A Metabolomic Approach to Assess the Toxicity of the Olive Tree Endophyte Bacillus sp. PTA13 Lipopeptides to the Aquatic Macrophyte Lemna minor L.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Cultivation of Lemna minor L.
2.3. Bioassays for the Assessment of the EC25 and EC50 Values of the Bacillus sp. PTA13 Lipopeptide (LP) Extract to Lemna minor L.
2.4. Mining the Effect of the Bacillus sp. PTA13 Lipopeptide Extract on the Metabolism of Lemna minor L. by GC/EI/MS Metabolomics
2.4.1. Experimental Design
2.4.2. Sample Preparation for GC/EI/MS Metabolomics Analysis
2.4.3. GC/EI/MS Analytical Conditions
2.4.4. Data Pre-Processing and Biomarker Discovery
3. Results and Discussion
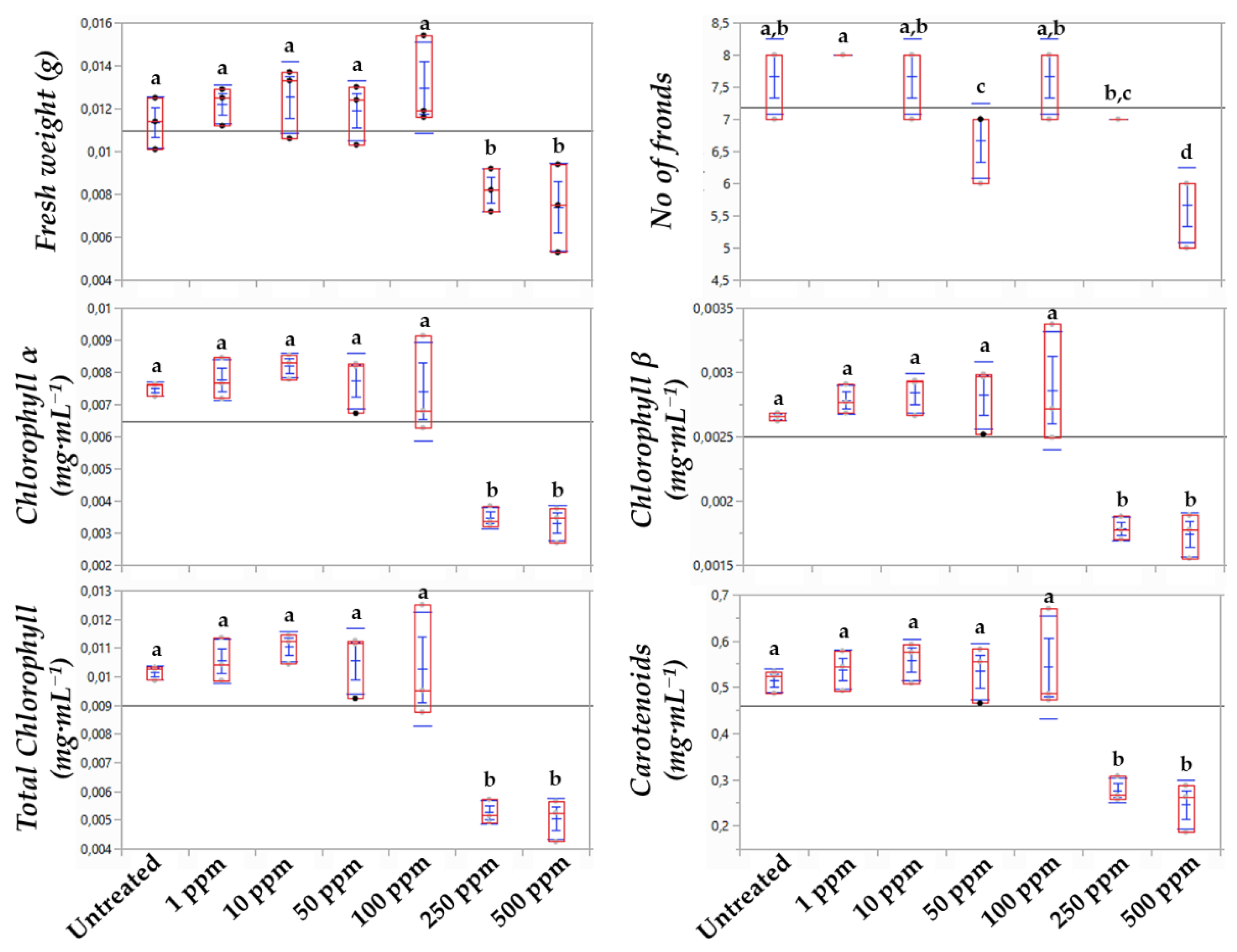
3.1. The Chlorophyl α Content Is the Most Sensitive Indicator of the Bacillus sp. Lipopeptide Extract’s Toxicity to Lemna minor L.
