobile Phase B (%) |
|---|---|---|---|
| Initial | 0.200 | 60.0 | 40.0 |
| 0.20 | 0.200 | 60.0 | 40.0 |
| 3.50 | 0.200 | 100.0 | 0.0 |
| 3.60 | 0.200 | 60.0 | 40.0 |
| 6.50 | 0.200 | 60.0 | 40.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Wang, X.; Zhao, W.; Liu, Y.; Liu, T.; Yang, P. The Migration Rules of Malathion during Indoor Simulated Lake Freezing. Toxics 2023, 11, 222. https://doi.org/10.3390/toxics11030222
Zhang Y, Wang X, Zhao W, Liu Y, Liu T, Yang P. The Migration Rules of Malathion during Indoor Simulated Lake Freezing. Toxics. 2023; 11(3):222. https://doi.org/10.3390/toxics11030222
Chicago/Turabian StyleZhang, Yan, Xiaozhuang Wang, Wanli Zhao, Yucan Liu, Tongshuai Liu, and Peiyuan Yang. 2023. "The Migration Rules of Malathion during Indoor Simulated Lake Freezing" Toxics 11, no. 3: 222. https://doi.org/10.3390/toxics11030222
APA StyleZhang, Y., Wang, X., Zhao, W., Liu, Y., Liu, T., & Yang, P. (2023). The Migration Rules of Malathion during Indoor Simulated Lake Freezing. Toxics, 11(3), 222. https://doi.org/10.3390/toxics11030222






