Abstract
This study was conducted to determine the optimal boiling time to reduce ptaquiloside (PTA) and to carry out a risk assessment for PTA, a representative toxic substance found in bracken fern (BF; Pteridium aquilinum), which is frequently consumed as food in East Asian countries. High-performance liquid chromatography showed that the concentration of PTA in BF was reduced by up to 99% after boiling for 20 min. Risk assessment results showed that the cancer margin of exposure (MOE; ≥ 25,000 = safe) to PTA for an average daily exposure scenario after boiling BF for 20 min was considered safe. In addition, the non-cancer MOE (≥ 300 = safe) to PTA under an average daily exposure scenario after BF boiling for 20 min was considered safe. However, human exposure to PTA was considered unsafe under the non-boiled BF exposure and maximum daily exposure scenarios. Therefore, boiling BF for at least 20 min is recommended before consumption, to reduce exposure to PTA as much as possible.
1. Introduction
Ferns are a group of plants of considerable interest [1], which are less studied than vascular plants for several scientific aspects, such as their taxonomy [2], ecology, habitat, and vegetation [3]. Bracken fern (BF; Pteridium aquilinum) is a terrestrial plant with a wide variety of species distributed worldwide [4]. The plants’ structure is divided into roots, rhizomes, and fronds, and immature plants have fiddleheads on the fronds [5] (Figure 1). The fiddlehead gradually spreads out as the BF grows, and after it matures, it spawns and spreads the spores necessary for reproduction [5] (Figure 1). BF is often consumed in conditions of food scarcity by livestock grazing in large areas [6,7], and is an indicator of the pasture presence. By contrast, BF is frequently consumed in stir-fries, soups, and stews in East Asian countries, including Korea, Japan, and China [8,9]. In particular, in Korea, young BF is generally stored after pretreatment and sun-drying, and is then consumed after processing, according to various recipes (Figure 1).
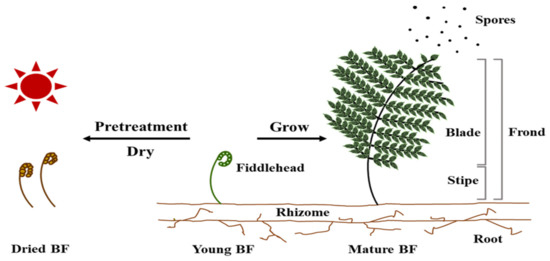
Figure 1.
The structure and fate of the bracken fern (BF; adapted from [5]).
However, BF can be poisonous and may cause esophageal and gastric cancers in humans [9,10,11]. As an example, in a case-control study on 98 patients with esophageal cancer and 476 controls, relative risks of 2.68 (95% confidence interval (CI) = 1.38–5.21) for a daily-BF-intake group and 1.53 (95% CI = 0.90–2.62) for an occasional-BF-intake group were observed, compared to a group with little or no BF intake [12,13,14]. In addition, animal experiments have shown that BF, processed BF, and BF extracts may result in leukemia and intestinal, lung, gastric, bladder, and mammary cancers in various animals, including rodents and cattle [7,9,12,13,15]. These results indicate the carcinogenicity of BF; however, the International Agency for Research on Cancer (IARC) stated that existing human studies had limitations, such as a lack of study details and a lack of consideration of cancer factors other than BF, and that the respective animal studies were conducted with small sample sizes and were reported incompletely [12,13]. Therefore, the IARC classified BF as IARC Group 2B (i.e., possibly carcinogenic to humans) based on intestinal and bladder cancers commonly observed in various animals [12,13].