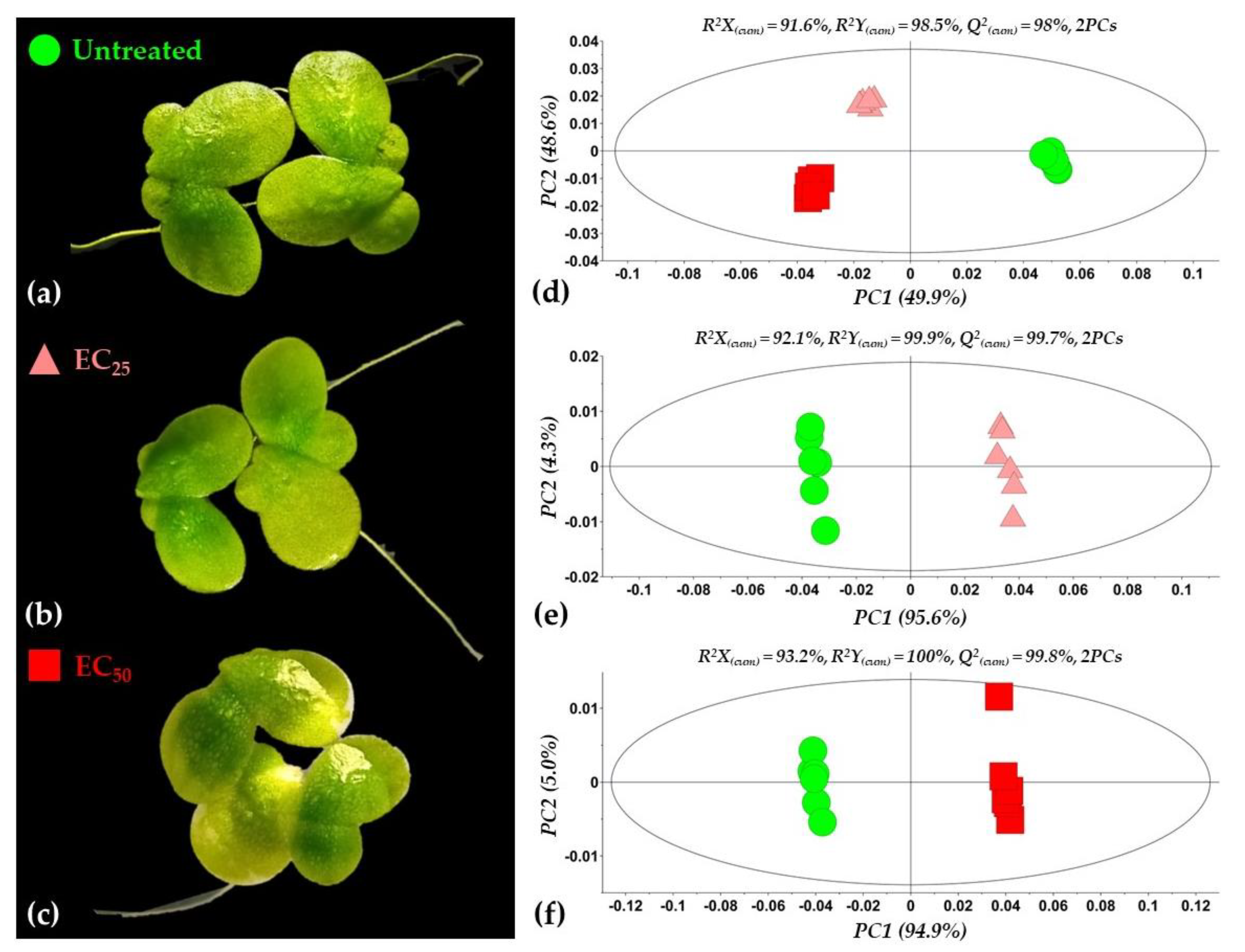
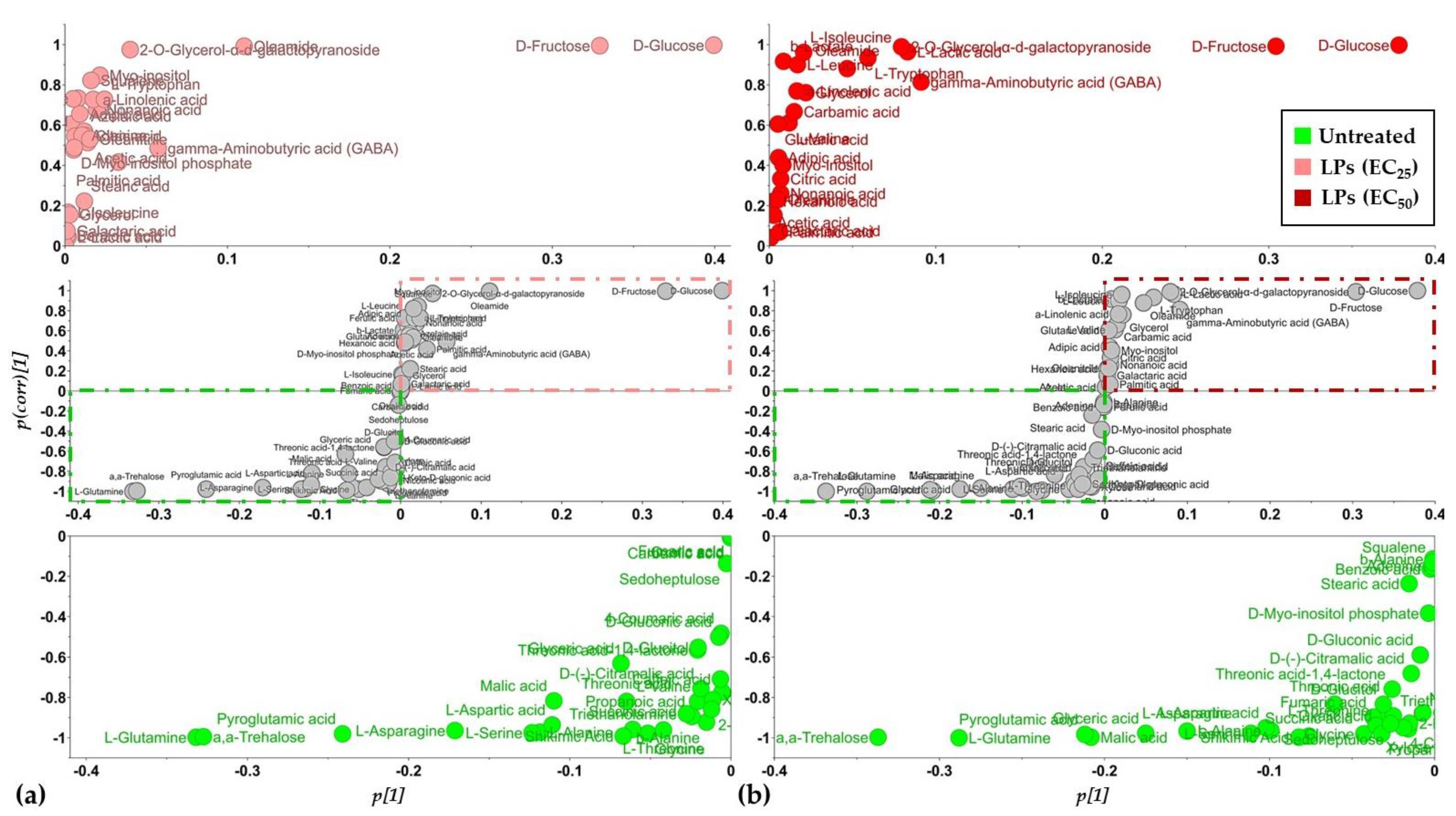
3.2. Overview of the Metabolomics Analysis
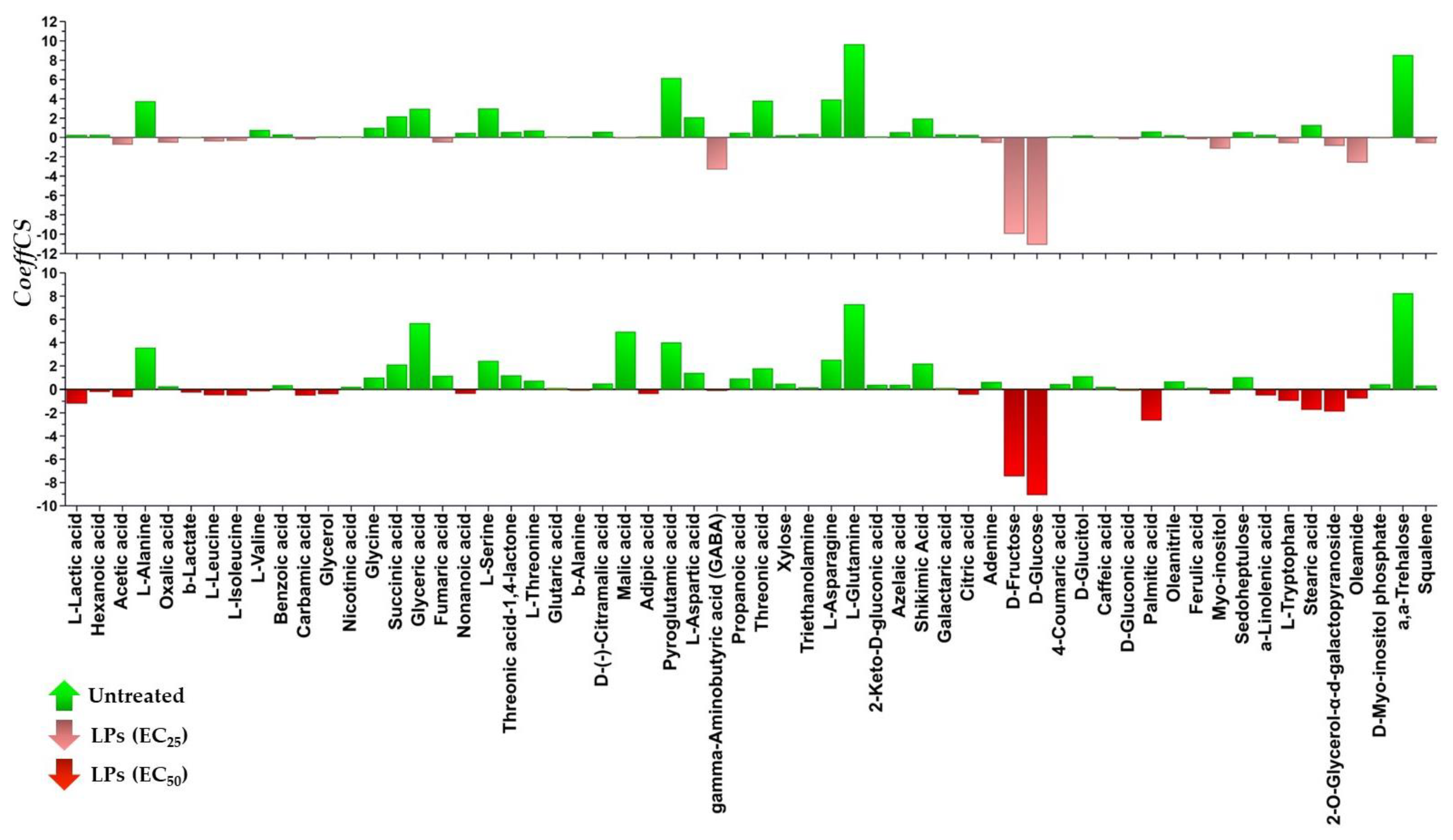
3.3. The Lipopeptide Extract of the Olive Tree Endophytic Bacillus sp. PTA13 Substantially Affects the Amino Acid Pool, the Energy Equilibrium and the Levels of Metabolites That Play Key Roles in the Physiology of Lemna minor L. Plants
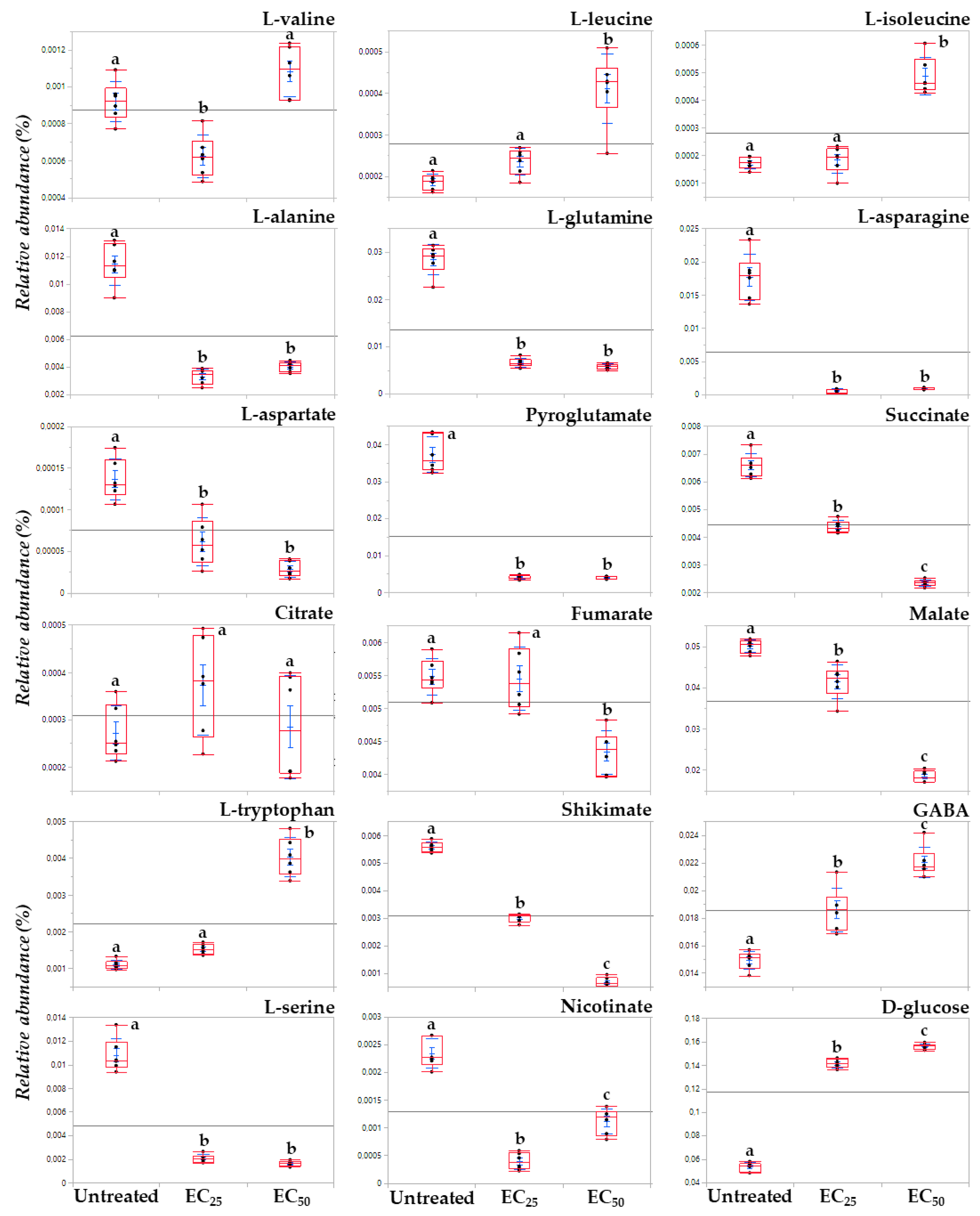
3.3.1. Fluctuation of the Amino Acid (AA) Pool of Lemna minor L.
3.3.2. Induction of Systemic Jasmonate-Mediated Signaling Mechanism of Lemna minor L.
3.3.3. Lipopeptides Affect the Energy Equilibrium of Lemna minor L.
3.3.4. Effect of Lipopeptides in the Content of the Plant in Metabolites with Key Role in Its Physiology
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lykogianni, M.; Bempelou, E.; Karamaouna, F.; Aliferis, K.A. Do pesticides promote or hinder sustainability in agriculture? The challenge of sustainable use of pesticides in modern agriculture. Sci. Total Environ. 2021, 795, 148625. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, F.P. Agriculture, pesticides, food security and food safety. Environ. Sci. Policy 2006, 9, 685–692. [Google Scholar] [CrossRef]
- Damalas, C.A. Safe food production with minimum and judicious use of pesticides. In Food Safety; Selamat, J., Iqbal, S.Z., Eds.; Springer Nature: Cham, Switzerland, 2016; pp. 43–55. [Google Scholar]
- Nicolopoulou-Stamati, P.; Maipas, S.; Kotampasi, C.; Stamatis, P.; Hens, L. Chemical pesticides and human health: The urgent need for a new concept in agriculture. Front. Public Health 2016, 4, 148. [Google Scholar] [CrossRef] [PubMed]
- Aliferis, K.A.; Chrysayi-Tokousbalides, M. Metabolomics in pesticide research and development: Review and future perspectives. Metabolomics 2011, 7, 35–53. [Google Scholar] [CrossRef]
