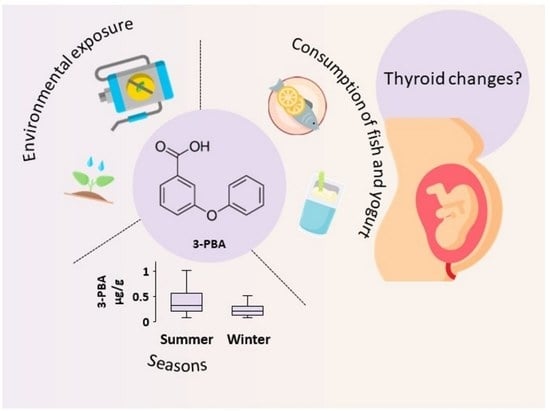
Association of 3-Phenoxybenzoic Acid Exposure during Pregnancy with Maternal Outcomes and Newborn Anthropometric Measures: Results from the IoMum Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
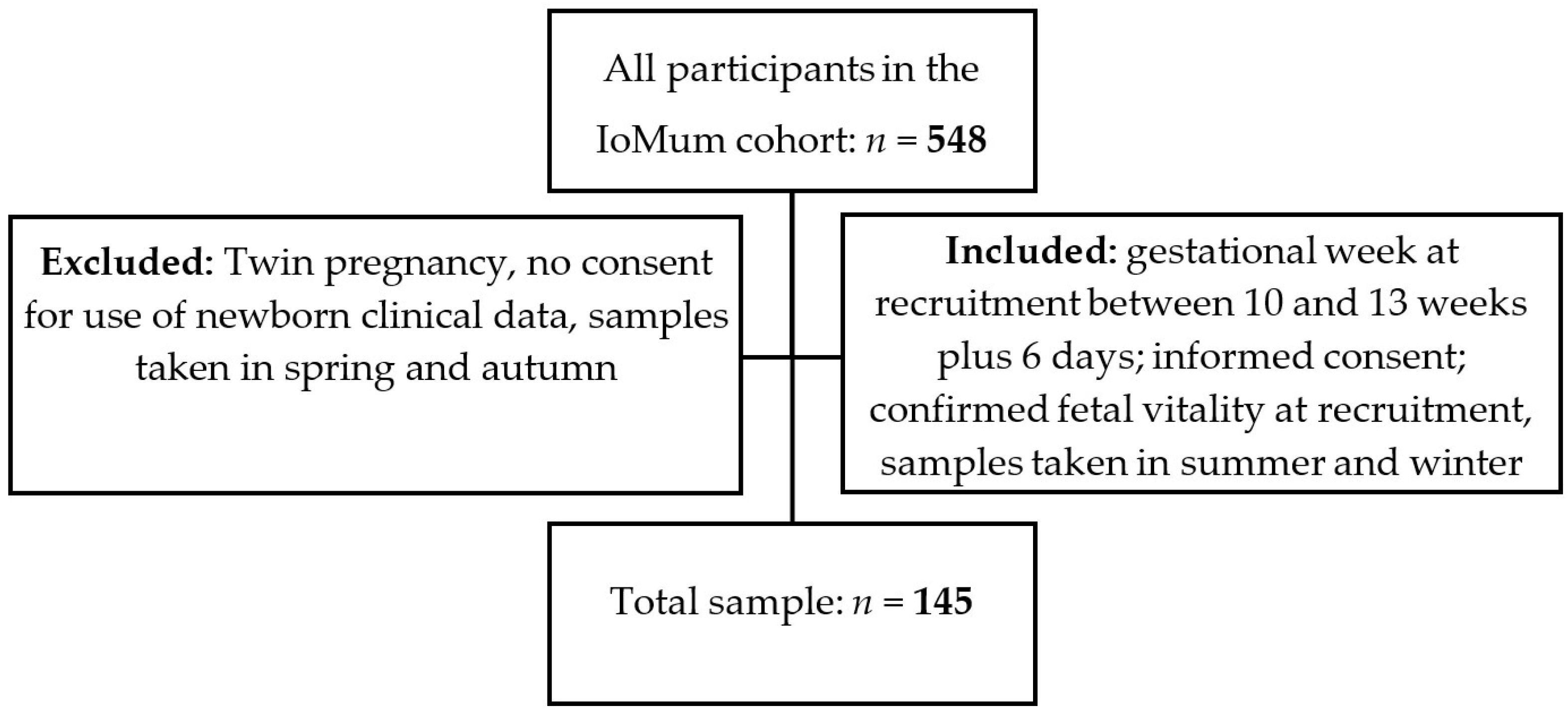
2.2. Study Design and Participants
2.3. Biochemical Analysis
2.3.1. Chemical Elements Quantification
2.3.2. Creatinine Quantification
2.4. Maternal Outcomes and Newborn Anthropometric Measures
- SGA: small for gestational age (below 10th percentile)
- AGA: appropriate for gestational age (between 10th percentile and 90th percentile)
- LGA: large for gestational age (above the 90th percentile)
2.5. Statistical Analysis
3. Results
3.1. Sociodemographic Data
3.2. 3-PBA Exposure by Seasons
3.3. 3-PBA Exposure in Association with Food Intake
3.4. 3-PBA Levels and Maternal Outcomes
3.5. Neonatal Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lewis, R.C.; Cantonwine, D.E.; Anzalota Del Toro, L.V.; Calafat, A.M.; Valentin-Blasini, L.; Davis, M.D.; Baker, S.E.; Alshawabkeh, A.N.; Cordero, J.F.; Meeker, J.D. Urinary biomarkers of exposure to insecticides, herbicides, and one insect repellent among pregnant women in Puerto Rico. Environ. Health 2014, 13, 97. [Google Scholar] [CrossRef] [PubMed]
- Braganca, I.; Lemos, P.C.; Delerue-Matos, C.; Domingues, V.F. Pyrethroid pesticide metabolite, 3-PBA, in soils: Method development and application to real agricultural soils. Environ. Sci. Pollut. Res. Int. 2019, 26, 2987–2997. [Google Scholar] [CrossRef]
- Balalian, A.A.; Liu, X.; Herbstman, J.B.; Daniel, S.; Whyatt, R.; Rauh, V.; Calafat, A.M.; Wapner, R.; Factor-Litvak, P. Prenatal exposure to organophosphate and pyrethroid insecticides and the herbicide 2,4-dichlorophenoxyacetic acid and size at birth in urban pregnant women. Environ. Res. 2021, 201, 111539. [Google Scholar] [CrossRef] [PubMed]
- EPA. Evironmental Protection Agency. Pesticide Science and Assessing Pesticide Risks; epa.gov.; EPA: Springfield, IL, USA, 2022.
- Braganca, I.; Lemos, P.C.; Barros, P.; Delerue-Matos, C.; Domingues, V.F. Phytotoxicity of pyrethroid pesticides and its metabolite towards Cucumis sativus. Sci. Total Environ. 2018, 619–620, 685–691. [Google Scholar] [CrossRef]
- Bragança, I.; Mucha, A.P.; Tomasino, M.P.; Santos, F.; Lemos, P.C.; Delerue-Matos, C.; Domingues, V.F. Deltamethrin impact in a cabbage planted soil: Degradation and effect on microbial community structure. Chemosphere 2019, 220, 1179–1186. [Google Scholar] [CrossRef]
- Li, W.; Morgan, M.K.; Graham, S.E.; Starr, J.M. Measurement of pyrethroids and their environmental degradation products in fresh fruits and vegetables using a modification of the quick easy cheap effective rugged safe (QuEChERS) method. Talanta 2016, 151, 42–50. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA); Medina-Pastor, P.; Triacchini, G. The 2018 European Union report on pesticide residues in food. EFSA J. 2020, 18, e06057. [Google Scholar] [PubMed]
