Investigation of the Relationship between Spontaneous Abortion, Serum Pesticides, and Polychlorinated Biphenyl Levels
Abstract
:1. Introduction
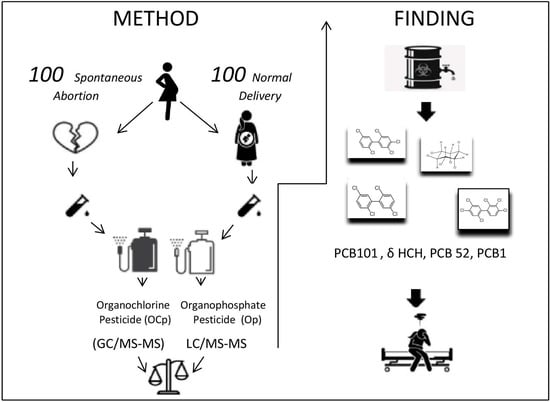
2. Materials and Methods
2.1. Study Design
2.2. Inclusion and Exclusion Criteria for Study Groups
2.3. Study Protocol
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rossen, L.M.; Ahrens, K.A.; Branum, A.M. Trends in Risk of Pregnancy Loss Among US Women, 1990–2011. Paediatr. Perinat. Epidemiol. 2018, 32, 19–29. [Google Scholar] [CrossRef] [PubMed]
- La, X.; Wang, W.; Zhang, M.; Liang, L. Definition and Multiple Factors of Recurrent Spontaneous Abortion. Adv. Exp. Med. Biol. 2021, 1300, 231–257. [Google Scholar] [CrossRef] [PubMed]
- Griebel, C.P.; Halvorsen, J.; Golemon, T.B.; Day, A.A. Management of spontaneous abortion. Am. Fam. Physician 2005, 72, 1243–1250. [Google Scholar] [PubMed]
- Mazziotta, C.; Pellielo, G.; Tognon, M.; Martini, F.; Rotondo, J.C. Significantly Low Levels of IgG Antibodies Against Oncogenic Merkel Cell Polyomavirus in Sera from Females Affected by Spontaneous Abortion. Front. Microbiol. 2021, 12, 789991. [Google Scholar] [CrossRef]
- Carlsson, I.; Breding, K.; Larsson, P.G. Complications related to induced abortion: A combined retrospective and longitudinal follow-up study. BMC Women’s Health 2018, 18, 158. [Google Scholar] [CrossRef]
- Tudi, M.; Daniel Ruan, H.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D.T. Agriculture Development, Pesticide Application and Its Impact on the Environment. Int. J. Environ. Res. Public Health 2021, 18, 1112. [Google Scholar] [CrossRef]
- Altıkat, A.; Turan, T.; Torun, F.E.; Bingül, Z. Türkiye’de pestisit kullanımı ve çevreye olan etkileri. Atatürk Üniv. Ziraat Fakültesi Derg. 2009, 40, 87–92. [Google Scholar]
- Zhu, W.; Zheng, H.; Liu, J.; Cai, J.; Wang, G.; Li, Y.; Shen, H.; Yang, J.; Wang, X.; Wu, J.; et al. The correlation between chronic exposure to particulate matter and spontaneous abortion: A meta-analysis. Chemosphere 2022, 286, 131802. [Google Scholar] [CrossRef]
- Foster, W.G.; Gannon, A.-M. Reproductive Toxicity of Environmental Contaminants in the Female. In Encyclopedia of Reproduction, 2nd ed.; Skinner, M.K., Ed.; Academic Press: Oxford, UK, 2018; pp. 702–706. [Google Scholar] [CrossRef]
- Llop, S.; Ballester, F.; Vizcaino, E.; Murcia, M.; Lopez-Espinosa, M.J.; Rebagliato, M.; Vioque, J.; Marco, A.; Grimalt, J.O. Concentrations and determinants of organochlorine levels among pregnant women in Eastern Spain. Sci. Total Environ. 2010, 408, 5758–5767. [Google Scholar] [CrossRef]
- Prahl, M.; Odorizzi, P.; Gingrich, D.; Muhindo, M.; McIntyre, T.; Budker, R.; Jagannathan, P.; Farrington, L.; Nalubega, M.; Nankya, F.; et al. Exposure to pesticides in utero impacts the fetal immune system and response to vaccination in infancy. Nat. Commun. 2021, 12, 132. [Google Scholar] [CrossRef]
