Environmental Risk Assessment of Oxaliplatin Exposure on Early Life Stages of Zebrafish (Danio rerio)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Solutions Preparation
2.2. Zebrafish Maintenance and Breeding
- (a)
- Live non-human vertebrate animals, including: (i) independently feeding larval forms; and (ii) foetal forms of mammals as from the last third of their normal development;
- (b)
- Live cephalopods.
2.3. Zebrafish Embryo Toxicity (ZFET) Assay
- (a)
- embryo coagulation—can also occur within a few hours of the start of exposure and indicates a generic acute toxic effect;
- (b)
- lack of somite formation—somite should be visible 12 h after fertilization; if absent, the embryo will not develop further, thus causing its death;
- (c)
- non-detachment of the tail—detachment of the tail from the yolk can be observed 24 h after fertilization, indicating normal growth of the embryo;
- (d)
- absence of heartbeat—the heartbeat is easily detectable 30 h after fertilization, its absence indicates the death of the embryo; embryo coagulation and absence of heartbeat were focused, as endpoints of mortality.
2.4. Histopathological Analysis
2.5. Total RNA Extraction and RT-PCR
2.6. Cell Death and Image Analysis
2.7. SOD and CAT Measurement
2.8. Data Analysis
3. Results
3.1. Mortality, Hatching Rate and Malformations
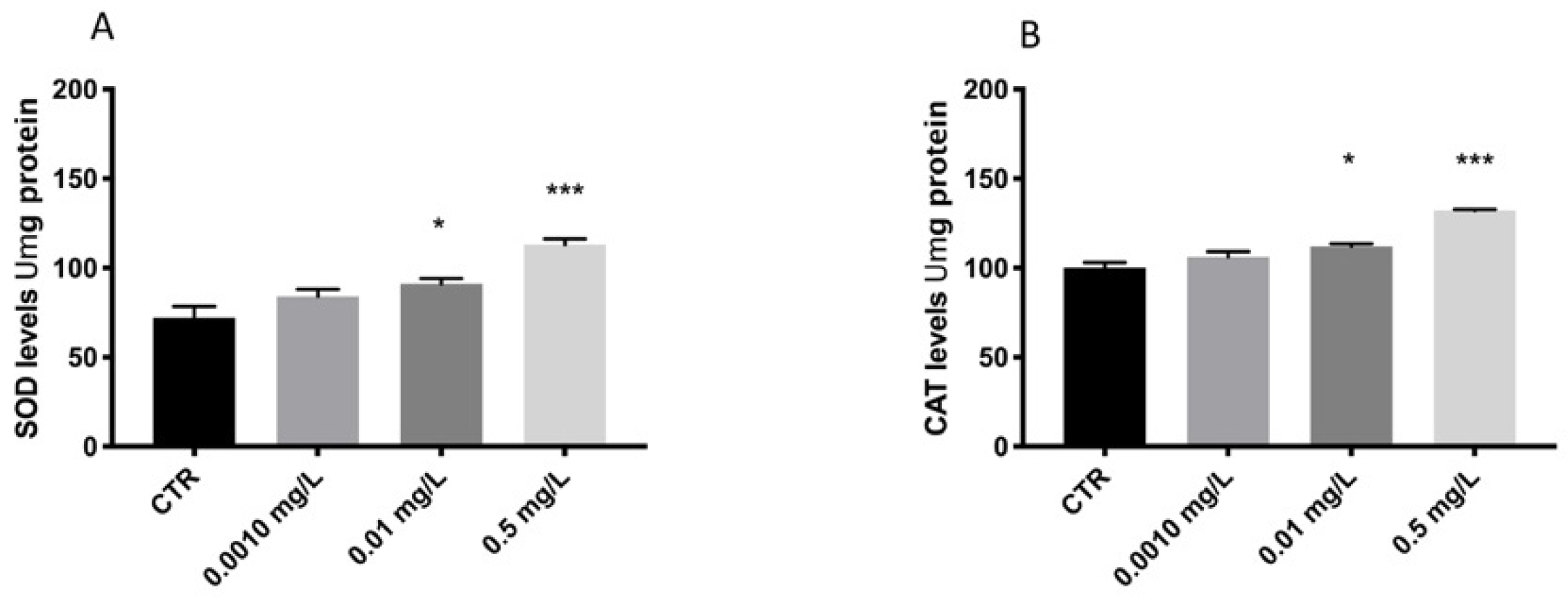
3.2. OXA Effect on Stress Oxidative Pathway
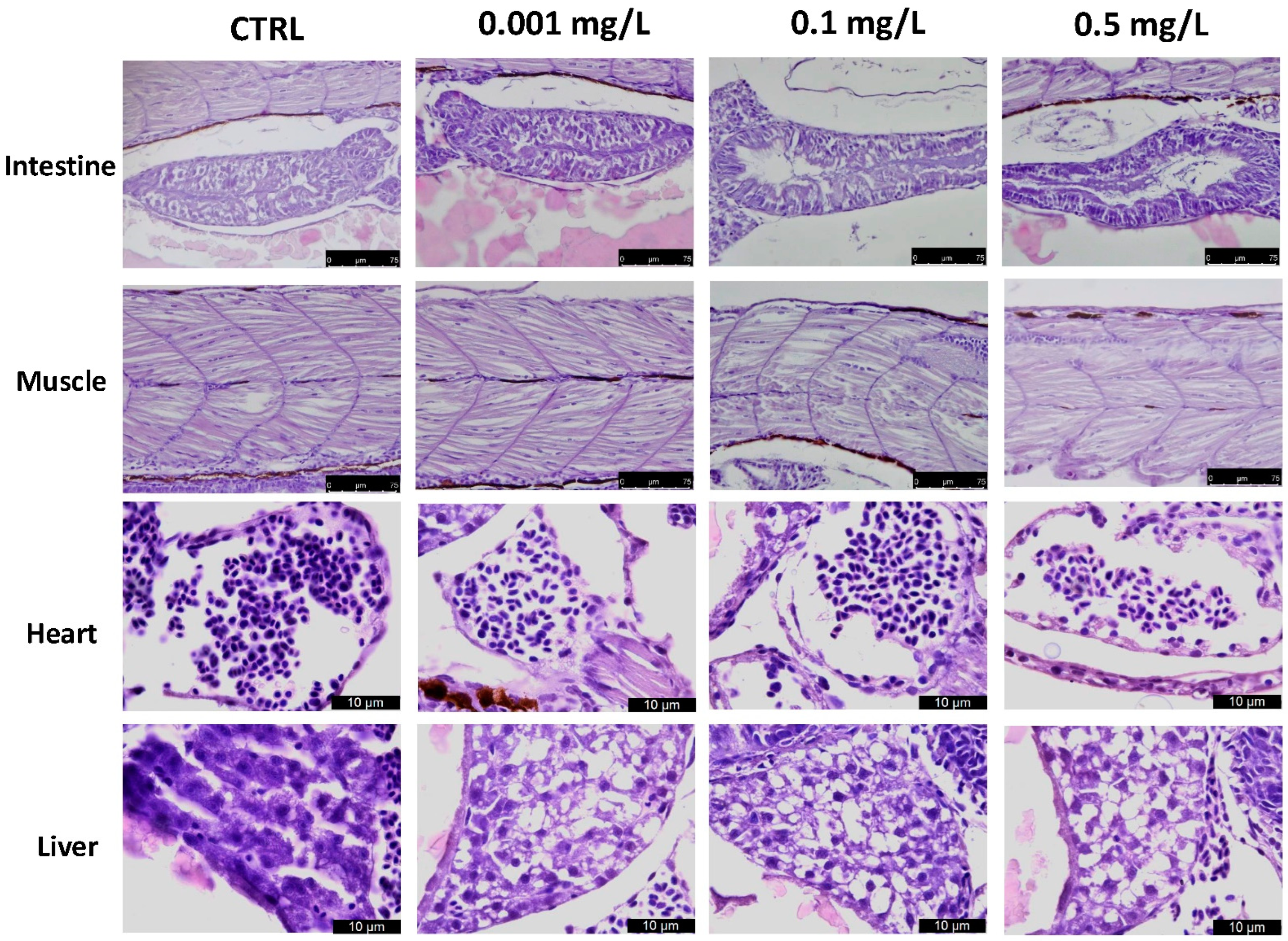
3.3. Histological Analysis
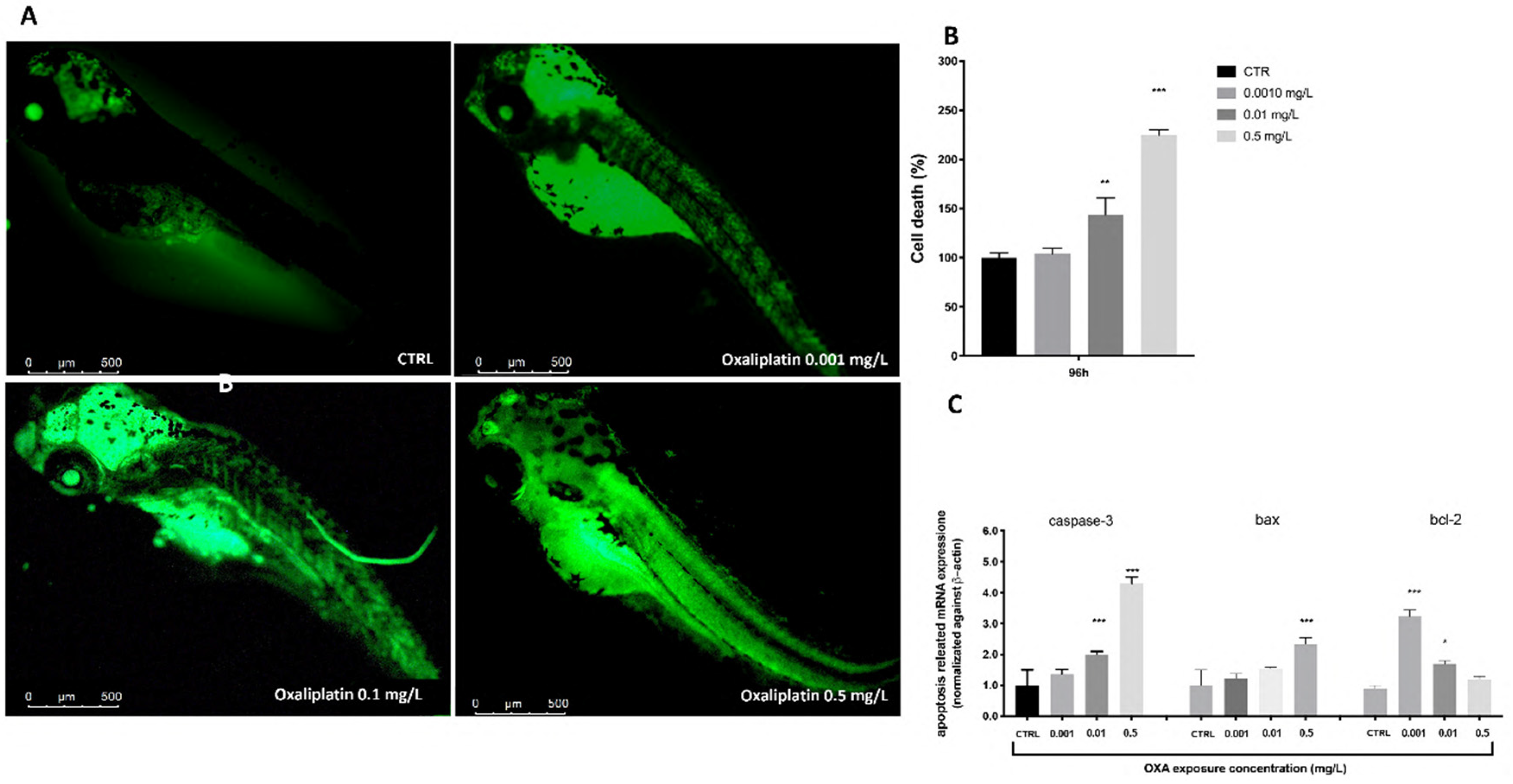
3.4. Cell Death Process
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Poklar, N.a.; Pilch, D.S.; Lippard, S.J.; Redding, E.A.; Dunham, S.U.; Breslauer, K.J. Influence of cisplatin intrastrand crosslinking on the conformation, thermal stability, and energetics of a 20-mer DNA duplex. Proc. Natl. Acad. Sci. USA 1996, 93, 7606–7611. [Google Scholar] [CrossRef]
- Rudd, G.; Hartley, J.; Souhami, R. Persistence of cisplatin-induced DNA interstrand crosslinking in peripheral blood mononuclear cells from elderly and young individuals. Cancer Chemother. Pharmacol. 1995, 35, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Osterauer, R.; Fassbender, C.; Braunbeck, T.; Kohler, H.R. Genotoxicity of platinum in embryos of zebrafish (Danio rerio) and ramshorn snail (Marisa cornuarietis). Sci. Total Environ. 2011, 409, 2114–2119. [Google Scholar] [CrossRef] [PubMed]
