Health Risk in a Geographic Area of Thailand with Endemic Cadmium Contamination: Focus on Albuminuria
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Collection and Analysis of Blood and Urine Samples
2.3. Estimated Glomerular Filtration Rates (eGFRs)
2.4. Normalization of ECd to Ecr and Ccr
2.5. Benchmark Dose Computation and Benchmark Response (BMR) Setting
2.6. Statistical Analysis
3. Results
3.1. Study Subjects Stratified by Residential Location
3.2. Study Subjects Stratified by Sex and eGFR
3.3. Multiple Regression Analysis of Albumin Excretion
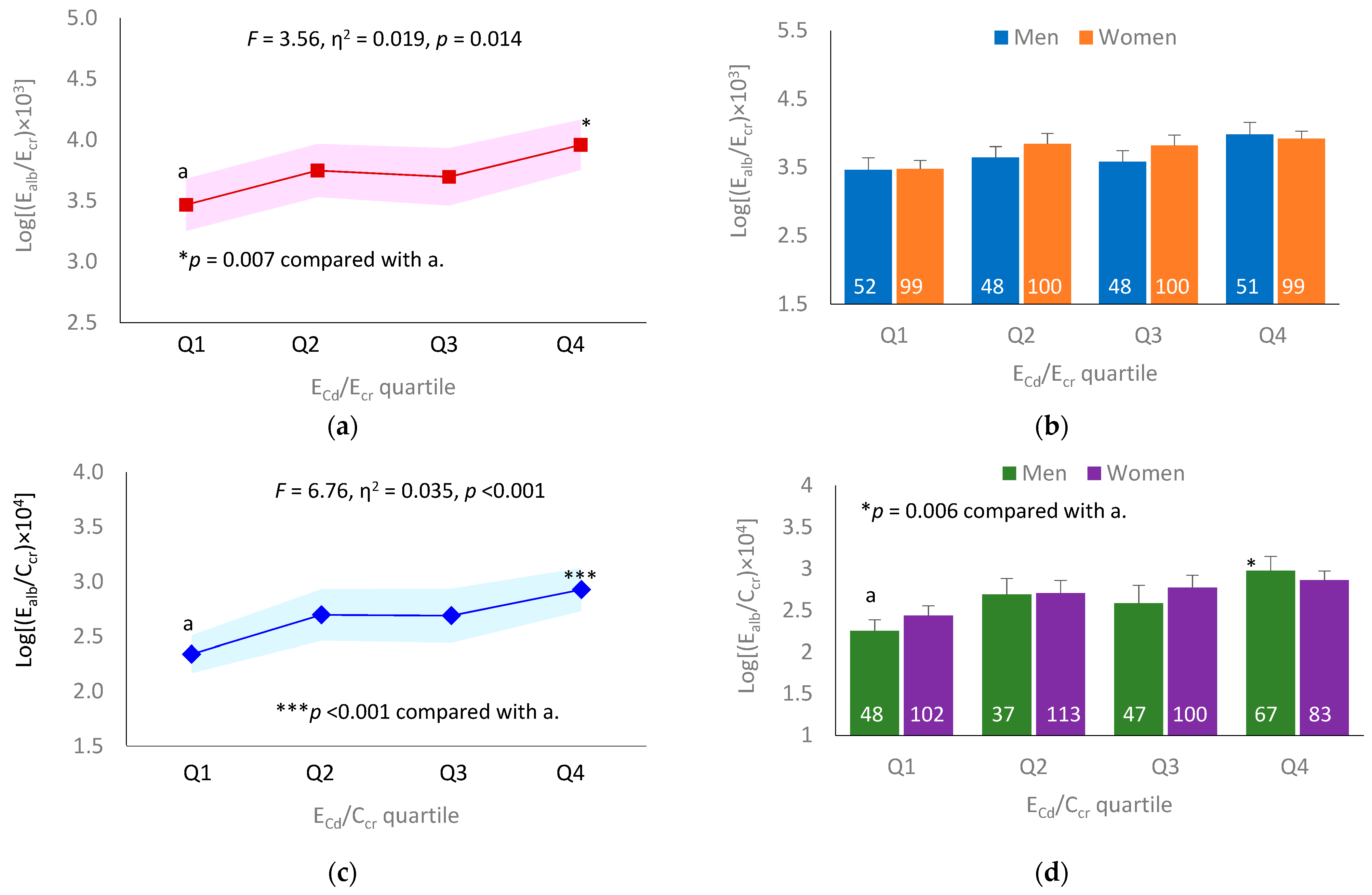
3.4. Dose–Effect Relationship between ECd and Ealb
3.5. Logistic Regression Analysis of Reduced eGFR
3.6. BMD Analysis of Albuminuria and Reduced eGFR according to Ecr-Normalized Data
3.7. BMD Analysis of Albuminuria and Reduced eGFR according to Ccr-Normalized Data
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Satarug, S.; Vesey, D.A.; Gobe, G.C. Current health risk assessment practice for dietary cadmium: Data from different countries. Food Chem. Toxicol. 2017, 106, 430–445. [Google Scholar] [CrossRef]
- Fechner, C.; Hackethal, C.; Höpfner, T.; Dietrich, J.; Bloch, D.; Lindtner, O.; Sarvan, I. Results of the BfR MEAL Study: In Germany, mercury is mostly contained in fish and seafood while cadmium, lead, and nickel are present in a broad spectrum of foods. Food Chem. X 2022, 14, 100326. [Google Scholar] [CrossRef]
- Boon, P.E.; Pustjens, A.M.; Te Biesebeek, J.D.; Brust, G.M.H.; Castenmiller, J.J.M. Dietary intake and risk assessment of elements for 1- and 2-year-old children in the Netherlands. Food Chem Toxicol. 2022, 161, 112810. [Google Scholar] [CrossRef] [PubMed]
- Arnich, N.; Sirot, V.; Rivière, G.; Jean, J.; Noël, L.; Guérin, T.; Leblanc, J.-C. Dietary exposure to trace elements and health risk assessment in the 2nd French total diet study. Food Chem. Toxicol. 2012, 50, 2432–2449. [Google Scholar] [CrossRef]
