Abstract
The efficient removal of Tetracycline Hydrochloride (TC) from wastewater, which is a difficult process, has attracted increasing attention. Aiming to synchronously achieve the goal of natural waste utilization and PMS activation, we have combined the MOFs material with waste coffee grounds (CG). The catalytic activity of the CG@ZIF-67 composite in the TC removal process was thoroughly evaluated, demonstrating that the TC removal rate could reach 96.3% within 30 min at CG@ZIF-67 composite dosage of 100 mg/L, PMS concertation of 1.0 mM, unadjusted pH 6.2, and contact temperate of 293.15 K. The 1O2 and ·SO4− in the CG@ZIF-67/PMS/TC system would play the crucial role in the TC degradation process, with 1O2 acting as the primary ROS. The oxygen-containing functional groups and graphite N on the surface of CG@ZIF-67 composite would play a major role in efficiently activating PMS and correspondingly degrading TC. In addition, the CG@ZIF-67/PMS/TC system could withstand a wide pH range (3–11). The application of CG in preparing MOF-based composites will provide a new method of removing emerging pollutants from an aqueous solution.
1. Introduction
In the water environment, the treatment of antibiotics pollutants, a typical kind of emerging pollutants [,], has received increasing attention [,,,,]. Among the antibiotics pollutants, as the second most used antibiotic in the world [], tetracycline hydrochloride (TC) has been widely used for many bacterial infections in humans and animals, since the majority of TC is hardly absorbed and up to 80–90% of the initial value or its metabolites can emit to the environment []. Though the concentration of TC in the environment is quite limited (from μg/L to ng/L) [], higher TC concentrations (100–500 mg/L) were detected in hospital and pharmaceutical plant wastewater []. Due to its high persistence, biological activity, antibiotic resistance, and toxic effects on ecosystems [], TC may lead to contamination of the environment, water resources, and soil. Many treatment methods to remove TC from an aqueous solution were investigated, e.g., adsorption [], photocatalysis [], electrocatalysis [], and the Fenton oxidation processes []. Among those TC removal routes, the advanced oxidation processes (AOPs) based on persulfate (PS) have attracted increasing attention in recent years [,]; this is because the sulfate radical (·SO4−)-based AOPs have superior oxidation potential (E0 = 2.5–3.1 V), a wider pH operating range, and a longer half-life time (30–40 μs) []. PMS with an asymmetric structure was usually water soluble and more oxidative []. PMS could be activated by UV, graphene oxide, transition metals, nanoscale magnetic, heat, and gamma radiation []. How to select an appropriate catalyst to efficiently activate the persulfates salt to generate reactive oxide species (ROS), correspondingly increase the degradation performance of organic pollutants was considered a crucial issue.
Metal-organic frames (MOFs) have been widely used in the activation of PMS to remove organic pollutants []. Considering its large pore sizes and high specific areas [], ample active centers, and plentiful derivatives [,], MOFs have garnered increasing interest in the catalytic field. Many scholars have prepared the MOFs materials, e.g., a core-heteroshell structural magnetic composite of ZIF-67/Vanadium-titanium magnetite (VTM) was synthesized through a solvothermal method, efficiently activating PMS for the removal of levofloxacin (LVF) (removal rate reaching 93.3%) []. The Co-Fe/NC@GCS was prepared by crosslinking the glutaraldehyde crosslinked chitosan (GCS) with Co-Fe nitrogen-doped carbon (derived from pyrolysis of MOF), efficiently removing 94.2% of sulfamethoxazole (SMX) within 60 min in the PMS solution []. The bone char (BBC) obtained through the pyrolysis of de-fatted swine bone could efficiently activate PS to the removal of 2,4-dichlorophenol (2,4-DCP) (removal rate reaching 85% within 30 min) []. The Fe-embedded biochar catalysts (Fe@HCBCs) originating from waste coffee grounds were used in the Fenton oxidation process for the removal of amaranth (AM) and sunset yellow (SY), with removal rates of 90.5% and 86.5%, respectively [], to activate the persulfates to treat the organic pollutant-containing solution. However, the common problems, e.g., the weak dispersion and the poor separation and recycling properties of the catalysts hinder their actual application in the removal of organic pollutants [,]. As a result, some scholars combined the MOFs material with metal particles [,] and metallic oxide [,,,,,,], aiming to improve the MOF’s actual application in wastewater treatment. However, the previously used metal or metallic oxide particles would usually need to be resynthesized, with more costs.
Herein, aiming to synchronously achieve the goal of the utilization of natural waste and activation of PMS, we have directly combined the MOFs material with waste coffee grounds (CG), analyzing the potential of CG in the forming of the MOF@CG composite. A series of material characterization methods including X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), Fourier transform infrared (FTIR), and Scanning electron microscopy (SEM) were conducted to analyze the structural performance of the prepared CG@ZIF-67. The primary reactive oxide species (ROS) in the degradation system were identified using electron paramagnetic resonance (EPR) analysis and free radical quenching experiments.
2. Materials and Methods
2.1. Raw Materials and Chemical Reagents
Tetracycline Hydrochloride (TC, C18H20FN3O4, 98%) and PMS (KHSO5⋅0.5KHSO4⋅0.5K2SO4) were applied by Aladdin Biochemical Co., Ltd., Shanghai, China. The coffee grounds (CG) were directly collected from a certain coffee shop in the city of Zhengzhou, Henan province, China. The collected raw CG material was firstly crushed through ball mill crushers and then washed using the deionized water several times, until the impurities (the residual oil and surface dust) were totally eliminated. Then, the coffee grounds were dried under 80 °C in the drying oven until the constant weight was reached. Finally, the dried coffee grounds were sieved through a 100-mesh sieve and stored for the following experiment.
The reagents of 2-Methylimidazole (2-MeIm, C4H6N2, 98%), Co(NO3)2·6H2O (98.5%) were purchased from Sinopharm Chemical Reagent Co., Ltd., Shanghai, China. The reagents of methyl alcohol (CH3OH), NaNO3, KH2PO4, tert-butyl alcohol (TBA, C4H10O), and furfuryl alcohol (FFA, C5H6O2) were also supplied by Sinopharm Chemical Reagent Co., Ltd., Shanghai, China, with analytical grades. The sodium chloride (NaCl, 99.5%), sodium hydroxide (NaOH, 96%), ethanol absolute (EtOH, C2H6O, 99.7%), sodium sulfate (Na2SO4, 99%), sodium bi-carbonate (NaHCO3, 99.5%), and sulfuric acid (H2SO4, 98%) were bought from Tianjin Kemiou Chemical Reagent Co., Ltd., Tianjin City, China. The deionized water used in this study was supplied by Wahaha Group Co., Ltd., Hangzhou, China. All the above reagents were directly supplied by the chemical reagent company, without further purification.
2.2. Synthesis of CG@ZIF-67 Composite
The proposed flowsheet of preparation of the CG@ZIF-67 composite was briefly depicted in Figure 1a. The CG@ZIF-67 composite was prepared through a facile solvothermal method []. The 2-MeIm (0.328 g) was firstly dissolved into 50 mL of the deionized water in a beaker (50 mL). Then, the CG (150 mg) was quickly added into the 2-MeIm solution and stirred in the thermostatic heating magnetic stirrer at 250 rpm for 5 min at 30 °C. Afterwards, the Co(NO3)2⋅6H2O (0.194 g) was directly added into the slurry containing CG and 2-MeIm solution and continuously stirred in the thermostatic heating magnetic stirrer at 250 rpm for 30 min. At the terminal of the reaction, the prepared CG@ZIF-67(5) composite was separated by centrifugation and then washed using methanol under ultrasound for a certain time, until the remaining supernatant was colorless. The prepared CG@ZIF-67(5) composite was finally dried in the vacuum drying oven at 60 °C for 12 h. For the change from Co(NO3)2⋅6H2O to CG samples as 5, 10, and 15, the prepared CG@ZIF-67 samples were labeled as CG@ZIF-67(1), CG@ZIF-67(10), and CG@ZIF-67(15), respectively.
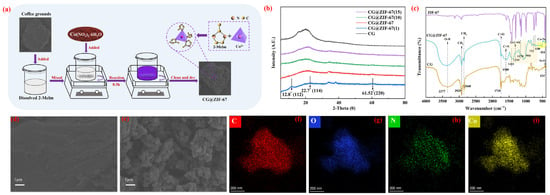
Figure 1.