- Karas, B.F.; Hotz, J.M.; Buckley, B.T.; Cooper, K.R. Cisplatin alkylating activity in zebrafish causes resistance to chorionic degradation and inhibition of osteogenesis. Aquat. Toxicol. 2020, 229, 105656. [Google Scholar] [CrossRef] [PubMed]
- Raymond, E.; Faivre, S.; Woynarowski, J.M.; Chaney, S.G. Oxaliplatin: Mechanism of action and antineoplastic activity. Semin. Oncol. 1998, 25, 4–12. [Google Scholar]
- Jerremalm, E. Biotransformation of the Antineoplastic Drug Oxaliplatin: Importance for Effects and Side Effects. Institutionen för onkologi-patologi/Department of Oncology-Pathology; Caroline Institute: Stockholm, Sweden, 4 April 2008. [Google Scholar]
- Mezzelani, M.; Gorbi, S.; Regoli, F. Pharmaceuticals in the aquatic environments: Evidence of emerged threat and future challenges for marine organisms. Mar. Environ. Res. 2018, 140, 41–60. [Google Scholar] [CrossRef]
- Aguirre-Martinez, G.V.; DelValls, T.A.; Martin-Diaz, M.L. General stress, detoxification pathways, neurotoxicity and genotoxicity evaluated in Ruditapes philippinarum exposed to human pharmaceuticals. Ecotoxicol. Environ. Saf. 2016, 124, 18–31. [Google Scholar] [CrossRef]
- Aherne, G.W.; Hardcastle, A.; Nield, A.H. Cytotoxic drugs and the aquatic environment: Estimation of bleomycin in river and water samples. J. Pharm. Pharmacol. 1990, 42, 741–742. [Google Scholar] [CrossRef]
- Lenz, K.; Koellensperger, G.; Hann, S.; Weissenbacher, N.; Mahnik, S.N.; Fuerhacker, M. Fate of cancerostatic platinum compounds in biological wastewater treatment of hospital effluents. Chemosphere 2007, 69, 1765–1774. [Google Scholar] [CrossRef]
- Lenz, K.; Hann, S.; Koellensperger, G.; Stefanka, Z.; Stingeder, G.; Weissenbacher, N.; Mahnik, S.N.; Fuerhacker, M. Presence of cancerostatic platinum compounds in hospital wastewater and possible elimination by adsorption to activated sludge. Sci. Total Environ. 2005, 345, 141–152. [Google Scholar] [CrossRef]
- Wildner, H. Kalibration, Probenzufuhr und Detektion fur diePlatinbestimmung im sub pg/g-Bereich. In Proceedings of the 3 Platin—Anwendertreffen, Munich, Germermy, 14–15 October 1996; Lustig, S., Schramel, P., Eds.; PGSP-Forschungszentrum, Institute fur Ecologische Chemie: Munich, Germermy. [Google Scholar]