- Sand, S.; Becker, W. Assessment of dietary cadmium exposure in Sweden and population health concern including scenario analysis. Food Chem. Toxicol. 2012, 50, 536–544. [Google Scholar] [CrossRef]
- Watanabe, T.; Kataoka, Y.; Hayashi, K.; Matsuda, R.; Uneyama, C. Dietary exposure of the Japanese general population to elements: Total diet study 2013–2018. Food Saf. 2022, 10, 83–101. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, H.; Oguma, E.; Sasaki, S.; Miyamoto, K.; Hosoi, Y.; Ono, A.; Kayama, F. Exposure assessment of cadmium in female farmers in cadmium-polluted areas in Northern Japan. Toxics 2020, 8, 44. [Google Scholar] [CrossRef] [PubMed]
- Nogawa, K.; Sakurai, M.; Ishizaki, M.; Kido, T.; Nakagawa, H.; Suwazono, Y. Threshold limit values of the cadmium concentration in rice in the development of itai-itai disease using benchmark dose analysis. J. Appl. Toxicol. 2017, 37, 962–966. [Google Scholar] [CrossRef] [PubMed]
- Nishijo, M.; Nogawa, K.; Suwazono, Y.; Kido, T.; Sakurai, M.; Nakagawa, H. Lifetime cadmium exposure and mortality for renal diseases in residents of the cadmium-polluted Kakehashi River Basin in Japan. Toxics 2020, 8, 81. [Google Scholar] [CrossRef] [PubMed]
- Codex Alimentarius, CODEX STAN 193–1995, General Standard for Contaminants and Toxins in Food and Feed. Available online: http://www.fao.org/fileadmin/user_upload/livestockgov/documents/1_CXS_193e.pdf (accessed on 16 December 2022).
- JECFA. In Proceedings of the Joint FAO/WHO Expert Committee on Food Additives and Contaminants, Seventy-Third Meeting, Geneva, Switzerland, 8–17 June 2010. In Summary and Conclusions; JECFA/73/SC; Food and Agriculture Organization of the United Nations/World Health Organization: Geneva, Switzerland, 2011; Available online: https://apps.who.int/iris/handle/10665/44521 (accessed on 16 December 2022).
