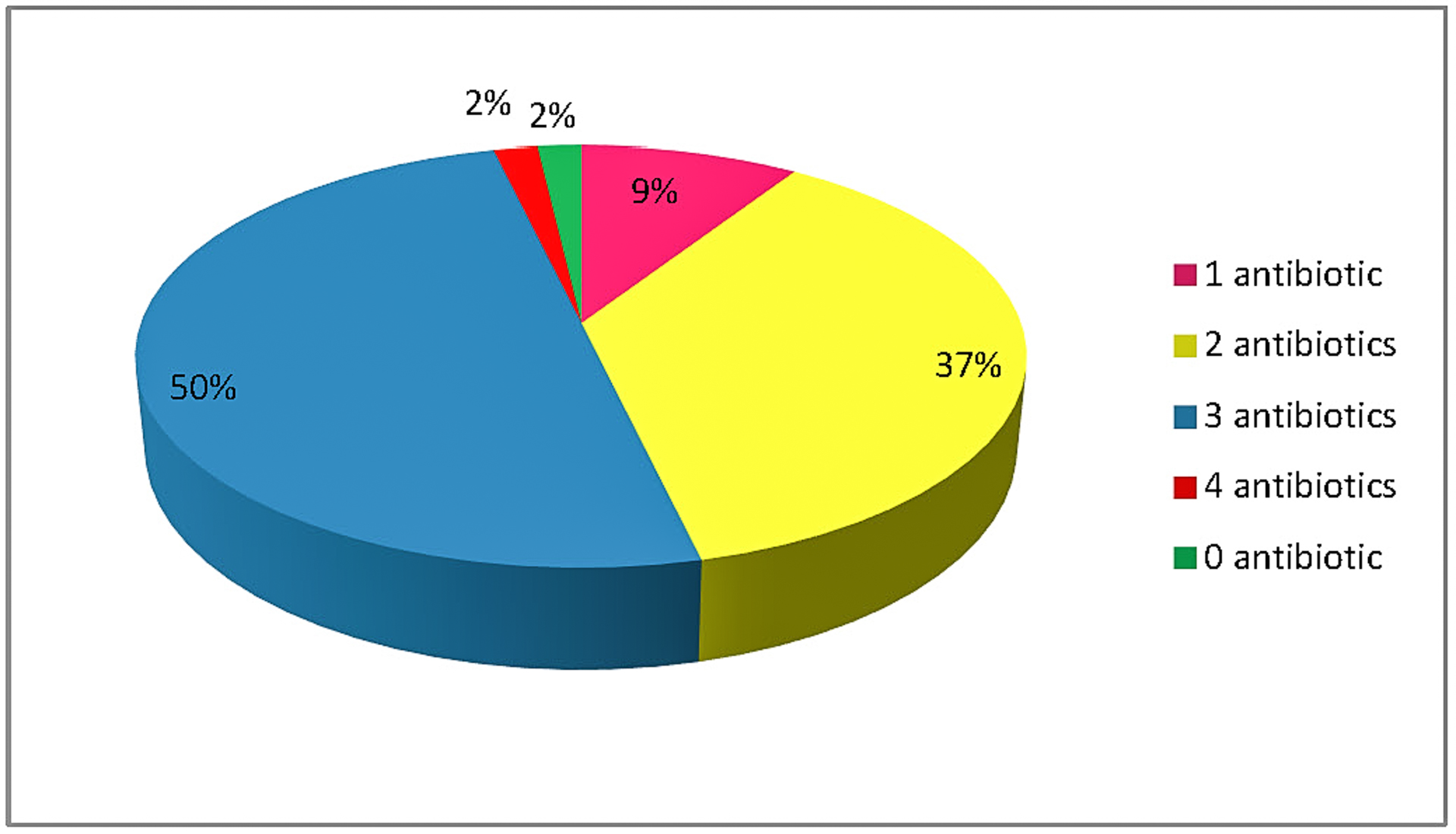
1. Introduction
The aim of antibiotics is to destroy bacteria and they are used in livestock and poultry production for therapeutic purposes to prevent, control and treat infectious diseases in animals, although some producers use antibiotics to improve meat production by increasing the rate of animal growth [
1]. Antibiotics as growth promoters are no longer used in European Union countries as there has been a legal ban from January 2006 [
2]. The widespread and prolonged use of antibiotics has contributed negatively to their effectiveness and thus the doses have been increased, alternative more powerful antibiotics have to be used and the times of administration have to be extended [
3]. In cases where antibiotics are misused and legal withdrawal periods (the time span from drug administration to animal slaughter and use of meat for human consumption) are not respected, the residues in edible tissues pose an increased risk for consumers [
4].
