Measuring TiO2N and AgHEC Airborne Particle Density during a Spray Coating Process
Abstract
:1. Introduction
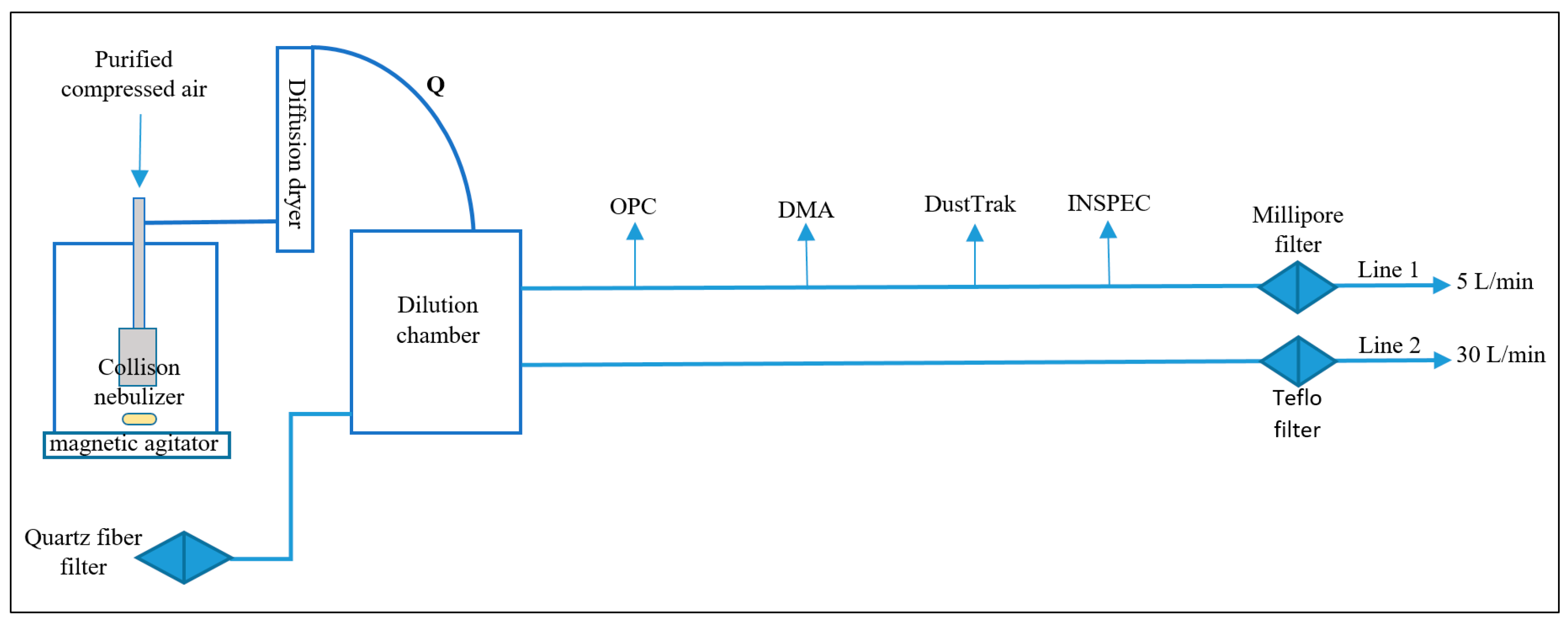
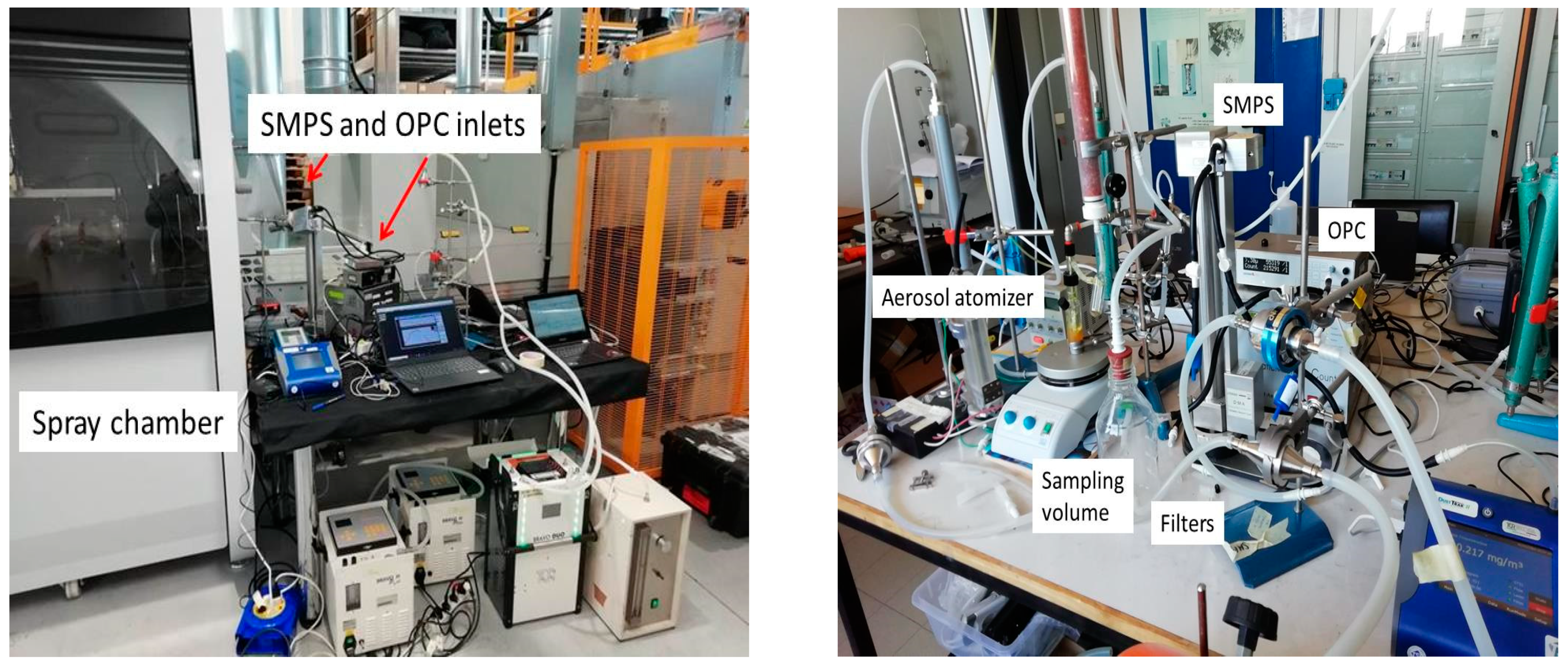
2. Materials and Methods
- (I)
- Mass-to-volume ratio: data from field measurements at the industrial pilot plant and on the laboratory scale
- (II)
- Direct single-particle density measurement: Inertial Spectrometer (INSPEC)
3. Results
3.1. Particle Density by Mass to Volume Ratio: Industrial Pilot Plant
3.2. Particle Density by Mass-to-Volume Ratio: Lab-Scale Atomizer
3.3. Direct Particle Density Measurements (INSPEC): Pilot Plant and Laboratory Scale
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paur, H.-R.; Cassee, F.R.; Teeguarden, J.; Fissan, H.; Diabate, S.; Aufderheide, M.; Kreyling, W.G.; Hänninen, O.; Kasper, G.; Riediker, M.; et al. In-Vitro Cell Exposure Studies for the Assessment of Nanoparticle Toxicity in the Lung—A Dialog between Aerosol Science and Biology. J. Aerosol Sci. 2011, 42, 668–692. [Google Scholar] [CrossRef]
- Hofmann, W. Modelling Inhaled Particle Deposition in the Human Lung—A Review. J. Aerosol Sci. 2011, 42, 693–724. [Google Scholar] [CrossRef]
- Koivisto, A.J.; Aromaa, M.; Mäkelä, J.M.; Pasanen, P.; Hussein, T.; Hämeri, K. Concept to Estimate Regional Inhalation Dose of Industrially Synthesized Nanoparticles. ACS Nano 2012, 6, 1195–1203. [Google Scholar] [CrossRef] [PubMed]