- Murton, M.; Goff-Leggett, D.; Bobrowska, A.; Garcia Sanchez, J.J.; James, G.; Wittbrodt, E.; Nolan, S.; Sörstadius, E.; Pecoits-Filho, R.; Tuttle, K. Burden of chronic kidney disease by KDIGO categories of glomerular filtration rate and albuminuria: A Systematic review. Adv. Ther. 2021, 38, 180–200. [Google Scholar] [CrossRef] [PubMed]
- Soveri, I.; Berg, U.B.; Björk, J.; Elinder, C.G.; Grubb, A.; Mejare, I.; Sterner, G.; Bäck, S.E.; SBU GFR Review Group. Measuring GFR: A systematic review. Am. J. Kidney Dis. 2014, 64, 411–424. [Google Scholar] [CrossRef]
- Levey, A.S.; Becker, C.; Inker, L.A. Glomerular filtration rate and albuminuria for detection and staging of acute and chronic kidney disease in adults: A systematic review. JAMA 2015, 313, 837–846. [Google Scholar] [CrossRef]
- Ferraro, P.M.; Costanzi, S.; Naticchia, A.; Sturniolo, A.; Gambaro, G. Low level exposure to cadmium increases the risk of chronic kidney disease: Analysis of the NHANES 1999–2006. BMC Publ. Health 2010, 10, 304. [Google Scholar] [CrossRef] [PubMed]
- Navas-Acien, A.; Tellez-Plaza, M.; Guallar, E.; Muntner, P.; Silbergeld, E.; Jaar, B.; Weaver, V. Blood cadmium and lead and chronic kidney disease in US adults: A joint analysis. Am. J. Epidemiol. 2009, 170, 1156–1164. [Google Scholar] [CrossRef]
- Li, Y.S.; Ho, W.C.; Caffrey, J.L.; Sonawane, B. Low serum zinc is associated with elevated risk of cadmium nephrotoxicity. Environ. Res. 2014, 134, 33–38. [Google Scholar]
- Madrigal, J.M.; Ricardo, A.C.; Persky, V.; Turyk, M. Associations between blood cadmium concentration and kidney function in the U.S. population: Impact of sex, diabetes and hypertension. Environ. Res. 2018, 169, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.J.; Wang, J.J.; Mao, J.H.; Shu, Q.; Du, L.Z. Relationships of cadmium, lead, and mercury levels with albuminuria in US adults: Results from the National Health and Nutrition Examination Survey Database, 2009–2012. Am. J. Epidemiol. 2019, 188, 1281–1287. [Google Scholar] [CrossRef]
- Grau-Perez, M.; Pichler, G.; Galan-Chilet, I.; Briongos-Figuero, L.S.; Rentero-Garrido, P.; Lopez-Izquierdo, R.; Navas-Acien, A.; Weaver, V.; García-Barrera, T.; Gomez-Ariza, J.L.; et al. Urine cadmium levels and albuminuria in a general population from Spain: A gene-environment interaction analysis. Environ. Int. 2017, 106, 27–36. [Google Scholar] [CrossRef]
- Feng, X.; Zhou, R.; Jiang, Q.; Wang, Y.; Chen, C. Analysis of cadmium accumulation in community adults and its correlation with low-grade albuminuria. Sci. Total Environ. 2022, 834, 155210. [Google Scholar] [CrossRef] [PubMed]
- Simmons, R.W.; Noble, A.D.; Pongsakul, P.; Sukreeyapongse, O.; Chinabut, N. Cadmium-hazard mapping using a general linear regression model (Irr-Cad) for rapid risk assessment. Environ. Geochem. Health 2009, 31, 71–79. [Google Scholar] [CrossRef]
