Associations of Long-Term Exposure to PM2.5 and Its Constituents with Erythrocytosis and Thrombocytosis in Rural Populations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection and Definition of Outcomes
2.3. Exposure Assessment
2.4. Statistical Analysis
3. Results
3.1. Characteristics of the Participants
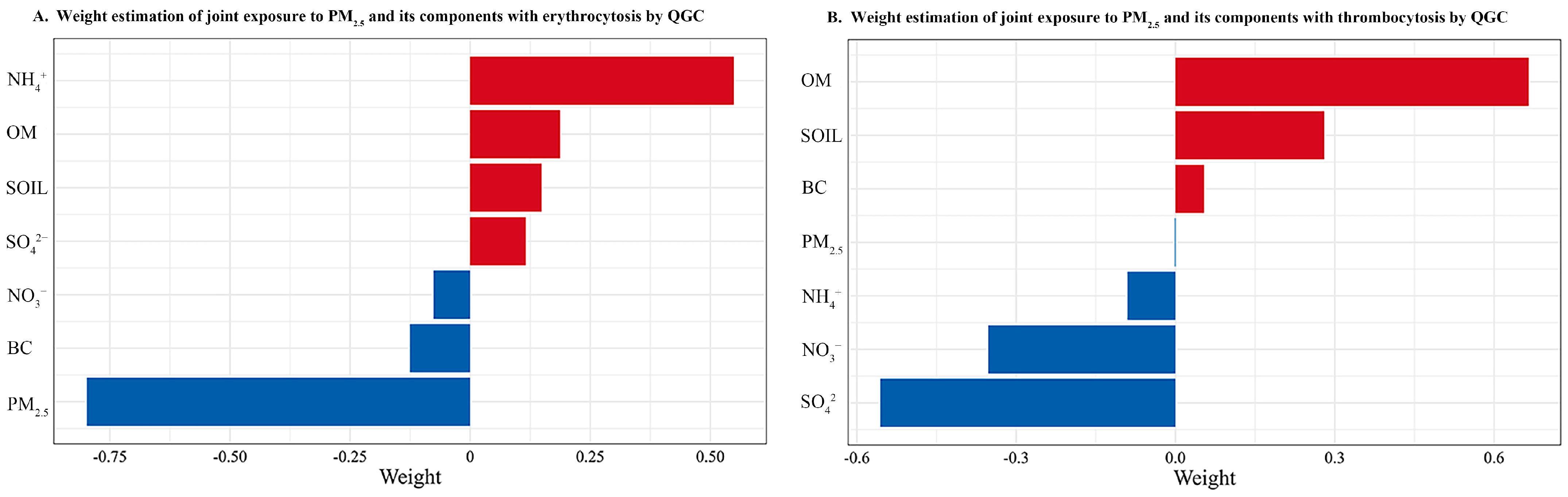
3.2. Associations of PM2.5 and Its Constituents with Erythrocytosis and Thrombocytosis
3.3. Stratified Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mithoowani, S.; Laureano, M.; Crowther, M.A.; Hillis, C.M. Investigation and management of erythrocytosis. CMAJ 2020, 192, E913–E918. [Google Scholar] [CrossRef] [PubMed]
- Schafer, A.I. Thrombocytosis. N. Engl. J. Med. 2004, 350, 1211–1219. [Google Scholar] [CrossRef]
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef] [PubMed]
- Wouters, H.; Mulder, R.; van Zeventer, I.A.; Schuringa, J.J.; van der Klauw, M.M.; van der Harst, P.; Diepstra, A.; Mulder, A.B.; Huls, G. Erythrocytosis in the general population: Clinical characteristics and association with clonal hematopoiesis. Blood Adv. 2020, 4, 6353–6363. [Google Scholar] [CrossRef] [PubMed]
- Gordeuk, V.R.; Key, N.S.; Prchal, J.T. Re-evaluation of hematocrit as a determinant of thrombotic risk in erythrocytosis. Haematologica 2019, 104, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Giannakeas, V.; Narod, S.A. Incidence of Cancer Among Adults with Thrombocytosis in Ontario, Canada. JAMA Netw. Open 2021, 4, e2120633. [Google Scholar] [CrossRef]
- Tsai, M.T.; Chen, Y.T.; Lin, C.H.; Huang, T.P.; Tarng, D.C. U-shaped mortality curve associated with platelet count among older people: A community-based cohort study. Blood 2015, 126, 1633–1635. [Google Scholar] [CrossRef]
- Cohen, A.J.; Brauer, M.; Burnett, R.; Anderson, H.R.; Frostad, J.; Estep, K.; Balakrishnan, K.; Brunekreef, B.; Dandona, L.; Dandona, R.; et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: An analysis of data from the Global Burden of Diseases Study 2015. Lancet 2017, 389, 1907–1918. [Google Scholar] [CrossRef]
- Jin, X.; Ma, Q.; Sun, Z.; Yang, X.; Zhou, Q.; Qu, G.; Liu, Q.; Liao, C.; Li, Z.; Jiang, G. Airborne Fine Particles Induce Hematological Effects through Regulating the Crosstalk of the Kallikrein-Kinin, Complement, and Coagulation Systems. Environ. Sci. Technol. 2019, 53, 2840–2851. [Google Scholar] [CrossRef]
- Mirabelli, M.C.; Vaidyanathan, A.; Flanders, W.D.; Qin, X.; Garbe, P. Outdoor PM2.5, Ambient Air Temperature, and Asthma Symptoms in the Past 14 Days among Adults with Active Asthma. Environ. Health Perspect. 2016, 124, 1882–1890. [Google Scholar] [CrossRef]