- Mojiri, A.; Zhou, J.L.; Robinson, B.; Ohashi, A.; Ozaki, N.; Kindaichi, T.; Farraji, H.; Vakili, M. Pesticides in aquatic environments and their removal by adsorption methods. Chemosphere 2020, 253, 126646. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Rey, M.; Tapie, N.; Le Menach, K.; Devier, M.-H.; Budzinski, H.; Bebianno, M.J. Occurrence of pharmaceutical compounds and pesticides in aquatic systems. Mar. Pollut. Bull. 2015, 96, 384–400. [Google Scholar] [CrossRef]
- deNoyelles, F.; Dewey, S.L.; Huggins, D.G.; Kettle, W.D. Aquatic mesocosms in ecological effects testing: Detecting direct and indirect effects of pesticides. In Aquatic Mesocosm Studies in Ecological Risk Assessment; Graney, R.L., Ed.; CRC Press: Boca Raton, FL, USA, 2020; pp. 577–603. [Google Scholar]
- Fadiji, A.E.; Babalola, O.O. Elucidating mechanisms of endophytes used in plant protection and other bioactivities with multifunctional prospects. Front. Bioeng. Biotechnol. 2020, 8, 467. [Google Scholar] [CrossRef]
- Dini-Andreote, F. Endophytes: The second layer of plant defense. Trends Plant Sci. 2020, 25, 319–322. [Google Scholar] [CrossRef]
- Bamisile, B.S.; Dash, C.K.; Akutse, K.S.; Keppanan, R.; Wang, L. Fungal endophytes: Beyond herbivore management. Front. Microbiol. 2018, 9, 544. [Google Scholar] [CrossRef]
- Gouda, S.; Das, G.; Sen, S.K.; Shin, H.-S.; Patra, J.K. Endophytes: A treasure house of bioactive compounds of medicinal importance. Front. Microbiol. 2016, 7, 1538. [Google Scholar] [CrossRef] [Green Version]
- De Silva, N.I.; Brooks, S.; Lumyong, S.; Hyde, K.D. Use of endophytes as biocontrol agents. Fungal Biol. Rev. 2019, 33, 133–148. [Google Scholar] [CrossRef]