- Simmons, R.W.; Pongsakul, P.; Saiyasitpanich, D.; Klinpholap, S. Elevated levels of cadmium and zinc in paddy soils and elevated levels of cadmium in rice grain downstream of a zinc mineralized area in Thailand: Implications for public health. Environ. Geochem. Health 2005, 27, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Suwatvitayakorn, P.; Ko, M.S.; Kim, K.W.; Chanpiwat, P. Human health risk assessment of cadmium exposure through rice consumption in cadmium-contaminated areas of the Mae Tao sub-district, Tak, Thailand. Environ. Geochem. Health 2020, 42, 2331–2344. [Google Scholar] [CrossRef]
- Nishijo, M.; Suwazono, Y.; Ruangyuttikarn, W.; Nambunmee, K.; Swaddiwudhipong, W.; Nogawa, K.; Nakagawa, H. Risk assessment for Thai population: Benchmark dose of urinary and blood cadmium levels for renal effects by hybrid approach of inhabitants living in polluted and non-polluted areas in Thailand. BMC Publ. Health 2014, 14, 702. [Google Scholar] [CrossRef]
- Swaddiwudhipong, W.; Mahasakpan, P.; Jeekeeree, W.; Funkhiew, T.; Sanjum, R.; Apiwatpaiboon, T.; Phopueng, I. Renal and blood pressure effects from environmental cadmium exposure in Thai children. Environ Res. 2015, 136, 82–87. [Google Scholar] [CrossRef]
- Swaddiwudhipong, W.; Nguntra, P.; Kaewnate, Y.; Mahasakpan, P.; Limpatanachote, P.; Aunjai, T.; Jeekeeree, W.; Punta, B.; Funkhiew, T.; Phopueng, I. Human health effects from cadmium exposure: Comparison between persons living in cadmium-contaminated and non-contaminated areas in northwestern Thailand. Southeast Asian J. Trop. Med. Publ. Health 2015, 46, 133–142. [Google Scholar]
- EFSA Scientific Committee. Update: Use of the benchmark dose approach in risk assessment. EFSA J. 2017, 15, 4658. [Google Scholar]
- Wong, C.; Roberts, S.M.; Saab, I.N. Review of regulatory reference values and background levels for heavy metals in the human diet. Regul. Toxicol. Pharmacol. 2022, 130, 105122. [Google Scholar] [CrossRef]
- Moffett, D.B.; Mumtaz, M.M.; Sullivan, D.W., Jr.; Whittaker, M.H. Chapter 13, General Considerations of Dose-Effect and Dose-Response Relationships. In Handbook on the Toxicology of Metals, 5th ed.; Volume I: General Considerations; Nordberg, G., Costa, M., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 299–317. [Google Scholar]
- Satarug, S.; Swaddiwudhipong, W.; Ruangyuttikarn, W.; Nishijo, M.; Ruiz, P. Modeling cadmium exposures in low- and high-exposure areas in Thailand. Environ. Health Perspect. 2013, 121, 531–536. [Google Scholar] [CrossRef]
- Yimthiang, S.; Pouyfung, P.; Khamphaya, T.; Kuraeiad, S.; Wongrith, P.; Vesey, D.A.; Gobe, G.C.; Satarug, S. Effects of environmental exposure to cadmium and lead on the risks of diabetes and kidney dysfunction. Int. J. Environ. Res. Public Health 2022, 19, 2259. [Google Scholar] [CrossRef]
- Bloch, M.J.; Basile, J.N. Review of recent literature in hypertension: Updated clinical practice guidelines for chronic kidney disease now include albuminuria in the classification system. J. Clin. Hypertens (Greenwich) 2013, 15, 865–867. [Google Scholar] [CrossRef]