(a) schematic diagram for the preparation of the CG@ZIF-67 composite. (b) XRD spectra of the CG and prepared CG@ZIF-67. (c) FTIR spectra of ZIF-67, CG and prepared CG@ZIF-67. The SEM image of original CG (d) and prepared CG@ZIF-67 (e). EDS-mapping of CG@ZIF-67 (f–i) (Symbols of f, g, h, and i were presenting the element of C, O, N, and Co, respectively.
2.3. TC Degradation Batch Experiment
During the TC degradation batch experiment, a certain amount of CG@ZIF-67 was added into the TC solution (20 mg/L, 100 mL) in a beaker (250 mL) and stirring at 300 rpm in the water bath thermostatic shaker at an indoor temperature (20 °C) to ensure the complete mixing between the CG@ZIF-67 and TC in the solution. Then, setting amounts of PMS solutions were added into the slurry (containing the TC and CG@ZIF-67) to start the TC degradation experiments. At the setting time intervals of 0, 1, 2, 3, 4, 5, 10, 15, 20, and 30 min, the slurry samples were immediately withdrawn from the reaction mixture. The withdrawn samples were quickly passed through a 0.45 μm syringe filter and immediately measured using the Ultraviolet-visible spectrophotometer (UV1800PC, Shanghai, China) at a wavelength of 357 nm (Figure S8). All the individual TC degradation experiments were repeated three times. The concentration of Co ions in the residual solution after TC degradation was measured using an inductively coupled plasma-atomic emission spectroscopy (ICP-OES, Optima 7000, PerkinElmer, Wellesley, MA, USA). The remaining PMS concentration in the solution was determined using the simple iodometric method by using KI and NaHCO3 as the reducing agents []. The main ROS (reactive oxygen species) acting in TC degradation were measured using electron paramagnetic resonance (EPR). The 5,5-dimethyl-1-pyrroline-N-oxide (DMPO) was used to quench the ·SO4− and ·OH, and the 2,2,6,6-tetramethyl-4-piperidine (TEMP) would quench the 1O2. The EPR spectra were obtained by using the JES-X320 EPR spectrometer (microwave frequency, 9.83 GHz; microwave power, 2.00 mW, JES-X320, JEOL, Tokyo, JPN).
2.3.1. Effect of CG@ZIF-67 Dosage on TC Degradation
The effects of the CG@ZIF-67 dosage on TC degradation were conducted through six samples. The six samples were conducted by adding a setting dosage of CG@ZIF-67 composite (0, 20, 60, 80, 100, and 120 mg/L) into the slurry containing TC (20 mg/L, 100 mL) in the 250 mL beakers. Then, the PMS (1.0 mM) solution was added into the slurry to start the TC degradation process. The contact temperature and initial pH were 293.15 K and 6.2.
2.3.2. Effect of PMS Dosage on TC Degradation
The effect of the PMS dosage on the TC removal performance were conducted by adding a setting dosage of PMS (0, 0.2, 0.6, 0.8, 1.0, and 1.2 mM) into the slurry containing CG@ZIF-67 composite (100 mg/L) and TC (20 mg/L, 100 mL) in the 250 mL beakers to start the TC degradation process. The contact temperature and initial pH were 293.15 K and 6.2, respectively.
2.3.3. Effect of Temperature and Initial pH on TC Degradation
The effect of temperature on the TC removal performance were investigated using four samples. The four samples were conducted by setting a series of temperatures (293.15, 303.15, 313.15, and 323.15 K). The factors including PMS concentration, initial TC concentration, and CG@ZIF-67 dosage were controlled as 1.0 mM, 20 mg/L, and 100 mg/L.
The effect of the initial pH on the TC removal performance were conducted using seven samples. The seven samples were controlled by setting a series of initial pH (3, 5, 6.2, 7, 9, 10, and 11) by adjusting H2SO4 (0.1 mM) and NaOH (0.1 mM) before the addition of PMS. Other conditions including the PMS concentration, CG@ZIF-67 dosage, and TC concentration were 1.0 mM, 100 mg/L, and 20 mg/L, respectively. The contact temperature was controlled as 293.15 K.
2.3.4. Effect of Co-Existing Ions on TC Degradation
The effects of the natural coexisting ions including Cl−, HCO3−, NO3−, SO42−, and H2PO4− on the TC degradation were investigated by separately adding coexisting ion solutions (1 mM, 5 mM, 10 mM, and 20 mM) into the TC/CG@ZIF-67/PMS system at an unadjusted pH 6.2. The contact temperature and time were determined as 293.15 K and 30 min, respectively.
2.3.5. Reusability of CG@ZIF-67 Composite
The reusability tests for the prepared CG@ZIF-67 were conducted for three cycles (Figure S4). The CG@ZIF-67 composite samples after every cycle of TC degradation were directly separated from the residual slurry and washed three times using the deionized water. The separated CG@ZIF-67 composite was then added into the TC solution to start the next cycle of TC degradation. Other conditions including the PMS dosage, contact temperature, and initial pH were kept as the first cycle.
2.4. Material Characterization of CG@ZIF-67 Composite
The X-ray diffraction (XRD) patterns of the prepared CG@ZIF-67 composite were obtained by using D/max 2500pc (Rigaku, Empyrean, The Netherlands) at 2θ ranging from 5 to 80°. The X-ray photoelectron spectroscopy (XPS, Escalab 250Xi, Thermo Scientific, Waltham, MA, USA) was used to analyze the chemical state of the main elementals of the prepared CG@ZIF-67. The morphology of the prepared CG@ZIF-67 was obtained by using a scanning electron microscope (SEM, Merlin compact, Zeiss, Jena, Germany). The total pore volume and pore size distribution of the CG@ZIF-67 composite were obtained through the N2 adsorption/desorption process (−196 °C) on the ASAP (2460, Micromeritics Instrument Ltd., Norcross, GA, USA).
3. Results
3.1. The Role of CG in the Prepared CG@ZIF-67 Composite
As shown in Figure 1b, the main characteristic peaks of the CG and CG@ZIF-67 composite were similar, confirming that CG was acting as the main component in the prepared CG@ZIF-67 composite. The peaks for CG@ZIF-67(1) located at 2θ of 12.8°and 22.7° were mainly related to the sodalite structure of ZIF-67 (pdf#43–0144). The peak observed 61.52° was mainly corresponding to the CoO (JCPDS, NO. 43-1004) []. However, at varying ratios of ZIF-67 to CG, the prepared CG@ZIF-67 was presenting a certain amorphous state, confirming that the stability of the prepared CG@ZIF-67 composite was needed to be improved. The FTIR spectra for CG and prepared CG@ZIF-67 composite were presented in Figure 1c. The bands observed at 1588 cm−1 and 752 cm−1 were related to the stretching of C=N and the vibration of the imidazole ring of 2-Melm (ZIF-67), respectively [,]. The vibrations observed at 600–1500 cm−1 were related to the stretching of the entire imidazole ring []. The band observed at 424 cm−1 was related to the stretching vibration of Co-N, attributing to the existence of ZIF-67 formed from the combination of the Co(NO3)2·6H2O and the 2-Melm []. The bands observed at 3377 cm−1, 1738 cm−1, and 1300 cm−1 were ascribed to the stretching vibration of O–H band, C=O band, and O-C=O band of CG, respectively []. All the characteristic peaks of ZIF-67 and CG were preserved for the CG@ZIF-67 composite, suggesting that the ZIF-67 was successfully loaded on the surface of CG. The SEM images of the original CG and prepared CG@ZIF-67 composite were shown in Figure 1d,e. Compared with the original CG, the layers of the ZIF-67 particles were grown on the surface of CG. The EDS-mapping images (Figure 1f–i) of the CG@ZIF-67 confirmed the distribution of C, N, and Co that the elements in the load layer (ZIF-67) were mainly C, N, O, and Co in the prepared CG@ZIF-67 composite. The specific surface areas of original CG and CG@ZIF-67 were determined as 0.11 and 4.02 m2 g-1 (Figure S1), respectively.
