Tolerance and Heavy Metal Accumulation Characteristics of Sasa argenteostriata (Regel) E.G. Camus under Zinc Single Stress and Combined Lead–Zinc Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Culture
2.2. Experimental Design
2.2.1. Zn Single-Stress Treatment
2.2.2. Combined Pb–Zn Stress Treatment
2.3. Determination of Plant Physiological Indexes
2.4. Determination of Metal Content in Plant Tissues
2.5. Statistical Analysis
3. Results and Analysis
3.1. Responses of Stress-Resistance Physiology and Heavy Metal Accumulation Characteristics of S. argenteostriata under Zn Stress
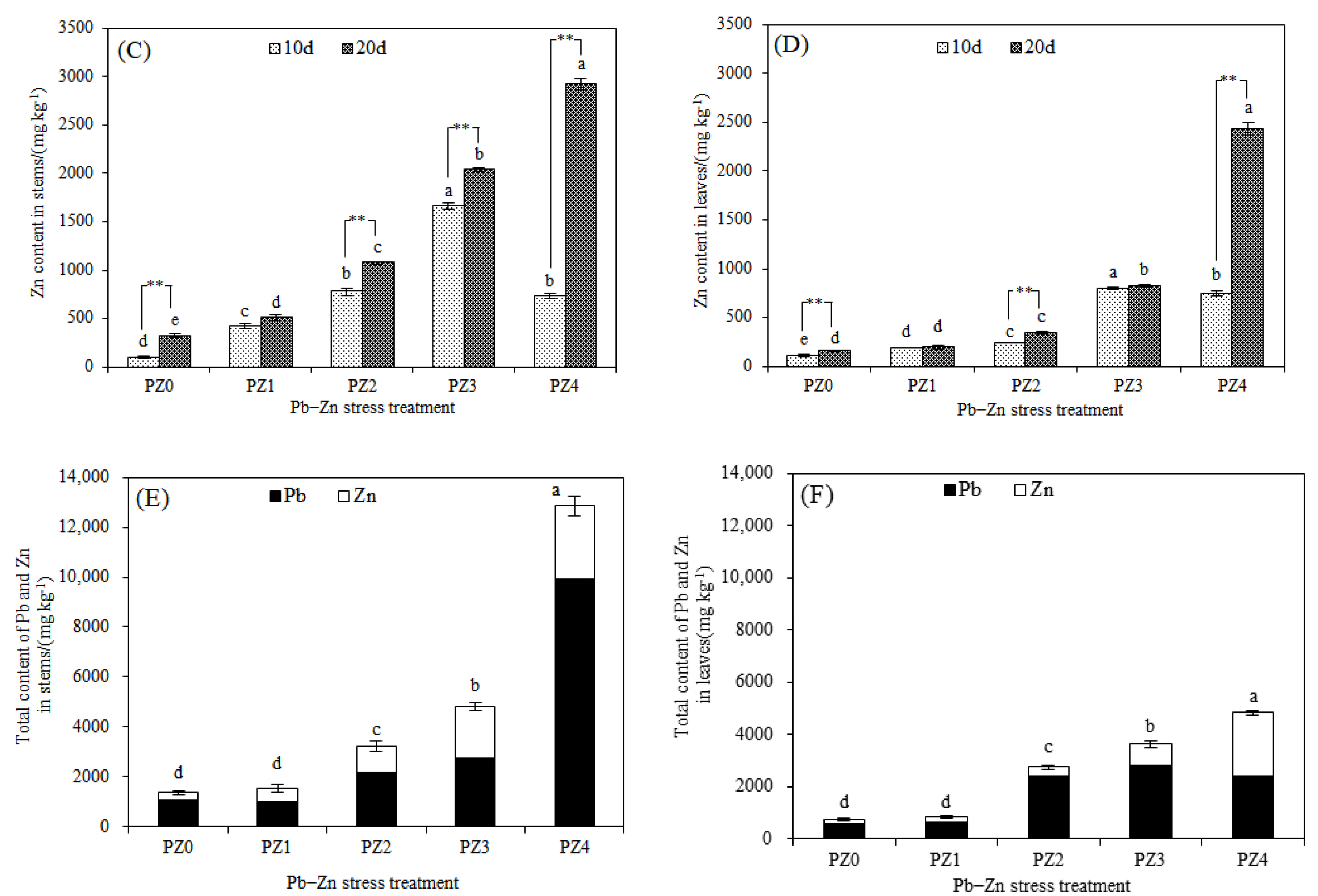
3.2. Responses of Stress-Resistance Physiology and Heavy Metal Accumulation Characteristics of S. argenteostriata under Combined Pb–Zn Stress
4. Discussion
4.1. Tolerance and Heavy Metal Accumulation Characteristics of S. argenteostriata under Zn Stress
4.2. Tolerance and Heavy Metal Accumulation Characteristics of S. argenteostriata under Combined Pb–Zn Stress
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dong, L.J.; Tong, X.J.; Li, X.B.; Zhou, J.; Wang, S.F.; Liu, B. Some developments and new insights of environmental problems and deep mining strategy for cleaner production in mines. J. Clean. Prod. 2019, 210, 1562–1578. [Google Scholar] [CrossRef]
- Obiora, S.C.; Chukwu, A.; Toteu, S.F.; Davies, T.C. Assessment of heavy metal contamination in soils around lead (Pb)-zinc (Zn) mining areas in Enyigba, southeastern Nigeria. J. Geol. Soc. India 2016, 87, 453–462. [Google Scholar] [CrossRef]
- Barac, N.; Skrivanj, S.; Mutic, J.; Manojlovic, D.; Bukumiric, Z.; Zivojinovic, D.; Petrovic, R.; Corac, A. Heavy Metals Fractionation in Agricultural Soils of Pb/Zn Mining Region and Their Transfer to Selected Vegetables. Water Air Soil Pollut. 2016, 227, 481. [Google Scholar] [CrossRef]
- Dong, J.; Yang, Q.W.; Sun, L.N.; Zeng, Q.; Liu, S.J.; Pan, J.; Liu, X.L. Assessing the concentration and potential dietary risk of heavy metals in vegetables at a Pb/Zn mine site, China. Environ. Earth Sci. 2011, 64, 1317–1321. [Google Scholar] [CrossRef]
- Kan, X.Q.; Dong, Y.Q.; Feng, L.; Zhou, M.; Hou, H.B. Contamination and health risk assessment of heavy metals in China’s lead-zinc mine tailings: A meta-analysis. Chemosphere 2021, 267, 128909. [Google Scholar] [CrossRef]