- Jaacks, L.M.; Diao, N.; Calafat, A.M.; Ospina, M.; Mazumdar, M.; Ibne Hasan, M.O.S.; Wright, R.; Quamruzzaman, Q.; Christiani, D.C. Association of prenatal pesticide exposures with adverse pregnancy outcomes and stunting in rural Bangladesh. Environ. Int. 2019, 133, 105243. [Google Scholar] [CrossRef] [PubMed]
- Kalliora, C.; Mamoulakis, C.; Vasilopoulos, E.; Stamatiades, G.A.; Kalafati, L.; Barouni, R.; Karakousi, T.; Abdollahi, M.; Tsatsakis, A. Association of pesticide exposure with human congenital abnormalities. Toxicol. Appl. Pharmacol. 2018, 346, 58–75. [Google Scholar] [CrossRef]
- Pascale, A.; Laborde, A. Impact of pesticide exposure in childhood. Rev. Environ. Health 2020, 35, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Settimi, L.; Spinelli, A.; Lauria, L.; Miceli, G.; Pupp, N.; Angotzi, G.; Fedi, A.; Donati, S.; Miligi, L.; Osborn, J. Spontaneous abortion and maternal work in greenhouses. Am. J. Ind. Med. 2008, 51, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Lu, T.; Hu, Y.; Duan, J.; Guan, Y.; Huang, X.; Zhou, J.; Huang, R.; Tang, M.; Sun, R.; et al. A metabolomic study on the effect of prenatal exposure to Benzophenone-3 on spontaneous fetal loss in mice. Ecotoxicol. Environ. Saf. 2022, 233, 113347. [Google Scholar] [CrossRef] [PubMed]
- He, Q.L.; Zhang, L.; Liu, S.Z. Effects of Polychlorinated Biphenyls on Animal Reproductive Systems and Epigenetic Modifications. Bull. Environ. Contam. Toxicol. 2021, 107, 398–405. [Google Scholar] [CrossRef]
- Björvang, R.D.; Hassan, J.; Stefopoulou, M.; Gemzell-Danielsson, K.; Pedrelli, M.; Kiviranta, H.; Rantakokko, P.; Ruokojärvi, P.; Lindh, C.H.; Acharya, G.; et al. Persistent organic pollutants and the size of ovarian reserve in reproductive-aged women. Environ. Int. 2021, 155, 106589. [Google Scholar] [CrossRef]
- Erdfelder, E.; Faul, F.; Buchner, A. GPOWER: A general power analysis program. Behav. Res. Methods Instrum. Comput. 1996, 28, 1–11. [Google Scholar] [CrossRef]
- Porta, M.; Pumarega, J.; Gasull, M. Number of persistent organic pollutants detected at high concentrations in a general population. Environ. Int. 2012, 44, 106–111. [Google Scholar] [CrossRef]
- Jain, D.; Kumar Verma, R.; Sharma, V.; Kaur, A.; Rai, A.R.; Kumari, P.; Nagar, V.; Singh Sankhla, M.; Parihar, K. Associations between high levels pesticide and adverse reproductive outcomes in females: A comprehensive review. Mater. Today Proc. 2023, in press. [Google Scholar] [CrossRef]
- Addissie, Y.A.; Kruszka, P.; Troia, A.; Wong, Z.C.; Everson, J.L.; Kozel, B.A.; Lipinski, R.J.; Malecki, K.M.C.; Muenke, M. Prenatal exposure to pesticides and risk for holoprosencephaly: A case-control study. Environ. Health 2020, 19, 65. [Google Scholar] [CrossRef]