- Honda, T.; Pun, V.C.; Manjourides, J.; Suh, H. Anemia prevalence and hemoglobin levels are associated with long-term exposure to air pollution in an older population. Environ. Int. 2017, 101, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Robertson, S.; Miller, M.R. Ambient air pollution and thrombosis. Part Fibre Toxicol. 2018, 15, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Guo, T.; Guo, H.; Chen, X.; Ma, Y.; Deng, H.; Yu, H.; Chen, Q.; Li, H.; Liu, Q.; et al. Ambient particulate air pollution, blood cell parameters, and effect modification by psychosocial stress: Findings from two studies in three major Chinese cities. Environ. Res. 2022, 210, 112932. [Google Scholar] [CrossRef] [PubMed]
- Kwag, Y.; Ye, S.; Oh, J.; Lee, D.W.; Yang, W.; Kim, Y.; Ha, E. Direct and Indirect Effects of Indoor Particulate Matter on Blood Indicators Related to Anemia. Int. J. Environ. Res. Public Health 2021, 18, 12890. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Jia, X.; Cui, L.; Liu, J.; Chen, J.; Wang, Y.; Niu, W.; Xu, J.; Miller, M.R.; Loh, M.; et al. Exposure to fine particulate matter promotes platelet activation and thrombosis via obesity-related inflammation. J. Hazard Mater. 2021, 413, 125341. [Google Scholar] [CrossRef]
- Sørensen, M.; Daneshvar, B.; Hansen, M.; Dragsted, L.O.; Hertel, O.; Knudsen, L.; Loft, S. Personal PM2.5 exposure and markers of oxidative stress in blood. Environ. Health Perspect. 2003, 111, 161–166. [Google Scholar] [CrossRef]
- Liu, M.; Guo, W.; Zhao, L.; Yang, H.; Fang, Q.; Li, M.; Shu, J.; Chen, S.; Lai, X.; Yang, L.; et al. Association of personal fine particulate matter and its respiratory tract depositions with blood pressure in children: From two panel studies. J. Hazard Mater. 2021, 416, 126120. [Google Scholar] [CrossRef]
- Kang, N.; Wu, R.; Liao, W.; Zhang, C.; Liu, X.; Mao, Z.; Huo, W.; Hou, J.; Zhang, K.; Tian, H.; et al. Association of long-term exposure to PM(2.5) constituents with glucose metabolism in Chinese rural population. Sci. Total Environ. 2023, 859 Pt 2, 160364. [Google Scholar] [CrossRef]
- Wang, J.; Li, T.; Fang, J.; Tang, S.; Zhang, Y.; Deng, F.; Shen, C.; Shi, W.; Liu, Y.; Chen, C.; et al. Associations between Individual Exposure to Fine Particulate Matter Elemental Constituent Mixtures and Blood Lipid Profiles: A Panel Study in Chinese People Aged 60–69 Years. Environ. Sci. Technol. 2022, 56, 13160–13168. [Google Scholar] [CrossRef]
- Xie, G.; Yue, J.; Yang, W.; Yang, L.; Xu, M.; Sun, L.; Zhang, B.; Guo, L.; Chung, M.C. Effects of PM(2.5) and its constituents on hemoglobin during the third trimester in pregnant women. Environ. Sci. Pollut. Res. Int. 2022, 29, 35193–35203. [Google Scholar] [CrossRef]