The parent substance of the antibiotics poses the highest toxicity; however, in the human it is metabolized and converted into an inactive and more easily excreted form [
5,
6]. Allergic reactions and other toxic effects have been observed and the risk is greater for hypersensitive individuals. The most common health effects of quinolones (QNLs) include effects on the central nervous system (CNS), such as anxiety, worry, nervousness and dizziness [
7]. In addition to seizures, other serious CNS reactions include delirium, delusions, psychosis, mania, encephalopathy and dysarthria [
8]. Recently, pharmacovigilance studies found a possible association between QNLs and peripheral nervous system toxicity [
9], including Guillain–Barré syndrome (GBS), a potentially severe form of acute peripheral polyneuropathy [
10]. In 2012, a study by a Canadian research team showed an increased risk of retinal detachment associated with the oral administration of QNLs [
11]. Gastrointestinal symptoms such as indigestion, nausea, vomiting and diarrhea are common side effects associated with QNL consumption [
12].
Allergic reactions associated with sulfonamides (SAs) include the full range of Gell–Coombs hypersensitivity reactions. In addition, there are reactions associated with immunoglobulin E (IgE), such as urticaria, angioedema and anaphylaxis [
13]. SAs have been correlated with hepatotoxicity and systemic hypersensitivity reactions [
14,
15].
Tetracyclines (TCs) can modify the normal intestinal flora, allowing the overproduction of Pseudomonas and Clostridium [
16], and cause nausea, diarrhea and even mortality. They are also found in the structure of newly formed teeth, if consumed during certain periods of pregnancy, such as the embryonic period (from the third through the eighth week after conception) [
17]. Hepatotoxicity occurs in patients with hepatic impairment or after intravenous administration of TCs and nephrotoxicity when administered concomitantly with diuretics [
18].
Streptomycines (STr) belong to the aminoglycosides (AGs) category of antibiotics. Patients receiving AGs may have reversible nephrotoxicity [
19] because AGs can enter the proximal tubule through megaline, a multiligand binding receptor. AG excretion from this intracellular compartment occurs very slowly and can take several days [
20]. Side effects include cochlear damage of the auditory nerve [
21], optic nerve dysfunction [
22], peripheral neuropathy [
23], arachnoiditis [
24] and encephalopathy [
25].
Μeat and dairy products constitute an important part of the diet. In 2013, global poultry meat production exceeded 109 million tons and global egg production was estimated at over 73 million tons. In 2014, global production of beef and pork was estimated at about 170 million tons [
26]. A major review by the Food and Agriculture Organization (FAO) of the United Nations, which makes extensive use of expert judgement, reported an increase of 76% in the total quantity of meat consumed by the mid-century. This includes a doubling in the consumption of poultry, a 69% increase in beef and a 42% increase in pork [
27]. In Europe, cheese and pig meat are the preferred animal-based protein sources, followed by poultry, milk and bovine meat. The EU citizen consumed an average of 2.2 kg less bovine meat in 2013 than in 2000 (decreased by 13%), but 3.0 kg more poultry (increased by 15%). Pork consumption remained nearly fixed throughout this period. According to FAOSTAT (Food and Agriculture Organization of the United Nations) [
28], in Greece the mean consumption of bovine meat in 2019 was 14.1 kg/capita/year, for pork 28.9 kg/capita/year and for poultry 25.6 kg/capita/year.
As noted by Arsène et al., antibiotic residues in food, such as meat, are likely to induce antibiotic resistance in bacteria and cause allergies and other more severe effects in humans [
29]. This fact, combined with the high positivity in food samples, leads to the assumption that increased meat consumption may be associated with a risk of antibiotic contamination. In addition, as the European Medicines Agency (EMA) describes, when the withdrawal period (“The time that must elapse between the last administration of a veterinary medicine and the slaughter or production of food from that animal”) is not respected then the antibiotic residues in meat can exceed the maximum residue levels (MRLs) [
30].
This study aims at screening the antibiotic residues in bovine, pork and chicken samples (muscle, liver and kidney) from the local Cretan market, assessing the exposure of the Cretan population to certain compounds due to meat consumption and ultimately estimating the risk for human health resulting from the dietary intake of multiple antibiotics through meat consumption, corrected for the aggregated dietary exposure.