- Saillenfait, A.M.; Ndiaye, D.; Sabaté, J.P. Pyrethroids: Exposure and health effects—An update. Int. J. Hyg. Environ. Health 2015, 218, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Kabir, E.; Jahan, S.A. Exposure to pesticides and the associated human health effects. Sci. Total Environ. 2017, 575, 525–535. [Google Scholar] [CrossRef]
- Wan, F.; Yu, T.; Hu, J.; Yin, S.; Li, Y.; Kou, L.; Chi, X.; Wu, J.; Sun, Y.; Zhou, Q.; et al. The pyrethroids metabolite 3-phenoxybenzoic acid induces dopaminergic degeneration. Sci. Total Environ. 2022, 838, 156027. [Google Scholar] [CrossRef]
- Personne, S.; Marcelo, P.; Pilard, S.; Baltora-Rosset, S.; Corona, A.; Robidel, F.; Lecomte, A.; Brochot, C.; Bach, V.; Zeman, F. Determination of maternal and foetal distribution of cis- and trans-permethrin isomers and their metabolites in pregnant rats by liquid chromatography tandem mass spectrometry (LC-MS/MS). Anal. Bioanal. Chem. 2019, 411, 8043–8052. [Google Scholar] [CrossRef]
- Connors, S.L.; Levitt, P.; Matthews, S.G.; Slotkin, T.A.; Johnston, M.V.; Kinney, H.C.; Johnson, W.G.; Dailey, R.M.; Zimmerman, A.W. Fetal mechanisms in neurodevelopmental disorders. Pediatr. Neurol. 2008, 38, 163–176. [Google Scholar] [CrossRef]
- Abreu-Villaça, Y.; Levin, E.D. Developmental neurotoxicity of succeeding generations of insecticides. Environ. Int. 2017, 99, 55–77. [Google Scholar] [CrossRef] [PubMed]
- Andersen, H.R.; David, A.; Freire, C.; Fernández, M.F.; D’Cruz, S.C.; Reina-Pérez, I.; Fini, J.B.; Blaha, L. Pyrethroids and developmental neurotoxicity - A critical review of epidemiological studies and supporting mechanistic evidence. Environ. Res. 2022, 214 Pt 2, 113935. [Google Scholar] [CrossRef]
- Hwang, M.; Lee, Y.; Choi, K.; Park, C. Urinary 3-phenoxybenzoic acid levels and the association with thyroid hormones in adults: Korean National Environmental Health Survey 2012-2014. Sci. Total Environ. 2019, 696, 133920. [Google Scholar] [CrossRef]
- Casals-Casas, C.; Desvergne, B. Endocrine disruptors: From endocrine to metabolic disruption. Annu. Rev. Physiol. 2011, 73, 135–162. [Google Scholar] [CrossRef]
- Zhang, J.; Yoshinaga, J.; Hisada, A.; Shiraishi, H.; Shimodaira, K.; Okai, T.; Koyama, M.; Watanabe, N.; Suzuki, E.; Shirakawa, M.; et al. Prenatal pyrethroid insecticide exposure and thyroid hormone levels and birth sizes of neonates. Sci. Total Environ. 2014, 488–489, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Du, G.; Shen, O.; Sun, H.; Fei, J.; Lu, C.; Song, L.; Xia, Y.; Wang, S.; Wang, X. Assessing hormone receptor activities of pyrethroid insecticides and their metabolites in reporter gene assays. Toxicol. Sci. 2010, 116, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhang, Z.; Qin, K.; Zhang, Y.; Pan, R.; Wang, Y.; Shi, R.; Gao, Y.; Tian, Y. Environmental pyrethroid exposure and thyroid hormones of pregnant women in Shandong, China. Chemosphere 2019, 234, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hisada, A.; Yoshinaga, J.; Shiraishi, H.; Shimodaira, K.; Okai, T.; Noda, Y.; Shirakawa, M.; Kato, N. Exposure to pyrethroids insecticides and serum levels of thyroid-related measures in pregnant women. Environ. Res. 2013, 127, 16–21. [Google Scholar] [CrossRef]