Potentially carcinogenic BFs contain various toxic substances, including ptaquiloside (PTA), shikimic acid, and thiaminase [9,15,16]. PTA is a norsesquiterpene glucoside and is considered a representative carcinogen in BF [7,9] (Figure 2). PTA can be converted into an unstable intermediate termed ptaquilodienone by the loss of glucose [7], and its epoxide structure can cause point mutations by alkylating selected deoxyribonucleic acid (DNA) bases in a specific codon [7,10]. Therefore, PTA has been reported to induce the activation of the Harvey rat sarcoma viral oncogene homolog (H-ras) [7,17,18]. Activation of H-ras is associated with abnormal cell proliferation, differentiation, and survival [19], thus BF likely elicits carcinogenic effects through PTA [7,17,20].
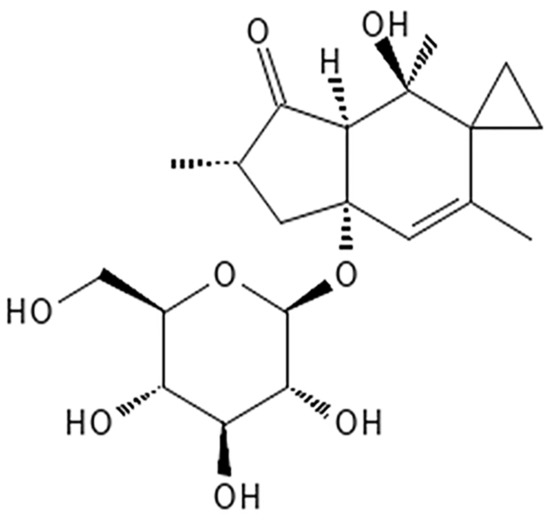
Figure 2.
Structural formula of ptaquiloside (PTA).
PTA is observed in the whole tissue of BF, low in roots and spores, and high in edible parts, crosiers [21,22]. Fortunately, PTA is degraded to a stable form by hydrothermal methods [23,24,25] and is expected to exhibit reduced toxicity. However, there is a lack of specific information to prove the reduction of toxicity (i.e., in vitro cytotoxicity test) [26] and degradation conditions such as temperature and heating time. Therefore, this study was conducted to identify the optimal boiling time to remove PTA in BF and to carry out a risk assessment by employing the concentration of PTA analyzed using high-performance liquid chromatography (HPLC). Non-cancer and cancer risk assessments for PTA in the BF were performed according to the European Food Safety Authority (EFSA) guidance [27,28,29].
2. Materials and Methods
2.1. Chemicals
PTA was purchased from Haihang Industry (Jinan, Shandong, China), and diclofenac sodium, used as an internal standard, was purchased from Sigma-Aldrich (St. Louis, MO, USA). The solvents used for HPLC analysis were of HPLC grade: acetonitrile (ACN) and methanol were purchased from J. T. Baker (Avantor, Radnor, PA, USA), and HPLC water was purchased from Sigma-Aldrich (St. Louis, MO, USA).
2.2. Pretreatment and Sampling of BF for HPLC Analysis
Twenty types of dried BF were purchased from retail stores in Korea. Dried BF was soaked in water for 4 h to obtain conditions similar to those of typical preparation for consumption. Subsequently, BF was boiled for 10 or 20 min or not boiled.
For HPLC sample preparation, 10 g of non-boiled or boiled BF was ground in 10 mL HPLC water and was centrifuged twice for 5 min at 3500 rpm. The supernatant was then filtered through a 0.2-μm Acrodisc syringe filter (Pall, Port Washington, NY, USA), and 200 μL was mixed with the same amount of ACN. The mixture was vortexed for 3 min and was centrifuged for 10 min at 5000 rpm. Then, 150 μL supernatant was mixed with 50 μL HPLC water to prepare 200 μL of the final HPLC sample. All sampling processes were performed at pH 7 ± 0.2 as PTA is stable at neutral pH [25].