- Khare, E.; Mishra, J.; Arora, N.K. Multifaceted interactions between endophytes and plant: Developments and prospects. Front. Microbiol. 2018, 9, 2732. [Google Scholar] [CrossRef]
- Bastías, D.A.; Gianoli, E.; Gundel, P.E. Fungal endophytes can eliminate the plant growth–defence trade-off. New Phytol. 2021, 230, 2105–2113. [Google Scholar] [CrossRef] [PubMed]
- Rai, N.; Kumari Keshri, P.; Verma, A.; Kamble, S.C.; Mishra, P.; Barik, S.; Kumar Singh, S.; Gautam, V. Plant associated fungal endophytes as a source of natural bioactive compounds. Mycology 2021, 12, 139–159. [Google Scholar] [CrossRef] [PubMed]
- Rustamova, N.; Bozorov, K.; Efferth, T.; Yili, A. Novel secondary metabolites from endophytic fungi: Synthesis and biological properties. Phytochem. Rev. 2020, 19, 425–448. [Google Scholar] [CrossRef]
- Gao, H.; Li, G.; Lou, H.-X. Structural diversity and biological activities of novel secondary metabolites from endophytes. Molecules 2018, 23, 646. [Google Scholar] [CrossRef]
- Papadopoulou, E.-A.; Angelis, A.; Antoniadi, L.; Aliferis, K.A.; Skaltsounis, A.-L. Discovering the Next-Generation Plant Protection Products: A Proof-of-Concept via the Isolation and Bioactivity Assessment of the Olive Tree Endophyte Bacillus sp. PTA13 Lipopeptides. Metabolites 2021, 11, 833. [Google Scholar] [CrossRef]
- Penha, R.O.; Vandenberghe, L.P.; Faulds, C.; Soccol, V.T.; Soccol, C.R. Bacillus lipopeptides as powerful pest control agents for a more sustainable and healthy agriculture: Recent studies and innovations. Planta 2020, 251, 1–15. [Google Scholar] [CrossRef]
- Fazle Rabbee, M.; Baek, K.-H. Antimicrobial activities of lipopeptides and polyketides of Bacillus velezensis for agricultural applications. Molecules 2020, 25, 4973. [Google Scholar] [CrossRef]
- Olishevska, S.; Nickzad, A.; Déziel, E. Bacillus and Paenibacillus secreted polyketides and peptides involved in controlling human and plant pathogens. Appl. Microbiol. Biotechnol. 2019, 103, 1189–1215. [Google Scholar] [CrossRef]