- Curl, C.L.; Porter, J.; Penwell, I.; Phinney, R.; Ospina, M.; Calafat, A.M. Effect of a 24-week randomized trial of an organic produce intervention on pyrethroid and organophosphate pesticide exposure among pregnant women. Environ. Int. 2019, 132, 104957. [Google Scholar] [CrossRef] [PubMed]
- Dereumeaux, C.; Saoudi, A.; Goria, S.; Wagner, V.; De Crouy-Chanel, P.; Pecheux, M.; Berat, B.; Zaros, C.; Guldner, L. Urinary levels of pyrethroid pesticides and determinants in pregnant French women from the Elfe cohort. Environ. Int. 2018, 119, 89–99. [Google Scholar] [CrossRef]
- Qi, X.; Zheng, M.; Wu, C.; Wang, G.; Feng, C.; Zhou, Z. Urinary pyrethroid metabolites among pregnant women in an agricultural area of the Province of Jiangsu, China. Int. J. Hyg. Environ. Health 2012, 215, 487–495. [Google Scholar] [CrossRef]
- Baker, S.E.; Barr, D.B.; Driskell, W.J.; Beeson, M.D.; Needham, L.L. Quantification of selected pesticide metabolites in human urine using isotope dilution high-performance liquid chromatography/tandem mass spectrometry. J. Expo. Anal. Environ. Epidemiol. 2000, 10, 789–798. [Google Scholar] [CrossRef]
- Skakkebaek, N.E.; Jørgensen, N.; Main, K.M.; Rajpert-De Meyts, E.; Leffers, H.; Andersson, A.M.; Juul, A.; Carlsen, E.; Mortensen, G.K.; Jensen, T.K.; et al. Is human fecundity declining? Int. J. Androl. 2006, 29, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Snijder, C.A.; te Velde, E.; Roeleveld, N.; Burdorf, A. Occupational exposure to chemical substances and time to pregnancy: A systematic review. Hum. Reprod. Update 2012, 18, 284–300. [Google Scholar] [CrossRef] [PubMed]
- Mehrpour, O.; Karrari, P.; Zamani, N.; Tsatsakis, A.M.; Abdollahi, M. Occupational exposure to pesticides and consequences on male semen and fertility: A review. Toxicol. Lett. 2014, 230, 146–156. [Google Scholar] [CrossRef]
- Shelton, J.F.; Geraghty, E.M.; Tancredi, D.J.; Delwiche, L.D.; Schmidt, R.J.; Ritz, B.; Hansen, R.L.; Hertz-Picciotto, I. Neurodevelopmental disorders and prenatal residential proximity to agricultural pesticides: The CHARGE study. Environ. Health Perspect. 2014, 122, 1103–1109. [Google Scholar] [CrossRef]
- Domingues, V.F.; Nasuti, C.; Piangerelli, M.; Correia-Sa, L.; Ghezzo, A.; Marini, M.; Abruzzo, P.M.; Visconti, P.; Giustozzi, M.; Rossi, G.; et al. Pyrethroid Pesticide Metabolite in Urine and Microelements in Hair of Children Affected by Autism Spectrum Disorders: A Preliminary Investigation. Int. J. Environ. Res. Public Health 2016, 13, 388. [Google Scholar] [CrossRef] [PubMed]
- Wagner-Schuman, M.; Richardson, J.R.; Auinger, P.; Braun, J.M.; Lanphear, B.P.; Epstein, J.N.; Yolton, K.; Froehlich, T.E. Association of pyrethroid pesticide exposure with attention-deficit/hyperactivity disorder in a nationally representative sample of U.S. children. Environ. Health 2015, 14, 44. [Google Scholar] [CrossRef] [PubMed]
- Furlong, M.A.; Barr, D.B.; Wolff, M.S.; Engel, S.M. Prenatal exposure to pyrethroid pesticides and childhood behavior and executive functioning. Neurotoxicology 2017, 62, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Šulc, L.; Janoš, T.; Figueiredo, D.; Ottenbros, I.; Šenk, P.; Mikeš, O.; Huss, A.; Čupr, P. Pesticide exposure among Czech adults and children from the CELSPAC-SPECIMEn cohort: Urinary biomarker levels and associated health risks. Environ. Res. 2022, 214, 114002. [Google Scholar] [CrossRef] [PubMed]