2.3. HPLC Analysis Conditions
Calibration plots were constructed from a standard sample spiked with an internal standard and eight PTA concentrations (1.56–200 μg/mL). The ultraviolet wavelength of PTA measured before HPLC analysis was 220 nm, and HPLC was performed using a Gilson (Middleton, WI, USA) instrument equipped with a C18 column (Synergi 4 µm Hydro-RP 80 Å, LC Column 250 × 4.6 mm; Phenomenex, Torrance, CA, USA). The HPLC samples were transferred at a flow rate of 0.8 mL/min at room temperature with an isocratic mobile phase consisting of a mixture of water and methanol at a ratio of 40:60 v/v, and the retention time of PTA was confirmed to be 6.68 min (Figure 3). The calibration curve was produced through weighted (1/x) least-squares linear regression, and the coefficient of determination (r2) for the calibration curve was 0.9983. The limit of detection of PTA was 0.05 ppm.
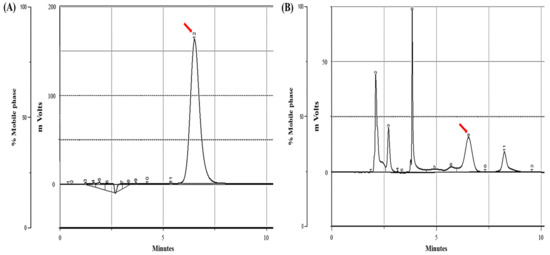
Figure 3.
High-performance liquid chromatography (HPLC) chromatograms of PTA. (A) PTA (200 μg/mL) standard sample (arrow) and (B) BF sample (arrow).
2.4. Human Exposure to PTA
Human exposure to PTA was calculated by considering daily BF intake, PTA concentration, oral absorption rate, and body weight, according to the following equation:
Human exposure (mg/kg/day) = (daily BF intake (g/day) × 1000 × concentration of PTA (%)/100 × oral absorption rate (%)/100) ÷ body weight (kg)
Daily BF intake was determined to be 2.32 g/day, corresponding to the average of the total population according to statistical data reported in Korea [30], and the concentration of PTA was determined as the median and maximal values of the analyzed concentrations to represent average and maximal exposure scenarios. The oral absorption rate was not reported previously; thus, it was assumed to be 100%, and the body weight was considered to be 70 kg, the default value for adults [27].
2.5. Non-Cancer and Cancer Risk Assessment
Risk assessment for PTA in BF was conducted based on the EFSA guidance [27,28,29]. In general, the non-cancer risk assessment results are expressed as the margin of exposure (MOE) = no observed adverse effect level (NOAEL)/human exposure, and if the MOE exceeds 100, it is considered safe [28]. However, if the lowest observed adverse effect level (LOAEL) is used instead of NOAEL, an uncertainty factor of three can be added [27]. By contrast, the cancer risk assessment results are expressed as the benchmark dose lower confidence limit for a 10% response (BMDL10)/human exposure, and if the MOE is greater than 10,000, it is considered safe [28]. However, if the data are not suitable for estimating BMDL10 (e.g., single linear model) [31], a chronic dose that causes tumors in a certain tissue region in 25% of the experimental animals after the rate is corrected with the spontaneous carcinogenesis rate (T25) can be used, and an uncertainty factor of 2.5 can be added [28,29,32].
3. Results
3.1. Determination of Optimal Boiling Time for Removal of PTA from BF
The optimal boiling time was investigated using five soaked BF batches. The concentration of PTA in BF boiled for 10 min was 10.82–33.61 ppm, which was decreased by 70.14–88.26% compared to non-boiled BF (Table 1). The concentration of PTA in BF boiled for 20 min was 2.48–6.54 ppm, which was reduced by 94.19–97.31% compared to the non-boiled BF (Table 1). The concentration of PTA in BF decreased as boiling time increased (Table 1), and 20 min of boiling with a PTA reduction rate of at least 94% was determined to be the appropriate boiling condition.

Table 1.
Analysis of the concentration of PTA in BF according to boiling time.