- Sharma, D.; Singh, S.S.; Baindara, P.; Sharma, S.; Khatri, N.; Grover, V.; Patil, P.B.; Korpole, S. Surfactin like broad spectrum antimicrobial lipopeptide co-produced with sublancin from Bacillus subtilis strain A52: Dual reservoir of bioactives. Front. Microbiol. 2020, 11, 1167. [Google Scholar] [CrossRef] [PubMed]
- Basaid, K.; Chebli, B.; Mayad, E.H.; Furze, J.N.; Bouharroud, R.; Krier, F.; Barakate, M.; Paulitz, T. Biological activities of essential oils and lipopeptides applied to control plant pests and diseases: A review. Int. J. Pest Manag. 2021, 67, 155–177. [Google Scholar] [CrossRef]
- Santos, V.S.V.; Silveira, E.; Pereira, B.B. Toxicity and applications of surfactin for health and environmental biotechnology. J. Toxicol. Environ. Health Part B 2018, 21, 382–399. [Google Scholar] [CrossRef]
- Kostopoulou, S.; Ntatsi, G.; Arapis, G.; Aliferis, K.A. Assessment of the effects of metribuzin, glyphosate, and their mixtures on the metabolism of the model plant Lemna minor L. applying metabolomics. Chemosphere 2020, 239, 124582. [Google Scholar] [CrossRef] [PubMed]
- Aliferis, K.A.; Materzok, S.; Paziotou, G.N.; Chrysayi-Tokousbalides, M. Lemna minor L. as a model organism for ecotoxicological studies performing 1H NMR fingerprinting. Chemosphere 2009, 76, 967–973. [Google Scholar] [CrossRef]
- OECD. Test No. 221: Lemna sp. Growth Inhibition Test; Organisation for Economic Co-operation and Development: Paris, France, 2006. [Google Scholar]
- ISO. Water Quality—Determination of the Toxic Effect of Water Constituents and Waste Water on Duckweed (Lemna minor)—Duckweed Growth Inhibition Test. Available online: https://www.iso.org/standard/34074.html (accessed on 7 August 2022).