- Kuhlbusch, T.A.; Asbach, C.; Fissan, H.; Göhler, D.; Stintz, M. Nanoparticle Exposure at Nanotechnology Workplaces: A Review. Part. Fibre Toxicol. 2011, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Jensen, A.C.Ø.; Harboe, H.; Brostrøm, A.; Jensen, K.A.; Fonseca, A.S. Nanoparticle Exposure and Workplace Measurements During Processes Related to 3D Printing of a Metal Object. Front. Public Health 2020, 8, 608718. [Google Scholar] [CrossRef]
- Koivisto, A.J.; Lyyränen, J.; Auvinen, A.; Vanhala, E.; Hämeri, K.; Tuomi, T.; Jokiniemi, J. Industrial Worker Exposure to Airborne Particles during the Packing of Pigment and Nanoscale Titanium Dioxide. Inhal. Toxicol. 2012, 24, 839–849. [Google Scholar] [CrossRef]
- Koivisto, A.J.; Palomäki, J.E.; Viitanen, A.-K.; Siivola, K.M.; Koponen, I.K.; Yu, M.; Kanerva, T.S.; Norppa, H.; Alenius, H.T.; Hussein, T.; et al. Range-Finding Risk Assessment of Inhalation Exposure to Nanodiamonds in a Laboratory Environment. Int. J. Environ. Res. Public Health 2014, 11, 5382–5402. [Google Scholar] [CrossRef]
- Koivisto, A.J.; Kling, K.I.; Fonseca, A.S.; Bluhme, A.B.; Moreman, M.; Yu, M.; Costa, A.L.; Giovanni, B.; Ortelli, S.; Fransman, W.; et al. Dip Coating of Air Purifier Ceramic Honeycombs with Photocatalytic TiO2 Nanoparticles: A Case Study for Occupational Exposure. Sci. Total Environ. 2018, 630, 1283–1291. [Google Scholar] [CrossRef]
- Koivisto, A.J.; Del Secco, B.; Trabucco, S.; Nicosia, A.; Ravegnani, F.; Altin, M.; Cabellos, J.; Furxhi, I.; Blosi, M.; Costa, A.; et al. Quantifying Emission Factors and Setting Conditions of Use According to ECHA Chapter R.14 for a Spray Process Designed for Nanocoatings—A Case Study. Nanomaterials 2022, 12, 596. [Google Scholar] [CrossRef]
- Fujitani, Y.; Sugaya, Y.; Hashiguchi, M.; Furuyama, A.; Hirano, S.; Takami, A. Particle Deposition Efficiency at Air–Liquid Interface of a Cell Exposure Chamber. J. Aerosol Sci. 2015, 81, 90–99. [Google Scholar] [CrossRef]
