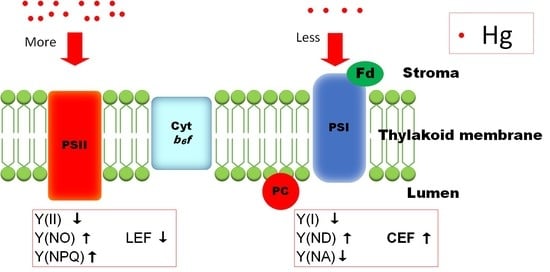
The Binding Ability of Mercury (Hg) to Photosystem I and II Explained the Difference in Its Toxicity on the Two Photosystems of Chlorella pyrenoidosa
Abstract
1. Introduction
2. Materials and Methods
2.1. Culture of Chlorella pyrenoidosa
2.2. Hg2+ Treatments
2.3. Measurement of Growth
2.4. Measurement of Pigments Content
2.5. Measurement of Activities of PSII and PSI
2.6. Measurements of Quantum Yield of Cyclic Electron Flow
2.7. Isolation of Photosystems and the Binding with Hg Ions
2.8. Statistics
3. Results
3.1. Effects of Hg2+ on Growth Rates
3.2. Effects of Hg2+ on Pigments Content
3.3. Effects of Hg2+ on the Chlorophyll a Fluorescence Parameters
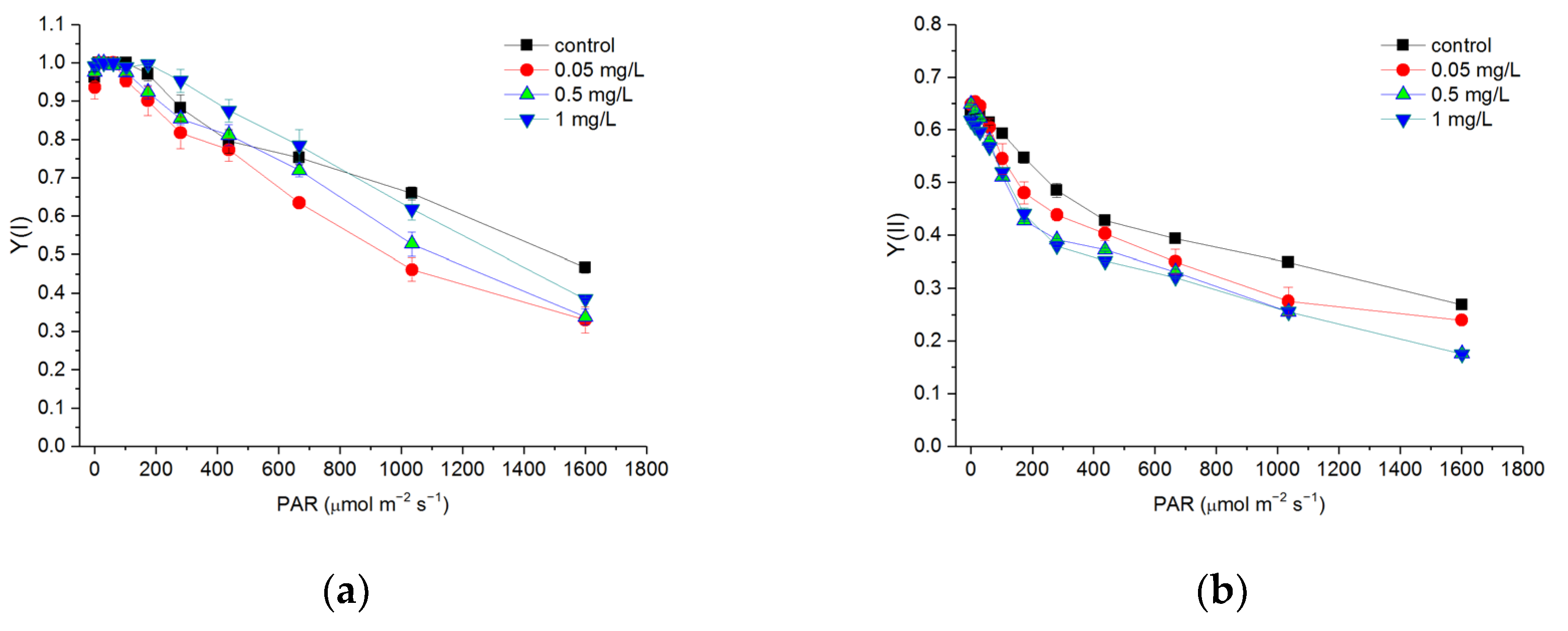
3.4. Effects of Hg2+ on Quantum Yields of PSI and PSII
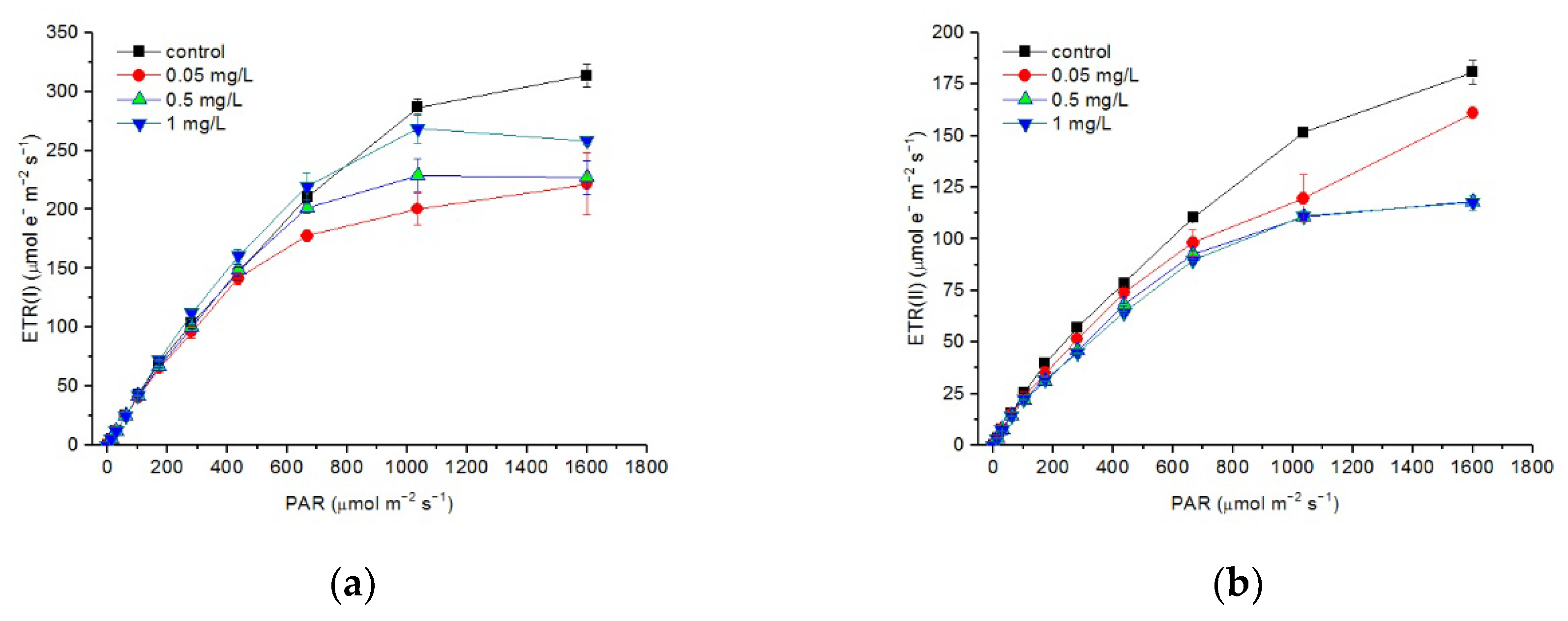
3.5. Effects of Hg2+ on Electron Transport Rates of PSI and PSII
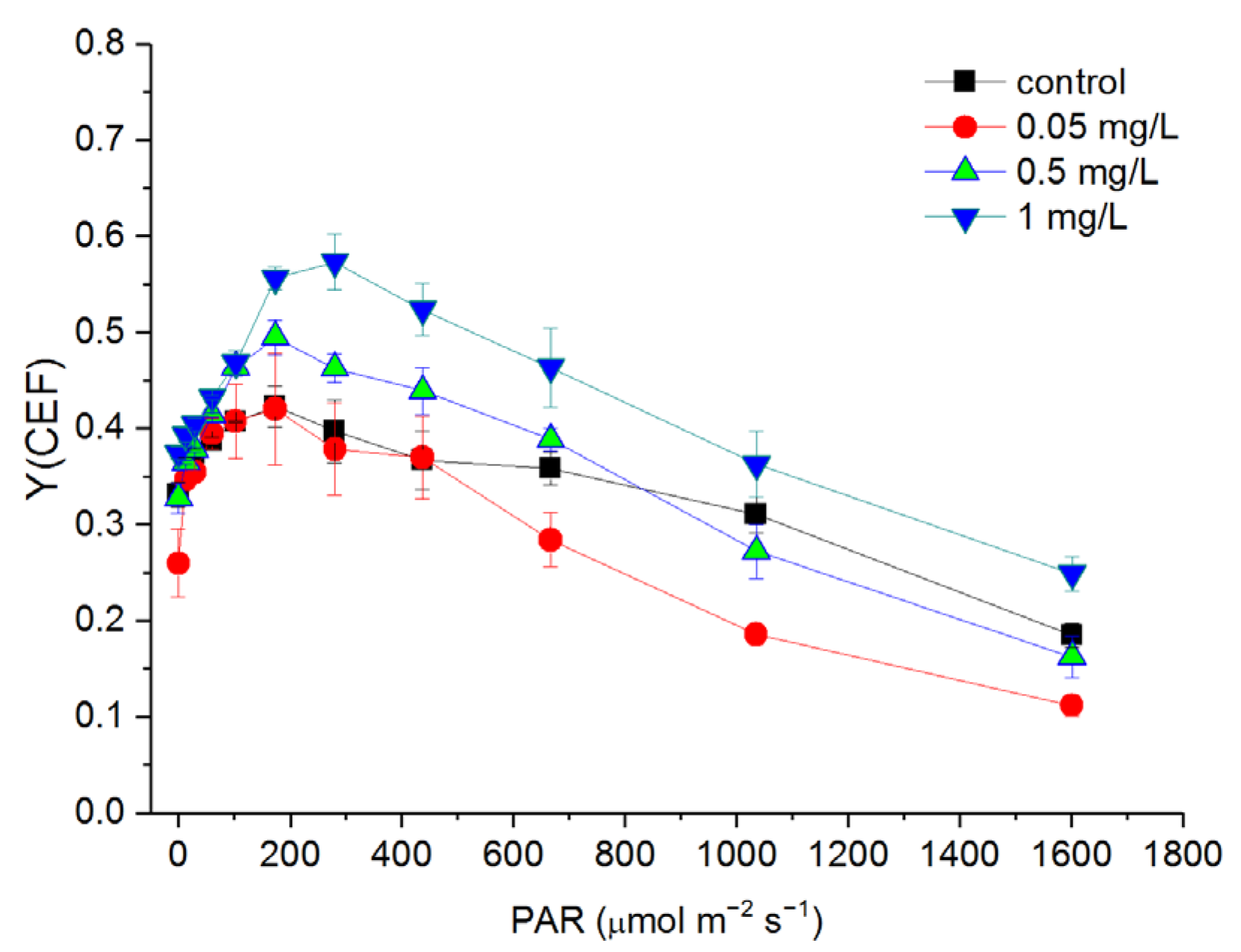
3.6. Response of Cyclic Electron Flow (CEF) to Hg2+ Treatments
3.7. Binding Ability of Hg Ions with Photosystems
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, F.; Zhang, S.; Cheng, P.; Zhang, S.; Sun, Y. Effects of soil amendments on heavy metal immobilization and accumulation by maize grown in a multiple-metal-contaminated soil and their potential for safe crop production. Toxics 2020, 8, 102. [Google Scholar] [CrossRef] [PubMed]
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.-Q. Heavy metals and pesticides toxicity in agricultural soil and plants: Ecological risks and human health implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Basu, N. The Minamata Convention on Mercury and the role for the environmental sciences community. Environ. Toxicol. Chem. 2018, 37, 2951–2952. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Liu, M.; He, Y.; Lin, H.; Yu, C.; Deng, C.; Wang, X. An experimental study of the impacts of solar radiation and temperature on mercury emission from different natural soils across China. Environ. Monit. Assess. 2019, 191, 545. [Google Scholar] [CrossRef]
- Enamorado-Montes, G.; Reino-Causil, B.; Urango-Cardenas, I.; Marrugo-Madrid, S.; Marrugo-Negrete, J. Mercury accumulation in commercial varieties of Oryza sativa L. cultivated in soils of La Mojana region, Colombia. Toxics 2021, 9, 304. [Google Scholar] [CrossRef]
- Feng, X.; Li, P.; Fu, X.; Wang, X.; Zhang, H.; Lin, C.-J. Mercury pollution in China: Implications on the implementation of the Minamata Convention. Environ. Sci. Proc. Impacts 2022, 24, 634–648. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, X.; Wang, Y.; Hou, Y. Evaluation and analysis of the toxicity of mercury (Hg2+) to allophycocyanin from Spirulina platensis in vitro. Environ. Sci. Pollut. Res. 2022. [Google Scholar] [CrossRef]