- Hornung, R.W.; Reed, L.D. Estimation of average concentration in the presence of nondetectable values. Appl. Occup. Environ. Hyg. 1990, 5, 46–51. [Google Scholar] [CrossRef]
- Phelps, K.R.; Gosmanova, E.O. A generic method for analysis of plasma concentrations. Clin. Nephrol. 2020, 94, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, T.; Jelsovsky, J.Z. Bootstrap estimation of benchmark doses and confidence limits with clustered quantal data. Risk Anal. 2007, 27, 447–465. [Google Scholar] [CrossRef]
- Slob, W.; Moerbeek, M.; Rauniomaa, E.; Piersma, A.H. A statistical evaluation of toxicity study designs for the estimation of the benchmark dose in continuous endpoints. Toxicol. Sci. 2005, 84, 167–185. [Google Scholar] [CrossRef]
- Slob, W.; Setzer, R.W. Shape and steepness of toxicological dose-response relationships of continuous endpoints. Crit. Rev. Toxicol. 2014, 44, 270–297. [Google Scholar] [CrossRef]
- Satarug, S.; Đorđević, A.B.; Yimthiang, S.; Vesey, D.A.; Gobe, G.C. The NOAEL equivalent of environmental cadmium exposure associated with GFR reduction and chronic kidney disease. Toxics 2022, 10, 614. [Google Scholar] [CrossRef] [PubMed]
- Gburek, J.; Konopska, B.; Gołąb, K. Renal handling of albumin-from early findings to current concepts. Int. J. Mol. Sci. 2021, 22, 5809. [Google Scholar] [CrossRef] [PubMed]
- Molitoris, B.A.; Sandoval, R.M.; Yadav, S.P.S.; Wagner, M.C. Albumin uptake and processing by the proximal tubule: Physiological, pathological, and therapeutic implications. Physiol. Rev. 2022, 102, 1625–1667. [Google Scholar] [CrossRef]
- Benzing, T.; Salant, D. 2021. Insights into glomerular filtration and albuminuria. N. Engl. J. Med. 2021, 384, 1437–1446. [Google Scholar] [CrossRef]
- Edwards, A.; Long, K.R.; Baty, C.J.; Shipman, K.E.; Weisz, O.A. Modelling normal and nephrotic axial uptake of albumin and other filtered proteins along the proximal tubule. J. Physiol. 2022, 600, 1933–1952. [Google Scholar] [CrossRef]
- Langelueddecke, C.; Roussa, E.; Fenton, R.A.; Wolff, N.A.; Lee, W.K.; Thévenod, F. Lipocalin-2 (24p3/neutrophil gelatinase-associated lipocalin (NGAL) receptor is expressed in distal nephron and mediates protein endocytosis. J. Biol. Chem. 2012, 287, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Dizi, E.; Hasler, U.; Nlandu-Khodo, S.; Fila, M.; Roth, I.; Ernandez, T.; Doucet, A.; Martin, P.Y.; Feraille, E.; de Seigneux, S. Albuminuria induces a proinflammatory and profibrotic response in cortical collecting ducts via the 24p3 receptor. Am. J. Physiol. Renal Physiol. 2013, 305, F1053–F1063. [Google Scholar] [CrossRef] [PubMed]
- Santoyo-Sánchez, M.P.; Pedraza-Chaverri, J.; Molina-Jijón, E.; Arreola-Mendoza, L.; Rodríguez-Muñoz, R.; Barbier, O.C. Impaired endocytosis in proximal tubule from subchronic exposure to cadmium involves angiotensin II type 1 and cubilin receptors. BMC Nephrol. 2013, 14, 211. [Google Scholar] [CrossRef] [PubMed]