- Ding, Y.; Weindl, P.; Lenz, A.-G.; Mayer, P.; Krebs, T.; Schmid, O. Quartz Crystal Microbalances (QCM) Are Suitable for Real-Time Dosimetry in Nanotoxicological Studies Using VITROCELL®Cloud Cell Exposure Systems. Part. Fibre Toxicol. 2020, 17, 44. [Google Scholar] [CrossRef] [PubMed]
- Teeguarden, J.G.; Hinderliter, P.M.; Orr, G.; Thrall, B.D.; Pounds, J.G. Particokinetics in Vitro: Dosimetry Considerations for in Vitro Nanoparticle Toxicity Assessments. Toxicol. Sci. 2007, 95, 300–312. [Google Scholar] [CrossRef] [PubMed]
- DeLoid, G.; Cohen, J.M.; Darrah, T.; Derk, R.; Rojanasakul, L.; Pyrgiotakis, G.; Wohlleben, W.; Demokritou, P. Estimating the Effective Density of Engineered Nanomaterials for in Vitro Dosimetry. Nat. Commun. 2014, 5, 3514. [Google Scholar] [CrossRef]
- Tadjiki, S.; Montaño, M.D.; Assemi, S.; Barber, A.; Ranville, J.; Beckett, R. Measurement of the Density of Engineered Silver Nanoparticles Using Centrifugal FFF-TEM and Single Particle ICP-MS. Anal. Chem. 2017, 89, 6056–6064. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.-M.; Chan, W.-H.; Lin, C.-W.; Huang, S.-H.; Chen, C.-C. Characterization of Vibrating Mesh Aerosol Generators. Aerosol Air Qual. Res. 2019, 19, 1678–1687. [Google Scholar] [CrossRef]
- Ding, Y.; Kuhlbusch, T.A.J.; van Tongeren, M.; Jiménez, A.S.; Tuinman, I.; Chen, R.; Alvarez, I.L.; Mikola, U.; Nickel, C.; Meyer, J.; et al. Airborne engineered nanomaterials in the workplace—A review of release and worker exposure during nanomaterial production and handling processes. J. Hazard. Mater. 2017, 322, 17–28. [Google Scholar] [CrossRef]