- Osaka, A.; Ueyama, J.; Kondo, T.; Nomura, H.; Sugiura, Y.; Saito, I.; Nakane, K.; Takaishi, A.; Ogi, H.; Wakusawa, S.; et al. Exposure characterization of three major insecticide lines in urine of young children in Japan-neonicotinoids, organophosphates, and pyrethroids. Environ. Res. 2016, 147, 89–96. [Google Scholar] [CrossRef] [PubMed]
- English, K.; Li, Y.; Jagals, P.; Ware, R.S.; Wang, X.; He, C.; Mueller, J.F.; Sly, P.D. Development of a questionnaire-based insecticide exposure assessment method and comparison with urinary insecticide biomarkers in young Australian children. Environ. Res. 2019, 178, 108613. [Google Scholar] [CrossRef]
- Wang, D.; Kamijima, M.; Imai, R.; Suzuki, T.; Kameda, Y.; Asai, K.; Okamura, A.; Naito, H.; Ueyama, J.; Saito, I.; et al. Biological monitoring of pyrethroid exposure of pest control workers in Japan. J. Occup. Health 2007, 49, 509–514. [Google Scholar] [CrossRef]
- Matta Coelho, C.; Guimarães, J.; Bracchi, I.; Xavier Moreira, N.; Pinheiro, C.; Ferreira, P.; Pestana, D.; Barreiros Mota, I.; Cortez, A.; Prucha, C.; et al. Noncompliance to iodine supplementation recommendation is a risk factor for iodine insufficiency in Portuguese pregnant women: Results from the IoMum cohort. J. Endocrinol. Investig. 2022, 45, 1865–1874. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, C.; Xavier Moreira, N.; Ferreira, P.; Matta Coelho, C.; Guimarães, J.; Pereira, G.; Cortez, A.; Bracchi, I.; Pestana, D.; Barreiros Mota, I.; et al. Iodine knowledge is associated with iodine status in Portuguese pregnant women: Results from the IoMum cohort study. Br. J. Nutr. 2021, 126, 1331–1339. [Google Scholar] [CrossRef]
- Ferreira, P.; Pinheiro, C.; Matta Coelho, C.; Guimarães, J.; Pereira, G.; Xavier Moreira, N.; Cortez, A.; Bracchi, I.; Pestana, D.; Barreiros Mota, I.; et al. The association of milk and dairy consumption with iodine status in pregnant women in Oporto region. Br. J. Nutr. 2021, 126, 1314–1322. [Google Scholar] [CrossRef]
- Richardson, D.B.; Ciampi, A. Effects of exposure measurement error when an exposure variable is constrained by a lower limit. Am. J. Epidemiol. 2003, 157, 355–363. [Google Scholar] [CrossRef]
- Schisterman, E.F.; Vexler, A.; Whitcomb, B.W.; Liu, A. The limitations due to exposure detection limits for regression models. Am. J. Epidemiol. 2006, 163, 374–383. [Google Scholar] [CrossRef]
- Govarts, E.G.L.; Rambaud, L.; Vogel, N.; Montazeri, P.; Berglund, M.; Santonen, T. Deliverable Report; Statistical Analysis Plan for the Co-Funded Studies of WP8; WP10—Data Management and Analysis; Deadline: March 2020 Upload by Coordinator: 10 December 2020; European Human Biomonitoring Initiative (HBM4EU) no.733032; 2020; Available online: https://www.hbm4eu.eu/work-packages/deliverable-10-12-update-statistical-analysis-plan-for-the-co-funded-studies-of-wp8/ (accessed on 18 December 2022).