- Gena, P.; Calamita, G.; Guggino, W.B. Cadmium impairs albumin reabsorption by down-regulating megalin and ClC5 channels in renal proximal tubule cells. Environ. Health Perspect. 2010, 118, 1551–1556. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Dong, F.; Xu, D.; Du, L.; Yan, S.; Hu, H.; Lobe, C.G.; Yi, F.; Kapron, C.M.; Liu, J. Short-term, low-dose cadmium exposure induces hyperpermeability in human renal glomerular endothelial cells. J. Appl. Toxicol. 2016, 36, 257–265. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, L.; Tao, T.; Su, W.; Guo, Y.; Yu, H.; Qin, J. Assessment of cadmium-induced nephrotoxicity using a kidney-on-a-chip device. Toxicol. Res. 2017, 6, 372–380. [Google Scholar] [CrossRef] [PubMed]
| Parameters | All Subjects n 603 | Residential Location | p | ||
|---|---|---|---|---|---|
| Pakpoon, n 75 | Mae Sot 1, n 313 | Mae Sot 2, n 215 | |||
| Females (%) | 66.3 | 78.7 | 72.2 | 53.5 | <0.001 |
| Smoking (%) | 44.9 | 10.7 | 40.9 | 62.8 | <0.001 |
| Diabetes (%) | 1.8 | 0 | 0 | 5.1 | <0.001 |
| Hypertension (%) | 37.1 | 42.7 | 46.0 | 22.3 | <0.001 |
| Age, years | 52.4 ± 9.8 | 61.1 ± 8.7 | 47.2 ± 4.7 | 57.0 ± 11.1 | <0.001 |
| BMI, kg/m2 | 23.4 ± 4.0 | 24.6 ± 4.5 | 24.5 ± 3.5 | 21.4 ± 3.6 | <0.001 |
| eGFR a, mL/min/1.73 m2 | 85 ± 20 | 78 ± 13 | 96 ± 16 | 72 ± 19 | <0.001 |
| eGFR range | 20−131 | 40−105 | 32−127 | 20−131 | |
| Reduced eGFR b (%) | 11.4 | 9.3 | 3.5 | 23.7 | <0.001 |
| Plasma creatinine, mg/dL | 0.90 ± 0.29 | 0.87 ± 0.16 | 0.79 ± 0.20 | 1.07 ± 0.35 | <0.001 |
| Urine creatinine, mg/dL | 119.5 ± 70.9 | 96.4 ± 53.2 | 125.8 ± 78.8 | 118.4 ± 62.2 | 0.016 |
| Urine albumin, mg/L | 19.0 ± 53.1 | 16.0 ± 49.4 | 15.2 ± 37.5 | 25.6 ± 70.6 | <0.001 |
| Urine Cd, µg/L | 7.37 ± 9.34 | 0.70 ± 1.09 | 5.89 ± 6.13 | 11.85 ± 12.28 | <0.001 |
| Normalized to Ecr as Ex/Ecr c | |||||
| Ealb/Ecr, mg/g creatinine | 17.6 ± 45.7 | 14.7 ± 33.8 | 14.5 ± 40.6 | 23.2 ± 55.1 | <0.001 |
| Ealb/Ecr ≥ 20 or 30 mg/g (%) d | 13.6 | 9.3 | 11.5 | 18.1 | 0.047 |
| ECd/Ecr, µg/g creatinine | 6.65 ± 6.61 | 1.00 ± 1.90 | 5.40 ± 4.51 | 10.43 ± 8.02 | <0.001 |
| Normalized to Ccr as Ex/Ccr e | |||||
| (Ealb/Ccr) × 100, mg/L filtrate | 18.8 ± 56.8 | 12.6 ± 31.6 | 13.2 ± 43.9 | 29.1 ± 76.0 | <0.001 |
| (Ealb/Ccr) × 100 ≥ 20 or 30 mg/L f (%) | 12.8 | 10.7 | 8.6 | 19.5 | 0.001 |
| (ECd/Ccr) × 100, µg/L filtrate | 6.33 ± 7.59 | 0.88 ± 1.66 | 4.25 ± 3.88 | 11.27 ± 9.89 | <0.001 |
| Parameters | Males (n 203) | Females (n 400) | ||
|---|---|---|---|---|
| eGFR > 60, n 175 | eGFR ≤ 60, n 28 | eGFR > 60, n 359 | eGFR ≤ 60, n 41 | |
| Smoking (%) | 77.7 | 85.7 | 26.2 | 41.5 # |
| Hypertension (%) | 28.0 | 42.9 | 41.2 | 36.6 |
| Diabetes (%) | 1.7 | 10.7 * | 1.1 | 2.4 |
| Age, years | 51.3 ± 9.2 | 67.6 ± 10.6 *** | 50.9 ± 8.3 | 60.2 ± 11.0 ### |
| BMI, kg/m2 | 22.3 ± 3.2 | 20.9 ± 3.3 | 24.1 ± 4.1 | 23.5 ± 4.5 |
| eGFR a, mL/min/1.73 m2 | 89 ± 16 | 44 ± 12 *** | 90 ± 16 | 50 ± 10 ### |
| Plasma creatinine, mg/dL | 0.97 ± 0.16 | 1.70 ± 0.45 *** | 0.76 ± 0.14 | 1.24 ± 0.29 ### |
| Urine creatinine, mg/dL | 136.9 ± 63.5 | 144.7 ± 68.3 | 110.6 ± 73.7 | 105.3 ± 60.7 |