- Shah, V.; Daverey, A. Phytoremediation: A multidisciplinary approach to clean up heavy metal contaminated soil. Environ. Technol. Innov. 2020, 18, 100774. [Google Scholar] [CrossRef]
- Ashraf, S.; Ali, Q.; Zahir, Z.A.; Ashraf, S.; Asghar, H.N. Phytoremediation: Environmentally sustainable way for reclamation of heavy metal polluted soils. Ecotoxicol. Environ. Saf. 2019, 174, 714–727. [Google Scholar] [CrossRef]
- Stefanowicz, A.M.; Stanek, M.; Woch, M.W.; Kapusta, P. The accumulation of elements in plants growing spontaneously on small heaps left by the historical Zn-Pb ore mining. Environ. Sci. Pollut. Res. 2016, 23, 6524–6534. [Google Scholar] [CrossRef] [Green Version]
- Chaabani, S.; Abdelmalek-Babbou, C.; Ben Ahmed, H.; Chaabani, A.; Sebei, A. Phytoremediation assessment of native plants growing on Pb–Zn mine site in Northern Tunisia. Environ. Earth. Sci. 2017, 76, 585. [Google Scholar] [CrossRef]
- Shikha, D.; Singh, P.K. In situ phytoremediation of heavy metal-contaminated soil and groundwater: A green inventive approach. Environ. Sci. Pollut. Res. 2021, 28, 4104–4124. [Google Scholar] [CrossRef]
- Sun, W.; Ji, B.; Khoso, S.A.; Tang, H.H.; Liu, R.Q.; Wang, L.; Hu, Y.H. An extensive review on restoration technologies for mining tailings. Environ. Sci. Pollut. Res. 2018, 25, 33911–33925. [Google Scholar] [CrossRef]
- Si, L.Q.; Zhang, J.T.; Hussain, A.; Qiao, Y.; Zhou, J.X.; Wang, X.P. Accumulation and translocation of food chain in soil-mulberry (Morus alba L.)-silkworm (Bombyx mori) under single and combined stress of lead and cadmium. Ecotoxicol. Environ. Saf. 2021, 208, 111582. [Google Scholar] [CrossRef]
- Qu, T.B.; Peng, Y.L.; Yang, C.X.; Du, X.; Guo, W.Q.; Zhang, J.F. Single and Combined Effects of Cadmium and Lead on Seed Germination and Early Seedling Growth in Rims typhina. Pol. J. Environ. Stud. 2021, 30, 823–831. [Google Scholar] [CrossRef]
- Murtaza, B.; Naeem, F.; Shahid, M.; Abbas, G.; Shah, N.S.; Amjad, M.; Bakhat, H.F.; Imran, M.; Niazi, N.K.; Murtaza, G. A multivariate analysis of physiological and antioxidant responses and health hazards of wheat under cadmium and lead stress. Environ. Sci. Pollut. Res. 2019, 26, 362–370. [Google Scholar] [CrossRef]
- Ahmad, P.; Ozturk, M.; Gucel, S. Oxidative damage and antioxidants induced by heavy metal stress in two cultivars of mustard (Brassica juncea L.) plants. Fresenius Environ. Bull. 2012, 21, 2953–2961. [Google Scholar]