- O’Brien, K.M.; Upson, K.; Buckley, J.P. Lipid and Creatinine Adjustment to Evaluate Health Effects of Environmental Exposures. Curr. Environ. Health Rep. 2017, 4, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Fossati, P.; Prencipe, L.; Berti, G. Enzymic creatinine assay: A new colorimetric method based on hydrogen peroxide measurement. Clin. Chem. 1983, 29, 1494–1496. [Google Scholar] [CrossRef]
- Alexander, G.R.; Kogan, M.D.; Himes, J.H. 1994-1996 U.S. singleton birth weight percentiles for gestational age by race, Hispanic origin, and gender. Matern. Child Health J. 1999, 3, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Leemans, M.; Couderq, S.; Demeneix, B.; Fini, J.B. Pesticides With Potential Thyroid Hormone-Disrupting Effects: A Review of Recent Data. Front. Endocrinol. 2019, 10, 743. [Google Scholar] [CrossRef] [PubMed]
- Report, D. Classification of Parishes on the Mainland into Rural and Non-Rural; Rural Development Program (PRODER); Portugal, 2013; p. 81. Available online: https://enrd.ec.europa.eu/country/portugal_en (accessed on 18 December 2022).
- Wielgomas, B.; Piskunowicz, M. Biomonitoring of pyrethroid exposure among rural and urban populations in northern Poland. Chemosphere 2013, 93, 2547–2553. [Google Scholar] [CrossRef] [PubMed]
- Leng, G.; Ranft, U.; Sugiri, D.; Hadnagy, W.; Berger-Preiss, E.; Idel, H. Pyrethroids used indoors--biological monitoring of exposure to pyrethroids following an indoor pest control operation. Int. J. Hyg. Environ. Health 2003, 206, 85–92. [Google Scholar] [CrossRef]
- Hardt, J.; Angerer, J. Biological monitoring of workers after the application of insecticidal pyrethroids. Int. Arch. Occup. Environ. Health 2003, 76, 492–498. [Google Scholar] [CrossRef]
- Berger-Preiss, E.; Levsen, K.; Leng, G.; Idel, H.; Sugiri, D.; Ranft, U. Indoor pyrethroid exposure in homes with woollen textile floor coverings. Int. J. Hyg. Environ. Health 2002, 205, 459–472. [Google Scholar] [CrossRef] [PubMed]
- Freire, C.; Suárez, B.; Vela-Soria, F.; Castiello, F.; Reina-Pérez, I.; Andersen, H.R.; Olea, N.; Fernández, M.F. Urinary metabolites of non-persistent pesticides and serum hormones in Spanish adolescent males. Environ. Res. 2021, 197, 111016. [Google Scholar] [CrossRef]
- Roca, M.; Miralles-Marco, A.; Ferré, J.; Pérez, R.; Yusà, V. Biomonitoring exposure assessment to contemporary pesticides in a school children population of Spain. Environ. Res. 2014, 131, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Viel, J.F.; Warembourg, C.; Le Maner-Idrissi, G.; Lacroix, A.; Limon, G.; Rouget, F.; Monfort, C.; Durand, G.; Cordier, S.; Chevrier, C. Pyrethroid insecticide exposure and cognitive developmental disabilities in children: The PELAGIE mother-child cohort. Environ. Int. 2015, 82, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Viel, J.F.; Rouget, F.; Warembourg, C.; Monfort, C.; Limon, G.; Cordier, S.; Chevrier, C. Behavioural disorders in 6-year-old children and pyrethroid insecticide exposure: The PELAGIE mother-child cohort. Occup. Environ. Med. 2017, 74, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Andersen, H.R.; Rambaud, L.; Riou, M.; Buekers, J.; Remy, S.; Berman, T.; Govarts, E. Exposure Levels of Pyrethroids, Chlorpyrifos and Glyphosate in EU-An Overview of Human Biomonitoring Studies Published since 2000. Toxics 2022, 10, 789. [Google Scholar] [CrossRef]