The XPS spectra of the prepared CG@ZIF-67 composite were shown in Figure 2a–c. The full survey XPS spectra of CG@ZIF-67 (Figure 2a) confirmed the main element state of C 1s, N 1s, O 1s, and Co 2p in the prepared CG@ZIF-67 composite. As shown in the C 1s spectra (Figure 2b), the peaks observed at 284.3, 284.8, 285.35, 286.5, and 288.1 eV were mainly related to sp2, C(graphite)/C-C/C-H, C=C, C=N, and COOH [,,,], respectively, further confirming of the presence of C=N (in ZIF-67) in the prepared CG@ZIF-67 composite. In the Co 2p spectra (Figure 2c), the two main peaks at 781.3 and 796.7 eV corresponded to the Co 2p3/2 and Co 2p1/2, respectively, with two satellite peaks of 2p3/2 observed at 786.5 eV and 802.8 eV (Sat.) [,]. The N 1s peak at 397.6 eV was corresponding to N of 2-Melm ligand coordinated with Co (Figure S2). The XPS spectra result illustrated that the Co was mainly presented as Co(II) in the prepared CG@ZIF-67. These above results confirmed that the ZIF-67 had part ly grown on the surface of the CG.
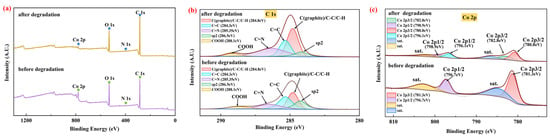
Figure 2.
XPS spectra of the CG@ZIF-67 composite: (a) full-range spectra, (b) C 1s spectra, (c) Co 2p spectra.
3.2. Catalytic Performance of CG@ZIF-67
The TC removal efficiency in different degradation systems were conducted and shown in Figure 3a. In the single PMS solution, 26.5% of the TC was removed, confirming that the single PMS could partially consume the TC in the aqueous solution. In the CG/PMS system, 27.9% of the TC was removed, indicating that the single CG could not efficiently active the PMS to realize the TC degradation in the solution. In the Co2+/PMS system, 71.4% of TC was removed (Figure S5). The TC removal ratio was determined as 96.3% in the CG@ZIF-67/PMS system within 30 min, confirming the complete consumption of TC in the CG@ZIF-67/PMS system. As shown in Figure 2f, the consumption ratio of PMS in the CG@ZIF-67/PMS system could reach 85.6% and 11.1% of TOC was removed (Figure S7), while only 15.8% of the PMS was consumed in the PMS/TC system, indicating that the CG@ZIF-67 catalyst could efficiently active PMS to degrade TC in an aqueous solution. The TC removal efficiencies of CG@ZIF-67(1), CG@ZIF-67(10), and CG@ZIF-67(15) in the PMS solution were determined as 96.1%, 95.9%, and 95.0%, thus indicating that different ratios of ZIF-67 in the prepared CG@ZIF-67 would not significantly change the TC removal rate.
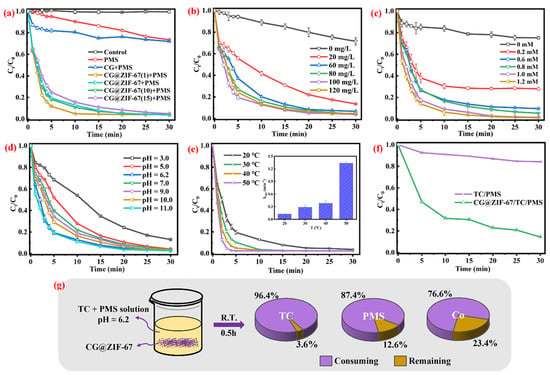
Figure 3.
(a) TC removal efficiency in different systems, (b) CG@ZIF-67 dosage, (c) PMS dosage, (d) effect of initial pH, and (e) contact temperature on TC removal efficiency (inserter figure was Kobs versus temperature); (f) the consumption of PMS of different systems within 30 min; (g) the consuming and remaining content of TC, PMS, and the Co ions within 30 min in the CG@ZIF-67/PMS/TC system. Other conditions: [TC] = 20 mg/L and unadjusted pH 6.2.
3.2.1. Effect of CG@ZIF-67 Composite and PMS Dosage
With the CG@ZIF-67 dosage ranging from 20 mg/L to 60 mg/L in the PMS solution (1.0 mM) (Figure 3b), the TC removal efficiency was increased from 86.5% to 95.9%, which was attributed to the catalytic active sites of PMS activation increasing the CG@ZIF-67 dosage. However, with the CG@ZIF-67 dosage further increasing to more than 60 mg/L, the TC degradation ratio was nearly kept constant. This result was mainly attributed to it, indicating that more catalyst would activate PMS to generate more ·SO4−, while the excess catalyst would undergo a self-quenching process or consume the remaining PMS, as shown in Equations (1) and (2) [,]. With the PMS dosage ranging from 0 to 1.2 mM (Figure 3c), the TC removal efficiency was increased from 24.8% to 98.9%. With the PMS dosage further increasing to more than 1.0 mM, the TC degradation efficiency remained nearly constant, which was attributed to the insufficient dosage of the CG@ZIF-67 catalyst.
SO42− + ·SO4− → S2O82−, k = 5 × 108 M−1s−1
S2O82−+ ·SO4− → ·S2O8−+ SO42−, k = 5.5 × 105 M−1s−1
3.2.2. Effect of Initial pH
The effect of pH on the TC removal efficiency was shown in Figure 3d. Under the natural pH (pH of 6.2), approximately 96.3% of the TC was removed in the CG@ZIF-67/PMS system within 30 min. At the more acidic pH of 3.0, the TC removal efficiency was decreased to 87.0%, which was attributed to the quenching of ·OH and ·SO4− by the excessive H+ (Equations (3) and (4)) [,,], accordance with the higher leaching rate of Co (at pH of 3) (Figure S6). At pH of 5.0, 7.0, 9.0, 10.0, and 11.0, the TC removal efficiency was all kept at a relatively higher value (more than 95.0%), presenting a high tolerance to pH in an aqueous solution because the PMS could be efficiently activated under strongly basic conditions. At a pH of 11.0 (>9.4), the PMS was decomposed into the strong oxidant of SO52−. The generated SO52− was easier to be activated by a catalyst, compared with PMS (HSO5−), further increasing the TC removal efficiency [,,]. Furthermore, the non-free radical of 1O2 was dominant in the CG@ZIF-67/PMS system, which was weakly dependent on the pH of the solution [].
H+ + ·OH + e− → H2O, k = 7 × 109 M−1s−1
·SO4− + H+ + e− →·HSO4−
3.2.3. Effect of Contact Temperature
The TC degradation process was accelerated with the increasing reaction temperature (Figure 3e), while the kobs of TC degradation gradually increased with the increasing temperature (Table S2). The higher temperature would provide more energy to overcome the energy barrier of PMS activation, promoting the generation of free radicals [].
The activation energies (Ea) of TC degradation in PMS activated by CG@ZIF-67 were evaluated by plotting ln (kobs) against 1/T (Figure S3, Equation S2). The calculated Ea (56.57 kJ/mol) was higher than that of the diffusion controlled reaction (ranging within 10–13 kJ/mol) [], implying the TC removal was mainly controlled by the chemical reaction, rather than the mass transfer process.
As shown in Figure 3g, the TC removal efficiency was 96.3%, while 87.4% of the PMS and 76.6% of the Co ions were consumed within 30 min in the CG@ZIF-67/PMS/TC system.
3.3. Effect of Co-Existing Substances
The effects of anions (Cl−, HCO3−, NO3−, SO42−, and H2PO4−) on the TC removal efficiency was shown in Figure 4. As shown as in Figure 4a, compared with the control group (TC removal efficiency of 96.3%), the TC removal efficiency was sharply decreased to 82.9% with the addition of Cl− (1 mM) to the CG@ZIF-67/PMS system, which was mainly attributing to the elimination of ·OH and ·SO4− by the added Cl−; this was calculated using Equations (5)–(8) [,,,]. Furthermore, the added Cl− could also consume the PMS, according to Equations (9) and (10) [,], thus decreasing the TC degradation efficiency.