- Guo, J.M.; Yang, J.X.; Yang, J.; Chen, T.B.; Li, H.E.; Xu, T.B.; Zhou, X.Y.; Ye, Y.; Yu, B. Interaction of Cd and Zn Affecting the Root Morphology and Accumulation of Heavy Metals in Sedum aizoon. Environ. Sci. 2019, 40, 470–479. [Google Scholar] [CrossRef]
- Sharma, R.K.; Agrawal, M.; Agrawal, S.B. Interactive effects of cadmium and zinc on carrots: Growth and biomass accumulation. J. Plant Nutr. 2007, 31, 19–34. [Google Scholar] [CrossRef]
- Xu, W.H.; Li, W.Y.; He, J.P.; Singh, B.; Xiong, Z.T. Effects of insoluble Zn, Cd, and EDTA on the growth, activities of antioxidant enzymes and uptake of Zn and Cd in Vetiveria zizanioides. J. Environ. Sci. 2009, 21, 186–192. [Google Scholar] [CrossRef]
- Liu, J.N.; Zhou, Q.X.; Sun, T.; Ma, L.Q.; Wang, S. Growth responses of three ornamental plants to Cd and Cd—Pb stress and their metal accumulation characteristics. J. Hazard. Mater. 2008, 151, 261–267. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Rehman, M.Z.U.; Maqbool, A. A critical review on the effects of zinc at toxic levels of cadmium in plants. Environ. Sci. Pollut. Res. 2019, 26, 6279–6289. [Google Scholar] [CrossRef]
- Mapodzeke, J.M.; Adil, M.F.; Wei, D.M.; Joan, H.I.; Ouyang, Y.N.; Shamsi, I.H. Modulation of key physio-biochemical and ultrastructural attributes after synergistic application of zinc and silicon on rice under cadmium stress. Plants 2021, 10, 87. [Google Scholar] [CrossRef] [PubMed]
- Bian, F.Y.; Zhong, Z.K.; Zhang, X.P.; Yang, C.B.; Gai, X. Bamboo—An untapped plant resource for the phytoremediation of heavy metal contaminated soils. Chemosphere 2020, 246, 125750. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.Y.; Jiang, M.Y.; Liao, J.R.; Yang, Y.X.; Li, N.F.; Cheng, Q.B.; Li, X.; Song, H.X.; Luo, Z.H.; Liu, S.L. Biomass allocation strategies and Pb-enrichment characteristics of six dwarf bamboos under soil Pb stress. Ecotoxicol. Environ. Saf. 2021, 207, 111500. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, J.R.; Islam, E.; Wang, Y.; Wu, J.S.; Ye, Z.Q.; Yan, W.B.; Peng, D.L.; Liu, D. Cadmium-induced oxidative stress, response of antioxidants and detection of intracellular cadmium in organs of moso bamboo (Phyllostachys pubescens) seedlings. Chemosphere 2016, 153, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Chen, J.R.; Mahmood, Q.; Li, S.; Wu, J.S.; Ye, Z.Q.; Peng, D.L.; Yan, W.B.; Lu, K.P. Effect of Zn toxicity on root morphology, ultrastructure, and the ability to accumulate Zn in Moso bamboo (Phyllostachys pubescens). Environ. Sci. Pollut. Res. 2014, 21, 13615–13624. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Li, S.; Islam, E.; Chen, J.R.; Wu, J.S.; Ye, Z.Q.; Peng, D.L.; Yan, W.B.; Lu, K.P. Lead accumulation and tolerance of Moso bamboo (Phyllostachys pubescens) seedlings: Applications of phytoremediation. J. Zhejiang Univ. Sci. B 2015, 16, 123–130. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.J.; Gao, J.; Cai, C.J.; Fan, S.H. Absorption and distribution of mineral nutrients in Pleioblastus fortunei under lead stress. Sci. Silvae Sin. 2011, 47, 153–157. [Google Scholar] [CrossRef]
- Jiang, M.Y.; Cai, X.Y.; Liao, J.R.; Yang, Y.X.; Chen, Q.B.; Gao, S.P.; Yu, X.F.; Luo, Z.H.; Lei, T.; Lv, B.Y.; et al. Different strategies for lead detoxification in dwarf bamboo tissues. Ecotoxicol. Environ. Saf. 2020, 193, 110329. [Google Scholar] [CrossRef]
- Liao, J.R.; Cai, X.Y.; Yang, Y.X.; Chen, Q.B.; Gao, S.P.; Liu, G.L.; Sun, L.X.; Luo, Z.H.; Lei, T.; Jiang, M.Y. Dynamic study of the lead (Pb) tolerance and accumulation characteristics of new dwarf bamboo in Pb-contaminated soil. Chemosphere 2021, 282, 131089. [Google Scholar] [CrossRef]
- Cai, X.Y.; Liao, J.R.; Yang, Y.X.; Li, N.F.; Xu, M.; Jiang, M.Y.; Chen, Q.B.; Li, X.; Liu, S.L.; Luo, Z.H.; et al. Physiological resistance of Sasa argenteostriata (Regel) E.G. Camus in response to high-concentration soil Pb stress. Acta Physiol. Plant. 2021, 43, 21. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplast I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid Determination of Free Proline for Water-Stress Studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Yan, H.; Filardo, F.; Hu, X.T.; Zhao, X.M.; Fu, D.H. Cadmium stress alters the redox reaction and hormone balance in oilseed rape (Brassica napus L.) leaves. Environ. Sci. Pollut. Res. 2016, 23, 3758–3769. [Google Scholar] [CrossRef]
- Barron, M.G. Bioconcentration: Will water-borne organic chemicals accumulate in aquatic animals? Environ. Sci. Technol. 1990, 24, 1612–1618. [Google Scholar] [CrossRef]