- Eljarrat, E. Pyrethroid Insecticides. In The Handbook of Environmental Chemistry; HEC: Paris, France, 2020; Volume 92. [Google Scholar]
- Yoo, M.; Lim, Y.H.; Kim, T.; Lee, D.; Hong, Y.C. Association between urinary 3-phenoxybenzoic acid and body mass index in Korean adults: 1(st) Korean National Environmental Health Survey. Ann. Occup. Environ. Med. 2016, 28, 2. [Google Scholar] [CrossRef] [PubMed]
- Dalsager, L.; Christensen, L.E.; Kongsholm, M.G.; Kyhl, H.B.; Nielsen, F.; Schoeters, G.; Jensen, T.K.; Andersen, H.R. Associations of maternal exposure to organophosphate and pyrethroid insecticides and the herbicide 2,4-D with birth outcomes and anogenital distance at 3 months in the Odense Child Cohort. Reprod. Toxicol. 2018, 76, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Stelmach-Mardas, M.; Kleiser, C.; Uzhova, I.; Peñalvo, J.L.; La Torre, G.; Palys, W.; Lojko, D.; Nimptsch, K.; Suwalska, A.; Linseisen, J.; et al. Seasonality of food groups and total energy intake: A systematic review and meta-analysis. Eur. J. Clin. Nutr. 2016, 70, 700–708. [Google Scholar] [CrossRef]
- Farag, M.R.; Alagawany, M.; Bilal, R.M.; Gewida, A.G.A.; Dhama, K.; Abdel-Latif, H.M.R.; Amer, M.S.; Rivero-Perez, N.; Zaragoza-Bastida, A.; Binnaser, Y.S.; et al. An Overview on the Potential Hazards of Pyrethroid Insecticides in Fish, with Special Emphasis on Cypermethrin Toxicity. Animals 2021, 11, 1880. [Google Scholar] [CrossRef] [PubMed]
- Riederer, A.M.; Hunter, R.E., Jr.; Hayden, S.W.; Ryan, P.B. Pyrethroid and organophosphorus pesticides in composite diet samples from Atlanta, USA adults. Environ. Sci. Technol. 2010, 44, 483–490. [Google Scholar] [CrossRef] [PubMed]
- McKinlay, R.; Plant, J.A.; Bell, J.N.; Voulvoulis, N. Endocrine disrupting pesticides: Implications for risk assessment. Environ. Int. 2008, 34, 168–183. [Google Scholar] [CrossRef]
- Lyssimachou, A.; Muilerman, H. Impact Assessment of the Criteria for Endocrine Disrupting Pesticides; Pesticide Action Network/PAN Europe: Brussels, Belgium, 2015–2016; p. 8. [Google Scholar]
- Orton, F.; Rosivatz, E.; Scholze, M.; Kortenkamp, A. Widely used pesticides with previously unknown endocrine activity revealed as in vitro antiandrogens. Environ. Health Perspect. 2011, 119, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Berbel, P.; Mestre, J.L.; Santamaría, A.; Palazón, I.; Franco, A.; Graells, M.; González-Torga, A.; de Escobar, G.M. Delayed neurobehavioral development in children born to pregnant women with mild hypothyroxinemia during the first month of gestation: The importance of early iodine supplementation. Thyroid 2009, 19, 511–519. [Google Scholar] [CrossRef]
- Jia, C.; Zhang, S.; Cheng, X.; An, J.; Zhang, X.; Li, P.; Li, W.; Wang, X.; Yuan, Y.; Zheng, H.; et al. Association between serum pyrethroid insecticide levels and incident type 2 diabetes risk: A nested case-control study in Dongfeng-Tongji cohort. Eur. J. Epidemiol. 2022, 37, 959–970. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Park, S.K.; Choi, Y.H. Environmental pyrethroid exposure and diabetes in U.S. adults. Environ. Res. 2019, 172, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.R.; Jørs, E.; Lander, F.; Condarco, G.; Schlünssen, V. Is cumulated pyrethroid exposure associated with prediabetes? A cross-sectional study. J. Agromed. 