Cl− + ·OH → ·ClOH−, k = 4.3 × 109 M−1s−1
·ClOH− → Cl−+ ·OH, k = 6.1 × 109 M−1s−1
Cl− + ·SO4− → SO42− + ·Cl
·Cl + Cl− → ·Cl2−, k=8 × 109 M−1s−1
Cl− + HSO5− → SO42− + HOCl
2Cl− + HSO5− + H+ → SO42− + Cl2 + H2O
HCO3− + HSO5− → ·SO4− + 2OH− + CO2
HCO3− + HSO5− → HSO4− + HCO4−
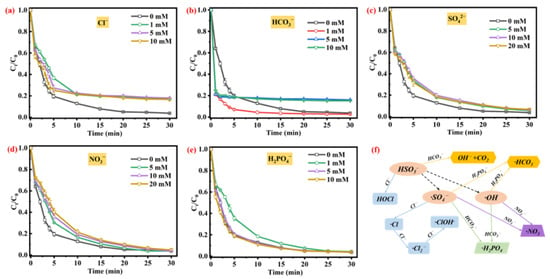
Figure 4.
Effects of co-existing substances (commonly found in natural environment) on TC removal efficiency: (a) Cl−, (b) HCO3−, (c) SO42−, (d) NO3−, and (e) H2PO4−. Conditions: [PMS] = 1.0 mM, [catalyst] = 100 mg/L, [TC] =20 mg/L, unadjusted pH 6.2, and contact temperature of 293.15 K. (f) The proposed mechanisms for inorganic ions affecting the active species generation in AOPs.
Compared with the control experiment, the TC removal ratio with the addition of 1 mM HCO3− was slightly increased to 97.6%, due to the generation of free radicals by the activation of HCO3− (Equation (11)) [,]. In addition, the HCO3− could react with PMS to form percarbonates (Equation (12)), which could selectively degrade organic pollutants [,]. An increased concentration of HCO3− (5 mM and 10 mM) significantly decreases the TC removal efficiency in the PMS/CG@ZIF-67 system (Figure 4b), which is mainly attributed to the consumption of ·OH and ·SO4− by the added HCO3− []. Other anions including NO3−, SO42−, and H2PO4− (Figure 4c–e) presented a weak effect on the TC removal process in the CG@ZIF-67/PMS. The NO3− could partially consume the free radicals []. The addition of SO42− would quench part of the ·SO4−, while the ·SO4− would be converted to S2O82− []. Furthermore, part of the ·OH would also be consumed by SO42− []. The H2PO4− could also consume part of the free radicals (·OH and ·SO4−) [,]. Furthermore, the H2PO4− could complex with Co(II), reducing the catalytic activity of CG@ZIF-67 [].
3.4. Identification of Reactive Specie
As shown in Figure 5a–c, with the addition of TBA (200 mM and 1000 mM), the TC removal efficiency has a slight drop (92.9% and 88.9%), respectively, which indicates that only a small amount of ·OH was generated in the CG@ZIF-67/PMS/TC system. With EtOH (200 mM and 1000 mM) added to the system, the TC removal efficiency was decreased to 61.1% and 46.6%, respectively. This result indicated the ·SO4− would facilitate the TC removal process. With addition of FFA (50 mL and 200 mM), the TC removal efficiency was sharply decreased from 37.7% and 14.6%, respectively, confirming the crucial role of 1O2 in the TC degradation process.
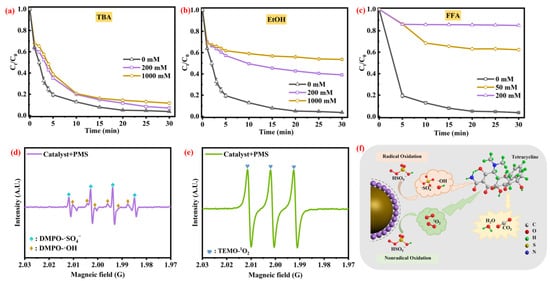
Figure 5.
Effects of different radical scavengers on the TC degradation in CG@ZIF-67/PMS system (a) TBA, (b) EtOH, and (c) FFA. EPR spectra of ·OH, ·SO4− (d), and 1O2 (e) in CG@ZIF-67/PMS/TC system. Conditions: [PMS] = 1.0 mM, [catalyst] = 100 mg/L, [TC] = 20 mg/L, unadjusted pH 6.2, and contact temperature of 293.15 K. (f) The proposed mechanism diagram for active species consuming the TC.
The EPR spectra conducted by using DMPO and TEMP as the spin-trapping agent were shown in Figure 5d,e, while the signals of DMPO-·OH and DMPO-·SO4− were both observed in the CG@ZIF-67/PMS system. The characteristic signals of DMPO-·OH (αN = αH = 14.9 G) confirmed the minor presence of ·OH in the TC degradation process []. The spectra of DMPO-·SO4− (αH = 9.6 G, αH = 1.48 G, αH = 0.78 G, and αN = 13.2 G) further confirmed the presence of ·SO4− in the CG@ZIF-67/PMS system []. The characteristic peaks of 1O2 EPR spectra (the characteristic peaks of 1O2 αN = 16.9 G) were clearly observed, further confirming the key role of 1O2 in the TC degradation process [,], which is in accordance with the quenching experiments result. Through those above analyses, the 1O2 would play a dominant role in the TC degradation process, with a non-radical activation route. Compared with the non-radical activation route, the ·SO4− would be a relatively weak driver of the TC degradation process; the reactive species contributing the most were 1O2 and ·SO4−.
3.5. Insight into the PMS Activation by CG@ZIF-67 Composite
The Co2+ ions (on the surface of CG@ZIF-67 composite) could directly activate PMS to form the ROS of ·OH and ·SO4−. In addition, from the XPS results (Figure 2c), the Co2+ captured water molecules to generate Co–OH and the CoOH+ of the CG@ZIF-67 composite would facilitate the redox cycle of PMS in the CG@ZIF-67/PMS/TC system, thus accelerating the activation of HSO5− to form ·SO4−, which was calculated using the following Equations (13)–(15) []. The 1O2 was firstly originated from the self-decomposition of PMS on the surface of CG@ZIF-67, where the generated ·OH and ·SO4− could decompose HSO5− to 1O2 as shown in Equations (16)–(18) [,]. Furthermore, ·SO5− would oxidize the HSO5− and H2O to form 1O2, which can be described by Equations (18) and (19) [,,,].
≡Co(II) + H2O → CoOH+ + H+
CoOH+ + HSO5− → ≡CoO+ + ·SO4− + H2O
CoO+ + 2H+ → ≡Co3+ + H2O
2·OH + HSO5− → HSO4− + 1O2 + H2O
·SO4− + HSO5− → SO42− + ·SO5− + H+, k < 1 × 105 M−1s−1
HSO5− + SO52− → 2SO42− + 1O2 + H+, k = 0.2 M−1s−1
2·SO5− + H2O → 2H+ + 2SO42− + 1.51O2
We should note that, from the XPS results (Figure 2b), the -COOH content of the CG@ZIF-67 decreased from 15.1% to 11.2% and the C(graphite)/C-C/C-H increased from 22.7% to 29.3% within the TC removal process (Table S1), indicating that the O-C=O bond could decompose the PMS to form ·SO4− [,,]. The content of the graphite N was decreased from 36.9% to 23.2%, while the C sp2 decreased from 20.5% to 13.3% within the TC removal process. Furthermore, the content of pyridinc N was enhanced from 47.8% to 60.4% (Table S1). The content change confirmed the participation of N (derived from CG) in driving the TC removal process. The graphite N of the CG@ZIF-67 composite might facilitate the electron transfer from the C atom to the N atom, further breaking the chemical inertia of sp2 carbon. The positive carbon atoms tend to adsorb HSO5− to form into ·OH and ·SO4− [,,,,].
4. Conclusions
In this paper, aiming to synchronously achieve the goals of natural coffee ground reuse and MOF material catalytic performance enhancement, the CG@MOF composites were successfully prepared and applied in the activation of PMS to TC degradation in an aqueous solution.
The material characterization routes including XRD, FTIR, XPS, and EDS-mapping confirmed the growth of ZIF-67 particles on the surface of the CG. The TC removal efficiency with CG@ZIF-67 composite acting in PMS activation could almost reach 96.3%, while the 1O2 and ·SO4− would play the crucial role to TC removal, withstanding a relatively wider pH range (3–11).