- Lin, Y.; Vogt, R.; Larssen, T. Environmental mercury in China: A review. Environ. Toxicol. Chem. 2012, 31, 2431–2444. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, W.-X. Differential acclimation of a marine diatom to inorganic mercury and methylmercury exposure. Aquat. Toxicol. 2013, 138–139, 52–59. [Google Scholar] [CrossRef]
- Barregard, L.; Fabricius-Lagging, E.; Lundh, T.; Mölne, J.; Wallin, M.; Olausson, M.; Modigh, C.; Sallsten, G. Cadmium, mercury, and lead in kidney cortex of living kidney donors: Impact of different exposure sources. Environ. Res. 2010, 110, 47–54. [Google Scholar] [CrossRef]
- Barone, G.; Storelli, A.; Garofalo, R.; Mallamaci, R.; Storelli, M.M. Residual levels of mercury, cadmium, lead and arsenic in some commercially key species from Italian coasts (Adriatic Sea): Focus on human health. Toxics 2022, 10, 223. [Google Scholar] [CrossRef]
- Deng, C.N.; Zhang, D.Y.; Pan, X.L.; Chang, F.Q.; Wang, S.Z. Toxic effects of mercury on PSI and PSII activities, membrane potential and transthylakoid proton gradient in Microsorium pteropus. J. Photochem. Photobiol. B 2013, 127, 1–7. [Google Scholar] [CrossRef]
- Lu, C.M.; Chau, C.W.; Zhang, J.H. Acute toxicity of excess mercury on the photosynthetic performance of cyanobacterium, S. platensis–assessment by chlorophyll fluorescence analysis. Chemosphere 2000, 41, 191–196. [Google Scholar] [CrossRef]
- Matorin, D.N.; Osipov, V.A.; Seifullina, N.K.; Venediktov, P.S.; Rubin, A.B. Increased toxic effect of methylmercury on Chlorella vulgaris under high light and cold stress conditions. Microbiology 2009, 78, 321–327. [Google Scholar] [CrossRef]
- Panda, S.; Panda, S. Effect of mercury ion on the stability of the lipid-protein complex of isolated chloroplasts. Indian J. Biochem. Biophys. 2009, 46, 405–408. [Google Scholar]
- Mishra, A.; Choudhuri, M.A. Monitoring of phytotoxicity of lead and mercury from germination and early seedling growth indices in two rice cultivars. Water Air Soil Pollut. 1999, 114, 339–346. [Google Scholar] [CrossRef]
- Liang, X.; Zhu, N.; Johs, A.; Chen, H.; Pelletier, D.A.; Zhang, L.; Yin, X.; Gao, Y.; Zhao, J.; Gu, B. Mercury reduction, uptake, and species transformation by freshwater alga Chlorella vulgaris under sunlit and dark conditions. Environ. Sci. Technol. 2022, 56, 4961–4969. [Google Scholar] [CrossRef]
- Singh, R.; Dubey, G.; Singh, V.P.; Srivastava, P.K.; Kumar, S.; Prasad, S.M. High light intensity augments mercury toxicity in cyanobacterium Nostoc muscorum. Biol. Trace Elem. Res. 2012, 149, 262–272. [Google Scholar] [CrossRef]
- Matson, R.S.; Mustoe, G.E.; Chang, S.B. Mercury inhibition on lipid biosynthesis in freshwater algae. Environ. Sci. Technol. 1972, 6, 158–160. [Google Scholar] [CrossRef]