2014, 19, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Berkowitz, G.S.; Wetmur, J.G.; Birman-Deych, E.; Obel, J.; Lapinski, R.H.; Godbold, J.H.; Holzman, I.R.; Wolff, M.S. In utero pesticide exposure, maternal paraoxonase activity, and head circumference. Environ. Health Perspect. 2004, 112, 388–391. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.; Cui, C.; Chen, L.; Gao, Y.; Zhou, Y.; Shi, R.; Tian, Y. Prenatal exposure to pyrethroid insecticides and birth outcomes in Rural Northern China. J. Expo. Sci. Environ. Epidemiol. 2015, 25, 264–270. [Google Scholar] [CrossRef]

| Residence Area, n (%) | ||
| Maia | 41 | (30) |
| Porto | 25 | (18) |
| Valongo | 35 | (26) |
| Outros | 35 | (26) |
| Maternal education level, n (%) | ||
| Low (≤12 years) | 62 | (45) |
| Medium (13–15 years) | 50 | (36) |
| High (≥16 years) | 26 | (19) |
| Age (years), n | 145 | |
| Mean | 32 | |
| SD | 5.2 | |
| Pre-pregnancy BMI (kg/m2), n | 142 | |
| Median | 24 | |
| P25; P75 | 21; 26 | |
| Minimum | 16 | |
| Maximum | 36 | |
| Pre-pregnancy BMI categories, n (%) | ||
| Low weight | 11 | (8) |
| Normal weight | 92 | (65) |
| Overweight | 24 | (17) |
| Obesity | 15 | (10) |
| Gestational age at recruitment (weeks), n | 145 | |
| Median | 12 | |
| P25; P75 | 12; 13 | |
| Primiparous, n (%) | ||
| No | 70 | (48) |
| Yes | 75 | (52) |
| Preterm (37 weeks), n (%) | ||
| No | 131 | (93) |
| Yes | 10 | (7) |
| Newborn sex, n (%) | ||
| Male | 68 | (48) |
| Female | 73 | (52) |
| Birth weight (grams), n | 141 | |
| Mean | 3152 | |
| SD | 477 | |
| Birth weight classification, n (%) | ||
| SGA | 12 | (9) |
| AGA | 122 | (86) |
| LGA | 7 | (5) |
| 3-PBA (µg/L), n | 145 | |
| Median | 0.182 | |
| P25; P75 | 0.182; 0.372 | |
| <LOD, n (%) | 103 | (71) |
| ≥LOD, n (%) | 42 | (29) |
| 3-PBA (µg/g), n | 145 | |
| Median | 0.263 | |
| P25; P75 | 0.167; 0.458 |
| n | (%) | P25 | Median | P75 | p | |
|---|---|---|---|---|---|---|
| Residence Area | ||||||
| Maia | 41 | (30) | 0.174 | 0.274 | 0.450 | 0.056 a |
| Porto | 25 | (18) | 0.131 | 0.174 | 0.294 | |
| Valongo | 35 | (26) | 0.172 | 0.278 | 0.368 | |
| Other | 35 | (26) | 0.172 | 0.333 | 0.507 | |
| Maternal education level | ||||||
| Low (≤12 years) | 62 | (45) | 0.166 | 0.278 | 0.525 | 0.242 a |
| Medium (13–15 years) | 50 | (36) | 0.172 | 0.310 | 0.410 | |
| High (≥16 years) | 26 | (19) | 0.139 | 0.189 | 0.368 | |
| Smoking habits | ||||||
| Non-Smoker | 94 | (66) | 0.172 | 0.276 | 0.497 | 0.185 a |
| Smoker | 23 | (16) | 0.169 | 0.254 | 0.309 | |
| Former smoker | 26 | (18) | 0.142 | 0.206 | 0.334 | |
| Pre-pregnancy BMI categories | ||||||
| Underweight | 11 | (8) | 0.152 | 0.291 | 0.328 | 0.049 a |
| Normal weight | 92 | (65) | 0.188 | 0.293 | 0.507 | |
| Overweight | 24 | (17) | 0.165 | 0.241 | 0.340 | |
| Obesity | 15 | (11) | 0.103 | 0.155 | 0.346 |
| Creatinine (mg/dL) | 3-PBA (µg/L) | 3-PBA (µg/g) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Seasons | n | (%) | P25 | Median | P75 | p | P25 | Median | P75 | (Min; Max) | p | P25 | Median | P75 | (Min; Max) | p |
| Summer | 74 | 51 | 46.06 | 79.61 | 121.81 | 0.397 a | 0.182 | 0.371 | 0.394 | (0.182; 0.553) | <0.001 a | 0.209 | 0.331 | 0.556 | (0.082; 2.166) | <0.001 a |