2. Materials and Methods
2.1. Reagents
Methanol (99.9%), formic acid (≥95%) and acetonitrile (≥99.9%) were purchased from Honeywell. Ethyl acetate (99.8%), NaCl (99.9%), n-hexane (99%) and phosphate buffer saline (PBS) tablets were from Sigma Aldrich (Saint Louis, MO, USA). Ultrapure water (Direct-Q 3UV), Na2HPO4 × 2H2O (99.5%) and NaOH (99%) were purchased from Merck (Darmstadt, Germany). ELISA kits (R3505 RIDASCREEN® Tetracyclin, R3004 RIDASCREEN® Sulfonamide, R3104 RIDASCREEN® Streptomycin, R3113 RIDASCREEN® Quinolones) were purchased from R-Biopharm (Darmstadt, Germany).
2.2. Sampling
A total of 54 samples of raw meat were randomly collected on November 2018 from butcheries in Crete, Greece. The samples were collected from the area of Crete but the animals originated from all over the country. Data concerning the age of the animals and the country of origin were collected. The collected samples were 16 (29.6%) bovine samples, 20 (37.0%) chicken and 18 (33.3%) pork. The collected samples consisted of 29 muscles (53.7%), 17 livers (31.5%) and 8 kidneys (14.8%). Out of the 29 samples there were 10 beef muscles, 6 beef livers, 10 pork muscles, 2 pork livers, 6 pork kidneys, 9 chicken muscles, 9 chicken livers and 2 chicken kidneys. Beef kidneys were not found in any Cretan butcher shop. The majority of the samples (81.5 %) came from animals of Greek origin. The average age of cattle was 15.5 ± 3.3, for pork 4.9 ± 2.0 and for chicken 2.3 ± 0.8 months. All samples were weighted and packed in properly labeled conical centrifuge tubes, sealed and kept at −20 °C, until the analysis.
2.3. Sample Preparation
Total SAs, TCs, STr and QNLs residues were detected using an ELISA test kit. The samples were cut into small pieces and then homogenized with a homogenizer of Janke & Kunkel, Ultraturrax T25 (Staufen, Germany). Then, they were placed in 50 mL Falcon tubes and stored in the freezer (−20 °C) until use. The sample preparation and analysis protocols were instructed from the manufacturer. Briefly, for SAs determination, the homogenized samples were weighed (1 g pork/bovine, 2 g chicken) and vortexed with organic solvent (2 mL methanol for pork/bovine, 6 mL acetonitrile/water 84:16 v/v for chicken). The mixture was centrifuged at 4000 rpm for 10 min and an aliquot of 1.5 mL of supernatant was evaporated to dryness. The dry residue was reconstituted in 0.5 mL buffer (provided by the kit) and 1 mL n-hexane was added. An aliquot of 50 μL of the lower phase was used for analysis. For chicken samples, 4 mL of the supernatant were transferred into a new centrifuge vial, 2 mL 2 M NaCl and 7 mL ethyl acetate were added and the mixture was shaken for 10 min. The mixture was centrifuged for 10 min at 3000 rpm (15 °C). The whole supernatant was evaporated to dryness and reconstituted in 1 mL sample buffer and 1 mL n-hexane. An aliquot of 50 μL of the lower phase was used for analysis.
For STr, 5 g of homogenized sample were mixed with 20 mL of wash buffer, vortexed for 10 s and shaken for 30 min. The mixture was centrifuged (10 min, 4000 rpm, 25 °C), the supernatant was diluted with wash buffer (1:10) and 50 μL were used for analysis.
For TCs, 1 g of homogenized sample and 9 mL 20 mM PBS buffer pH 7.4 were transferred into a centrifuge vial and shaken 10 min for extraction. Then, the mixture was centrifuged (10 min, 4000 rpm, 25 °C) and 1 mL of supernatant was transferred and mixed with 2 mL of n-hexane. An aliquot of 50 µL of the lower aqueous phase was used per well in the assay.
For QNLs, 1 g of homogenized sample and 4 mL methanol/water (70/30, v/v) were mixed vigorously for 10 min and centrifuged (10 min, 4000 rpm, 25 °C). The supernatant was diluted with washing buffer (1:2) and 50 µL were used for analysis.