- El-Baz, M.A.H.; Amin, A.F.; Mohany, K.M. Exposure to pesticide components causes recurrent pregnancy loss by increasing placental oxidative stress and apoptosis: A case-control study. Sci. Rep. 2023, 13, 9147. [Google Scholar] [CrossRef] [PubMed]
- Aktar, W.; Sengupta, D.; Chowdhury, A. Impact of pesticides use in agriculture: Their benefits and hazards. Interdiscip. Toxicol. 2009, 2, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kapeleka, J.A.; Sauli, E.; Sadik, O.; Ndakidemi, P.A. Biomonitoring of Acetylcholinesterase (AChE) Activity among Smallholder Horticultural Farmers Occupationally Exposed to Mixtures of Pesticides in Tanzania. J. Environ. Public Health 2019, 2019, 3084501. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Jaiswar, S.P.; Ansari, N.G.; Deo, S.; Sankhwar, P.; Pant, S.; Upadhyay, S. Pesticide Risk and Recurrent Pregnancy Loss in Females of Subhumid Region of India. Niger. Med. J. 2020, 61, 55–59. [Google Scholar] [CrossRef]
- Arbuckle, T.E.; Lin, Z.; Mery, L.S. An exploratory analysis of the effect of pesticide exposure on the risk of spontaneous abortion in an Ontario farm population. Environ. Health Perspect. 2001, 109, 851–857. [Google Scholar] [CrossRef]
- Rita, P.; Reddy, P.P.; Reddy, S.V. Monitoring of workers occupationally exposed to pesticides in grape gardens of andhra pradesh. Environ. Res. 1987, 44, 1–5. [Google Scholar] [CrossRef]
- Piazza, M.J.; Urbanetz, A.A. Environmental toxins and the impact of other endocrine disrupting chemicals in women’s reproductive health. JBRA Assist. Reprod. 2019, 23, 154–164. [Google Scholar] [CrossRef]
- Öztekin, O.; Köken, R.; Bulut, S.; Alpay, F. Quantification of Pesticides Levels in Mother Milk in the City of Afyonkarahisar and Its Epidemiological Influence. Turk. Klin. J. Pediatr. 2011, 20, 113–118. [Google Scholar]
- Korrick, S.A.; Chen, C.; Damokosh, A.I.; Ni, J.; Liu, X.; Cho, S.-I.; Altshul, L.; Ryan, L.; Xu, X. Association of DDT with spontaneous abortion: A case-control study. Ann. Epidemiol. 2001, 11, 491–496. [Google Scholar] [CrossRef]
- Longnecker, M.P.; Klebanoff, M.A.; Dunson, D.B.; Guo, X.; Chen, Z.; Zhou, H.; Brock, J.W. Maternal serum level of the DDT metabolite DDE in relation to fetal loss in previous pregnancies. Environ. Res. 2005, 97, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Ruan, F.; Liu, C.; Hu, W.; Ruan, J.; Ding, X.; Zhang, L.; Yang, C.; Zuo, Z.; He, C.; Huang, J. Early life PCB138 exposure induces kidney injury secondary to hyperuricemia in male mice. Environ. Pollut. 2022, 301, 118977. [Google Scholar] [CrossRef] [PubMed]