Compared with advanced conventional material oxidation technology, the TC could be effectively degraded at a wide pH range, thus confirming that the CG@ZIF-67/PMS system could achieve a satisfactory catalytic performance over a wide range of pH values and further ensure the possibility of its practical applications.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/toxics11020088/s1, Figure S1: N2 adsorption–desorption isotherms of the original CG (a) and prepared CG@ZIF-67 composite (b). Figure S2: XPS spectra of the CG@ZIF-67 composite: (a) N 1s and (b) O 1s. Figure S3: Reaction activation energy of temperature on TC removal efficiency. Figure S4: Recyclability of CG@ZIF-67 (a) and the Co ions concentration in three batch experiments (b). Figure S5: The TC removal efficiency in the Co2+/PMS system. Figure S6: The correlation between Co leaching and the pH. Figure S7: TOC removal after 30 min reaction in CG@ZIF-67/PMS/TC system. Figure S8: The comparison for the display data between the HPLC and UV-Vis tests. Table S1: The element content of C, N, O, and Co (calculated using XPS analysis) in CG@ZIF-67 before and after TC removal process. Table S2: The Kobs of TC removal process in CG@ZIF-67/PMS system at different temperatures. Text S1. Determination of PMS concentration in the solution.
Author Contributions
W.Z.: writing—review and editing; J.L.: methodology and writing—original draft preparation; S.L.: methodology; C.W.: methodology; Q.Z.: writing—review and editing; L.G.: writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Modern Analysis and Gene Sequencing Center of Zhengzhou University. We would also thank the Natural Sciences Foundation of China (grant No. 52000163), the Youth Talent Promotion Project of Henan province (2023HYTP035), the technology Think Tank Project of Henan province (grant No. HNKJZK-2023-06B), the Youth Talent Plan of China Association for Science and Technology (20220615ZZ07110090), the Open Research Fund of Henan Key Laboratory of Water Resources Conservation and Intensive Utilization in the Yellow River Basin (grant NO. HAKF202101), and the Open fund from Henan Key Laboratory of Water Pollution Control and Rehabilitation Technology (CJSP2022001).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank the Modern Analytical Computing Center of Zhengzhou University for the support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Patel, M.; Kumar, R.; Kishor, K.; Mlsna, T.; Pittman, C.U., Jr.; Mohan, D. Pharmaceuticals of Emerging Concern in Aquatic Systems: Chemistry, Occurrence, Effects, and Removal Methods. Chem. Rev. 2019, 119, 3510–3673. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, X.; Sun, Q.; Yuan, M.; Sun, Z.; Xia, S.; Zhao, J. Enhanced visible light photo-Fenton-like degradation of tetracyclines by expanded perlite supported FeMo3Ox/g-C3N4 floating Z-scheme catalyst. J. Hazard. Mater. 2022, 424, 127387. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Li, Y.; Chai, S.; Zhang, W.; Zuo, Q.; He, C. Enhanced catalytic activation of H2O2 by CNTs/SCH through rapid Fe(III)/Fe(II) redox couple circulation: Insights into the role of functionalized multiwalled CNTs. Sep. Purif. Technol. 2022, 282, 120000. [Google Scholar] [CrossRef]
- Cui, Q.; Zhang, W.; Chai, S.; Zuo, Q.; Kim, K.H. The potential of green biochar generated from biogas residue as a heterogeneous persulfate activator and its non-radical degradation pathways: Adsorption and degradation of tetracycline. Environ. Res. 2022, 204, 112335. [Google Scholar] [CrossRef] [PubMed]
- Sheng, X.; Wang, J.; Cui, Q.; Zhang, W.; Zhu, X. A feasible biochar derived from biogas residue and its application in the efficient adsorption of tetracycline from an aqueous solution. Environ. Res. 2022, 207, 112175. [Google Scholar] [CrossRef]
- Sheng, X.; Wang, J.; Zhang, W.; Zuo, Q. The Potential for PE Microplastics to Affect the Removal of Carbamazepine Medical Pollutants from Aqueous Environments by Multiwalled Carbon Nanotubes. Toxics 2021, 9, 139. [Google Scholar] [CrossRef]
- Wan, H.; Yan, J.; Guo, C.; Cui, Q.; Zhang, W. Synthesis of core-heteroshell structure for ZIF-67/VTM and its efficient activation of peroxymonosulfate in treatment of levofloxacin from an aqueous solution. Environ. Res. 2022, 204, 111986. [Google Scholar] [CrossRef]
- He, D.; Sun, Y.; Xin, L.; Feng, J. Aqueous tetracycline degradation by non-thermal plasma combined with nano-TiO2. Chem. Eng. J. 2014, 258, 18–25. [Google Scholar] [CrossRef]
- Yang, X.; Wang, W.; Feng, S.; Jin, L.; Lu, T.J.; Chai, Y.; Zhang, Q. Thermal Analysis of Cold Storage: The Role of Porous Metal Foam. Energy Procedia 2016, 88, 566–573. [Google Scholar] [CrossRef]
- Watkinson, A.J.; Murby, E.J.; Costanzo, S.D. Removal of antibiotics in conventional and advanced wastewater treatment: Implications for environmental discharge and wastewater recycling. Water Res. 2007, 41, 4164–4176. [Google Scholar] [CrossRef]
- Larsson, D.G.; de Pedro, C.; Paxeus, N. Effluent from drug manufactures contains extremely high levels of pharmaceuticals. J. Hazard. Mater. 2007, 148, 751–755. [Google Scholar] [CrossRef]
- Ahmadi, M.; Ramezani Motlagh, H.; Jaafarzadeh, N.; Mostoufi, A.; Saeedi, R.; Barzegar, G.; Jorfi, S. Enhanced photocatalytic degradation of tetracycline and real pharmaceutical wastewater using MWCNT/TiO(2) nano-composite. J. Environ. Manag. 2017, 186, 55–63. [Google Scholar] [CrossRef]
- Wan, D.; Wu, L.; Liu, Y.; Chen, J.; Zhao, H.; Xiao, S. Enhanced Adsorption of Aqueous Tetracycline Hydrochloride on Renewable Porous Clay-Carbon Adsorbent Derived from Spent Bleaching Earth via Pyrolysis. Langmuir 2019, 35, 3925–3936. [Google Scholar] [CrossRef]
- Wan, X.; Mo, G.; Luo, J. Metal–organic frameworks-derived TiO2 for photocatalytic degradation of tetracycline hydrochloride. Can. J. Chem. Eng. 2022, 1, 24550. [Google Scholar] [CrossRef]
- Xie, T.; Hu, H.; Chen, D.; Sun, P. Electrochemical Degradation of Tetracycline Hydrochloride in Aqueous Medium by (B4C/C)-β-PbO2 Electrode. Bull. Korean Chem. Soc. 2017, 38, 756–762. [Google Scholar] [CrossRef]
- Li, Q.; Ren, Z.; Liu, Y.; Zhang, C.; Liu, J.; Zhou, R.; Bu, Y.; Mao, F.; Wu, H. Petal-like hierarchical Co3O4/N-doped porous carbon derived from Co-MOF for enhanced peroxymonosulfate activation to remove tetracycline hydrochloride. Chem. Eng. J. 2023, 452, 139545. [Google Scholar] [CrossRef]
- Giannakis, S.; Lin, K.-Y.A.; Ghanbari, F. A review of the recent advances on the treatment of industrial wastewaters by Sulfate Radical-based Advanced Oxidation Processes (SR-AOPs). Chem. Eng. J. 2021, 406, 127083. [Google Scholar] [CrossRef]