3.2. Concentration of PTA after Boiling BF for 20 Min
The PTA concentration was measured using 20 soaked BF samples. The concentration of PTA in non-boiled BF was 12.50–1995.38 ppm, with a median of 409.55 ppm (Table 2). The concentration of PTA in BF boiled for 20 min was 2.31–132.74 ppm, with a median of 5.78 ppm (Table 2). The concentration of PTA in BF thus decreased by 79.61ȁ99.24% after boiling, with a median decrease of 95.51% (Table 2).

Table 2.
Analysis of the concentration of PTA in BF after boiling for 20 min.
3.3. Human Exposure to PTA
Human exposure to PTA was calculated for average and maximum exposure scenarios. Human exposure to PTA was 1.36 × 10−2 mg/kg/day for non-boiled BF and 1.92 × 10−4 mg/kg/day for BF boiled for 20 min in the average exposure scenario (Table 3). Under the maximum exposure scenario, human exposure to PTA was 6.63 × 10−2 mg/kg/day for non-boiled BF and 4.41 × 10−3 mg/kg/day for BF boiled for 20 min (Table 3).

Table 3.
Human exposure to PTA.
3.4. Determination of Non-Cancer and Cancer Toxicity Values for PTA
The toxicity values for PTA were derived from the most appropriate study on PTA toxicity [14]. The non-cancer toxicity value was derived from a different study [33], and the cancer toxicity value was derived from a further study [34]. The details of the selected toxicity data and methods for calculating toxicity values are summarized in Table 4.

Table 4.
Determination of non-cancer and cancer toxicity values for PTA.
3.5. Non-Cancer and Cancer Risk Assessment for PTA
The results of the non-cancer and cancer risk assessments were derived by calculating the MOE. The non-cancer MOE was 7.65 for non-boiled BF and 541.67 for BF boiled for 20 min for the average exposure scenario (Table 5). The cancer MOE was 415.59 for non-boiled BF and 29,437.50 for BF boiled for 20 min (Table 5). Under the maximal exposure scenario, the non-cancer MOE was 1.57 for non-boiled BF and 23.58 for BF boiled for 20 min (Table 5). The cancer MOE was 85.25 for non-boiled BF and 1281.63 for BF boiled for 20 min (Table 5). It was thus considered unsafe, except for the non-cancer and cancer risk assessment results for BF boiled for 20 min under the average exposure scenario (Table 5).

Table 5.
Non-cancer and cancer risk assessment results for PTA.
4. Discussion
Plants contain various substances, including toxic compounds [36,37,38], and risk assessment of these substances is considered important for risk management [39,40,41,42,43]. The present study is the first to conduct non-cancer and cancer risk assessments of PTA in BF. For the risk assessment, the concentration of PTA in BF was measured using HPLC, and the toxicity value of PTA was derived from animal toxicity data. In addition, human exposure to PTA was calculated according to an equation, and the EFSA guidance was referred to [27,28,29].
The concentration of PTA in BF decreased as boiling time increased (Table 1), and the reduction rate of PTA after 20 min of boiling was determined as the optimal boiling condition was up to 99.24% (Table 2). These results were compared with those of a previous study [23], which predicted the concentration of PTA in BF using pterosin B (PTB), a stable metabolite of PTA; this previous study showed that the PTB reduction rates for raw BF boiled for 5 and 10 min were 60% and 66.4%, respectively. In addition, the reduction rate of PTB was 88% for raw BF soaked in water for 12 h after boiling for 5 min [23]. These results indicated that the concentration of PTA in BF decreased with boiling time, which was in line with our results (Table 1), and that it also decreased with soaking time and the number of water exchanges. This explains why BF is typically soaked in water and boiled before consumption.
Human exposure to PTA was calculated using a formula and was found to be lower for BF boiled for 20 min than for non-boiled BF (Table 3). Our results were compared with those of human exposure to PTA in drinking water as examined in a previous study [14], where the 99th percentile lifetime average human exposure was calculated as 0.5 μg/kg/day considering the maximal exposure scenario. This value was 132.6-fold and 8.82-fold lower than that for non-boiled BF and BF boiled for 20 min, respectively, under the maximal exposure scenario (Table 3). Comparative results showed that drinking water is not the main route of human exposure to PTA and that dietary exposure is more prevalent [14]. In addition, humans can be exposed to PTA through milk and soil [7,44,45].