| Urine albumin, mg/L | 18.0 ± 45.4 | 61.5 ± 80.4 *** | 15.2 ± 51.9 | 27.8 ± 59.9 ## |
| Urine Cd, µg/L | 7.94 ± 10.65 | 16.42 ± 16.22 ** | 9.11 ± 11.90 | 10.49 ± 12.46 # |
| Normalized to Ecr as Ex/Ecr b | ||||
| EAlb/Ecr, mg/g creatinine | 16.7 ± 49.1 | 53.7 ± 83.0 *** | 14.1 ± 38.7 | 28.2 ± 42.5 ## |
| Albuminuria c | 12.0 | 50.0 *** | 9.7 | 29.3 ### |
| ECd/Ecr, µg/g creatinine | 6.20 ± 6.95 | 10.36 ± 7.07 *** | 6.15 ± 5.67 | 10.36 ± 9.97 # |
| Normalized to Ccr as Ex/Ccr d | ||||
| EAlb/Ccr × 100, mg/L filtrate | 16.6 ± 48.8 | 105.1 ± 167.5 *** | 11.3 ± 34.6 | 34.5 ± 51.3 ### |
| ECd/Ccr × 100, µg/L filtrate | 6.16 ± 6.98 | 17.98 ± 13.38 *** | 4.74 ± 4.66 | 13.05 ± 13.25 ### |
| Independent Variables/ Factors | Urinary Excretion of Albumin a | |||||
|---|---|---|---|---|---|---|
| All Subjects, n 603 | Males, n 203 | Females, n 400 | ||||
| β b | p | β | p | β | p | |
| Model 1 | ||||||
| Age, years | 0.162 | <0.001 | 0.173 | 0.022 | 0.137 | 0.011 |
| BMI, kg/m2 | 0.036 | 0.426 | 0.014 | 0.852 | 0.041 | 0.450 |
| Log [(ECd/Ecr) × 103], µg/g creatinine | 0.186 | <0.001 | 0.279 | <0.001 | 0.130 | 0.020 |
| Diabetes | −0.057 | 0.158 | −0.017 | 0.810 | −0.086 | 0.083 |
| Sex | −0.067 | 0.147 | − | − | − | − |
| Hypertension | −0.106 | 0.011 | −0.134 | 0.060 | −0.087 | 0.086 |
| Smoking | −0.089 | 0.063 | −0.001 | 0.990 | −0.122 | 0.020 |
| Adjusted R2 | 0.067 | <0.001 | 0.102 | <0.001 | 0.046 | <0.001 |
| Model 2 | ||||||
| Age, years | 0.203 | <0.001 | 0.198 | 0.007 | 0.182 | 0.001 |
| BMI, kg/m2 | 0.048 | 0.278 | 0.019 | 0.798 | 0.056 | 0.300 |
| Log [(ECd/Ccr) × 105], µg/L filtrate | 0.239 | <0.001 | 0.342 | <0.001 | 0.174 | 0.001 |
| Diabetes | −0.007 | 0.880 | −0.017 | 0.809 | −0.092 | 0.061 |
| Sex | −0.060 | 0.132 | − | − | − | − |
| Hypertension | −0.106 | 0.009 | −0.137 | 0.047 | −0.085 | 0.089 |
| Smoking | −0.073 | 0.121 | 0.017 | 0.806 | −0.113 | 0.029 |
| Adjusted R2 | 0.105 | <0.001 | 0.164 | <0.001 | 0.070 | <0.001 |
| Independent Variables/ Factors | Number of Subjects | Reduced eGFR a | ||||
|---|---|---|---|---|---|---|
| β Coefficients | POR | 95% CI | p | |||
| (SE) | Lower | Upper | ||||
| Model 1 | ||||||
| Age, years | 597 | −0.123 (0.015) | 0.885 | 0.859 | 0.912 | <0.001 |
| BMI, kg/m2 | 597 | −0.071 (0.038) | 0.932 | 0.865 | 1.003 | 0.061 |
| Diabetes | 11 | −0.780 (0.829) | 0.458 | 0.090 | 2.325 | 0.346 |
| Hypertension | 224 | −0.276 (0.319) | 0.759 | 0.406 | 1.418 | 0.386 |
| Sex (females) | 398 | −0.032 (0.355) | 0.968 | 0.483 | 1.943 | 0.928 |
| Smoking | 269 | −0.193 (0.361) | 0.825 | 0.407 | 1.672 | 0.593 |
| Quartile of ECd/Ecr in µg/g creatinine | ||||||
| Q1: 0.03−2.41 | 151 | Referent | ||||
| Q2: 2.42−4.64 | 148 | 0.651 (0.377) | 1.917 | 0.915 | 4.015 | 0.085 |
| Q3: 4.65−8.36 | 148 | 1.470 (0.482) | 4.349 | 1.692 | 11.183 | 0.002 |
| Q4: 8.37−57.6 | 150 | 1.446 (0.440) | 4.245 | 1.792 | 10.055 | 0.001 |
| Model 2 | ||||||
| Age, years | 597 | −0.131 (0.017) | 0.878 | 0.849 | 0.906 | <0.001 |