- Montano, L.; Pironti, C.; Pinto, G.; Ricciardi, M.; Buono, A.; Brogna, C.; Venier, M.; Piscopo, M.; Amoresano, A.; Motta, O. Polychlorinated Biphenyls (PCBs) in the Environment: Occupational and Exposure Events, Effects on Human Health and Fertility. Toxics 2022, 10, 365. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, T.; Fréour, T.; Duval, G.; Ploteau, S.; Marchand, P.; Le Bizec, B.; Antignac, J.-P.; Cano-Sancho, G. Associations between internal concentrations of fluorinated and organochlorinated chemicals in women and in vitro fertilization outcomes: A multi-pollutant study. Environ. Pollut. 2022, 313, 120087. [Google Scholar] [CrossRef]
- Meng, Y.; Zhong, K.; Chen, S.; Huang, Y.; Wei, Y.; Wu, J.; Liu, J.; Xu, Z.; Guo, J.; Liu, F.; et al. Cardiac toxicity assessment of pendimethalin in zebrafish embryos. Ecotoxicol. Environ. Saf. 2021, 222, 112514. [Google Scholar] [CrossRef]
- El-Sharkawy, N.I.; Reda, R.M.; El-Araby, I.E. Assessment of Stomp®(Pendimethalin) toxicity on Oreochromis niloticus. J. Am. Sci. 2011, 7, 568–576. [Google Scholar]
- Giglio, A.; Vommaro, M.L. Dinitroaniline herbicides: A comprehensive review of toxicity and side effects on animal non-target organisms. Environ. Sci. Pollut. Res. Int. 2022, 29, 76687–76711. [Google Scholar] [CrossRef]
- Hou, L.; Lee, W.J.; Rusiecki, J.; Hoppin, J.A.; Blair, A.; Bonner, M.R.; Lubin, J.H.; Samanic, C.; Sandler, D.P.; Dosemeci, M.; et al. Pendimethalin exposure and cancer incidence among pesticide applicators. Epidemiology 2006, 17, 302–307. [Google Scholar] [CrossRef]
- Li, Y.; Macdonald, R. Sources and pathways of selected organochlorine pesticides to the Arctic and the effect of pathway divergence on HCH trends in biota: A review. Sci. Total Environ. 2005, 342, 87–106. [Google Scholar] [CrossRef]
- Agrahari, A.; Singh, A.; Srivastava, A.; Jha, R.R.; Patel, D.K.; Yadav, S.; Srivastava, V.; Parmar, D. Overexpression of cerebral cytochrome P450s in prenatally exposed offspring modify the toxicity of lindane in rechallenged offspring. Toxicol. Appl. Pharmacol. 2019, 371, 20–37. [Google Scholar] [CrossRef]
- Ben Mukiibi, S.; Nyanzi, S.A.; Kwetegyeka, J.; Olisah, C.; Taiwo, A.M.; Mubiru, E.; Tebandeke, E.; Matovu, H.; Odongo, S.; Abayi, J.J.M.; et al. Organochlorine pesticide residues in Uganda’s honey as a bioindicator of environmental contamination and reproductive health implications to consumers. Ecotoxicol. Environ. Saf. 2021, 214, 112094. [Google Scholar] [CrossRef]
- Pathak, R.; Mustafa, M.D.; Ahmed, R.S.; Tripathi, A.K.; Guleria, K.; Banerjee, B.D. Association between recurrent miscarriages and organochlorine pesticide levels. Clin. Biochem. 2010, 43, 131–135. [Google Scholar] [CrossRef]
- Arbuckle, T.E.; Savitz, D.A.; Mery, L.S.; Curtis, K.M. Exposure to Phenoxy Herbicides and the Risk of Spontaneous Abortion. Epidemiology 1999, 10, 752–760. [Google Scholar] [CrossRef]