| Winter | 71 | 49 | 58.47 | 85.35 | 133.75 | 0.182 | 0.182 | 0.182 | (0.182; 0.390) | 0.139 | 0.218 | 0.311 | (0.780; 1.064) | |||
| Food Intake | 3-PBA Summer (µg/g) | 3-PBA Winter (µg/g) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | (%) | Median | (P25; P75) | p | n | (%) | Median | (P25; P75) | p | |
| Fish | ||||||||||
| ≤3 times a week | 41 | (32) | 0.318 | (0.228; 0.608) | 0.533 a | 67 | (52) | 0.213 | (0.139; 0.301) | 0.002 a |
| >3 times a week | 17 | (13) | 0.325 | (0.155; 0.650) | 3 | (2) | 0.976 | (0.343; 1.064) | ||
| Yogurt | ||||||||||
| ≤6 times a week | 40 | (28) | 0.350 | (0.236; 0.521) | 0.938 a | 40 | (28) | 0.180 | (0.133; 0.270) | 0.015 a |
| ≥1 time a day | 33 | (23) | 0.328 | (0.174; 0.618) | 30 | (21) | 0.269 | (0.170; 0.462) | ||
| Newborn Sex | n | (%) | P25 | Median | P75 | (Min; Max) µg/g | p |
|---|---|---|---|---|---|---|---|
| Male | 68 | (48) | 0.161 | 0.271 | 0.401 | (0.078; 1.428) | 0.463 a |
| Female | 73 | (52) | 0.170 | 0.270 | 0.507 | (0.780; 2.166) |
| Birth Size Categories | n | (%) | P25 | Median | P75 | p |
|---|---|---|---|---|---|---|
| Birth weight | ||||||
| SGA | 12 | (8) | 0.196 | 0.303 | 0.356 | |
| AGA | 122 | (87) | 0.167 | 0.261 | 0.462 | 0.786 a |
| LGA | 7 | (5) | 0.174 | 0.301 | 0.497 | |
| Birth head circumference | ||||||
| SGA | 13 | (10) | 0.203 | 0.278 | 0.333 | 0.973 a |
| AGA | 112 | (83) | 0.166 | 0.263 | 0.463 | |
| LGA | 10 | (7) | 0.168 | 0.257 | 0.456 | |
| Birth length | ||||||
| SGA | 9 | (6) | 0.221 | 0.263 | 0.291 | 0.171 a |
| AGA | 131 | (93) | 0.168 | 0.274 | 0.476 | |
| LGA | 1 | (1) | 0.101 | 0.101 | 0.101 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guimarães, J.; Bracchi, I.; Pinheiro, C.; Moreira, N.X.; Coelho, C.M.; Pestana, D.; Prucha, M.d.C.; Martins, C.; Domingues, V.F.; Delerue-Matos, C.; et al. Association of 3-Phenoxybenzoic Acid Exposure during Pregnancy with Maternal Outcomes and Newborn Anthropometric Measures: Results from the IoMum Cohort Study. Toxics 2023, 11, 125. https://doi.org/10.3390/toxics11020125
Guimarães J, Bracchi I, Pinheiro C, Moreira NX, Coelho CM, Pestana D, Prucha MdC, Martins C, Domingues VF, Delerue-Matos C, et al. Association of 3-Phenoxybenzoic Acid Exposure during Pregnancy with Maternal Outcomes and Newborn Anthropometric Measures: Results from the IoMum Cohort Study. Toxics. 2023; 11(2):125. https://doi.org/10.3390/toxics11020125
Chicago/Turabian StyleGuimarães, Juliana, Isabella Bracchi, Cátia Pinheiro, Nara Xavier Moreira, Cláudia Matta Coelho, Diogo Pestana, Maria do Carmo Prucha, Cristina Martins, Valentina F. Domingues, Cristina Delerue-Matos, and et al. 2023. "Association of 3-Phenoxybenzoic Acid Exposure during Pregnancy with Maternal Outcomes and Newborn Anthropometric Measures: Results from the IoMum Cohort Study" Toxics 11, no. 2: 125. https://doi.org/10.3390/toxics11020125
APA StyleGuimarães, J., Bracchi, I., Pinheiro, C., Moreira, N. X., Coelho, C. M., Pestana, D., Prucha, M. d. C., Martins, C., Domingues, V. F., Delerue-Matos, C., Dias, C. C., Azevedo, L. F. R., Calhau, C., Leite, J. C., Ramalho, C., Keating, E., & Fernandes, V. C. (2023). Association of 3-Phenoxybenzoic Acid Exposure during Pregnancy with Maternal Outcomes and Newborn Anthropometric Measures: Results from the IoMum Cohort Study. Toxics, 11(2), 125. https://doi.org/10.3390/toxics11020125