- Yi, X.-H.; Ji, H.; Wang, C.-C.; Li, Y.; Li, Y.-H.; Zhao, C.; Wang, A.; Fu, H.; Wang, P.; Zhao, X.; et al. Photocatalysis-activated SR-AOP over PDINH/MIL-88A(Fe) composites for boosted chloroquine phosphate degradation: Performance, mechanism, pathway and DFT calculations. Appl. Catal. B Environ. 2021, 293, 120229. [Google Scholar] [CrossRef]
- Bose, S.; Kumar, M. Microwave-assisted persulfate/peroxymonosulfate process for environmental remediation. Curr. Opin. Chem. Eng. 2022, 36, 100826. [Google Scholar] [CrossRef]
- Hassani, A.; Scaria, J.; Ghanbari, F.; Nidheesh, P.V. Sulfate radicals-based advanced oxidation processes for the degradation of pharmaceuticals and personal care products: A review on relevant activation mechanisms, performance, and perspectives. Environ. Res. 2022, 217, 114789. [Google Scholar] [CrossRef]
- Honarmandrad, Z.; Sun, X.; Wang, Z.; Naushad, M.; Boczkaj, G. Activated persulfate and peroxymonosulfate based advanced oxidation processes (AOPs) for antibiotics degradation–A review. Water Resour. Ind. 2022, 29, 100194. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Wang, J.; Gascon, J.; Li, J.; Van der Bruggen, B. Metal-organic frameworks based membranes for liquid separation. Chem. Soc. Rev. 2017, 46, 7124–7144. [Google Scholar] [CrossRef] [PubMed]
- Rowsell, J.L.C.; Yaghi, O.M. Metal–organic frameworks: A new class of porous materials. Microporous Mesoporous Mater. 2004, 73, 3–14. [Google Scholar] [CrossRef]
- Wu, Y.N.; Zhou, M.; Li, S.; Li, Z.; Li, J.; Wu, B.; Li, G.; Li, F.; Guan, X. Magnetic metal-organic frameworks: Gamma-Fe2O3@MOFs via confined in situ pyrolysis method for drug delivery. Small 2014, 10, 2927–2936. [Google Scholar] [CrossRef]
- Li, S.; Wang, C.; Liu, Y.; Liu, Y.; Cai, M.; Zhao, W.; Duan, X. S-scheme MIL-101(Fe) octahedrons modified Bi2WO6 microspheres for photocatalytic decontamination of Cr(VI) and tetracycline hydrochloride: Synergistic insights, reaction pathways, and toxicity analysis. Chem. Eng. J. 2023, 455, 140943. [Google Scholar] [CrossRef]
- Wang, A.; Ni, J.; Wang, W.; Liu, D.; Zhu, Q.; Xue, B.; Chang, C.-C.; Ma, J.; Zhao, Y. MOF Derived Co−Fe nitrogen doped graphite carbon@crosslinked magnetic chitosan Micro−nanoreactor for environmental applications: Synergy enhancement effect of adsorption−PMS activation. Appl. Catal. B Environ. 2022, 319, 121926. [Google Scholar] [CrossRef]
- Zhou, X.; Zeng, Z.; Zeng, G.; Lai, C.; Xiao, R.; Liu, S.; Huang, D.; Qin, L.; Liu, X.; Li, B.; et al. Persulfate activation by swine bone char-derived hierarchical porous carbon: Multiple mechanism system for organic pollutant degradation in aqueous media. Chem. Eng. J. 2020, 383, 123091. [Google Scholar] [CrossRef]
- Shin, J.; Bae, S.; Chon, K. Fenton oxidation of synthetic food dyes by Fe-embedded coffee biochar catalysts prepared at different pyrolysis temperatures: A mechanism study. Chem. Eng. J. 2021, 421, 129943. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, P.; Zhan, T.; Yu, X.; Wen, Y.; Liu, X.; Gao, H.; Wang, P.; She, X. In situ growth of ZIF-67 on ultrathin CoAl layered double hydroxide nanosheets for electrochemical sensing toward naphthol isomers. J. Colloid Interface Sci. 2020, 576, 313–321. [Google Scholar] [CrossRef]
- Yu, Y.; You, S.; Du, J.; Xing, Z.; Dai, Y.; Chen, H.; Cai, Z.; Ren, N.; Zou, J. ZIF-67-derived CoO (tetrahedral Co2+)@nitrogen-doped porous carbon protected by oxygen vacancies-enriched SnO2 as highly active catalyst for oxygen reduction and Pt co-catalyst for methanol oxidation. Appl. Catal. B Environ. 2019, 259, 118043. [Google Scholar] [CrossRef]
- Iqbal, R.; Ali, S.; Saleem, A.; Majeed, M.K.; Hussain, A.; Rauf, S.; Rehman Akbar, A.; Xu, H.; Qiao, L.; Zhao, W. Electrically Conductive Pt-MOFs for Acidic Oxygen Reduction: Optimized Performance via Altering Conjugated Ligands. Chem. Eng. J. 2022, 455, 140799. [Google Scholar] [CrossRef]
- Guo, C.; Cheng, F.; Liang, G.; Zhang, S.; Jia, Q.; He, L.; Duan, S.; Fu, Y.; Zhang, Z.; Du, M. Copper-based polymer-metal–organic framework embedded with Ag nanoparticles: Long-acting and intelligent antibacterial activity and accelerated wound healing. Chem. Eng. J. 2022, 435, 134915. [Google Scholar] [CrossRef]
- Cheng, H.; Xu, G.; Zhu, C.; Alhalili, Z.; Du, X.; Gao, G. Porous MOF derived TiO2/ZnO/C@CNTs composites for enhancing lithium storage performance. Chem. Eng. J. 2023, 454, 140454. [Google Scholar] [CrossRef]
- Haris, M.; Khan, M.W.; Zavabeti, A.; Mahmood, N.; Eshtiaghi, N. Self-assembly of C@FeO nanopillars on 2D-MOF for simultaneous removal of microplastic and dissolved contaminants from water. Chem. Eng. J. 2022, 140390. [Google Scholar] [CrossRef]
- Li, X.; Wang, Z.; Zhang, B.; Rykov, A.I.; Ahmed, M.A.; Wang, J. Fe Co3−O4 nanocages derived from nanoscale metal–organic frameworks for removal of bisphenol A by activation of peroxymonosulfate. Appl. Catal. B Environ. 2016, 181, 788–799. [Google Scholar] [CrossRef]
- Yang, S.; Qiu, X.; Jin, P.; Dzakpasu, M.; Wang, X.C.; Zhang, Q.; zhang, L.; Yang, L.; Ding, D.; Wang, W.; et al. MOF-templated synthesis of CoFe2O4 nanocrystals and its coupling with peroxymonosulfate for degradation of bisphenol A. Chem. Eng. J. 2018, 353, 329–339. [Google Scholar] [CrossRef]
- Ru, Z.; Zhang, X.; Zhang, M.; Mi, J.; Cao, C.; Yan, Z.; Ge, M.; Liu, H.; Wang, J.; Zhang, W.; et al. Bimetallic-MOF-Derived Zn(x)Co(3−x)O(4)/Carbon Nanofiber Composited Sorbents for High-Temperature Coal Gas Desulfurization. Environ. Sci. Technol. 2022, 56, 17288–17297. [Google Scholar] [CrossRef]
- Wang, K.; Huang, Z.; Jin, X.; Zhang, D.; Wang, J. MOF–derived hollow porous ZnFe2O4/AgCl/Ag/C nanotubes with magnetic–dielectric synergy as high–performance photocatalysts for hydrogen evolution reaction. Chem. Eng. J. 2021, 422, 130140. [Google Scholar] [CrossRef]
- Zheng, Y.; Su, Y.; Pang, C.; Yang, L.; Song, C.; Ji, N.; Ma, D.; Lu, X.; Han, R.; Liu, Q. Interface-Enhanced Oxygen Vacancies of CoCuO(x) Catalysts In Situ Grown on Monolithic Cu Foam for VOC Catalytic Oxidation. Environ. Sci. Technol. 2022, 56, 1905–1916. [Google Scholar] [CrossRef]
- Wu, Y.N.; Zhou, M.; Zhang, B.; Wu, B.; Li, J.; Qiao, J.; Guan, X.; Li, F. Amino acid assisted templating synthesis of hierarchical zeolitic imidazolate framework-8 for efficient arsenate removal. Nanoscale 2014, 6, 1105–1112. [Google Scholar] [CrossRef]
- Wahba, N.; El Asmar, M.; El Sadr, M. Iodometric method for determination of persulfates. Anal. Chem. 1959, 31, 1870–1871. [Google Scholar] [CrossRef]