- Archer, J.; Walker, J.S.; Gregson, F.K.A.; Hardy, D.A.; Reid, J.P. Drying Kinetics and Particle Formation from Dilute Colloidal Suspensions in Aerosol Droplets. Langmuir 2020, 36, 12481–12493. [Google Scholar] [CrossRef]
- Vehring, R. Pharmaceutical Particle Engineering via Spray Drying. Pharm. Res. 2008, 25, 999–1022. [Google Scholar] [CrossRef]
- Malamatari, M.; Charisi, A.; Malamataris, S.; Kachrimanis, K.; Nikolakakis, I. Spray Drying for the Preparation of Nanoparticle-Based Drug Formulations as Dry Powders for Inhalation. Processes 2020, 8, 788. [Google Scholar] [CrossRef]
- Bickley, R.I.; González-Carreno, T.; Lees, J.S.; Palmisano, L.; Tilley, R.J.D. A structural inves-tigation of titanium dioxide photocatalysts. J. Solid State Chem. 1991, 92, 178–190. [Google Scholar] [CrossRef]
- Lee, S.Y.; Park, S.J. TiO2 photocatalyst for water treatment applications. J. Ind. Eng. Chem. 2013, 19, 1761–1769. [Google Scholar] [CrossRef]
- Nomiya, K.; Yoshizawa, A.; Tsukagoshi, K.; Chikaraishi Kasuga, N.; Hirakawa, S.; Watanabe, J. Synthesis and structural characterization of silver(I), aluminium(III) and cobalt(II) complexes with 4-isopropyltropolone (hinoki-tiol) showing noteworthy biological activities. Action of silver(I)-oxygen bonding complexes on the antimicrobial activities. J. Inorg. Biochem. 2004, 98, 46–60. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Silver, S. Silver as a biocide: Will resistance become a problem? Nat. Biotechnol. 1998, 16, 888. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.L.; Blosi, M. Process for the Preparation of Nanoparticles of Noble Metals in Hydrogel and Nanoparticles Thus Obtained. WIPO Patent WO2016125070A1, 11 August 2016. [Google Scholar]