- Wang, S.; Kaur, M.; Li, T.; Pan, F. Effect of Different Pollution Parameters and Chemical Components of PM(2.5) on Health of Residents of Xinxiang City, China. Int. J. Environ. Res. Public Health 2021, 18, 6821. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Mao, Z.; Li, Y.; Wu, W.; Zhang, X.; Huo, W.; Yu, S.; Shen, L.; Li, L.; Tu, R. Cohort Profile: The Henan Rural Cohort: A prospective study of chronic non-communicable diseases. Int. J. Epidemiol. 2019, 48, 1756-1756j. [Google Scholar] [CrossRef] [PubMed]
- Chinese Nutrition Society. The Dietary Guidelines for Chinese Residents; The Tibet people’s Publishing House: Lhasa, China, 2011; pp. 97, 197, 198. [Google Scholar]
- McMullin, M.F.; Bareford, D.; Campbell, P.; Green, A.R.; Harrison, C.; Hunt, B.; Oscier, D.; Polkey, M.I.; Reilly, J.T.; Rosenthal, E.; et al. Guidelines for the diagnosis, investigation and management of polycythaemia/erythrocytosis. Br. J. Haematol. 2005, 130, 174–195. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Martin, R.V.; van Donkelaar, A.; Boys, B.L.; Hammer, M.S.; Xu, J.W.; Marais, E.A.; Reff, A.; Strum, M.; Ridley, D.A.; et al. Trends in Chemical Composition of Global and Regional Population-Weighted Fine Particulate Matter Estimated for 25 Years. Environ. Sci. Technol. 2017, 51, 11185–11195. [Google Scholar] [CrossRef]
- Available online: http://wiki.seas.harvard.edu/geos-chem/index.php/GEOS-Chem_overview (accessed on 23 March 2023).
- Available online: http://wiki.seas.harvard.edu/geos-chem/index.php/Carbonaceous_aerosols (accessed on 24 March 2023).
- Available online: http://wiki.seas.harvard.edu/geos-chem/index.php/Mineral_dust_aerosols (accessed on 24 March 2023).
- Available online: http://wiki.seas.harvard.edu/geos-chem/index.php/Sea_salt_aerosols (accessed on 24 March 2023).
- Zhang, L.; Kok, J.F.; Henze, D.; Li, Q.; Zhao, C. Improving simulations of fine dust surface concentrations over the western United States by optimizing the particle size distribution. Geophys. Res. Letts. 2013, 40, 3270–3275. [Google Scholar] [CrossRef]
- Hammer, M.S.; van Donkelaar, A.; Li, C.; Lyapustin, A.; Sayer, A.M.; Hsu, N.C.; Levy, R.C.; Garay, M.J.; Kalashnikova, O.V.; Kahn, R.A.; et al. Global Estimates and Long-Term Trends of Fine Particulate Matter Concentrations (1998–2018). Environ. Sci. Technol. 2020, 54, 7879–7890. [Google Scholar] [CrossRef]
- Liu, C.; Cai, J.; Qiao, L.; Wang, H.; Xu, W.; Li, H.; Zhao, Z.; Chen, R.; Kan, H. The Acute Effects of Fine Particulate Matter Constituents on Blood Inflammation and Coagulation. Environ. Sci. Technol. 2017, 51, 8128–8137. [Google Scholar] [CrossRef]
- Mostofsky, E.; Schwartz, J.; Coull, B.A.; Koutrakis, P.; Wellenius, G.A.; Suh, H.H.; Gold, D.R.; Mittleman, M.A. Modeling the association between particle constituents of air pollution and health outcomes. Am. J. Epidemiol. 2012, 176, 317–326. [Google Scholar] [CrossRef]