- Protopopov, F.F.; Todorenko, D.A.; Nikolaev, I.N.; Alekseev, A.A.; Bratkovskaya, L.B.; Matorin, D.N. The fluorescence of phytoplankton chlorophyll from the Moskva River in the presence of mercury ions. Biophysics 2021, 66, 779–785. [Google Scholar] [CrossRef]
- Ahmad, J.; Ali, A.A.; Iqbal, M.; Ahmad, A.; Qureshi, M.I. Proteomics of mercury-induced responses and resilience in plants: A review. Environ. Chem. Lett. 2022. [Google Scholar] [CrossRef]
- Patra, M.; Bhowmik, N.; Bandopadhyay, B.; Sharma, A. Comparison of mercury, lead and arsenic with respect to genotoxic effects on plant systems and the development of genetic tolerance. Environ. Exp. Bot. 2004, 52, 199–223. [Google Scholar] [CrossRef]
- Klughammer, C.; Schreiber, U. Complementary PS II quantum yields calculated from simple fluorescence parameters measured by PAM fluorometry and the Saturation Pulse method. PAM Appl. Notes 2008, 1, 27–35. [Google Scholar]
- Klughammer, C.; Schreiber, U. Saturation Pulse method for assessment of energy conversion in PS I. PAM Appl. Notes 2008, 1, 11–14. [Google Scholar]
- Wang, S.Z.; Zhang, D.Y.; Pan, X.L. Effects of cadmium on the activities of photosystems of Chlorella pyrenoidosa and the protective role of cyclic electron flow. Chemosphere 2013, 93, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Z.; Chen, F.L.; Mu, S.Y.; Zhang, D.Y.; Pan, X.L.; Lee, D.-J. Simultaneous analysis of photosystem responses of Microcystis aeruginoga under chromium stress. Ecotoxicol. Environ. Saf. 2013, 88, 163–168. [Google Scholar] [CrossRef]
- Li, C.; Zheng, C.; Fu, H.; Zhai, S.; Hu, F.; Naveed, S.; Zhang, C.; Ge, Y. Contrasting detoxification mechanisms of Chlamydomonas reinhardtii under Cd and Pb stress. Chemosphere 2021, 274, 129771. [Google Scholar] [CrossRef] [PubMed]
- Stanier, R.Y.; Kunisawa, R.; Mandel, M.; Cohen-Bazire, G. Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol. Rev. 1971, 35, 171–205. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.-H.; Yang, C.-Y.; An, Y.-J. Effects of antimony on aquatic organisms (larva and embryo of Oryzias latipes, Moina macrocopa, Simocephalus mixtus, and Pseudokirchneriella subcapitata). Chemosphere 2009, 75, 889–893. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Wada, S.; Amako, K.; Miyake, C. Identification of a novel mutation exacerbated the PSI photoinhibition in pgr5/pgrl1 mutants; Caution for overestimation of the phenotypes in Arabidopsis pgr5-1 mutant. Cells 2021, 10, 2884. [Google Scholar] [CrossRef]
- Li, T.-Y.; Shi, Q.; Sun, H.; Yue, M.; Zhang, S.-B.; Huang, W. Diurnal response of Photosystem I to fluctuating light is affected by stomatal conductance. Cells 2021, 10, 3128. [Google Scholar] [CrossRef]
- Suzuki, K.; Ohmori, Y.; Ratel, E. High root temperature blocks both linear and cyclic electron transport in the dark during chilling of the leaves of rice seedlings. Plant Cell Physiol. 2011, 52, 1697–1707. [Google Scholar] [CrossRef]