- Del Secco, B.; Trabucco, S.; Ravegnani, F.; Koivisto, A.J.; Zanoni, I.; Blosi, M.; Ortelli, S.; Altin, M.; Bartolini, G.; Costa, A.L.; et al. Particles Emission from an Industrial Spray Coating Process Using Nano-Materials. Nanomaterials 2022, 12, 313. [Google Scholar] [CrossRef]
- Nicosia, A.; Manodori, L.; Trentini, A.; Ricciardelli, I.; Bacco, D.; Poluzzi, V.; Di Matteo, L.; Belosi, F. Field Study of a Soft X-Ray Aerosol Neutralizer Combined with Electrostatic Classifiers for Nanoparticle Size Distribution Measurements. Particuology 2018, 37, 99–106. [Google Scholar] [CrossRef]
- Gormley, P.G.; Kennedy, M. Diffusion from a Stream Flowing through a Cylindrical Tube. Proc. R. Ir. Academy. Sect. A Math. Phys. Sci. 1948, 52, 163–169. [Google Scholar]
- Prodi, V.; Melandri, C.; Tarroni, G.; de Zaiacomo, T.; Formignani, M.; Hochrainer, D. An Inertial Spectrometer for Aerosol Particles. J. Aerosol Sci. 1979, 10, 411–419. [Google Scholar] [CrossRef]
- Belosi, F.; Prodi, V. Particle Deposition within the Inertial Spectrometer. J. Aerosol Sci. 1987, 18, 37–42. [Google Scholar] [CrossRef]
- Hinds, W.C. Aerosol Technology; John Wiley and Sons: Hoboken, NJ, USA, 1999; p. 54. [Google Scholar]
- Santi, E.; Belosi, F.; Santachiara, G.; Prodi, F.; Berico, M. Real-Time Aerosol Photometer and Optical Particle Counter Compa-rison. Nuovo Cim. B 2010, 125, 969–981. [Google Scholar] [CrossRef]