- Hiscox, J.; Israelstam, G. A method for the extraction of chlorophyll from leaf tissue without maceration. Can. J. Bot. 1979, 57, 1332–1334. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1. [Google Scholar] [CrossRef]
- Kirk, J.; Allen, R. Dependence of chloroplast pigment synthesis on protein synthesis: Effect of actidione. Biochem. Biophys. Res. Commun. 1965, 21, 523–530. [Google Scholar] [CrossRef]
- Bekele, E.A.; Annaratone, C.E.; Hertog, M.L.; Nicolai, B.M.; Geeraerd, A.H. Multi-response optimization of the extraction and derivatization protocol of selected polar metabolites from apple fruit tissue for GC–MS analysis. Anal. Chim. Acta 2014, 824, 42–56. [Google Scholar] [CrossRef]
- Fiehn, O.; Robertson, D.; Griffin, J.; van der Werf, M.; Nikolau, B.; Morrison, N.; Sumner, L.W.; Goodacre, R.; Hardy, N.W.; Taylor, C. The metabolomics standards initiative (MSI). Metabolomics 2007, 3, 175–178. [Google Scholar] [CrossRef]
- Tsugawa, H.; Cajka, T.; Kind, T.; Ma, Y.; Higgins, B.; Ikeda, K.; Kanazawa, M.; VanderGheynst, J.; Fiehn, O.; Arita, M. MS-DIAL: Data-independent MS/MS deconvolution for comprehensive metabolome analysis. Nat. Methods 2015, 12, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Gitelson, A. Towards a generic approach to remote non-invasive estimation of foliar carotenoid-to-chlorophyll ratio. J. Plant Physiol. 2020, 252, 153227. [Google Scholar] [CrossRef] [PubMed]
- Fekete-Kertész, I.; Kunglné-Nagy, Z.; Gruiz, K.; Magyar, Á.; Farkas, É.; Molnár, M. Assessing toxicity of organic aquatic micropollutants based on the total chlorophyll content of Lemna minor as a sensitive endpoint. Period. Polytech. Chem. Eng. 2015, 59, 262–271. [Google Scholar] [CrossRef]
- Hildebrandt, T.M.; Nesi, A.N.; Araújo, W.L.; Braun, H.-P. Amino acid catabolism in plants. Mol. Plant 2015, 8, 1563–1579. [Google Scholar] [CrossRef]
- Wahman, R.; Cruzeiro, C.; Graßmann, J.; Schröder, P.; Letzel, T. The changes in Lemna minor metabolomic profile: A response to diclofenac incubation. Chemosphere 2022, 287, 132078. [Google Scholar] [CrossRef]
- Li, R.; Luo, C.; Qiu, J.; Li, Y.; Zhang, H.; Tan, H. Metabolomic and transcriptomic investigation of the mechanism involved in enantioselective toxicity of imazamox in Lemna minor. J. Hazard. Mater. 2022, 425, 127818. [Google Scholar] [CrossRef]
- Rai, V. Role of amino acids in plant responses to stresses. Biol. Plant. 2002, 45, 481–487. [Google Scholar] [CrossRef]
- Rodrigues-Corrêa, K.C.d.S.; Fett-Neto, A.G. Abiotic stresses and non-protein amino acids in plants. Crit. Rev. Plant Sci. 2019, 38, 411–430. [Google Scholar] [CrossRef]
- Galili, G. The aspartate-family pathway of plants: Linking production of essential amino acids with energy and stress regulation. Plant Signal. Behav. 2011, 6, 192–195. [Google Scholar] [CrossRef]
- Okumoto, S.; Funck, D.; Trovato, M.; Forlani, G. Amino acids of the glutamate family: Functions beyond primary metabolism. Front. Plant Sci. 2016, 7, 318. [Google Scholar] [CrossRef] [Green Version]
- Brzezowski, P.; Richter, A.S.; Grimm, B. Regulation and function of tetrapyrrole biosynthesis in plants and algae. Biochim. Biophys. Acta Bioenerg. 2015, 1847, 968–985. [Google Scholar] [CrossRef] [PubMed]
- Kirma, M.; Araújo, W.L.; Fernie, A.R.; Galili, G. The multifaceted role of aspartate-family amino acids in plant metabolism. J. Exp. Bot. 2012, 63, 4995–5001. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Zhang, C.; Suglo, P.; Sun, S.; Wang, M.; Su, T. L-Aspartate: An essential metabolite for plant growth and stress acclimation. Molecules 2021, 26, 1887. [Google Scholar] [CrossRef] [PubMed]
- Ros, R.; Muñoz-Bertomeu, J.; Krueger, S. Serine in plants: Biosynthesis, metabolism, and functions. Trends Plant Sci. 2014, 19, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.-L.; Saito, K. Molecular biology of the plastidic phosphorylated serine biosynthetic pathway in Arabidopsis thaliana. Amino Acids 2001, 20, 243–259. [Google Scholar] [CrossRef]
- Cascales-Miñana, B.; Muñoz-Bertomeu, J.; Flores-Tornero, M.; Anoman, A.D.; Pertusa, J.; Alaiz, M.; Osorio, S.; Fernie, A.R.; Segura, J.; Ros, R. The phosphorylated pathway of serine biosynthesis is essential both for male gametophyte and embryo development and for root growth in Arabidopsis. Plant Cell 2013, 25, 2084–2101. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Dudareva, N. The shikimate pathway and aromatic amino acid biosynthesis in plants. Annu. Rev. Plant Biol. 2012, 63, 73–105. [Google Scholar] [CrossRef]