- Pfündel, E.; Klughammer, C.; Schreiber, U. Monitoring the effects of reduced PS II antenna size on quantum yields of photosystems I and II using the Dual-PAM-100 measuring system. PAM Appl. Notes 2008, 1, 21–24. [Google Scholar]
- Chiu, Y.-F.; Chen, Y.-H.; Roncel, M.; Dilbeck, P.L.; Huang, J.-Y.; Ke, S.-C.; Ortega, J.M.; Burnap, R.L.; Chu, H.-A. Spectroscopic and functional characterization of cyanobacterium Synechocystis PCC 6803 mutants on the cytoplasmic-side of cytochrome b559 in photosystem II. Biochim. Biophys. Acta Bioenerg. 2013, 1827, 507–519. [Google Scholar] [CrossRef]
- Platt, T.; Gallegos, C.L.; Harrison, W.G. Photoinhibition of photosynthesis in natural assemblages of marine phytoplankton. J. Mar. Res. 1980, 38, 687–701. [Google Scholar]
- Kühl, M.; Glud, R.N.; Borum, J.; Roberts, R.; Rysgaard, S. Photosynthetic performance of surface-associated algae below sea ice as measured with a pulse-amplitude-modulated (PAM) fluorometer and O2 microsensors. Mar. Ecol. Prog. Ser. 2001, 223, 1–14. [Google Scholar] [CrossRef]
- Nitschke, U.; Connan, S.; Stengel, D.B. Chlorophyll a fluorescence responses of temperate Phaeophyceae under submersion and emersion regimes: A comparison of rapid and steady-state light curves. Photosynth. Res. 2012, 114, 29–42. [Google Scholar] [CrossRef]
- Ralph, P.J.; Gademann, R. Rapid light curves: A powerful tool to assess photosynthetic activity. Aquat. Bot. 2005, 82, 222–237. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, S.-B.; Cao, K.-F. Stimulation of cyclic electron flow during recovery after chilling-induced photoinhibition of PSII. Plant Cell Physiol. 2010, 51, 1922–1928. [Google Scholar] [CrossRef]
- Coopman, R.E.; Fuentes-Neira, F.P.; Briceño, V.F.; Cabrera, H.M.; Corcuera, L.J.; Bravo, L.A. Light energy partitioning in photosystems I and II during development of Nothofagus nitida growing under different light environments in the Chilean evergreen temperate rain forest. Trees 2010, 24, 247–259. [Google Scholar] [CrossRef]
- Cullen, M.; Ray, N.; Husain, S.; Nugent, J.; Nield, J.; Purton, S. A highly active histidine-tagged Chlamydomonas reinhardtii Photosystem II preparation for structural and biophysical analysis. Photochem. Photobiol. Sci. 2007, 6, 1177–1183. [Google Scholar] [CrossRef] [PubMed]
- Marco, P.; Elman, T.; Yacoby, I. Binding of ferredoxin NADP+ oxidoreductase (FNR) to plant photosystem I. Biochim. Biophys. Acta Bioenerg. 2019, 1860, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Bals, T.; Schünemann, D. Isolation of Arabidopsis thylakoid membranes and their use for in vitro protein insertion or transport assays. In Chloroplast Research in Arabidopsis: Methods and Protocols; Jarvis, R.P., Walker, J.M., Eds.; Methods in Molecular Biology; Humana Press: New York, NY, USA, 2011; Volume I, pp. 321–338. [Google Scholar]