- Shackira, A.M.; Puthur, J.T.; Salim, E.N. Acanthus ilicifolius L. a promising candidate for phytostabilization of zinc. Environ. Monit. Assess. 2017, 189, 282. [Google Scholar] [CrossRef]
- Subba, P.; Mukhopadhyay, M.; Mahato, S.K.; Bhutia, K.D.; Mondal, T.K.; Ghosh, S.K. Zinc stress induces physiological, ultra-structural and biochemical changes in mandarin orange (Citrus reticulata Blanco) seedlings. Physiol. Mol. Biol. Plants 2014, 20, 461–473. [Google Scholar] [CrossRef] [Green Version]
- Kulbat-Warycha, K.; Georgiadou, E.C.; Mankowska, D.; Smolinska, B.; Fotopoulos, V.; Leszczynska, J. Response to stress and allergen production caused by metal ions (Ni, Cu and Zn) in oregano (Origanum vulgare L.) plants. J. Biotechnol. 2020, 324, 171–182. [Google Scholar] [CrossRef]
- Parlak, K.U.; Yilmaz, D.D. Response of antioxidant defences to Zn stress in three duckweed species. Ecotoxicol. Environ. Saf. 2012, 85, 52–58. [Google Scholar] [CrossRef]
- Grassi, C.; Cecchi, S.; Baldi, A.; Zanchi, C.A.; Orlandini, S.; Pardini, A.; Napoli, M. Crop suitability assessment in remediation of Zn contaminated soil. Chemosphere 2020, 246, 125706. [Google Scholar] [CrossRef]
- Lin, Y.F.; Aarts, M.G.M. The molecular mechanism of zinc and cadmium stress response in plants. Cell. Mol. Life. Sci. 2012, 69, 3187–3206. [Google Scholar] [CrossRef]
- Bian, F.Y.; Zhong, Z.K.; Zhang, X.P.; Yang, C.B. Phytoremediation potential of moso bamboo (Phyllostachys pubescens) intercropped with Sedum plumbizincicola in metal-contaminated soil. Environ. Sci. Pollut. Res. 2017, 24, 27244–27253. [Google Scholar] [CrossRef]
- Embaye, K.; Weih, M.; Ledin, S.; Christersson, L. Biomass and nutrient distribution in a highland bamboo forest in southwest Ethiopia: Implications for management. For. Ecol. Manag. 2005, 204, 159–169. [Google Scholar] [CrossRef]
- Wei, C.; Jiao, Q.J.; Agathokleous, E.; Liu, H.T.; Li, G.Z.; Zhang, J.J.; Fahad, S.; Jiang, Y. Hormetic effects of zinc on growth and antioxidant defense system of wheat plants. Sci. Total Environ. 2022, 807, 150992. [Google Scholar] [CrossRef]
- Santos, M.L.S.D.; Almeida, A.A.F.D.; Silva, N.M.D.; Oliveira, B.R.M.; Silva, J.V.S.; Souza, J.O.; Ahnert, D.; Baligar, V.C. Mitigation of cadmium toxicity by zinc in juvenile cacao: Physiological, biochemical, molecular and micromorphological responses. Environ. Exp. Bot. 2020, 179, 104201. [Google Scholar] [CrossRef]