After samples/standards were loaded, 50 μL of antibody solution were added in each well and plates were incubated for 1 h at room temperature. The wells were washed with 250 μL buffer three times, 100 μL of substrate/chromogen was added and incubated for 15 min at room temperature in the dark. Finally, 100 µL of the stop solution were added to each well and the absorbance was measured at 450 nm.
The LC–MS-based methodology for the detection of antibiotics residues was carried out according to a previously published method [
31]. Briefly, 500 µL of EDTA 150 mM were added in 5 g of homogenized meat and vortexed for 10 minutes. Extraction was carried out with 5 mL acidified acetonitrile (0.1% formic acid) for 10 minutes and then the mixtures were placed in the freezer (−20 °C) for 30 minutes. Then extracts were centrifuged (10 min, 4000 rpm), the supernatant was collected and the extraction was repeated. The combined supernatants were evaporated to dryness and the dry residue was reconstituted in 500 µL of the mobile phase.
2.4. Instrumental Analysis
A Shimadzu LC-MS-2010EV (Kyoto, Japan) was used for the detection and quantification of the analytes after the separation of the analytes on a Supelco Discovery C18 column (25 cm × 4.6 mm, 5 μm) (Sigma-Aldrich, Saint Louis, MO, USA). The oven was set at 30 °C and the flow rate was 0.6 mL/min. The mobile phase was water with 0.1% formic acid (Solvent A) and acetonitrile with 0.1% formic acid (Solvent B). The mass spectrometer was coupled with an ESI (electrospray ionization) ion source and the detection was achieved in selected ion monitoring (SIM) in positive mode. The retention times and m/z ions were for MBX: 8.66 min and m/z 362.1, for OTC: 8.90 min and m/z 461.15, for ENR: 9.21 min and m/z 360.1, for DOX: 10.48 min and m/z 445.05, for SDZ: 8.01 min and m/z 251.0/272.9 and for SMX: 11.11 min and m/z 254.0/275.9, respectively
2.5. Exposure Assessment
Exposure of the general population was assessed for each one of the four antibiotic groups (SAs, TCs, QNLs and STr). The daily dietary intake of antibiotics derives from the antibiotic concentration in food consumed and the daily food consumption.
Consumption data for the Greek population for all food items were retrieved from the FAOSTAT database [
28] and 2019 data are represented (
Table 1). The estimated daily intake of antibiotics from meat, and specifically bovine meat, pig meat and poultry meat (EDImeat) (μg/kg body weight/day), was calculated using the following equation:
where cantibiotic is the concentration of antibiotics in meat tissue determined in this study (bovine meat, pig meat and poultry meat), expressed as the median concentration (μg/kg meat, on fresh weight basis), Wmeat (g meat/capita) represents the daily average consumption of meat (bovine meat, pig meat and poultry meat) per person and BW is the mean body weight for an adult consumer (70 kg).
2.6. Risk Characterization
Risk characterization was conducted following the approach of the source-related hazard quotient (HQ) and hazard index (HI) initially proposed in Goumenou and Tsatsakis [
32], and application of the methodology is presented in details in relevant case studies [
33,
34,
35,
36,
37]. Using this approach, the source-related hazard quotient (HQ) is assessed, after accounting for the correction factor for meat (CFm). The CFm expresses the contribution of meat to the total antibiotic dietary daily intake and it is equal with the ratio of the maximum permitted daily intake through meat consumption MPDIm (meat consumption × maximum residue level (MRL) in meat) to the maximum permitted daily intake through the whole diet, MPDIA (SUM of MPDIi = SUM (food
i consumption × MRL in the food
i), where food
i represents each food item with considerable contribution in the overall exposure).
More specifically, CFm = (consumption data for the meat × MRL for meat)/SUM (consumption data for relevant foodi × MRL in relevant foodi).
The corrected EDImeat is calculated with the formula:
ADI and MRL values in relevant food items were extracted from official databases, such as the European Commission [
38] and FAO/WHO [
39]. According to FAO/WHO, the ADI for SAs and STr is 50 μg/kg bw/day whereas the corresponding value for tetracyclines is 30 μg/kg bw/day. The ADI for quinolones is referred to as 6.2 μg/kg bw/day and specifically for enrofloxacin, selected as the most conservative value [
40]. MRLs for TCs, SAs and QNLs were set to be 100 μg/kg, whereas for STr the MRL is 600 μg/kg. The food groups contributing the most to the dietary antibiotic intake we considered from the FAOSTAT database [
28] were: honey, bovine meat, mutton and goat meat, pig meat, poultry meat, meat, other, offals, edible butter, ghee, cream, eggs, milk—excluding butter, freshwater fish, demersal fish, pelagic fish, marine fish, other.