- Wu, X.; Zhao, X.; Deng, Z.; Liang, X.; Fang, S. Investigation of interactions between zein and natamycin by fluorescence spectroscopy and molecular dynamics simulation. J. Mol. Liq. 2021, 327, 114873. [Google Scholar] [CrossRef]
- Nowicka, B. Heavy metal–induced stress in eukaryotic algae—mechanisms of heavy metal toxicity and tolerance with particular emphasis on oxidative stress in exposed cells and the role of antioxidant response. Environ. Sci. Pollut. Res. 2022, 29, 16860–16911. [Google Scholar] [CrossRef] [PubMed]
- Grajek, H.; Rydzyński, D.; Piotrowicz-Cieślak, A.; Herman, A.; Maciejczyk, M.; Wieczorek, Z. Cadmium ion-chlorophyll interaction–Examination of spectral properties and structure of the cadmium-chlorophyll complex and their relevance to photosynthesis inhibition. Chemosphere 2020, 261, 127434. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Han, X.; Liu, C.-Q.; Guo, L.; Ding, H.; Lang, Y. Differences in the spectroscopic characteristics of wetland dissolved organic matter binding with Fe3+, Cu2+, Cd2+, Cr3+ and Zn2+. Sci. Total Environ. 2021, 800, 149476. [Google Scholar] [CrossRef]
- Zhang, D.; Pan, X.; Mostofa, K.M.G.; Chen, X.; Mu, G.; Wu, F.; Liu, J.; Song, W.; Yang, J.; Liu, Y.; et al. Complexation between Hg(II) and biofilm extracellular polymeric substances: An application of fluorescence spectroscopy. J. Hazard. Mater. 2010, 175, 359–365. [Google Scholar] [CrossRef]
- Duarte, B.; Gameiro, C.; Utkin, A.B.; Matos, A.R.; Caçador, I.; Fonseca, V.; Cabrita, M.T. A multivariate approach to chlorophyll a fluorescence data for trace element ecotoxicological trials using a model marine diatom. Estuar. Coast. Shelf Sci. 2021, 250, 107170. [Google Scholar] [CrossRef]
- Shen, J.; Li, X.; Zhu, X.; Ding, Z.; Huang, X.; Chen, X.; Jin, S. Molecular and photosynthetic performance in the yellow leaf mutant of Torreya grandis according to transcriptome sequencing, chlorophyll a fluorescence, and modulated 820 nm reflection. Cells 2022, 11, 431. [Google Scholar] [CrossRef]
- Jamla, M.; Khare, T.; Joshi, S.; Patil, S.; Penna, S.; Kumar, V. Omics approaches for understanding heavy metal responses and tolerance in plants. Curr. Plant Biol. 2021, 27, 100213. [Google Scholar] [CrossRef]
- Barón-Sola, Á.; Toledo-Basantes, M.; Arana-Gandía, M.; Martínez, F.; Ortega-Villasante, C.; Dučić, T.; Yousef, I.; Hernández, L.E. Synchrotron Radiation-Fourier Transformed Infrared microspectroscopy (μSR-FTIR) reveals multiple metabolism alterations in microalgae induced by cadmium and mercury. J. Hazard. Mater. 2021, 419, 126502. [Google Scholar] [CrossRef]
- Wu, Y.; Zeng, Y.; Qu, J.Y.; Wang, W.-X. Mercury effects on Thalassiosira weissflogii: Applications of two-photon excitation chlorophyll fluorescence lifetime imaging and flow cytometry. Aquat. Toxicol. 2012, 110–111, 133–140. [Google Scholar] [CrossRef]