The toxicity values for PTA, LOAEL, and T25 were derived from animal toxicity data, however, these data contained limited information (Table 4). Non-cancer and cancer studies were conducted with only one variable dose group for a small number of animals, and the cancer study was a 207-day study rather than a two-year study (Table 4). However, this result was expected because the concentration of PTA in BF is not constant, and PTA is unstable [33,34].
The risk assessment results showed that human exposure to PTA is only safe for BF boiled for 20 min under the average exposure scenario for non-cancer and cancer risk assessment (Table 5). These results may be because exposure was assessed using an oral absorption rate of 100% due to the lack of data. However, human exposure to PTA may require caution under a conservative risk assessment approach that considers a conservative assessment factor and a maximal exposure scenario. Similar to our results, when non-cancer and cancer risk assessments were evaluated using human exposure to PTA through 0.5 μg/kg/day of drinking water [14], it was calculated as non-cancer MOE 208 and cancer MOE 11,304 and was considered unsafe.
PTA is a toxic substance contained in BF that can be readily absorbed by consumers through the diet. Therefore, it is suggested that BF should be sufficiently boiled, i.e., for at least 20 min, before consumption to reduce human exposure to PTA. In addition, further studies, in addition to the proposed method, are needed to avert human exposure to PTA. Finally, in future studies, it seems necessary to establish a database for optimal risk reduction conditions for each food containing toxic substances.
Author Contributions
Conceptualization, M.K.K. and J.S.K.; methodology, M.K.K. and J.S.K.; software, A.K.; validation, A.K.; formal analysis, M.K.K.; investigation, M.K.K. and J.S.K.; resources, M.K.K. and J.S.K.; data curation, M.K.K. and J.S.K.; writing—original draft preparation, M.K.K. and J.S.K.; writing—review and editing, H.S.K. and B.-M.L.; visualization, M.K.K. and J.S.K.; supervision, B.-M.L.; project administration, B.-M.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Sungkyunkwan University in 2019.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mannan, M.M.; Maridass, M.; Victor, B. A Review on the Potential Uses of Ferns. Ethnobot. Leafl. 2008, 12, 281–285. [Google Scholar]
- Cárdenas, G.G.; Lehtonen, S.; Tuomisto, H. Taxonomy and evolutionary history of the neotropical fern genus Salpichlaena (Blechnaceae). Blumea Biodivers. Evol. Biogeogr. Plants 2019, 64, 1–22. [Google Scholar] [CrossRef]
- Perrino, E.V.; Tomaselli, V.; Wagensommer, R.P.; Silletti, G.N.; Esposito, A.; Stinca, A. Ophioglossum lusitanicum L.: New Records of Plant Community and 92/43/EEC Habitat in Italy. Agronomy 2022, 12, 3188. [Google Scholar] [CrossRef]