- Keil, A.P.; Buckley, J.P.; O’Brien, K.M.; Ferguson, K.K.; Zhao, S.; White, A.J. A Quantile-Based g-Computation Approach to Addressing the Effects of Exposure Mixtures. Environ. Health Perspect. 2020, 128, 47004. [Google Scholar] [CrossRef]
- Yang, M.; Cheng, H.; Shen, C.; Liu, J.; Zhang, H.; Cao, J.; Ding, R. Effects of long-term exposure to air pollution on the incidence of type 2 diabetes mellitus: A meta-analysis of cohort studies. Environ. Sci. Pollut. Res. Int. 2020, 27, 798–811. [Google Scholar] [CrossRef]
- Yang, T.; Chen, R.; Gu, X.; Xu, J.; Yang, L.; Zhao, J.; Zhang, X.; Bai, C.; Kang, J.; Ran, P.; et al. Association of fine particulate matter air pollution and its constituents with lung function: The China Pulmonary Health study. Environ. Int. 2021, 156, 106707. [Google Scholar] [CrossRef]
- Beelen, R.; Raaschou-Nielsen, O.; Stafoggia, M.; Andersen, Z.J.; Weinmayr, G.; Hoffmann, B.; Wolf, K.; Samoli, E.; Fischer, P.; Nieuwenhuijsen, M.; et al. Effects of long-term exposure to air pollution on natural-cause mortality: An analysis of 22 European cohorts within the multicentre ESCAPE project. Lancet 2014, 383, 785–795. [Google Scholar] [CrossRef]
- Wang, S.; Wang, L.; Fan, X.; Wang, N.; Ma, S.; Zhang, R. Formation pathway of secondary inorganic aerosol and its influencing factors in Northern China: Comparison between urban and rural sites. Sci. Total Environ. 2022, 840, 156404. [Google Scholar] [CrossRef]
- Li, N.; Zhang, H.; Zhu, S.; Liao, H.; Hu, J.; Tang, K.; Feng, W.; Zhang, R.; Shi, C.; Xu, H.; et al. Secondary PM(2.5) dominates aerosol pollution in the Yangtze River Delta region: Environmental and health effects of the Clean air Plan. Environ. Int. 2023, 171, 107725. [Google Scholar] [CrossRef]
- Anenberg, S.C.; Schwartz, J.; Shindell, D.; Amann, M.; Faluvegi, G.; Klimont, Z.; Janssens-Maenhout, G.; Pozzoli, L.; Van Dingenen, R.; Vignati, E.; et al. Global air quality and health co-benefits of mitigating near-term climate change through methane and black carbon emission controls. Environ. Health Perspect. 2012, 120, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, Y.; Yang, Z.; Luo, S.; Zhang, Y. Long-term exposure to fine particulate constituents and cardiovascular diseases in Chinese adults. J. Hazard Mater. 2021, 416, 126051. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Who Publishes New Global Data on the Use of Clean and Polluting Fuels for Cooking by Fuel Type. 2022. Available online: https://www.who.int/news/item/20-01-2022-who-publishes-new-global-data-on-the-use-of-clean-and-polluting-fuels-for-cooking-by-fuel-type (accessed on 11 April 2023).