- Le Faucheur, S.; Campbell, P.G.C.; Fortin, C.; Slaveykova, V.I. Interactions between mercury and phytoplankton: Speciation, bioavailability, and internal handling. Environ. Toxicol. Chem. 2014, 33, 1211–1224. [Google Scholar] [CrossRef]
- Juneau, P.; Dewez, D.; Matsui, S.; Kim, S.-G.; Popovic, R. Evaluation of different algal species sensitivity to mercury and metolachlor by PAM-fluorometry. Chemosphere 2001, 45, 589–598. [Google Scholar] [CrossRef]
- Dong, L.-L.; Zhang, G.-Q.; Li, W.; Ding, T.; Wang, H.-X.; Zhang, G. Effects of Cu2+ and Hg2+ on growth and photosynthesis of two Scenedesmus species. Pol. J. Environ. Stud. 2020, 29, 1129–1135. [Google Scholar] [CrossRef]
- Essemine, J.; Govindachary, S.; Ammar, S.; Bouzid, S.; Carpentier, R. Abolition of photosystem I cyclic electron flow in Arabidopsis thaliana following thermal-stress. Plant Physiol. Biochem. 2011, 49, 235–243. [Google Scholar] [CrossRef]
- Elbaz, A.; Wei, Y.Y.; Meng, Q.; Zheng, Q.; Yang, Z.M. Mercury-induced oxidative stress and impact on antioxidant enzymes in Chlamydomonas reinhardtii. Ecotoxicology 2010, 19, 1285–1293. [Google Scholar] [CrossRef]
- Souri, Z.; Cardoso, A.A.; da-Silva, C.J.; de Oliveira, L.M.; Dari, B.; Sihi, D.; Karimi, N. Heavy metals and photosynthesis: Recent developments. In Photosynthesis, Productivity and Environmental Stress; Ahmad, P., Ahanger, M.A., Alyemeni, M.N., Alam, P., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2019; pp. 107–134. [Google Scholar]
- Zhang, T.; Lu, Q.; Su, C.; Yang, Y.; Hu, D.; Xu, Q. Mercury induced oxidative stress, DNA damage, and activation of antioxidative system and Hsp70 induction in duckweed (Lemna minor). Ecotoxicol. Environ. Saf. 2017, 143, 46–56. [Google Scholar] [CrossRef]
- Fernández-Marín, B.; Míguez, F.; Becerril, J.; García-Plazaola, J. Dehydration-mediated activation of the xanthophyll cycle in darkness: Is it related to desiccation tolerance? Planta 2011, 234, 579–588. [Google Scholar] [CrossRef]
- Pinto, E.; Sigaud-kutner, T.C.S.; Leitão, M.A.S.; Okamoto, O.K.; Morse, D.; Colepicolo, P. Heavy metal–induced oxidative stress in algae. J. Phycol. 2003, 39, 1008–1018. [Google Scholar] [CrossRef]
- Canonico, M.; Konert, G.; Crepin, A.; Šedivá, B.; Kaňa, R. Gradual response of cyanobacterial thylakoids to acute high-light stress—importance of carotenoid accumulation. Cells 2021, 10, 1916. [Google Scholar] [CrossRef] [PubMed]
- Šeršeň, F.; Král’ová, K. New facts about CdCl2 action on the photosynthetic apparatus of spinach chloroplasts and its comparison with HgCl2 action. Photosynthetica 2001, 39, 575–580. [Google Scholar] [CrossRef]