Finally, the source-related hazard quotients (HQs) for each antibiotic group (SAs, TCs, QNLs and STr) were calculated with the following formula
and the HI was calculated as the sum of all HQs.
For considering no risk it should be: CFmi > Hqi, where i is the respective antibiotic group/antibiotic.
4. Discussion
The results of the present study are compared with similar data in literature in
Table 9. A study conducted in southern Italy determined OTC levels in beef muscle and liver samples, using LC–MS [
42]. Although the number of samples was greater than the present study, very low frequencies were reported (3% in muscle, 7% in liver). The more positive liver samples compared to muscle and the higher liver concentrations of 31.5 µg/kg (23.9–40.2 µg/kg) compared to muscle concentrations of 15.9 µg/kg (15.0–28.6 µg/kg), show a similar trend that was observed in the present study (83% positive bovine liver samples, range: 66.60–102.1 µg/kg and 30% bovine muscle samples, range: 4.31–17.17 µg/kg).
Higher frequencies as well as higher concentrations may be due to inappropriate use of antibiotics and may depend on the rate of drug administration and amounts used. Oxytetracycline is used for pneumonia and some mouth infections. It has been reported that disease burden can vary between seasons depending on humidity [
43]. Furthermore, the rate of metabolism of drugs from the body depends on weather and seasonal variations [
44]. It should be noted that the seasons when the samples were collected for the present study were autumn and winter.
Panzenhagen et al. screened ENR in muscles, livers and kidneys from chickens with liquid chromatography [
45]. Based on their results, 23% of the muscle samples (mean: 12.3 µg/kg), 17% of liver samples (mean: 45.4 µg/kg) and 17% of kidney samples (mean: 17.4 µg/kg) were positive for ENR. Although higher frequencies of detection (44% in muscle, 33% in liver and 100 in kidney samples) were depicted in the current study, the detected mean values of all type of samples were much lower than those reported in the above study.
Table 9.
Comparison between current results and data from other monitoring studies in literature.
Table 9.
Comparison between current results and data from other monitoring studies in literature.
| Reference | Country | Method | N | Samples | Compounds | Mean (μg/kg) | Range | % Positive Samples |
|---|
| Present study | Greece | LC–MS | 16 beef | Muscle | OTC | 10.1 | 4.3–17.2 | 30 |
| | | | | Liver | | 77.5 | 66.6–102.1 | 83 |
| Cammilleri et al., 2019 [42] | Italy | LC–MS | 369 beef | Muscle | OTC | 15.9 | 15.0–28.6 | 3 |
| | | | | Liver | | 31.5 | 23.9–40.2 | 7 |
| Present study | Greece | ELISA | 18 pork | Muscle | SAs | 6.3 | 2.8–18.9 | 100 |
| | | | | Liver | | 47.2 | 8.4–86.0 | 100 |
| | | | | Kidney | | 14.0 | 2.5–31.9 | 83 |
| | | | 20 chicken | Muscle | | 22.9 | 1.8–157.3 | 89 |
| | | | | Liver | | 4.8 | 2.5–7.2 | 100 |
| | | | 16 beef | Muscle | | 7.4 | 2.5–30.0 | 90 |
| | | | | Liver | | 23.8 | 2.1–77.5 | 100 |
| Ramatla et al., 2017 [46] | Africa | ELISA | 50 pork | Muscle | SAs | 0 | - | 0 |
| | | | | Liver | | 58.5 | 48.2–69.9 | 9 |
| | | | | Kidney | | 72.7 | 52.8–92.8 | 36 |
| | | | 50 chicken | Muscle | | 47.5 | 32.5–65.9 | 12 |
| | | | | Liver | | 73.4 | 45.8–81.6 | 28 |
| | | | 32 beef | Muscle | | 65.3 | - | 7 |
| | | | | Liver | | 51.6 | 19.8–87.9 | 29 |
| Present study | Greece | LC–MS | 20 chicken | Muscle | ENR | 3.4 | 0.4–9.3 | 44 |
| | | | | Liver | | 7.8 | 6.4–9.5 | 33 |
| | | | | Kidney | | 2.1 | 1.4–2.8 | 100 |
| Panzenhagen et al., 2016 [45] | Brazil | LC–MS | 72 chicken | Muscle | ENR | 12.3 | 0.96–35.8 | 23 |
| | | | | Liver | | 45.4 | - | 17 |
| | | | | Kidney | | 17.4 | - | 17 |
| Present study | Greece | ELISA | 16 Beef | Muscle | STr | 169.8 | 135.6–191.5 | 30 |
| Abdullah et al., 2012 [47] | Iraq | ELISA | 23 Beef | Muscle | STr | 59.6 | 26.0–282.2 | 61 |
In South Africa, Ramatla et al. measured sulfonamide residues in pork samples (muscle, liver and kidney) using the ELISA [
46]. No sulfonamides were detected in the pork muscle samples, whereas 9% of pork liver samples and 36% of pork kidney samples were positive. The mean concentrations were 58.5 µg/kg (48.2–69.9 µg/kg) and 72.7 µg/kg (52.8–92.8 µg/kg), respectively. The results of the present study are in agreement with Ramatla et al., as higher concentrations of SAs in pork liver/kidney were found compared to pork muscle. In contrast with the literature, higher detection frequencies were found in the present study and particularly all samples of pork muscle were positive.