- Matongera, T.N.; Mutanga, O.; Dube, T.; Lottering, R.T. Detection and mapping of bracken fern weeds using multispectral remotely sensed data: A review of progress and challenges. Geocarto Int. 2018, 33, 209–224. [Google Scholar] [CrossRef]
- Marrs, R.H.; Watt, A.S. Biological Flora of the British Isles: Pteridium aquilinum (L.) Kuhn. J. Ecol. 2006, 94, 1272–1321. [Google Scholar] [CrossRef]
- Aranha, P.C.D.R.; Rasmussen, L.H.; Wolf-Jäckel, G.A.; Jensen, H.M.E.; Hansen, H.C.B.; Friis, C. Fate of ptaquiloside-A bracken fern toxin-In cattle. PLoS ONE 2019, 14, e0218628. [Google Scholar] [CrossRef]
- Virgilio, A.; Sinisi, A.; Russo, V.; Gerado, S.; Santoro, A.; Galeone, A.; Taglialatela-Scafati, O.; Roperto, F. Ptaquiloside, the major carcinogen of bracken fern, in the pooled raw milk of healthy sheep and goats: An underestimated, global concern of food safety. J. Agric. Food Chem. 2015, 63, 4886–4892. [Google Scholar] [CrossRef]
- Liu, Y.; Wujisguleng, W.; Long, C. Food uses of ferns in China: A review. Acta Soc. Bot. Pol. 2012, 81, 263–270. [Google Scholar] [CrossRef]
- Tourchi-Roudsari, M. Multiple effects of bracken fern under in vivo and in vitro conditions. Asian Pac. J. Cancer Prev. 2014, 15, 7505–7513. [Google Scholar] [CrossRef]
- Alonso-Amelot, M.E.; Avendaño, M. Human carcinogenesis and bracken fern: A review of the evidence. Curr. Med. Chem. 2002, 9, 675–686. [Google Scholar] [CrossRef]
- Marliére, C.A.; Wathern, P.; Castro, M.C.F.M.; O’Connor, P.; Galvao, M.A. Bracken fern (Pteridium aquilinum) ingestion and oesophageal and stomach cancer. IARC Sci. Publ. 2002, 156, 379–380. [Google Scholar]
- IARC. Bracken fern (Pteridium aquilinum) and some of its constituents. IARC Monogr. Eval. Carcinog. Risk Chem. Hum. 1986, 40, 47–65. [Google Scholar]
- IARC. Overall evaluations of carcinogenicity: An updating of IARC Monographs volumes 1 to 42. In IARC Working Group on the Evaluation of the Carcinogenic Risk of Chemicals to Humans; WHO: Geneva, Switzerland, 1987; Volume 7, pp. 1–440. [Google Scholar]
- Ramwell, C.T.; van Beinum, W.; Rowbothan, A.; Parry, H.; Parsons, S.A.; Luo, W.; Evans, G. Ptaquiloside & Other Bracken Toxins: A Preliminary Risk Assessment. The Food and Environment Research Agency (FERA). Available online: https://www.dwi.gov.uk/research/completed-research/risk-assessment-chemical/ptaquiloside-other-bracken-toxins-a-preliminary-risk-assessment/ (accessed on 28 May 2010).
- Wilson, D.; Donaldson, L.J.; Sepai, O. Should we be frightened of bracken? A review of the evidence. J. Epidemiol. Community Health 1998, 52, 812–817. [Google Scholar] [CrossRef]
- Vetter, J. A biological hazard of our age: Bracken fern [Pteridium aquilinum (L.) Kuhn]—A review. Acta Vet. Hung. 2009, 57, 183–196. [Google Scholar] [CrossRef]
- Prakash, A.S.; Pereira, T.N.; Smith, B.L.; Shaw, G.; Seawright, A.A. Mechanism of bracken fern carcinogenesis: Evidence for H-ras activation via initial adenine alkylation by ptaquiloside. Nat. Toxins 1996, 4, 221–227. [Google Scholar] [CrossRef]