- Shi, D.; Li, D.; Zhang, Y.; Li, X.; Tao, Y.; Yan, Z.; Ao, Y. Effects of Pseudomonas alkylphenolica KL28 on immobilization of Hg in soil and accumulation of Hg in cultivated plant. Biotechnol. Lett. 2019, 41, 1343–1354. [Google Scholar] [CrossRef] [PubMed]
- Clijsters, H.; Van Assche, F. Inhibition of photosynthesis by heavy metals. Photosynth. Res. 1985, 7, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Z.; Zhang, D.Y.; Pan, X.L. Effects of arsenic on growth and photosystem II (PSII) activity of Microcystis aeruginosa. Ecotoxicol. Environ. Saf. 2012, 84, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Z.; Pan, X.L. Effects of sb(V) on growth and chlorophyll fluorescence of Microcystis aeruginosa (FACHB-905). Curr. Microbiol. 2012, 65, 733–741. [Google Scholar] [CrossRef]
- Aggarwal, A.; Sharma, I.; Tripathi, B.; Munjal, A.; Baunthiyal, M.; Sharma, V. Metal toxicity and photosynthesis. In Photosynthesis: Overviews on Recent Progress and Future Perspectives; Itoh, S., Mohanty, P., Guruprasad, K.N., Eds.; IK International Publishing Hous: New Delhi, India, 2012; pp. 229–236. [Google Scholar]
- Solti, Á.; Sárvári, É.; Tóth, B.; Mészáros, I.; Fodor, F. Incorporation of iron into chloroplasts triggers the restoration of cadmium induced inhibition of photosynthesis. J. Plant Physiol. 2016, 202, 97–106. [Google Scholar] [CrossRef][Green Version]
- Bernier, M.; Popovic, R.; Carpentier, R. Mercury inhibition at the donor side of photosystem II is reversed by chloride. FEBS Lett. 1993, 321, 19–23. [Google Scholar] [CrossRef]
- Suresh Kumar, K.; Dahms, H.-U.; Lee, J.-S.; Kim, H.C.; Lee, W.C.; Shin, K.-H. Algal photosynthetic responses to toxic metals and herbicides assessed by chlorophyll a fluorescence. Ecotoxicol. Environ. Saf. 2014, 104, 51–71. [Google Scholar] [CrossRef]
- Samson, G.; Morissette, J.-C.; Popovic, R. Determination of four apparent mercury interaction sites in photosystem II by using a new modification of the stern-volmer analysis. Biochem. Bioph. Res. Commun. 1990, 166, 873–878. [Google Scholar] [CrossRef]
- Zeng, Z.-L.; Sun, H.; Wang, X.-Q.; Zhang, S.-B.; Huang, W. Regulation of leaf angle protects Photosystem I under fluctuating light in tobacco young leaves. Cells 2022, 11, 252. [Google Scholar] [CrossRef]
- Munekage, Y.; Hojo, M.; Meurer, J.; Endo, T.; Tasaka, M.; Shikanai, T. PGR5 is involved in cyclic electron flow around photosystem I and is essential for photoprotection in Arabidopsis. Cell 2002, 110, 361–371. [Google Scholar] [CrossRef]
- Huang, W.; Yang, S.J.; Zhang, S.B.; Zhang, J.L.; Cao, K.F. Cyclic electron flow plays an important role in photoprotection for the resurrection plant Paraboea rufescens under drought stress. Planta 2012, 235, 819–828. [Google Scholar] [CrossRef]
- Johnson, G.N. Physiology of PSI cyclic electron transport in higher plants. Biochim. Biophys. Acta 2011, 1807, 384–389. [Google Scholar] [CrossRef]