- Liao, J.; Li, Y.; Wang, X.; Zhang, B.; Xia, W.; Peng, Y.; Zhang, W.; Cao, Z.; Zhang, Y.; Liang, S.; et al. Prenatal exposure to fine particulate matter, maternal hemoglobin concentration, and fetal growth during early pregnancy: Associations and mediation effects analysis. Environ. Res. 2019, 173, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Kampfrath, T.; Maiseyeu, A.; Ying, Z.; Shah, Z.; Deiuliis, J.A.; Xu, X.; Kherada, N.; Brook, R.D.; Reddy, K.M.; Padture, N.P.; et al. Chronic fine particulate matter exposure induces systemic vascular dysfunction via NADPH oxidase and TLR4 pathways. Circ. Res. 2011, 108, 716–726. [Google Scholar] [CrossRef]
- Beaulieu, L.M.; Freedman, J.E. The role of inflammation in regulating platelet production and function: Toll-like receptors in platelets and megakaryocytes. Thromb. Res. 2010, 125, 205–209. [Google Scholar] [CrossRef]
- Hou, J.; Duan, Y.; Liu, X.; Li, R.; Tu, R.; Pan, M.; Dong, X.; Mao, Z.; Huo, W.; Chen, G.; et al. Associations of long-term exposure to air pollutants, physical activity and platelet traits of cardiovascular risk in a rural Chinese population. Sci. Total Environ. 2020, 738, 140182. [Google Scholar] [CrossRef]
- Lim, S.; Bassey, E.; Bos, B.; Makacha, L.; Varaden, D.; Arku, R.E.; Baumgartner, J.; Brauer, M.; Ezzati, M.; Kelly, F.J.; et al. Comparing human exposure to fine particulate matter in low and high-income countries: A systematic review of studies measuring personal PM(2.5) exposure. Sci. Total Environ. 2022, 833, 155207. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Xu, R.; Gao, C.X.; Yu, W.; Zhang, Y.; Han, K.; Yu, P.; Guo, Y.; Li, S. Socioeconomic disparity in the association between long-term exposure to PM(2.5) and mortality in 2640 Chinese counties. Environ. Int. 2021, 146, 106241. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Guo, Y.; Cai, M.; Qian, Z.M.; Zhang, S.; Zhang, Z.; Yang, Y.; Vaughn, M.G.; Aaron, H.E.; Wu, F.; et al. Constituents of fine particulate matter and asthma in 6 low- and middle-income countries. J. Allergy Clin. Immunol. 2022, 150, 214–222.e5. [Google Scholar] [CrossRef] [PubMed]

| Variables | Erythrocytosis | Non-Erythrocytosis | p-Value | Thrombocytosis | Non-Thrombocytosis | p-Value |
|---|---|---|---|---|---|---|
| (n = 3499) | (n = 30,086) | (n = 333) | (n = 33,252) | |||
| Age (year), mean ± SD | 52.68 ± 13.06 | 55.58 ± 12.15 | <0.001 | 52.54 ± 12.54 | 55.31 ± 12.28 | <0.001 |
| Gender, n (%) | <0.001 | <0.001 | ||||
| Men | 2926 (83.62) | 10,648 (35.39) | 56 (16.82) | 13,518 (40.65) | ||
| Women | 573 (16.38) | 19,438 (64.61) | 277 (83.18) | 19,734 (59.35) | ||
| Marital status, n (%) | 0.021 | 0.897 | ||||
| Married/cohabiting | 3194 (91.28) | 27,093 (90.05) | 301 (90.39) | 29,986 (90.18) | ||
| Unmarried/divorced/widowed | 305 (8.72) | 2993 (9.95) | 32 (9.61) | 3266 (9.82) | ||
| Education levels, n (%) | <0.001 | 0.172 | ||||