- Bouche, N.; Fromm, H. GABA in plants: Just a metabolite? Trends Plant Sci. 2004, 9, 110–115. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, C. Fengycins, cyclic lipopeptides from marine Bacillus subtilis strains, kill the plant-pathogenic fungus Magnaporthe grisea by inducing reactive oxygen species production and chromatin condensation. Appl. Environ. Microbiol. 2018, 84, e00445-18. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, C.; Liang, J.; Wu, L.; Gao, W.; Jiang, J. Iturin A extracted from Bacillus subtilis WL-2 affects Phytophthora infestans via cell structure disruption, oxidative stress, and energy supply dysfunction. Front. Microbiol. 2020, 11, 536083. [Google Scholar] [CrossRef]
- Weber, H. Fatty acid-derived signals in plants. Trends Plant Sci. 2002, 7, 217–224. [Google Scholar] [CrossRef]
- Aliferis, K.A.; Faubert, D.; Jabaji, S. A metabolic profiling strategy for the dissection of plant defense against fungal pathogens. PLoS ONE 2014, 9, e111930. [Google Scholar]
- Wasternack, C. Action of jasmonates in plant stress responses and development-applied aspects. Biotechnol. Adv. 2014, 32, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.; Zhou, Y.; Zhou, M.; Yan, J.; Khurshid, M.; Weng, W.; Cheng, J.; Zhang, K. Jasmonic acid signaling pathway in plants. Int. J. Mol. Sci. 2019, 20, 2479. [Google Scholar] [CrossRef]
- Ghorbel, M.; Brini, F.; Sharma, A.; Landi, M. Role of jasmonic acid in plants: The molecular point of view. Plant Cell Rep. 2021, 40, 1471–1494. [Google Scholar] [CrossRef]
- Wang, J.; Song, L.; Gong, X.; Xu, J.; Li, M. Functions of jasmonic acid in plant regulation and response to abiotic stress. Int. J. Mol. Sci. 2020, 21, 1446. [Google Scholar] [CrossRef]
- Henry, G.; Deleu, M.; Jourdan, E.; Thonart, P.; Ongena, M. The bacterial lipopeptide surfactin targets the lipid fraction of the plant plasma membrane to trigger immune-related defence responses. Cell. Microbiol. 2011, 13, 1824–1837. [Google Scholar] [CrossRef]
- Farace, G.; Fernandez, O.; Jacquens, L.; Coutte, F.; Krier, F.; Jacques, P.; Clément, C.; Barka, E.A.; Jacquard, C.; Dorey, S. Cyclic lipopeptides from Bacillus subtilis activate distinct patterns of defence responses in grapevine. Mol. Plant Pathol. 2015, 16, 177–187. [Google Scholar] [CrossRef]
- Gond, S.K.; Bergen, M.S.; Torres, M.S.; White, J.F., Jr. Endophytic Bacillus spp. produce antifungal lipopeptides and induce host defence gene expression in maize. Microbiol. Res. 2015, 172, 79–87. [Google Scholar] [CrossRef]
- Pérez-García, A.; Romero, D.; De Vicente, A. Plant protection and growth stimulation by microorganisms: Biotechnological applications of Bacilli in agriculture. Curr. Opin. Biotechnol. 2011, 22, 187–193. [Google Scholar] [CrossRef]
- Falardeau, J.; Wise, C.; Novitsky, L.; Avis, T.J. Ecological and mechanistic insights into the direct and indirect antimicrobial properties of Bacillus subtilis lipopeptides on plant pathogens. J. Chem. Ecol. 2013, 39, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Schulze, J.; Tesfaye, M.; Litjens, R.; Bucciarelli, B.; Trepp, G.; Miller, S.; Samac, D.; Allan, D.; Vance, C. Malate plays a central role in plant nutrition. Plant Soil 2002, 247, 133–139. [Google Scholar] [CrossRef]
- Guo, H.; Chen, H.; Hong, C.; Jiang, D.; Zheng, B. Exogenous malic acid alleviates cadmium toxicity in Miscanthus sacchariflorus through enhancing photosynthetic capacity and restraining ROS accumulation. Ecotoxicol. Environ. Saf. 2017, 141, 119–128. [Google Scholar] [CrossRef]
- Araújo, W.L.; Nunes-Nesi, A.; Fernie, A.R. Fumarate: Multiple functions of a simple metabolite. Phytochemistry 2011, 72, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Kumar, V. Quantitative systems pharmacology: Lessons from fumaric acid and herbal remedies. Drug Des. 2017, 6, 1000152. [Google Scholar] [CrossRef]
- Ryan, D.G.; Murphy, M.P.; Frezza, C.; Prag, H.A.; Chouchani, E.T.; O’Neill, L.A.; Mills, E.L. Coupling Krebs cycle metabolites to signalling in immunity and cancer. Nat. Metab. 2019, 1, 16–33. [Google Scholar] [CrossRef]
- Erdemir, U.S.; Arslan, H.; Guleryuz, G.; Yaman, M.; Gucer, S. Manganese tolerance in Verbascum olympicum Boiss. affecting elemental uptake and distribution: Changes in nicotinic acid levels under stress conditions. Environ. Sci. Pollut. Res. 2018, 25, 29129–29143. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, Z.; Bashir, K.; Matsui, A.; Tanaka, M.; Sasaki, R.; Oikawa, A.; Hirai, M.Y.; Zu, Y.; Kawai-Yamada, M.; Rashid, B. Overexpression of nicotinamidase 3 (NIC3) gene and the exogenous application of nicotinic acid (NA) enhance drought tolerance and increase biomass in Arabidopsis. Plant Mol. Biol. 2021, 107, 63–84. [Google Scholar] [CrossRef]
- Staykov, N.S.; Angelov, M.; Petrov, V.; Minkov, P.; Kanojia, A.; Guinan, K.J.; Alseekh, S.; Fernie, A.R.; Sujeeth, N.; Gechev, T.S. An Ascophyllum nodosum-derived biostimulant protects model and crop plants from oxidative stress. Metabolites 2020, 11, 24. [Google Scholar] [CrossRef]
- Graf, E. Antioxidant potential of ferulic acid. Free Radic. Biol. Med. 1992, 13, 435–448. [Google Scholar] [CrossRef]