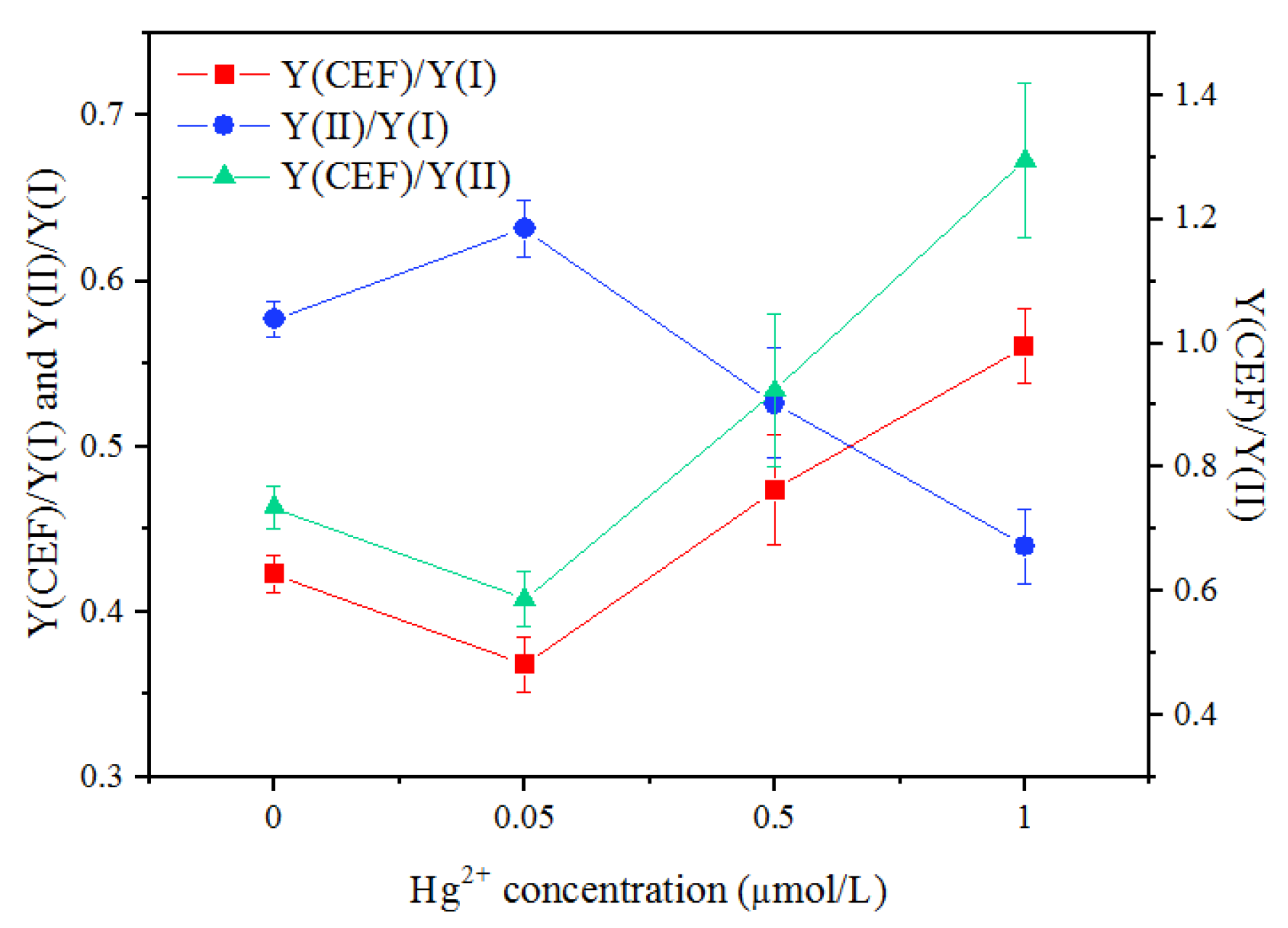

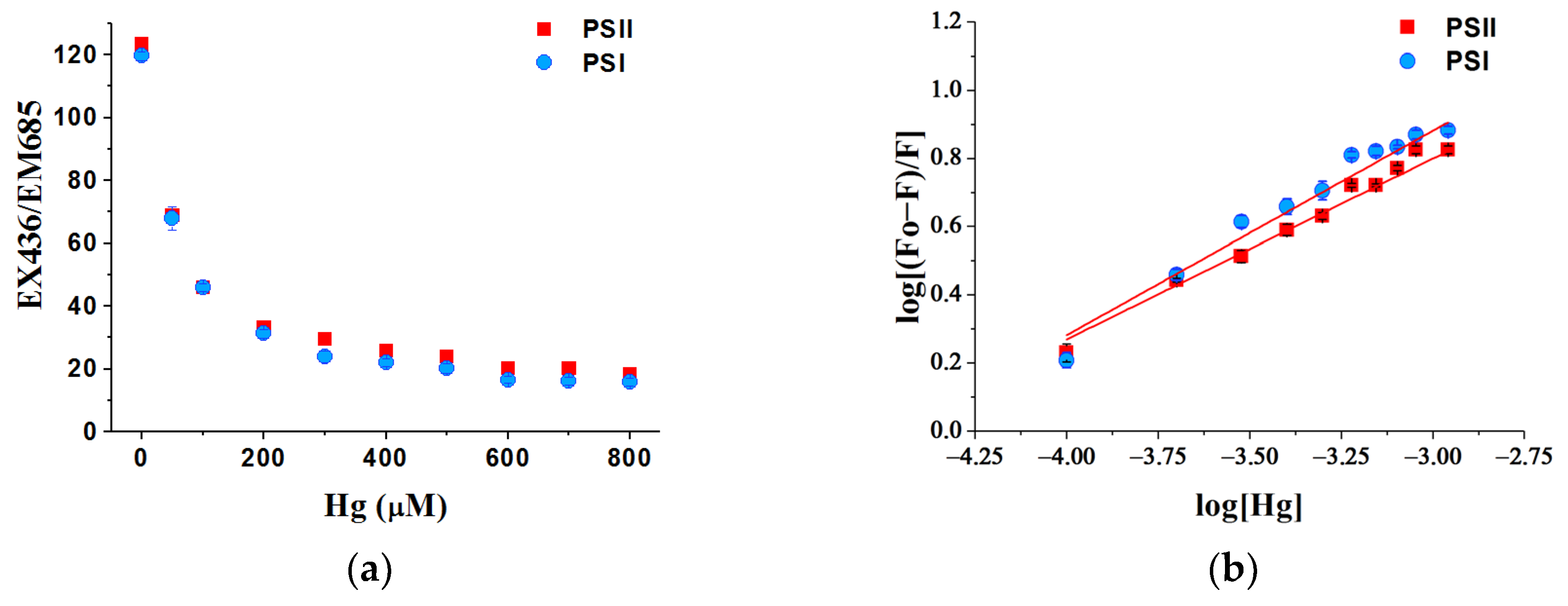
| Hg2+ Concentration (mg/L) | Quantum Yields in PSI | Quantum Yields in PSII | ||||
|---|---|---|---|---|---|---|
| Y(I) | Y(ND) | Y(NA) | Y(II) | Y(NO) | Y(NPQ) | |
| 0 | 0.467 ± 0.014 a | 0.342 ± 0.035 b | 0.178 ± 0.037 a | 0.270 ± 0.008 a | 0.653 ± 0.001 c | 0.040 ± 0.014 c |
| 0.05 | 0.330 ± 0.040 b | 0.670 ± 0.050 a | 0.040 ± 0.024 b | 0.240 ± 0.001 b | 0.790 ± 0.029 a | 0.023 ± 0.009 c |
| 0.5 | 0.338 ± 0.021 b | 0.660 ± 0.039 a | 0.027 ± 0.019 b | 0.176 ± 0.002 c | 0.740 ± 0.004 ab | 0.086 ± 0.003 b |
| 1 | 0.384 ± 0.009 b | 0.603 ± 0.037 a | 0.035 ± 0.022 b | 0.175 ± 0.005 c | 0.693 ± 0.005 b | 0.137 ± 0.005 a |
| Hg2+ Concentration (mg L−1) | Parameters of RLCs of ETR(I) | Parameters of RLCs of ETR(II) | ||||
|---|---|---|---|---|---|---|
| Ik (μmol Photon m−2 s−1) | α (e− Photon−1) | ETRmax (μmol e− m−2 s−1) | Ik (μmol Photon m−2 s−1) | α (e− Photon−1) | ETRmax (μmol e− m−2 s−1) | |
| 0 | 765.90 ± 64.41 a | 0.440 ± 0.025 a | 333.52 ± 12.28 a | 901.65 ± 74.58 a | 0.230 ± 0.003 a | 207.42 ± 17.00 a |
| 0.05 | 549.97 ± 89.22 b | 0.430 ± 0.028 a | 231.13 ± 24.95 b | 715.70 ± 8.50 b | 0.232 ± 0.001 a | 165.85 ± 1.55 b |
| 0.5 | 493.92 ± 22.89 b | 0.477 ± 0.008 a | 235.62 ± 12.30 b | 564.00 ± 10.78 b | 0.211 ± 0.002 b | 118.98 ± 1.36 c |
| 1 | 552.00 ± 14.93 b | 0.489 ± 0.024 a | 277.20 ±15.40 b | 588.58 ± 24.41 b | 0.202 ± 0.003 c | 118.75 ± 3.60 c |
| Heavy Metal | PSI Particles | PSII Particles | ||
|---|---|---|---|---|
| Ka (×104 M−1) | n | Ka (×104 M−1) | n | |
| Hg | 2.68 ± 0.19 | 0.69 ± 0.09 | 2.72 ± 0.12 | 0.79 ± 0.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Duo, J.; Wufuer, R.; Li, W.; Pan, X. The Binding Ability of Mercury (Hg) to Photosystem I and II Explained the Difference in Its Toxicity on the Two Photosystems of Chlorella pyrenoidosa. Toxics 2022, 10, 455. https://doi.org/10.3390/toxics10080455
Wang S, Duo J, Wufuer R, Li W, Pan X. The Binding Ability of Mercury (Hg) to Photosystem I and II Explained the Difference in Its Toxicity on the Two Photosystems of Chlorella pyrenoidosa. Toxics. 2022; 10(8):455. https://doi.org/10.3390/toxics10080455
Chicago/Turabian StyleWang, Shuzhi, Jia Duo, Rehemanjiang Wufuer, Wenfeng Li, and Xiangliang Pan. 2022. "The Binding Ability of Mercury (Hg) to Photosystem I and II Explained the Difference in Its Toxicity on the Two Photosystems of Chlorella pyrenoidosa" Toxics 10, no. 8: 455. https://doi.org/10.3390/toxics10080455
APA StyleWang, S., Duo, J., Wufuer, R., Li, W., & Pan, X. (2022). The Binding Ability of Mercury (Hg) to Photosystem I and II Explained the Difference in Its Toxicity on the Two Photosystems of Chlorella pyrenoidosa. Toxics, 10(8), 455. https://doi.org/10.3390/toxics10080455