- Shahin, M.; Moore, M.R.; Worrall, S.; Smith, B.L.; Seawright, A.A.; Prakash, A.S. H-ras activation is an early event in the ptaquiloside-induced carcinogenesis: Comparison of acute and chronic toxicity in rats. Biochem. Biophys. Res. Commun. 1998, 250, 491–497. [Google Scholar] [CrossRef]
- Schubbert, S.; Shannon, K.; Bollag, G. Hyperactive Ras in developmental disorders and cancer. Nat. Rev. Cancer 2007, 7, 295–308. [Google Scholar] [CrossRef]
- Yamada, K.; Ojika, M.; Kigoshi, H. Ptaquiloside, the major toxin of bracken, and related terpene glycosides: Chemistry, biology and ecology. Nat. Prod. Rep. 2007, 24, 798–813. [Google Scholar] [CrossRef]
- Sharma, R.; Bhat, T.K.; Sharma, O.P. The environmental and human effects of ptaquiloside-induced enzootic bovine hematuria: A tumorous disease of cattle. Rev. Environ. Contam. Toxicol. 2013, 224, 53–95. [Google Scholar] [CrossRef]
- Vetter, J. The Norsesquiterpene Glycoside Ptaquiloside as a Poisonous, Carcinogenic Component of Certain Ferns. Molecules 2022, 27, 6662. [Google Scholar] [CrossRef]
- Lee, H.H.; Kim, A.G.; Lee, M.G.; Choi, S.Y.; Seo, J.J.; Kim, E.S.; Seo, K.W.; Cho, B.S. Study on Processing Methods to Remove Toxic Ptaquiloside from Bracken Fern. J. Food Hyg. Saf. 2017, 32, 217–221. [Google Scholar] [CrossRef]
- Potter, D.M.; Baird, M.S. Carcinogenic effects of ptaquiloside in bracken fern and related compounds. Br. J. Cancer 2000, 83, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Nagao, T.; Matoba, M.; Koyama, K.; Natori, S.; Murakami, T.; Saiki, Y. Chemical assay of ptaquiloside, the carcinogen of pteridium aquilinum, and the distribution of related compounds in the pteridaceae. Phytochemistry 1989, 28, 1605–1611. [Google Scholar] [CrossRef]
- Chen, L.Y.; Hu, A.; Chang, C.J. The Degradation Mechanism of Toxic Atractyloside in Herbal Medicines by Decoction. Molecules 2013, 18, 2018–2028. [Google Scholar] [CrossRef]
- EFSA. Guidance on selected default values to be used by the EFSA Scientific Committee, Scientific Panels and Units in the absence of actual measured data. EFSA J. 2012, 10, 1–32. [Google Scholar] [CrossRef]
- EFSA. Opinion of the Scientific Committee on a request from EFSA related to A Harmonised Approach for Risk Assessment of Substances Which are both Genotoxic and Carcinogenic. EFSA J. 2005, 3, 1–31. [Google Scholar] [CrossRef]
- Fürst, P.; Milana, M.R.; Pfaff, K.; Tlustos, C.; Vleminckx, C.; Arcella, D.; Barthélémy, E.; Colombo, P.; Goumperis, T.; Roldán Torres, R.; et al. Risk evaluation of chemical contaminants in food in the context of RASFF notifications. EFSA Support. Publ. 2019, 16, 1–108. [Google Scholar] [CrossRef]
- KHIDI. Amount of Intake by Food. Korea Health Industry Development Institute (KHIDI). Available online: https://www.khidi.or.kr/kps/dhraStat/result2?menuId=MENU01653&year=2018 (accessed on 14 March 2020).