In a study in Iraq, STr levels in 23 beef muscle samples were determined by ELISA [
47]. A total of 61% of the samples were positive with a mean concentration of 59.60 µg/kg (26.0–282.2 µg/kg). However, in our study the results differ significantly as 30% of the samples were positive with a mean concentration of 169.76 µg/kg (135.62–191.5 µg/kg).
The observed differences between the results of the present study and others in literature [
46,
47] may be due to the way that antibiotics were administered, for example intramuscularly, intravenously or administration via food and drinking water. Furthermore, the long-term use of antibiotics before sampling and the short time between last antibiotic administration and slaughter may be significant parameters for the detection rate of the compounds. According to Yamaguchi et al. [
48], the sampling period affected significantly the detected concentrations of antibiotics in chicken samples. Higher or lesser amounts were detected during five separate occasions.
Exposure and risk assessment analysis in the present study showed that the antibiotics levels in chicken, pork and beef from the Cretan market pose no actual risk for human health. To the best of our knowledge, this is the first study for antibiotics in meat from the Greek market although there are others similar in literature. A recent work by Oyedeji et al. [
49] presented the concentrations of nineteen antibiotic residues in imported poultry products (turkey muscle and gizzard and chicken muscle) in Nigeria. The risk assessment analysis with the conventional method showed that the dietary exposure to antibiotics per meat type was within safe levels for adults and children. Vragovic et al. examined streptomycin and tetracyclines presence in meat samples of the Croatian market [
50]. Similar to the present study, EDI was significantly higher for streptomycin (5.56 μg/person/day or 0.080 μg/kg bw/day) than TCs (0.21 μg/person/day or 0.003 μg/kg bw/day). The same trend was observed in our results too, as performing the LC–MS method for TCs led to EDI approximately two orders of magnitude lower than STr.
In 2017, Wang et al. investigated livestock and poultry meat samples from Shanghai for TCs, QNLs and SAs presence [
51]. Estimated daily exposure dose was below 1 μg/kg bw/day, whereas according to the authors aquatic products were a more importance source of these antibiotics than meat or milk. Kyriakides et al. examined the differences in exposure to antibiotics between children and adolescents in Cyprus from the consumption of pork meat for the years from 2012 to 2017 [
52]. EDI values were far below ADI and notably higher in children aged 6–9 years old compared to adolescents aged 10–17 years old. All HI values were below 0.056 and indicated low risk exposure for all participants.
A different approach was followed by Zhang et al. [
53], who calculated EDI from the urinary levels of the excreted antibiotics to estimate initial exposure of the Chinese. They found that 14.7% of the children had HI greater than 1 as well as 23.6% of the parents and 11.8% of the grandparents, with ciprofloxacin being the major contributor to exposure among all participants. Lately, researchers aimed to describe the antibiotic exposure in Shanghai primary school students [
54]. Fluoroquinolones, lincosamides, sulfonamides and tetracyclines were examined and the totally daily exposure dose was found to be below 1 μg/kg bw/day. Finally, the study concluded that intake frequency of white meat (poultry meat) is positively associated with TCS and intake frequency of dairy products with enrofloxacin (QNLs).