- Dai, P.; Yao, Y.; Hu, E.; Xu, D.; Li, Z.; Wang, C. Self-assembled ZIF-67@graphene oxide as a cobalt-based catalyst precursor with enhanced catalytic activity toward methanolysis of sodium borohydride. Appl. Surf. Sci. 2021, 546, 149128. [Google Scholar] [CrossRef]
- Gross, A.F.; Sherman, E.; Vajo, J.J. Aqueous room temperature synthesis of cobalt and zinc sodalite zeolitic imidizolate frameworks. Dalton Trans. 2012, 41, 5458–5460. [Google Scholar] [CrossRef]
- Hu, Y.; Kazemian, H.; Rohani, S.; Huang, Y.; Song, Y. In situ high pressure study of ZIF-8 by FTIR spectroscopy. Chem. Commun. 2011, 47, 12694–12696. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gao, X.; Ai, L.; Jiang, J. Mechanistic insight into the interaction and adsorption of Cr(VI) with zeolitic imidazolate framework-67 microcrystals from aqueous solution. Chem. Eng. J. 2015, 274, 238–246. [Google Scholar] [CrossRef]
- Son, J.; Kim, B.; Park, J.; Yang, J.; Lee, J.W. Wet in situ transesterification of spent coffee grounds with supercritical methanol for the production of biodiesel. Bioresour. Technol. 2018, 259, 465–468. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Y.; Tang, L.; Wang, J.; Yu, J.; Zhang, H.; Yu, M.; Zou, J.; Xie, Q. Egg shell biochar-based green catalysts for the removal of organic pollutants by activating persulfate. Sci. Total Environ. 2020, 745, 141095. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, X.; Ngo, H.H.; Guo, W.; Li, Z.; Wang, X.; Zhang, J.; Long, T. A new spent coffee grounds based biochar—Persulfate catalytic system for enhancement of urea removal in reclaimed water for ultrapure water production. Chemosphere 2022, 288, 132459. [Google Scholar] [CrossRef]
- Hong, H.; Liu, J.; Huang, H.; Atangana Etogo, C.; Yang, X.; Guan, B.; Zhang, L. Ordered Macro-Microporous Metal-Organic Framework Single Crystals and Their Derivatives for Rechargeable Aluminum-Ion Batteries. J. Am. Chem. Soc. 2019, 141, 14764–14771. [Google Scholar] [CrossRef]
- Cho, D.W.; Tsang, D.C.W.; Kim, S.; Kwon, E.E.; Kwon, G.; Song, H. Thermochemical conversion of cobalt-loaded spent coffee grounds for production of energy resource and environmental catalyst. Bioresour. Technol. 2018, 270, 346–351. [Google Scholar] [CrossRef]
- McIntyre, N.; Cook, M. X-ray photoelectron studies on some oxides and hydroxides of cobalt, nickel, and copper. Anal. Chem. 1975, 47, 2208–2213. [Google Scholar] [CrossRef]
- Lee, Y.C.; Li, Y.F.; Chen, M.J.; Chen, Y.C.; Kuo, J.; Lo, S.L. Efficient decomposition of perfluorooctanic acid by persulfate with iron-modified activated carbon. Water Res. 2020, 174, 115618. [Google Scholar] [CrossRef]
- Clifton, C.L.; Huie, R.E. Rate constants for hydrogen abstraction reactions of the sulfate radical, SO4? Alcohols. Int. J. Chem. Kinet. 1989, 21, 677–687. [Google Scholar] [CrossRef]
- Wang, H.; Chen, T.; Chen, D.; Zou, X.; Li, M.; Huang, F.; Sun, F.; Wang, C.; Shu, D.; Liu, H. Sulfurized oolitic hematite as a heterogeneous Fenton-like catalyst for tetracycline antibiotic degradation. Appl. Catal. B Environ. 2020, 260, 118203. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, Y.; Chen, L.; Zhang, H. Goethite as an efficient heterogeneous Fenton catalyst for the degradation of methyl orange. Catal. Today 2015, 252, 107–112. [Google Scholar] [CrossRef]
- Tang, W.Z.; Huang, C.P. 2,4-Dichlorophenol Oxidation Kinetics by Fenton’s Reagent. Environ. Technol. 1996, 17, 1371–1378. [Google Scholar] [CrossRef]
- Chen, C.; Liu, L.; Li, Y.; Li, W.; Zhou, L.; Lan, Y.; Li, Y. Insight into heterogeneous catalytic degradation of sulfamethazine by peroxymonosulfate activated with CuCo2O4 derived from bimetallic oxalate. Chem. Eng. J. 2020, 384, 123257. [Google Scholar] [CrossRef]
- Duan, X.; Ao, Z.; Zhou, L.; Sun, H.; Wang, G.; Wang, S. Occurrence of radical and nonradical pathways from carbocatalysts for aqueous and nonaqueous catalytic oxidation. Appl. Catal. B Environ. 2016, 188, 98–105. [Google Scholar] [CrossRef]
- Guan, Y.H.; Ma, J.; Ren, Y.M.; Liu, Y.L.; Xiao, J.Y.; Lin, L.Q.; Zhang, C. Efficient degradation of atrazine by magnetic porous copper ferrite catalyzed peroxymonosulfate oxidation via the formation of hydroxyl and sulfate radicals. Water Res. 2013, 47, 5431–5438. [Google Scholar] [CrossRef]
- Zhang, S.; Huo, X.; Xu, S.; Zhang, Y.; Zhang, B.; Wang, M.; Wang, Q.; Zhang, J. Original sulfur-doped carbon materials synthesized by coffee grounds for activating persulfate to BPA degradation: The key role of electron transfer. Process Saf. Environ. Prot. 2022, 168, 1219–1234. [Google Scholar] [CrossRef]
- Lin, S.-S.; Gurol, M.D. Catalytic decomposition of hydrogen peroxide on iron oxide: Kinetics, mechanism, and implications. Environ. Sci. Technol. 1998, 32, 1417–1423. [Google Scholar] [CrossRef]
- Minakata, D.; Kamath, D.; Maetzold, S. Mechanistic Insight into the Reactivity of Chlorine-Derived Radicals in the Aqueous-Phase UV-Chlorine Advanced Oxidation Process: Quantum Mechanical Calculations. Environ. Sci. Technol. 2017, 51, 6918–6926. [Google Scholar] [CrossRef] [PubMed]
- Jayson, G.; Parsons, B.; Swallow, A.J. Some simple, highly reactive, inorganic chlorine derivatives in aqueous solution. Their formation using pulses of radiation and their role in the mechanism of the Fricke dosimeter. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1973, 69, 1597–1607. [Google Scholar] [CrossRef]
- Atinault, E.; De Waele, V.; Schmidhammer, U.; Fattahi, M.; Mostafavi, M. Scavenging of es− and OH radicals in concentrated HCl and NaCl aqueous solutions. Chem. Phys. Lett. 2008, 460, 461–465. [Google Scholar] [CrossRef]
- Liang, C.; Wang, Z.S.; Mohanty, N. Influences of carbonate and chloride ions on persulfate oxidation of trichloroethylene at 20 degrees C. Sci. Total Environ. 2006, 370, 271–277. [Google Scholar] [CrossRef]
- Wang, Z.; Feng, M.; Fang, C.; Huang, Y.; Ai, L.; Yang, F.; Xue, Y.; Liu, W.; Liu, J. Both degradation and AOX accumulation are significantly enhanced in UV/peroxymonosulfate/4-chlorophenol/Cl− system: Two sides of the same coin? RSC Adv. 2017, 7, 12318–12321. [Google Scholar] [CrossRef]
- Lente, G.; Kalmár, J.; Baranyai, Z.; Kun, A.; Kék, I.; Bajusz, D.; Takács, M.; Veres, L.; Fábián, I. One-versus two-electron oxidation with peroxomonosulfate ion: Reactions with iron (II), vanadium (IV), halide ions, and photoreaction with cerium (III). Inorg. Chem. 2009, 48, 1763–1773. [Google Scholar] [CrossRef]
- Yang, S.; Wang, P.; Yang, X.; Shan, L.; Zhang, W.; Shao, X.; Niu, R. Degradation efficiencies of azo dye Acid Orange 7 by the interaction of heat, UV and anions with common oxidants: Persulfate, peroxymonosulfate and hydrogen peroxide. J. Hazard. Mater. 2010, 179, 552–558. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Effect of inorganic anions on the performance of advanced oxidation processes for degradation of organic contaminants. Chem. Eng. J. 2021, 411, 128392. [Google Scholar] [CrossRef]