- Chakrabarti, B.; Fine, P.M.; Delfino, R.; Sioutas, C. Performance Evaluation of the Active-Flow Personal DataRAM PM2.5 Mass Monitor (Thermo Anderson PDR-1200) Designed for Continuous Personal Exposure Measurements. Atmos. Environ. 2004, 38, 3329–3340. [Google Scholar] [CrossRef]
- Knight, M.; Petrucci, G.A. Study of Residual Particle Concentrations Generated by the Ultrasonic Nebulization of Deionized Water Stored in Different Container Types. Anal. Chem. 2003, 75, 4486–4492. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.; Kournikakis, B.; Gunning, A.; Fildes, J. Submicron Aerosol Characterization of Water by a Differential Mobility Particle Sizer. J. Aerosol Sci. 1988, 19, 1425–1428. [Google Scholar] [CrossRef]
- Willeke, K.; Baron, P.A. Aerosol Measurement Principles and Techniques; Van Nostrand Reinhold: New York, NY, USA, 1993; p. 501. [Google Scholar]
- Hales, T.C. An Overview of the Kepler Conjecture. arXiv 1998, arXiv:math/9811071. [Google Scholar]
- Mueller, R.; Kleinebudde, P. Comparison of a Laboratory and a Production Coating Spray Gun with Respect to Scale-Up. AAPS PharmSciTech 2007, 8, E21–E31. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Pulli, K. Numerical and Experimental Investigation on the Spray Coating Process Using a Pneumatic Atomizer: Influences of Operating Conditions and Target Geometries. Coatings 2017, 7, 13. [Google Scholar] [CrossRef]
- Vehring, R.; Foss, W.R.; Lechuga-Ballesteros, D. Particle Formation in Spray Drying. J. Aerosol Sci. 2007, 38, 728–746. [Google Scholar] [CrossRef]
- Lieber, C.; Melekidis, S.; Koch, R.; Bauer, H.-J. Insights into the Evaporation Characteristics of Saliva Droplets and Aerosols: Levitation Experiments and Numerical Modeling. J. Aerosol Sci. 2021, 154, 105760. [Google Scholar] [CrossRef]