- Alt, F.; Eschnauer, H.; Mergler, B.; Messerschmidt, J.; Tölg, G. A contribution to the ecology and enology of platinum. Fresenius’ J. Anal. Chem. 1997, 357, 1013–1019. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, J.; Cheng, L.; Zheng, Y.; Xu, J. Sediment and salinity effects on the bioaccumulation of sulfamethoxazole in zebrafish (Danio rerio). Chemosphere 2017, 180, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Grabicova, K.; Lindberg, R.H.; Östman, M.; Grabic, R.; Randak, T.; Larsson, D.J.; Fick, J. Tissue-specific bioconcentration of antidepressants in fish exposed to effluent from a municipal sewage treatment plant. Sci. Total Environ. 2014, 488, 46–50. [Google Scholar] [CrossRef]
- Nakamura, Y.; Yamamoto, H.; Sekizawa, J.; Kondo, T.; Hirai, N.; Tatarazako, N. The effects of pH on fluoxetine in Japanese medaka (Oryzias latipes): Acute toxicity in fish larvae and bioaccumulation in juvenile fish. Chemosphere 2008, 70, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Grabicova, K.; Grabic, R.; Fedorova, G.; Fick, J.; Cerveny, D.; Kolarova, J.; Turek, J.; Zlabek, V.; Randak, T. Bioaccumulation of psychoactive pharmaceuticals in fish in an effluent dominated stream. Water Res. 2017, 124, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, A.J.; Brain, R.A.; Usenko, S.; Mottaleb, M.A.; O’Donnell, J.G.; Stahl, L.L.; Wathen, J.B.; Snyder, B.D.; Pitt, J.L.; Perez-Hurtado, P. Occurrence of pharmaceuticals and personal care products in fish: Results of a national pilot study in the United States. Environ. Toxicol. Chem. 2009, 28, 2587–2597. [Google Scholar] [CrossRef]
- Steinbach, C.; Fedorova, G.; Prokes, M.; Grabicova, K.; Machova, J.; Grabic, R.; Valentova, O.; Kroupova, H.K. Toxic effects, bioconcentration and depuration of verapamil in the early life stages of common carp (Cyprinus carpio L.). Sci. Total Environ. 2013, 461, 198–206. [Google Scholar] [CrossRef]
- Li, D.; Chen, H.; Liu, H.; Schlenk, D.; Mu, J.; Lacorte, S.; Ying, G.-G.; Xie, L. Anticancer drugs in the aquatic ecosystem: Environmental occurrence, ecotoxicological effect and risk assessment. Environ. Int. 2021, 153, 106543. [Google Scholar] [CrossRef]