| Primary school or below | 1048 (29.95) | 13,634 (45.32) | 149 (44.74) | 14,533 (43.71) | ||
| Junior high school | 1623 (46.38) | 11,798 (39.21) | 142 (42.64) | 13,279 (39.93) | ||
| Senior high school or above | 828 (23.67) | 4654 (15.47) | 42 (12.62) | 5440 (16.36) | ||
| Per capita monthly income, n (%) | <0.001 | 0.467 | ||||
| <500 RMB | 1111 (31.75) | 10,772 (35.80) | 122 (36.64) | 11,761 (35.37) | ||
| 500–999 RMB | 1143 (32.67) | 9789 (32.54) | 98 (29.43) | 10,834 (32.58) | ||
| ≥1000 RMB | 1245 (35.58) | 9525 (31.66) | 113 (33.93) | 10,657 (32.05) | ||
| Smoking status, n (%) | <0.001 | <0.001 | ||||
| Never | 1384 (39.56) | 22,851 (75.95) | 287 (86.19) | 23,948 (72.02) | ||
| Ever | 513 (14.66) | 2151 (7.15) | 20 (6.01) | 2644 (7.95) | ||
| Current | 1602 (45.78) | 5084 (16.90) | 26 (7.80) | 6660 (20.03) | ||
| Drinking status, n (%) | <0.001 | <0.001 | ||||
| Never | 1756 (50.18) | 24,183 (80.38) | 301 (90.39) | 25,638 (77.1) | ||
| Ever | 276 (7.89) | 1310 (4.35) | 5 (1.50) | 1581 (4.75) | ||
| Current | 1467 (41.93) | 4593 (15.27) | 27 (8.11) | 6033 (18.14) | ||
| Physical activity, n (%) | <0.001 | 0.013 | ||||
| Low | 1353 (38.67) | 9368 (31.14) | 129 (38.74) | 10,592 (31.85) | ||
| Moderate | 1065 (30.44) | 11,611 (38.59) | 104 (31.23) | 12,572 (37.81) | ||
| High | 1081 (30.89) | 9107 (30.27) | 100 (30.03) | 10,088 (30.34) | ||
| High fat diet, n (%) | 898 (25.66) | 5388 (17.91) | <0.001 | 46 (13.81) | 6240 (18.77) | 0.021 |
| Adequate vegetable and fruit intake, n (%) | 1363 (38.95) | 13,716 (45.59) | <0.001 | 129 (38.74) | 14,950 (44.96) | 0.023 |
| BMI (kg/m2, mean ± SD) | 25.44 ± 3.47 | 24.71 ± 3.56 | <0.001 | 25.79 ± 3.43 | 24.78 ± 3.56 | <0.001 |
| HGB (g/L, mean ± SD) | 166.86 ± 16.92 | 135.17 ± 14.62 | <0.001 | 119.90 ± 24.77 | 138.66 ± 17.57 | <0.001 |
| HCT (%, mean ± SD) | 52.00 ± 4.94 | 42.10 ± 3.89 | <0.001 | 39.03 ± 6.47 | 43.17 ± 4.99 | <0.001 |
| PLT (109/L, mean ± SD) | 206.62 ± 64.60 | 216.15 ± 71.75 | <0.001 | 437.78 ± 28.40 | 212.92 ± 67.80 | <0.001 |
| PM2.5 (μg/m3, mean ± SD) | 77.69 ± 8.99 | 73.50 ± 9.66 | <0.001 | 77.75 ± 8.77 | 73.90 ± 9.68 | <0.001 |
| Constituents | ||||||
| BC (μg/m3, mean ± SD) | 5.48 ± 0.92 | 5.05 ± 0.98 | <0.001 | 5.52 ± 0.89 | 5.09 ± 0.98 | <0.001 |
| NH4+ (μg/m3, mean ± SD) | 10.95 ± 1.31 | 10.36 ± 1.35 | <0.001 | 10.85 ± 1.22 | 10.42 ± 1.36 | <0.001 |
| NO3− (μg/m3, mean ± SD) | 18.49 ± 2.37 | 17.43 ± 2.50 | <0.001 | 18.40 ± 2.28 | 17.54 ± 2.51 | <0.001 |
| OM (μg/m3, mean ± SD) | 16.22 ± 1.62 | 15.55 ± 1.90 | <0.001 | 16.49 ± 1.81 | 15.61 ± 1.88 | <0.001 |
| SO42− (μg/m3, mean ± SD) | 14.95 ± 1.70 | 14.19 ± 1.71 | <0.001 | 14.74 ± 1.57 | 14.26 ± 1.73 | <0.001 |
| SOIL (μg/m3, mean ± SD) | 10.31 ± 1.67 | 9.69 ± 1.69 | <0.001 | 10.57 ± 1.63 | 9.75 ± 1.70 | <0.001 |
| Variables | Mean | SD | Minimum | Median | Maximum | IQR |
|---|---|---|---|---|---|---|
| PM2.5 (μg/m3) | 73.93 | 9.67 | 48.49 | 77.87 | 94.09 | 17.61 |