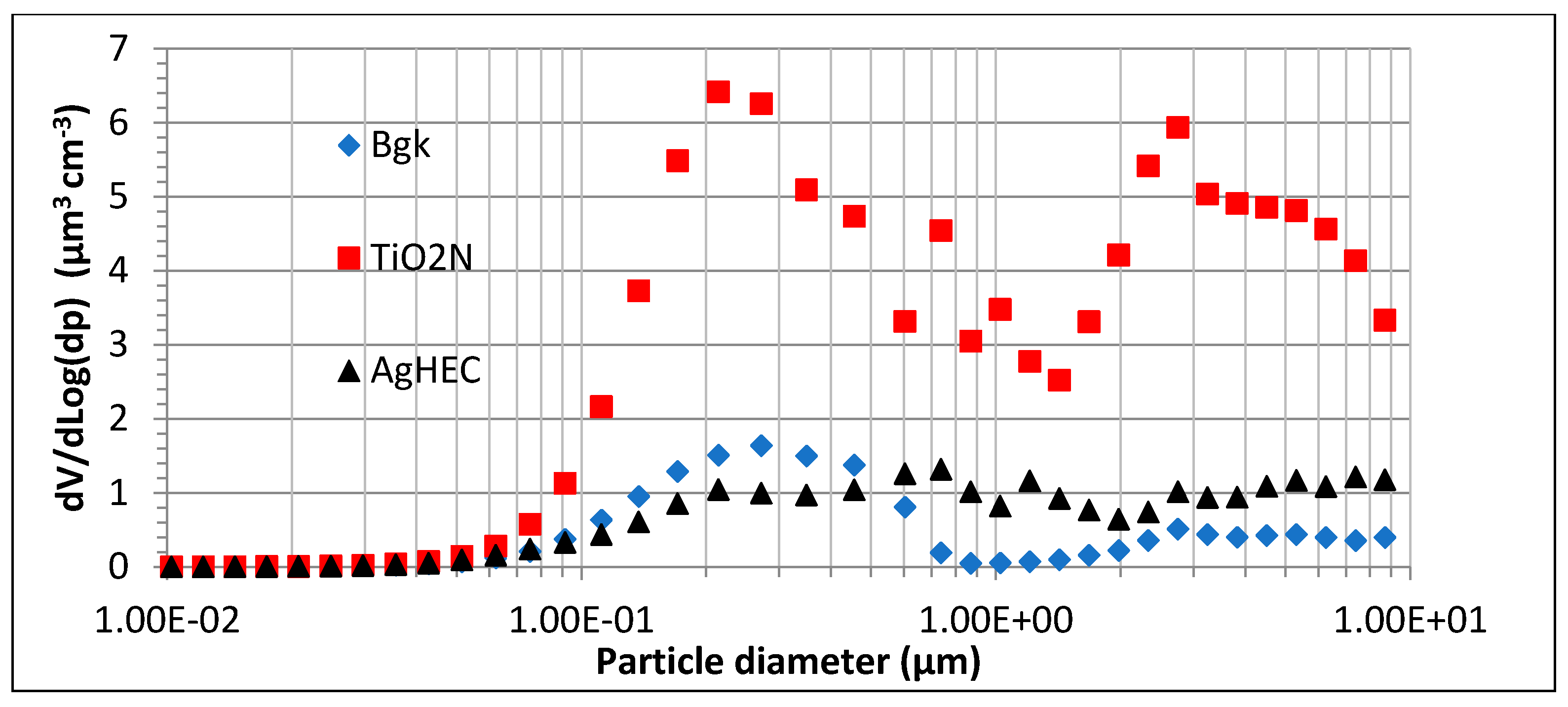
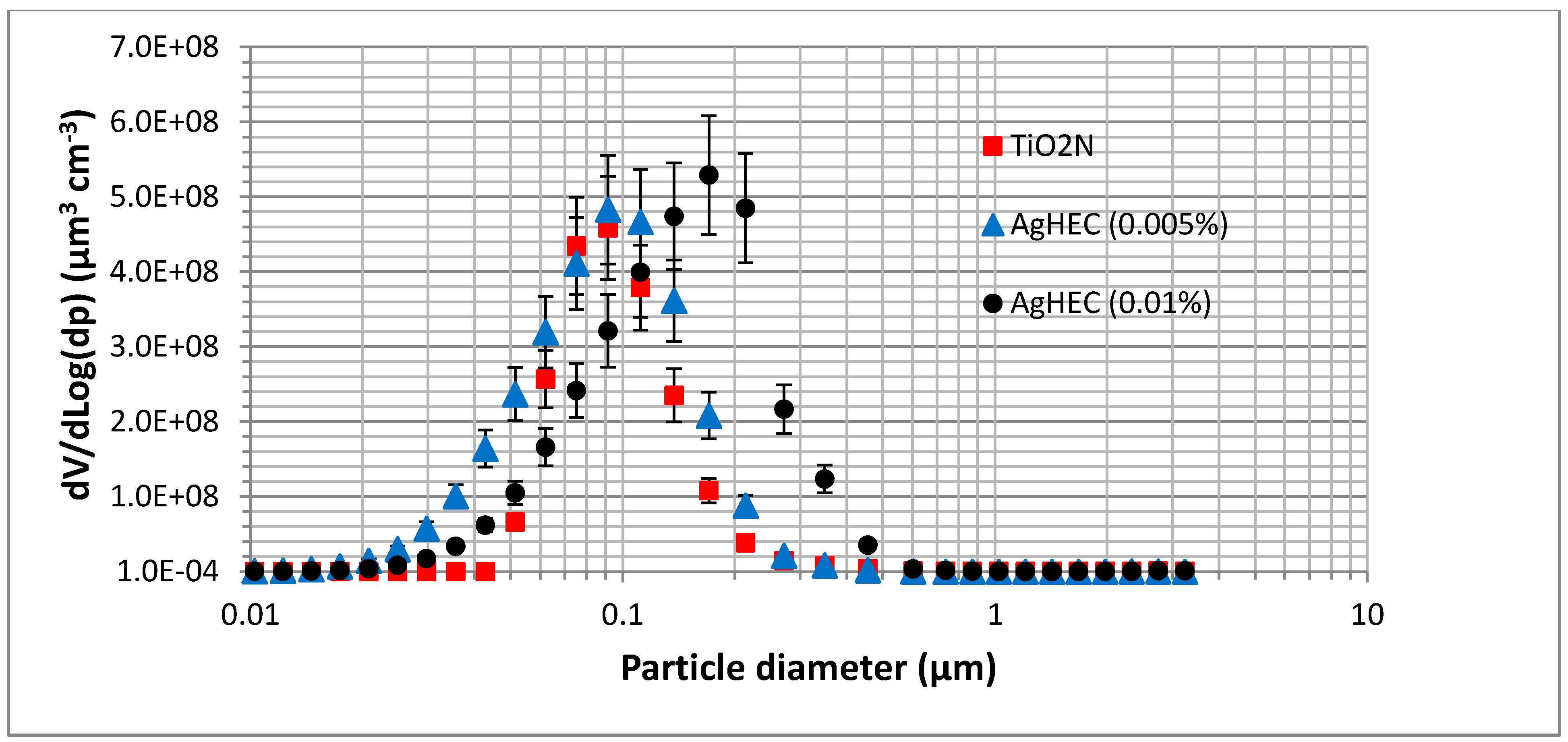
| Spray | PM | ΣniVi | ρ |
|---|---|---|---|
| Gravimetric | (µm3/cm3) | (g/cm3) | |
| (µg/m3) | |||
| Background | 28 ± 1 | 15 ± 6 | 1.9 ± 0.8 |
| (15/02/2021) | |||
| TiO2N1 | 92 ± 1 | 91 ± 50 | 1.0 ± 0.6 |
| (Test 1–6) | |||
| AgHEC 1 | 36 ± 1 | 12 ± 2 | 3.0 ± 0.5 |
| (Test 7–13) |
| Spray | Aerosol | ΣniVi | ρ |
|---|---|---|---|
| (TiO2N) | Photometer | (µm3/cm3) | (g/cm3) |
| (µg/m3) | |||
| Test 1 (200 mL/min-PMMA) | 38 | 22 | 1.7 |
| Test 2 (400 mL/min-PMMA) | 47 | 31 | 1.6 |
| Test 3 (800 mL/min-PMMA) | 212 | 123 | 1.7 |
| Test 4 (200 mL/min-Textile) | 49 | 17 | 2.9 |
| Test 5 (400 mL/min-Textile) | 100 | 38 | 2.6 |
| Test 6 (800mL/min-Textile) | 162 | 124 | 1.3 |
| Spray | Aerosol | ΣniVi | ρ |
|---|---|---|---|
| (AgHEC) | Photometer | (µm3/cm3) | (g/cm3) |
| (µg/m3) | |||
| Test 7 (200 mL/min-0.01% Textile) | 10.0 | 2.5 | 4.0 |
| Test 8 (400 mL/min-0.01% Textile) | 16.0 | 3.6 | 4.4 |
| Test 9 (200 mL/min-0.05% Textile) | 18.6 | 5.8 | 3.2 |
| Test 10 (400 mL/min-0.05% Textile) | 30.3 | 9.5 | 3.2 |
| Deposition Section mm | Aerodynamic Diameter (µm) | TiO2-N | Ag-HEC |
|---|---|---|---|
| Experimental Density (g/cm3) | Experimental Density (g/cm3) | ||
| Witek field campaign | |||
| 23–30 | 3.3 | 1.5 | 1.1 |
| 30–42 | 2.1 | 1.6 | 1.2 |
| 42–48 | 1.3 | 2.1 | 1.2 |
| Averaged density (g/cm3) | 1.7 ± 0.3 | 1.2 ± 0.1 | |
| Suspension | TiO2N | AgHEC |
|---|---|---|
| Density (g/cm3) | Density (g/cm3) | |
| Bulk | 4.2 | 1.4 |
| Agglomeration (Keplero cong.) | 3.1 | 1.0 |
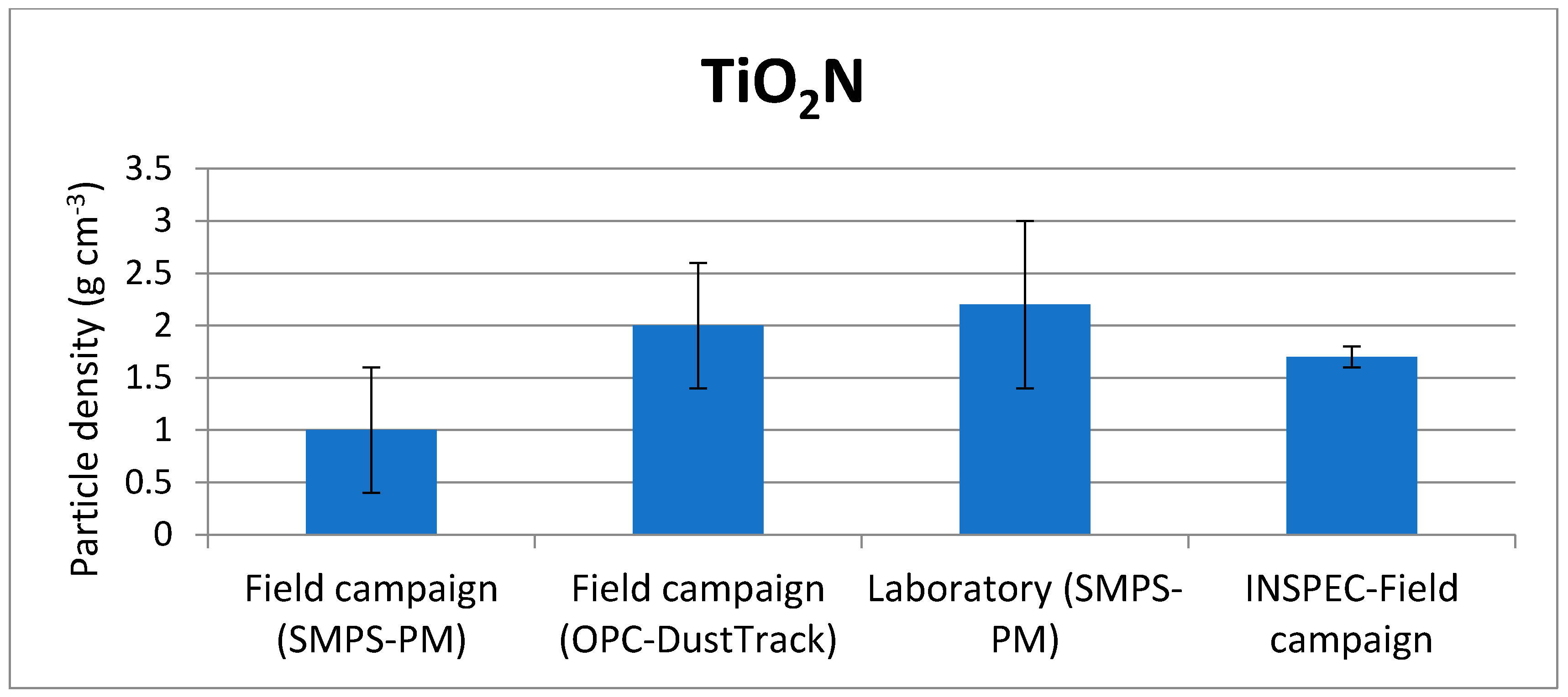