- Zhang, X.; Fan, J.; Chen, Y.; Wang, J.; Song, Z.; Zhao, J.; Li, Z.; Wu, X.; Hu, Y. Cytogenetic Analysis of the Products of Conception after Spontaneous Abortion in the First Trimester. Cytogenet. Genome Res. 2021, 161, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Öztekin, L. Şeftali ve Şeftali Sularında Bazı Organik Fosforlu ve Bromlu Pestisit Kalıntılarının Saptanması. Ph.D. Dissertation, Bursa Uludag University, Bursa, Turkey, 2005. [Google Scholar]
- Nguyen Dang Giang, C.; Le, D.B.C.; Nguyen, V.H.; Hoang, T.L.; Tran, T.V.T.; Huynh, T.P.L.; Nguyen, T.Q.T. Assessment of pesticide use and pesticide residues in vegetables from two provinces in Central Vietnam. PLoS ONE 2022, 17, e0269789. [Google Scholar] [CrossRef]
- Çatak, H.; Polat, B.; Tiryaki, O. Farklı yıkama uygulamaları ile kapya biberlerde pirimiphos-methyl kalıntısının giderilmesi. Anadolu Tarım Bilim. Derg. 2020, 35, 97–105. [Google Scholar] [CrossRef]
- Yang, S.-J.; Mun, S.; Kim, H.J.; Han, S.J.; Kim, D.W.; Cho, B.-S.; Kim, A.G.; Park, D.W. Effectiveness of Different Washing Strategies on Pesticide Residue Removal: The First Comparative Study on Leafy Vegetables. Foods 2022, 11, 2916. [Google Scholar] [CrossRef] [PubMed]

| Variables | The Group with Spontaneous Abortions (n = 100) | The Group with Normal Deliveries (n = 100) | p Value |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| Age | 27.73 ± 4.5 | 27.99 ± 4.5 | 0.5 |
| Weight | 68.6 ± 8.0 | 70.5 ± 9.2 | 0.2 |
| BMI (kg/m2) | 25.9 ± 2.3 | 26.8 ± 3.2 | 0.021 |
| Total Number of Pregnancies | 3.01 ± 1.09 | 2.67 ± 0.5 | 0.027 |
| Knowledge Level | 12.3 ± 2.3 | 11.94 ± 2.6 | 0.3 |
| Total Number of Chemicals Identified | 5.05 ± 1.97 | 4.1 ± 2.06 | 0.007 |
| Descriptive Characteristics | Groups | p Value | |
|---|---|---|---|
| The Group with Spontaneous Abortions | The Group with Normal Deliveries | ||
| Settlements Near Agricultural Lands (n/%) | 49 (62.8%) * | 50 (42%) | p < 0.004 |
| Settlements Far from Agricultural Lands (n/%) | 29 (37.2%) | 69 (58%) | |
| Pesticide Exposure History | 69 (42.9%) | 31 (79.5%) | p < 0.000 |
| 92 (57.1%) | 8 (20.5%) | ||
| Educational Status (n/%) | Literate 2 (2%) | Literate 4 (4%) | p > 0.05 |
| Primary school graduate 43 (43%) | Primary school graduate 34 (34%) | ||
| Secondary/high school graduate 52 (52%) | Secondary/high school graduate 55 (55%) | ||
| Undergraduate education and above 3(3%) | Undergraduate education and above 7 (7%) | ||
| Spontaneous Abortion Before 12 weeks Spontaneous Abortion After 12 weeks | 73(73%) 27(27%) | ||
| Birth Weight | - | <2500 g- 5 (%5) 2500–3000 g- 6 (%6) 3000–3500 g- 50 (%50) >3500 g- 39 (%39) | |
| Total | 100 | 100 | |
| OC Pesticides– PCBs | The Group with Spontaneous Abortions | The Group with Normal Deliveries | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD (ng/mL) | 25% | 50% | 75% | Mean ± SD (ng/mL) | 25% | 50% | 75% | p Value | |
| γ-HCH | 51.5 ± 42.5 | 25.0 | 30.8 | 57.3 | 32.6 ± 44.7 | 0.0 | 21.8 | 37.3 | 0.799 |