- Mandal, S.M.; Chakraborty, D.; Dey, S. Phenolic acids act as signaling molecules in plant-microbe symbioses. Plant Signal. Behav. 2010, 5, 359–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lattanzio, V.; Lattanzio, V.M.; Cardinali, A. Role of phenolics in the resistance mechanisms of plants against fungal pathogens and insects. Phytochem. Adv. Res. 2006, 661, 23–67. [Google Scholar]
- Goddijn, O.J.; van Dun, K. Trehalose metabolism in plants. Trends Plant Sci. 1999, 4, 315–319. [Google Scholar] [CrossRef]
- Elbein, A.D.; Pan, Y.; Pastuszak, I.; Carroll, D. New insights on trehalose: A multifunctional molecule. Glycobiology 2003, 13, 17R–27R. [Google Scholar] [CrossRef] [PubMed]
- Eastmond, P.J.; Van Dijken, A.J.; Spielman, M.; Kerr, A.; Tissier, A.F.; Dickinson, H.G.; Jones, J.D.; Smeekens, S.C.; Graham, I.A. Trehalose-6-phosphate synthase 1, which catalyses the first step in trehalose synthesis, is essential for Arabidopsis embryo maturation. Plant J. 2002, 29, 225–235. [Google Scholar] [CrossRef]
- Satoh-Nagasawa, N.; Nagasawa, N.; Malcomber, S.; Sakai, H.; Jackson, D. A trehalose metabolic enzyme controls inflorescence architecture in maize. Nature 2006, 441, 227–230. [Google Scholar] [CrossRef]
- Gómez, L.D.; Baud, S.; Gilday, A.; Li, Y.; Graham, I.A. Delayed embryo development in the Arabidopsis trehalose-6-phosphate synthase 1 mutant is associated with altered cell wall structure, decreased cell division and starch accumulation. Plant J. 2006, 46, 69–84. [Google Scholar] [CrossRef]
- John, R.; Raja, V.; Ahmad, M.; Jan, N.; Majeed, U.; Ahmad, S.; Yaqoob, U.; Kaul, T. Trehalose: Metabolism and role in stress signaling in plants. In Stress Signaling in Plants: Genomics and Proteomics Perspective Volume 2; Sarwat, M., Ahmad, A., Abdin, M.Z., Ibrahim, M.M., Eds.; Springer Nature: Cham, Switzerland, 2016; pp. 261–275. [Google Scholar]
- Banat, I.M.; Satpute, S.K.; Cameotra, S.S.; Patil, R.; Nyayanit, N.V. Cost effective technologies and renewable substrates for biosurfactants’ production. Front. Microbiol. 2014, 5, 697. [Google Scholar] [CrossRef]
- Chen, W.C.; Juang, R.S.; Wei, Y.H. Applications of a lipopeptide biosurfactant, surfactin, produced by microorganisms. Biochem. Eng. J. 2015, 103, 158–169. [Google Scholar] [CrossRef]
- Zhi, Y.; Wu, Q.; Xu, Y. Genome and transcriptome analysis of surfactin biosynthesis in Bacillus amyloliquefaciens MT45. Sci. Rep. 2017, 7, 40976. [Google Scholar] [CrossRef]
- Paraszkiewicz, K.; Bernat, P.; Kuśmierska, A.; Chojniak, J.; Płaza, G. Structural identification of lipopeptide biosurfactants produced by Bacillus subtilis strains grown on the media obtained from renewable natural resources. J. Environ. Manage. 2018, 209, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Yu, K.O.; Ramzi, A.B.; Choe, S.H.; Kim, S.W.; Han, S.O. Improvement of surfactin production in Bacillus subtilis using synthetic wastewater by overexpression of specific extracellular signaling peptides, comX and phrC. Biotechnol. Bioeng. 2012, 109, 2349–2356. [Google Scholar] [CrossRef] [PubMed]
- Jiao, S.; Li, X.; Yu, H.; Yang, H.; Li, X.; Shen, Z. In situ enhancement of surfactin biosynthesis in Bacillus subtilis using novel artificial inducible promoters. Biotechnol. Bioeng. 2017, 114, 832–842. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yu, H.; Wang, M.; Yang, H.; Shen, Z. Enhanced biosynthesis and characterization of surfactin isoforms with engineered Bacillus subtilis through promoter replacement and Vitreoscilla hemoglobin co-expression. Process Biochem. 2018, 70, 36–44. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papadopoulou, E.-A.; Giaki, K.; Angelis, A.; Skaltsounis, A.-L.; Aliferis, K.A. A Metabolomic Approach to Assess the Toxicity of the Olive Tree Endophyte Bacillus sp. PTA13 Lipopeptides to the Aquatic Macrophyte Lemna minor L. Toxics 2022, 10, 494. https://doi.org/10.3390/toxics10090494
Papadopoulou E-A, Giaki K, Angelis A, Skaltsounis A-L, Aliferis KA. A Metabolomic Approach to Assess the Toxicity of the Olive Tree Endophyte Bacillus sp. PTA13 Lipopeptides to the Aquatic Macrophyte Lemna minor L. Toxics. 2022; 10(9):494. https://doi.org/10.3390/toxics10090494
Chicago/Turabian StylePapadopoulou, Evgenia-Anna, Katerina Giaki, Apostolis Angelis, Alexios-Leandros Skaltsounis, and Konstantinos A. Aliferis. 2022. "A Metabolomic Approach to Assess the Toxicity of the Olive Tree Endophyte Bacillus sp. PTA13 Lipopeptides to the Aquatic Macrophyte Lemna minor L." Toxics 10, no. 9: 494. https://doi.org/10.3390/toxics10090494
APA StylePapadopoulou, E.-A., Giaki, K., Angelis, A., Skaltsounis, A.-L., & Aliferis, K. A. (2022). A Metabolomic Approach to Assess the Toxicity of the Olive Tree Endophyte Bacillus sp. PTA13 Lipopeptides to the Aquatic Macrophyte Lemna minor L. Toxics, 10(9), 494. https://doi.org/10.3390/toxics10090494