| BMI, kg/m2 | 597 | −0.093 (0.041) | 0.912 | 0.842 | 0.987 | 0.023 |
| Diabetes | 11 | −0.450 (0.826) | 0.638 | 0.126 | 3.218 | 0.586 |
| Hypertension | 224 | −0.408 (0.334) | 0.665 | 0.346 | 1.279 | 0.221 |
| Sex (females) | 398 | −0.295 (0.375) | 0.745 | 0.357 | 1.555 | 0.433 |
| Smoking | 269 | 0.012 (0.368) | 1.012 | 0.492 | 2.083 | 0.974 |
| Quartile of (ECd/Ccr) × 100, µg/L filtrate | ||||||
| Q1: 0.03−1.95 | 150 | Referent | ||||
| Q2: 1.96−3.88 | 150 | 1.683 (0.432) | 5.382 | 2.310 | 12.543 | <0.001 |
| Q3: 3.89−7.68 | 147 | 1.573 (0.426) | 4.820 | 2.090 | 11.115 | <0.001 |
| Q4: 7.69−63.2 | 150 | 3.154 (0.603) | 23.429 | 7.179 | 76.464 | <0.001 |
| Endpoints | Males | Females | ||||
|---|---|---|---|---|---|---|
| BMDL | BMDU | U/L | BMDL | BMDU | U/L | |
| 5% increase of Ealb/Ecr | 4.16 × 10−6 | 2.29 | 5.5 × 105 | 3.89 × 10−4 | 3.41 | 8.8 × 103 |
| 5% increase in prevalence of albuminuria a | 3.06 × 10−3 | 36.7 | 1.2 × 102 | 1.22 × 10−2 | 3.05 × 105 | 2.5 × 107 |
| 10% increase in prevalence of albuminuria | 0.55 | 337 | 612 | 2.52 | 1.74 × 106 | 6.7 × 105 |
| 5% Decrease of eGFR | 2.07 | 6.93 | 3.35 | 6.82 | 21.7 | 3.81 |
| 5% increase in prevalence of reduced eGFR b | 1.47 | 10.6 | 7.21 | 1.93 | 15.6 | 8.08 |
| 10% increase in prevalence of reduced eGFR | 3.92 | 15.7 | 4.00 | 5.31 | 23.6 | 4.44 |
| Endpoints | Males | Females | ||||
|---|---|---|---|---|---|---|
| BMDL | BMDU | U/L | BMDL | BMDU | U/L | |
| 5% increase of Ealb/Ccr × 100 | 0.00053 | 1.11 | 2094 | 0.00357 | 2.09 | 585 |
| 5% increase in prevalence of albuminuria a | 0.163 | 13 | 80 | 0.718 | 154 | 214 |
| 10% increase in prevalence of albuminuria | 1.65 | 20 | 12 | 3.55 | 212 | 60 |
| 5% Decrease of eGFR | 2.15 | 6.71 | 3.12 | 2.15 | 6.56 | 3.05 |
| 5% increase in prevalence of reduced eGFR b | 3.22 | 9.64 | 2.99 | 3.33 | 9.2 | 2.76 |
| 10% increase in prevalence of reduced eGFR | 5.61 | 13.4 | 2.39 | 5.88 | 12.9 | 2.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Satarug, S.; Vesey, D.A.; Gobe, G.C.; Yimthiang, S.; Buha Đorđević, A. Health Risk in a Geographic Area of Thailand with Endemic Cadmium Contamination: Focus on Albuminuria. Toxics 2023, 11, 68. https://doi.org/10.3390/toxics11010068
Satarug S, Vesey DA, Gobe GC, Yimthiang S, Buha Đorđević A. Health Risk in a Geographic Area of Thailand with Endemic Cadmium Contamination: Focus on Albuminuria. Toxics. 2023; 11(1):68. https://doi.org/10.3390/toxics11010068
Chicago/Turabian StyleSatarug, Soisungwan, David A. Vesey, Glenda C. Gobe, Supabhorn Yimthiang, and Aleksandra Buha Đorđević. 2023. "Health Risk in a Geographic Area of Thailand with Endemic Cadmium Contamination: Focus on Albuminuria" Toxics 11, no. 1: 68. https://doi.org/10.3390/toxics11010068
APA StyleSatarug, S., Vesey, D. A., Gobe, G. C., Yimthiang, S., & Buha Đorđević, A. (2023). Health Risk in a Geographic Area of Thailand with Endemic Cadmium Contamination: Focus on Albuminuria. Toxics, 11(1), 68. https://doi.org/10.3390/toxics11010068