- Barlow, S.; Schlatter, J. Risk assessment of carcinogens in food. Toxicol. Appl. Pharmacol. 2010, 243, 180–190. [Google Scholar] [CrossRef]
- Dybing, E.; Sanner, T.; Roelfzema, H.; Kroese, D.; Tennant, R.W. T25: A simplified carcinogenic potency index: Description of the system and study of correlations between carcinogenic potency and species/site specificity and mutagenicity. Pharmacol. Toxicol. 1997, 80, 272–279. [Google Scholar] [CrossRef]
- Gounalan, S.; Somvanshi, R.; Kumar, R.; Dash, S.; Devi, V. Clinico-pathological effects of bracken fern (Pteridium aquilinum) feeding in laboratory rats. Indian J. Anim. Sci. 1999, 69, 385–388. [Google Scholar]
- Hirono, I.; Ogino, H.; Fujimoto, M.; Yamada, K.; Yoshida, Y.; Ikagawa, M.; Okumura, M. Induction of tumors in ACI rats given a diet containing ptaquiloside, a bracken carcinogen. J. Natl. Cancer Inst. 1987, 79, 1143–1149. [Google Scholar] [CrossRef]
- Dybing, E.; O’Brien, J.; Renwick, A.G.; Sanner, T. Risk assessment of dietary exposures to compounds that are genotoxic and carcinogenic—An overview. Toxicol. Lett. 2008, 180, 110–117. [Google Scholar] [CrossRef]
- Ahmad, M.H.; Zezi, A.U.; Anafi, S.B.; Alshargi, O.Y.; Mohammed, M.; Mustapha, S.; Bala, A.A.; Muhammad, S.; Julde, S.M.; Wada, A.S.; et al. Sub-acute toxicity study on hydromethanolic leaves extract of Combretum hypopilinum (Combretaceae) Diels in Wistar rats. Toxicol. Res. 2022, 38, 487–502. [Google Scholar] [CrossRef]
- Da Silva, S.P.; da Costa, C.B.L.; de Freitas, A.F.S.; da Silva, J.D.F.; Costa, W.K.; da Silva, W.S.F.L.; Machado, J.C.B.; da Silva, S.M.S.; Ferreira, M.R.A.; Soares, L.A.L.; et al. Saline extract of Portulaca elatior leaves with photoprotective and antioxidant activities does not show acute oral and dermal toxicity in mice. Toxicol. Res. 2022. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, V.; Akhouri, V.; Kumar, R.; Ali, M.; Rashmi, T.; Chand, G.B.; Singh, S.K.; Ghosh, A.K. Protective efficacy of Coriandrum sativum seeds against arsenic induced toxicity in Swiss albino mice. Toxicol. Res. 2022, 38, 437–447. [Google Scholar] [CrossRef]
- Bai, Z.; He, Y.; Han, Z.; Wu, F. Leaching Mechanism and Health Risk Assessment of As and Sb in Tailings of Typical Antimony Mines: A Case Study in Yunnan and Guizhou Province, Southwest China. Toxics 2022, 10, 777. [Google Scholar] [CrossRef]
- Kim, K.B.; Kwack, S.J.; Lee, J.Y.; Kacew, S.; Lee, B.M. Current opinion on risk assessment of cosmetics. J. Toxicol. Environ. Health B Crit. Rev. 2021, 24, 137–161. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, J.W.; Kim, J.Y.; Park, J.H.; Park, S.H.; Kim, K.B.; Lee, B.M.; Kwon, S. Current trends in read-across applications for chemical risk assessments and chemical registrations in the Republic of Korea. J. Toxicol. Environ. Health B Crit. Rev. 2022, 25, 393–404. [Google Scholar] [CrossRef]
- Qi, H.; Liu, Y.; Li, L.; Zhao, B. Optimization of Cancer Risk Assessment Models for PM2.5-Bound PAHs: Application in Jingzhong, Shanxi, China. Toxics 2022, 10, 761. [Google Scholar] [CrossRef]
- Wang, Y.; Xin, C.; Yu, S.; Xie, Y.; Zhang, W.; Fu, R. Health Risk Assessment Based on Source Identification of Heavy Metal(loid)s: A Case Study of Surface Water in the Lijiang River, China. Toxics 2022, 10, 726. [Google Scholar] [CrossRef]
- Alonso-Amelot, M.E.; Castillo, U.; Smith, B.L.; Lauren, D.R. Bracken ptaquiloside in milk. Nature 1996, 382, 587. [Google Scholar] [CrossRef] [PubMed]
- García-Jorgensen, D.B.; Hansen, H.C.B.; Abrahamsen, P.; Diamantopoulos, E. A novel model concept for modelling the leaching of natural toxins: Results for the case of ptaquiloside. Environ. Sci. Process Impacts 2020, 22, 1768–1779. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).