- Isidori, M.; Lavorgna, M.; Russo, C.; Kundi, M.; Žegura, B.; Novak, M.; Filipič, M.; Mišík, M.; Knasmueller, S.; de Alda, M.L. Chemical and toxicological characterisation of anticancer drugs in hospital and municipal wastewaters from Slovenia and Spain. Environ. Pollut. 2016, 219, 275–287. [Google Scholar] [CrossRef]
- Zhang, C.; Willett, C.; Fremgen, T. Zebrafish: An animal model for toxicological studies. Curr. Protoc. Toxicol. 2003, 17, 1–7. [Google Scholar] [CrossRef]
- McGrath, P.; Li, C.-Q. Zebrafish: A predictive model for assessing drug-induced toxicity. Drug Discov. Today 2008, 13, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Parenti, C.C.; Ghilardi, A.; Della Torre, C.; Magni, S.; Del Giacco, L.; Binelli, A. Evaluation of the infiltration of polystyrene nanobeads in zebrafish embryo tissues after short-term exposure and the related biochemical and behavioural effects. Environ. Pollut. 2019, 254, 112947. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, K.; Hassan, H.M.; Guo, H.; Ding, P.; Han, L.; He, Q.; Chen, W.; Hsiao, C.-D.; Zhang, L. Liver fatty acid binding protein deficiency provokes oxidative stress, inflammation, and apoptosis-mediated hepatotoxicity induced by pyrazinamide in zebrafish larvae. Antimicrob. Agents Chemother. 2016, 60, 7347–7356. [Google Scholar] [CrossRef]
- Zou, Y.; Fu, X.; Liu, N.; Duan, D.; Wang, X.; Xu, J.; Gao, X. The synergistic anti-inflammatory activities of agaro-oligosaccharides with different degrees of polymerization. J. Appl. Phycol. 2019, 31, 2547–2558. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Wei, Y.; Zhang, H.; Xu, M.; Dai, J. Induction of time-dependent oxidative stress and related transcriptional effects of perfluorododecanoic acid in zebrafish liver. Aquat. Toxicol. 2008, 89, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Zhang, X.; Shu, L.; Chen, L.; Sun, L.; Qian, H.; Liu, W.; Fu, Z. Oxidative stress response and gene expression with atrazine exposure in adult female zebrafish (Danio rerio). Chemosphere 2010, 78, 846–852. [Google Scholar] [CrossRef]
- Jin, Y.; Wang, L.; Ruan, M.; Liu, J.; Yang, Y.; Zhou, C.; Xu, B.; Fu, Z. Cypermethrin exposure during puberty induces oxidative stress and endocrine disruption in male mice. Chemosphere 2011, 84, 124–130. [Google Scholar] [CrossRef]
- Stucki, G.E.; Alexander, M.A. Role of dissolution rate and solubility in biodegradation of aromatic compounds. Appl. Environ. Microbiol. 1987, 53, 2603. [Google Scholar] [CrossRef]