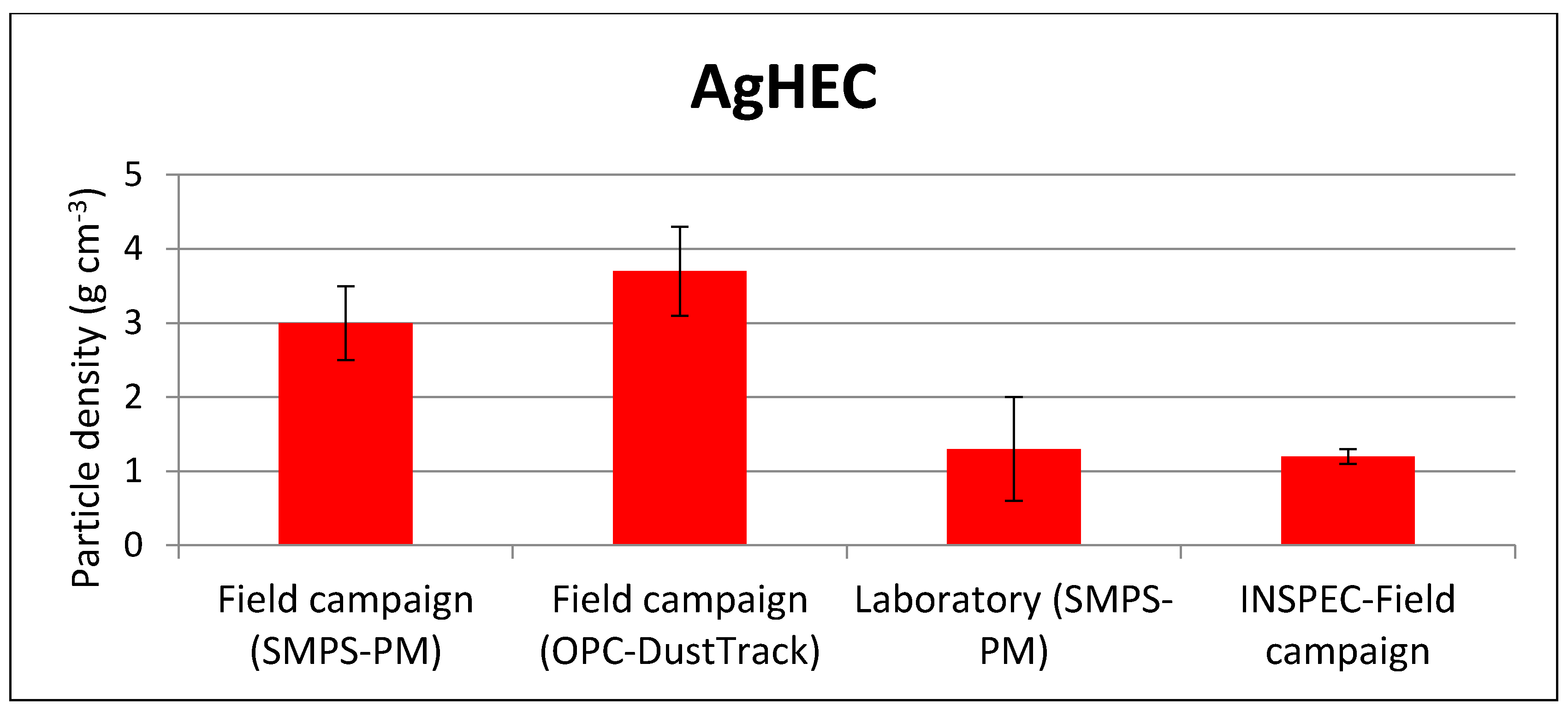
| Field campaign (SMPS-PM) | 1.0 ± 0.6 | 3.0 ± 0.5 |
| Field campaign (OPC-DustTrack) | 2.0 ± 0.6 | 3.7 ± 0.6 |
| Laboratory (SMPS-PM) | 2.2 ± 0.8 | 1.3 ± 0.7 |
| Direct measurement—Field campaign (INSPEC) | 1.7 ± 0.1 | 1.2 ± 0.1 |
| Particle Density | TiO2N | AgHEC | ||
|---|---|---|---|---|
| (g cm−3) | (µg m−3) | (µg m−3) | ||
| <1 µm | <4 µm | <1 µm | <4 µm | |
| 1.8 (present work) | 71 | 129 | ||
| 2.1 [9] | 83 | 150 | ||
| 3 (present work) | 10 | 25 | ||
| 6.5 [9] | 22 | 54 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trabucco, S.; Koivisto, A.J.; Ravegnani, F.; Ortelli, S.; Zanoni, I.; Blosi, M.; Costa, A.L.; Belosi, F. Measuring TiO2N and AgHEC Airborne Particle Density during a Spray Coating Process. Toxics 2022, 10, 498. https://doi.org/10.3390/toxics10090498
Trabucco S, Koivisto AJ, Ravegnani F, Ortelli S, Zanoni I, Blosi M, Costa AL, Belosi F. Measuring TiO2N and AgHEC Airborne Particle Density during a Spray Coating Process. Toxics. 2022; 10(9):498. https://doi.org/10.3390/toxics10090498
Chicago/Turabian StyleTrabucco, Sara, Antti Joonas Koivisto, Fabrizio Ravegnani, Simona Ortelli, Ilaria Zanoni, Magda Blosi, Anna Luisa Costa, and Franco Belosi. 2022. "Measuring TiO2N and AgHEC Airborne Particle Density during a Spray Coating Process" Toxics 10, no. 9: 498. https://doi.org/10.3390/toxics10090498
APA StyleTrabucco, S., Koivisto, A. J., Ravegnani, F., Ortelli, S., Zanoni, I., Blosi, M., Costa, A. L., & Belosi, F. (2022). Measuring TiO2N and AgHEC Airborne Particle Density during a Spray Coating Process. Toxics, 10(9), 498. https://doi.org/10.3390/toxics10090498