| β-HCH | 7.8 ±2.9 | 5.2 | 7.7 | 10.6 | 8.6 ± 3.0 | 6.4 | 6.7 | 11.9 | 0.327 |
| δ-HCH | 31.7 ± 26.6 | 7.8 | 33.9 | 54.5 | 35.4 ± 57.9 | 7.3 | 11.1 | 75.7 | 0.754 |
| PCB-52 | 20.8 ± 26.1 | 5.7 | 10.0 | 28.5 | 13.5 ± 11.9 | 6.4 | 9.7 | 22.6 | 1.0 |
| PCB-28 | 10.5 ± 5.5 | 7.4 | 9.1 | 11.8 | 8.8 ± 5.0 | 7.0 | 8.9 | 10.1 | 0.39 |
| PCB-118 | 18.7 ± 14.2 | 9.1 | 11.6 | 31.8 | 29.0 ± 46.1 | 7.2 | 14.2 | 25.4 | 1.0 |
| PCB-101 | 11.5 ± 5.4 | 8.1 | 9.5 | 14.4 | 5.5 ± 0.7 | 5.5 | 5.0 | - | 0.059 |
| PCB-153 | 122 ± 72.6 | 50 | 135 | 192 | 169 ± 74.2 | 94.7 | 194 | 232 | 0.291 |
| PCB-138 | 11.3 ± 6.7 | 6.1 | 10.5 | 14.1 | 13.1 ± 8.2 | 7.0 | 8.6 | 20.4 | 0.590 |
| PCB-180 | 11.1 ± 8.5 | 6.4 | 8.4 | 11.8 | 8.1 ± 2.9 | 5.9 | 7.4 | 9.3 | 0.252 |
| PCB-202 | 7.9 ± 2.2 | 6.4 | 7.2 | 9.8 | 9.6 ± 2.8 | 7.0 | 9.9 | 12.0 | 0.123 |
| op′-DDE | 26.7 ± 32.8 | 9.7 | 16.31 | 29.1 | 27.8 ± 12.4 | 20.3 | 25.5 | 31.7 | 0.128 |
| op′-DDT | 61.6 ± 27.7 | 42.0 | 61.6 | 0 | 19.4 ± 9.7 | 10.9 | 18.8 | 28.5 | 0.064 |
| op′-DDD | 22.5 ± 15.9 | 11.5 | 17.5 | 31.6 | 34.6 ± 32.7 | 11.8 | 23.6 | 43.4 | 0.468 |
| pp′-DDE | 30.7 | 30.7 | 30.7 | 30.7 | 6.1 | 6.1 | 6.1 | 6.1 | 0.317 |
| pp′-DDT | 37.39 ± 45.7 | 12.8 | 19.4 | 42.5 | 59.5 ± 44.3 | 21.2 | 42.4 | 79.6 | 0.053 |
| pp′-DDD | 52.1 ± 57.2 | 14.5 | 26.2 | 78.1 | 66.6 ± 69.0 | 11.7 | 39.2 | 103.6 | 0.574 |
| Pesticides and PCBs | Groups | n | % | ERR * | p Value ** |
|---|---|---|---|---|---|
| γ-HCH | The group with spontaneous abortions | 29 | 67.4% | 0.62 | 0.01 |
| The group with normal deliveries | 14 | 32.6% | |||
| PCB-52 | The group with spontaneous abortions | 18 | 69.2% | 0.39 | 0.036 |
| The group with normal deliveries | 8 | 30.8% | |||
| PCB-101 | The group with spontaneous abortions | 13 | 86.7% | 0.13 | 0.003 |
| The group with normal deliveries | 2 | 14.3% | |||
| PCB-138 | The group with spontaneous abortions | 26 | 63.4% | 0.50 | 0.05 |
| The group with normal deliveries | 15 | 36.6% | |||
| Pentimanthalin | The group with spontaneous abortions | 0 | - | 0.013 | |
| The group with normal deliveries | 6 | 6% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akgöl, J.; Kanat Pektaş, M. Investigation of the Relationship between Spontaneous Abortion, Serum Pesticides, and Polychlorinated Biphenyl Levels. Toxics 2023, 11, 884. https://doi.org/10.3390/toxics11110884
Akgöl J, Kanat Pektaş M. Investigation of the Relationship between Spontaneous Abortion, Serum Pesticides, and Polychlorinated Biphenyl Levels. Toxics. 2023; 11(11):884. https://doi.org/10.3390/toxics11110884
Chicago/Turabian StyleAkgöl, Jale, and Mine Kanat Pektaş. 2023. "Investigation of the Relationship between Spontaneous Abortion, Serum Pesticides, and Polychlorinated Biphenyl Levels" Toxics 11, no. 11: 884. https://doi.org/10.3390/toxics11110884