- Jin, Y.; Liu, Z.; Liu, F.; Ye, Y.; Peng, T.; Fu, Z. Embryonic exposure to cadmium (II) and chromium (VI) induce behavioral alterations, oxidative stress and immunotoxicity in zebrafish (Danio rerio). Neurotoxicol. Teratol. 2015, 48, 9–17. [Google Scholar] [CrossRef]
- Nastrucci, C.; Cesario, A.; Russo, P. Anticancer drug discovery from the marine environment. Recent Pat. Anticancer Drug Discov. 2012, 7, 218–232. [Google Scholar] [CrossRef]
- Hylland, K.; Robinson, C.D.; Burgeot, T.; Martínez-Gómez, C.; Lang, T.; Svavarsson, J.; Thain, J.E.; Vethaak, A.D.; Gubbins, M.J. Integrated chemical and biological assessment of contaminant impacts in selected European coastal and offshore marine areas. Mar. Environ. Res. 2017, 124, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Vethaak, A.D.; Davies, I.M.; Thain, J.E.; Gubbins, M.J.; Martínez-Gómez, C.; Robinson, C.D.; Moffat, C.F.; Burgeot, T.; Maes, T.; Wosniok, W. Integrated indicator framework and methodology for monitoring and assessment of hazardous substances and their effects in the marine environment. Mar. Environ. Res. 2017, 124, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Klatte, S.; Schaefer, H.-C.; Hempel, M. Pharmaceuticals in the environment—A short review on options to minimize the exposure of humans, animals and ecosystems. Sustain. Chem. Pharm. 2017, 5, 61–66. [Google Scholar] [CrossRef]
- Oliveira, R.; Domingues, I.; Grisolia, C.K.; Soares, A.M. Effects of triclosan on zebrafish early-life stages and adults. Environ. Sci. Pollut. Res. 2009, 16, 679–688. [Google Scholar] [CrossRef]
- Jiao, L.; Li, D.-D.; Yang, C.-L.; Peng, R.-Q.; Guo, Y.-Q.; Zhang, X.-S.; Zhu, X.-F. Reactive oxygen species mediate oxaliplatin-induced epithelial-mesenchymal transition and invasive potential in colon cancer. Tumor Biol. 2016, 37, 8413–8423. [Google Scholar] [CrossRef]
- Samaee, S.-M.; Rabbani, S.; Jovanović, B.; Mohajeri-Tehrani, M.R.; Haghpanah, V. Efficacy of the hatching event in assessing the embryo toxicity of the nano-sized TiO2 particles in zebrafish: A comparison between two different classes of hatching-derived variables. Ecotoxicol. Environ. Saf. 2015, 116, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhu, Y.; Luo, G.-Z.; Wang, X.; Yue, Y.; Wang, X.; Zong, X.; Chen, K.; Yin, H.; Fu, Y. Abundant DNA 6mA methylation during early embryogenesis of zebrafish and pig. Nat. Commun. 2016, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Papiya, S.; Kanamadi, R. Effect of mercurial fungicide Emisan®-6 on the embryonic developmental stages of zebrafish, Brachydanio (Danio rerio). J. Adv. Zool. 2000, 21, 12–18. [Google Scholar]