| Constituents | ||||||
| BC (μg/m3) | 5.09 | 0.98 | 3.11 | 5.49 | 7.11 | 2.00 |
| NH4+ (μg/m3) | 10.42 | 1.35 | 6.74 | 10.68 | 13.65 | 2.21 |
| NO3− (μg/m3) | 17.54 | 2.51 | 9.97 | 18.89 | 23.41 | 3.82 |
| OM (μg/m3) | 15.61 | 1.88 | 8.74 | 15.38 | 19.74 | 3.00 |
| SO42− (μg/m3) | 14.26 | 1.72 | 10.33 | 13.86 | 18.36 | 3.07 |
| SOIL (μg/m3) | 9.75 | 1.70 | 6.94 | 8.96 | 14.03 | 3.56 |
| Variables | Single-Pollutant Model a | Constituent-PM2.5 Model b | Constituent Residual Model c |
|---|---|---|---|
| Erythrocytosis | |||
| PM2.5 | 1.049 (1.044, 1.054) | ||
| Constituents | |||
| BC | 1.598 (1.528, 1.671) | 1.072 (0.852, 1.348) | 1.054 (0.839, 1.325) |
| NH4+ | 1.407 (1.363, 1.452) | 1.317 (1.149, 1.508) | 1.521 (1.318, 1.756) |
| NO3− | 1.206 (1.185, 1.227) | 1.137 (1.058, 1.220) | 1.125 (1.044, 1.211) |
| OM | 1.230 (1.199, 1.261) | 0.818 (0.771, 0.869) | 0.777 (0.736, 0.821) |
| SO42− | 1.296 (1.265, 1.328) | 1.152 (1.059, 1.254) | 1.310 (1.202, 1.428) |
| SOIL | 1.196 (1.168, 1.224) | 0.979 (0.946, 1.013) | 0.980 (0.947, 1.015) |
| Thrombocytosis | |||
| PM2.5 | 1.040 (1.027, 1.054) | ||
| Constituents | |||
| BC | 1.568 (1.372, 1.792) | 4.316 (2.259, 8.246) | 4.053 (2.216, 7.412) |
| NH4+ | 1.220 (1.118, 1.332) | 0.228 (0.141, 0.369) | 0.158 (0.092, 0.272) |
| NO3− | 1.134 (1.080, 1.191) | 0.729 (0.594, 0.895) | 0.692 (0.559, 0.856) |
| OM | 1.346 (1.245, 1.456) | 1.680 (1.416, 1.992) | 1.547 (1.316, 1.819) |
| SO42− | 1.135 (1.061, 1.214) | 0.433 (0.346, 0.542) | 0.434 (0.347, 0.541) |
| SOIL | 1.292 (1.205, 1.385) | 1.232 (1.114, 1.364) | 1.257 (1.134, 1.392) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Y.; He, Y.; Kang, N.; Zhang, C.; Liao, W.; Yuchi, Y.; Liu, X.; Hou, J.; Mao, Z.; Huo, W.; et al. Associations of Long-Term Exposure to PM2.5 and Its Constituents with Erythrocytosis and Thrombocytosis in Rural Populations. Toxics 2023, 11, 885. https://doi.org/10.3390/toxics11110885
Zheng Y, He Y, Kang N, Zhang C, Liao W, Yuchi Y, Liu X, Hou J, Mao Z, Huo W, et al. Associations of Long-Term Exposure to PM2.5 and Its Constituents with Erythrocytosis and Thrombocytosis in Rural Populations. Toxics. 2023; 11(11):885. https://doi.org/10.3390/toxics11110885
Chicago/Turabian StyleZheng, Yiquan, Yaling He, Ning Kang, Caiyun Zhang, Wei Liao, Yinghao Yuchi, Xiaotian Liu, Jian Hou, Zhenxing Mao, Wenqian Huo, and et al. 2023. "Associations of Long-Term Exposure to PM2.5 and Its Constituents with Erythrocytosis and Thrombocytosis in Rural Populations" Toxics 11, no. 11: 885. https://doi.org/10.3390/toxics11110885
APA StyleZheng, Y., He, Y., Kang, N., Zhang, C., Liao, W., Yuchi, Y., Liu, X., Hou, J., Mao, Z., Huo, W., Zhang, K., Tian, H., Lin, H., & Wang, C. (2023). Associations of Long-Term Exposure to PM2.5 and Its Constituents with Erythrocytosis and Thrombocytosis in Rural Populations. Toxics, 11(11), 885. https://doi.org/10.3390/toxics11110885