- Rayaroth, M.P.; Aravind, U.K.; Aravindakumar, C.T. Effect of inorganic ions on the ultrasound initiated degradation and product formation of triphenylmethane dyes. Ultrason. Sonochem. 2018, 48, 482–491. [Google Scholar] [CrossRef]
- Hassani, A.; Eghbali, P.; Mahdipour, F.; Wacławek, S.; Lin, K.-Y.A.; Ghanbari, F. Insights into the synergistic role of photocatalytic activation of peroxymonosulfate by UVA-LED irradiation over CoFe2O4-rGO nanocomposite towards effective Bisphenol A degradation: Performance, mineralization, and activation mechanism. Chem. Eng. J. 2023, 453, 139556. [Google Scholar] [CrossRef]
- Ma, J.; Yang, Y.; Jiang, X.; Xie, Z.; Li, X.; Chen, C.; Chen, H. Impacts of inorganic anions and natural organic matter on thermally activated persulfate oxidation of BTEX in water. Chemosphere 2018, 190, 296–306. [Google Scholar] [CrossRef]
- Zhang, Z.; Zheng, Z.; Wong, K.C.J.; Lung, C.W.; Khan, M.; He, J.; Kumar, A.; Lo, I. Unraveling Singlet Oxygen Dominant Antibiotics Degradation from Drinking Water in the Photoelectrocatalytic Activation of Peroxymonosulfate Using Mos2 Embedded Carbon Substrate. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4150344 (accessed on 13 January 2023). [CrossRef]
- Wu, X.; Gu, X.; Lu, S.; Qiu, Z.; Sui, Q.; Zang, X.; Miao, Z.; Xu, M. Strong enhancement of trichloroethylene degradation in ferrous ion activated persulfate system by promoting ferric and ferrous ion cycles with hydroxylamine. Sep. Purif. Technol. 2015, 147, 186–193. [Google Scholar] [CrossRef]
- Mártire, D.O.; Gonzalez, M.C. Aqueous phase kinetic studies involving intermediates of environmental interest: Phosphate radicals and their reactions with substituted benzenes. Prog. React. Kinet. Mech. 2001, 26, 201–218. [Google Scholar] [CrossRef]
- Hammes, G.G.; Morrell, M.L. A study of nickel (II) and cobalt (II) phosphate complexes. J. Am. Chem. Soc. 1964, 86, 1497–1502. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Y.; Liu, Y.; Cheng, X.; Tang, W.; Zhao, C.; Guo, H. Kinetic performance of peroxymonosulfate activated by Co/Bi25FeO40: Radical and non-radical mechanism. J. Taiwan Inst. Chem. Eng. 2019, 100, 56–64. [Google Scholar] [CrossRef]
- Xie, J.; Luo, X.; Chen, L.; Gong, X.; Zhang, L.; Tian, J. ZIF-8 derived boron, nitrogen co-doped porous carbon as metal-free peroxymonosulfate activator for tetracycline hydrochloride degradation: Performance, mechanism and biotoxicity. Chem. Eng. J. 2022, 440, 135760. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y.; Li, W.; Zhong, R.; Lan, Y.; Guo, J. The efficient degradation of sulfisoxazole by singlet oxygen ((1)O2) derived from activated peroxymonosulfate (PMS) with Co3O4-SnO2/RSBC. Environ. Res. 2020, 187, 109665. [Google Scholar] [CrossRef]
- Zhu, S.; Li, X.; Kang, J.; Duan, X.; Wang, S. Persulfate Activation on Crystallographic Manganese Oxides: Mechanism of Singlet Oxygen Evolution for Nonradical Selective Degradation of Aqueous Contaminants. Environ. Sci. Technol. 2019, 53, 307–315. [Google Scholar] [CrossRef]
- Deng, J.; Feng, S.; Zhang, K.; Li, J.; Wang, H.; Zhang, T.; Ma, X. Heterogeneous activation of peroxymonosulfate using ordered mesoporous Co3O4 for the degradation of chloramphenicol at neutral pH. Chem. Eng. J. 2017, 308, 505–515. [Google Scholar] [CrossRef]
- Fang, Z.; Qi, J.; Xu, Y.; Liu, Y.; Qi, T.; Xing, L.; Dai, Q.; Wang, L. Promoted generation of singlet oxygen by hollow-shell CoS/g-C3N4 catalyst for sulfonamides degradation. Chem. Eng. J. 2022, 441, 136051. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, M.; Zhao, X.; Cao, D.; Guo, T.; Yang, B. Insights into heterogeneous catalysis of peroxymonosulfate activation by boron-doped ordered mesoporous carbon. Carbon 2018, 135, 238–247. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, H.; Zhang, Y.; Tang, W.; Cheng, X.; Li, W. Heterogeneous activation of peroxymonosulfate by sillenite Bi25FeO40: Singlet oxygen generation and degradation for aquatic levofloxacin. Chem. Eng. J. 2018, 343, 128–137. [Google Scholar] [CrossRef]
- Nguyen, T.-B.; Huang, C.P.; Doong, R.-a.; Wang, M.-H.; Chen, C.-W.; Dong, C.-D. Manipulating the morphology of 3D flower-like CoMn2O4 bimetallic catalyst for enhancing the activation of peroxymonosulfate toward the degradation of selected persistent pharmaceuticals in water. Chem. Eng. J. 2022, 436, 135244. [Google Scholar] [CrossRef]
- Zhang, X.; Bai, B.; Li Puma, G.; Wang, H.; Suo, Y. Novel sea buckthorn biocarbon SBC@β-FeOOH composites: Efficient removal of doxycycline in aqueous solution in a fixed-bed through synergistic adsorption and heterogeneous Fenton-like reaction. Chem. Eng. J. 2016, 284, 698–707. [Google Scholar] [CrossRef]
- Sun, H.; Liu, S.; Zhou, G.; Ang, H.M.; Tade, M.O.; Wang, S. Reduced graphene oxide for catalytic oxidation of aqueous organic pollutants. ACS Appl. Mater. Interfaces 2012, 4, 5466–5471. [Google Scholar] [CrossRef]
- Sun, H.; Kwan, C.; Suvorova, A.; Ang, H.M.; Tadé, M.O.; Wang, S. Catalytic oxidation of organic pollutants on pristine and surface nitrogen-modified carbon nanotubes with sulfate radicals. Appl. Catal. B Environ. 2014, 154, 134–141. [Google Scholar] [CrossRef]
- Duan, X.; Ao, Z.; Sun, H.; Indrawirawan, S.; Wang, Y.; Kang, J.; Liang, F.; Zhu, Z.H.; Wang, S. Nitrogen-doped graphene for generation and evolution of reactive radicals by metal-free catalysis. ACS Appl. Mater. Interfaces 2015, 7, 4169–4178. [Google Scholar] [CrossRef]
- Wang, C.; Kang, J.; Sun, H.; Ang, H.M.; Tadé, M.O.; Wang, S. One-pot synthesis of N-doped graphene for metal-free advanced oxidation processes. Carbon 2016, 102, 279–287. [Google Scholar] [CrossRef]
- Choi, C.H.; Park, S.H.; Woo, S.I. Binary and ternary doping of nitrogen, boron, and phosphorus into carbon for enhancing electrochemical oxygen reduction activity. ACS Nano 2012, 6, 7084–7091. [Google Scholar] [CrossRef]
- Oh, W.-D.; Lisak, G.; Webster, R.D.; Liang, Y.-N.; Veksha, A.; Giannis, A.; Moo, J.G.S.; Lim, J.-W.; Lim, T.-T. Insights into the thermolytic transformation of lignocellulosic biomass waste to redox-active carbocatalyst: Durability of surface active sites. Appl. Catal. B Environ. 2018, 233, 120–129. [Google Scholar] [CrossRef]
- Briggs, D.; Beamson, G. XPS studies of the oxygen 1s and 2s levels in a wide range of functional polymers. Anal. Chem. 1993, 65, 1517–1523. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).