- Ismail, A.; Yusof, S. Effect of mercury and cadmium on early life stages of Java medaka (Oryzias javanicus): A potential tropical test fish. Mar. Pollut. Bull. 2011, 63, 347–349. [Google Scholar] [CrossRef]
- Baillie, T.A.; Rettie, A.E. Role of biotransformation in drug-induced toxicity: Influence of intra-and inter-species differences in drug metabolism. Drug Metab. Pharmacokinet. 2011, 26, 15–29. [Google Scholar] [CrossRef]
- Leung, L.; Kalgutkar, A.S.; Obach, R.S. Metabolic activation in drug-induced liver injury. Drug Metab. Rev. 2012, 44, 18–33. [Google Scholar] [CrossRef] [PubMed]
- Wijesinghe, W.; Jeon, Y.J.; Ramasamy, P.; Wahid, M.E.A.; Vairappan, C.S. Anticancer activity and mediation of apoptosis in human HL-60 leukaemia cells by edible sea cucumber (Holothuria edulis) extract. Food Chem. 2013, 139, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Franco, R.; Sánchez-Olea, R.; Reyes-Reyes, E.M.; Panayiotidis, M.I. Environmental toxicity, oxidative stress and apoptosis: Menage a trois. Mutat. Res. Genet. Toxicol. Environ. Mutagenesis 2009, 674, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Ozben, T. Oxidative stress and apoptosis: Impact on cancer therapy. J. Pharm. Sci. 2007, 96, 2181–2196. [Google Scholar] [CrossRef]
- Choi, J.E.; Kim, S.; Ahn, J.H.; Youn, P.; Kang, J.S.; Park, K.; Yi, J.; Ryu, D.-Y. Induction of oxidative stress and apoptosis by silver nanoparticles in the liver of adult zebrafish. Aquat. Toxicol. 2010, 100, 151–159. [Google Scholar] [CrossRef]
- Xia, Q.; Wei, L.; Zhang, Y.; Kong, H.; Shi, Y.; Wang, X.; Chen, X.; Han, L.; Liu, K. Psoralen induces developmental toxicity in zebrafish embryos/larvae through oxidative stress, apoptosis, and energy metabolism disorder. Front. Pharmacol. 2018, 9, 1457. [Google Scholar] [CrossRef]
- Faivre, S.; Chan, D.; Salinas, R.; Woynarowska, B.; Woynarowski, J.M. DNA strand breaks and apoptosis induced by oxaliplatin in cancer cells. Biochem. Pharmacol. 2003, 66, 225–237. [Google Scholar] [CrossRef]
- Arango, D.; Wilson, A.; Shi, Q.; Corner, G.; Aranes, M.; Nicholas, C.; Lesser, M.; Mariadason, J.; Augenlicht, L. Molecular mechanisms of action and prediction of response to oxaliplatin in colorectal cancer cells. Br. J. Cancer 2004, 91, 1931–1946. [Google Scholar] [CrossRef]
- William-Faltaos, S.; Rouillard, D.; Lechat, P.; Bastian, G. Cell cycle arrest and apoptosis induced by oxaliplatin (L-OHP) on four human cancer cell lines. Anticancer Res. 2006, 26, 2093–2099. [Google Scholar]

| Gene | Primer Orientation | Nucleotide Sequence |
|---|---|---|
| b-actin | forward | 5′-AGAGCTATGAGCTGCCTGACG-3′ |
| reverse | 5′-CCGCAAGATTCCATACCCA-3′ | |
| casp-3 | forward | 5′-CCGCTGCCCATCACTA-3′ |
| reverse | 5′-ATCCTTTCACGACCATCT-3′ | |
| Bax | forward | 5′-GGCTATTTCAACCAGGGTTCC-3′ |
| reverse | 5′-TGCGAATCACCAATGCTGT-3′ | |
| bcl-2 | forward | 5′-TCACTCGTTCAGACCCTCAT-3′ |
| reverse | 5′-ACGCTTTCCACGCACAT-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Paola, D.; Capparucci, F.; Abbate, J.M.; Cordaro, M.; Crupi, R.; Siracusa, R.; D’Amico, R.; Fusco, R.; Genovese, T.; Impellizzeri, D.; et al. Environmental Risk Assessment of Oxaliplatin Exposure on Early Life Stages of Zebrafish (Danio rerio). Toxics 2022, 10, 81. https://doi.org/10.3390/toxics10020081
Di Paola D, Capparucci F, Abbate JM, Cordaro M, Crupi R, Siracusa R, D’Amico R, Fusco R, Genovese T, Impellizzeri D, et al. Environmental Risk Assessment of Oxaliplatin Exposure on Early Life Stages of Zebrafish (Danio rerio). Toxics. 2022; 10(2):81. https://doi.org/10.3390/toxics10020081
Chicago/Turabian StyleDi Paola, Davide, Fabiano Capparucci, Jessica Maria Abbate, Marika Cordaro, Rosalia Crupi, Rosalba Siracusa, Ramona D’Amico, Roberta Fusco, Tiziana Genovese, Daniela Impellizzeri, and et al. 2022. "Environmental Risk Assessment of Oxaliplatin Exposure on Early Life Stages of Zebrafish (Danio rerio)" Toxics 10, no. 2: 81. https://doi.org/10.3390/toxics10020081
APA StyleDi Paola, D., Capparucci, F., Abbate, J. M., Cordaro, M., Crupi, R., Siracusa, R., D’Amico, R., Fusco, R., Genovese, T., Impellizzeri, D., Cuzzocrea, S., Spanò, N., Gugliandolo, E., & Peritore, A. F. (2022). Environmental Risk Assessment of Oxaliplatin Exposure on Early Life Stages of Zebrafish (Danio rerio). Toxics, 10(2), 81. https://doi.org/10.3390/toxics10020081