- Feng, P.; Sun, L.; Shen, X.H.; Li, R.L.; Jiang, C.; Zheng, H.Y.; Li, Z.J.; Li, Z.M.; Guo, W.; Han, X.D.; et al. Response and accumulation ability of perennial ryegrass to plumbum and cadmium stress. Fresenius Environ. Bull. 2017, 26, 598–606. [Google Scholar]
- Benáková, M.; Ahmadi, H.; Ducaiova, Z.; Tylova, E.; Clemens, S.; Tuma, J. Effects of Cd and Zn on physiological and anatomical properties of hydroponically grown Brassica napus plants. Environ. Sci. Pollut. Res. 2017, 24, 20705–20716. [Google Scholar] [CrossRef]
- Sbartai, H.; Djebar, M.R.; Sbartai, I.; Berrabbah, H. Bioaccumulation of cadmium and zinc in tomato (Lycopersicon esculentum L.). Comptes Rendus Biol. 2012, 335, 585–593. [Google Scholar] [CrossRef]
- Jin, C.; Zhao, Z.J.; Nan, Z.R.; Wang, S.L.; Wu, W.F.; Wang, H.C. Speciation Characteristics and Bioavailability of Heavy Metals in Oasis Soil Under Pb, Zn Combined Stress. Environ. Sci. 2015, 36, 1870–1876. [Google Scholar] [CrossRef]
- Adamczyk-Szabela, D.; Lisowska, K.; Romanowska-Duda, Z.; Wolf, W.M. Combined cadmium-zinc interactions alter manganese, lead, copper uptake by Melissa officinalis. Sci. Rep. 2020, 10, 1675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, G.H.; Kumari, J.P. Heavy Metal Lead Influative Toxicity and Its Assessment in Phytoremediating Plants—A Review. Water Air Soil Pollut. 2015, 226, 324. [Google Scholar] [CrossRef]
- Pace, R.; Liberati, D.; Sconocchia, P.; Angelis, P.D. Lead transfer into the vegetation layer growing naturally in a Pb-contaminated site. Environ. Geochem. Health 2020, 42, 2321–2329. [Google Scholar] [CrossRef]
- Bahraminia, M.; Zarei, M.; Ronaghi, A.; Ghasemi-Fasaei, R. Effectiveness of arbuscular mycorrhizal fungi in phytoremediation of lead- contaminated soil by vetiver grass. Int. J. Phytorem. 2016, 18, 730–737. [Google Scholar] [CrossRef]
- Chen, Y.H.; Shen, Z.G.; Li, X.D. The use of vetiver grass (Vetiveria zizanioides) in the phytoremediation of soils contaminated with heavy metals. Appl. Geochem. 2004, 19, 1553–1565. [Google Scholar] [CrossRef] [Green Version]
- Lamb, D.T.; Ming, H.; Megharaj, M.; Naidu, R. Phytotoxicity and Accumulation of Lead in Australian Native Vegetation. Arch. Environ. Contam. Toxicol. 2010, 58, 613–621. [Google Scholar] [CrossRef]

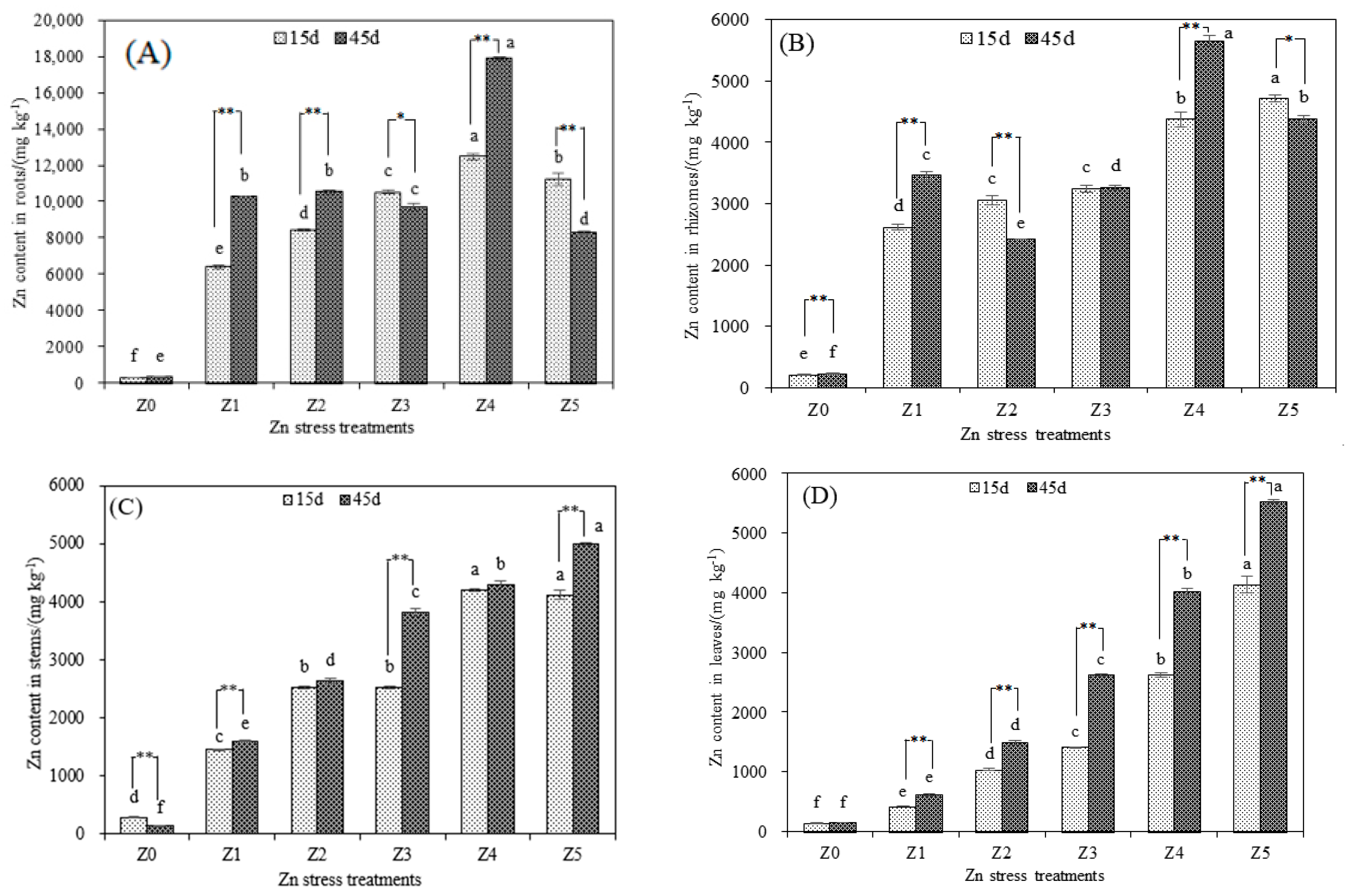
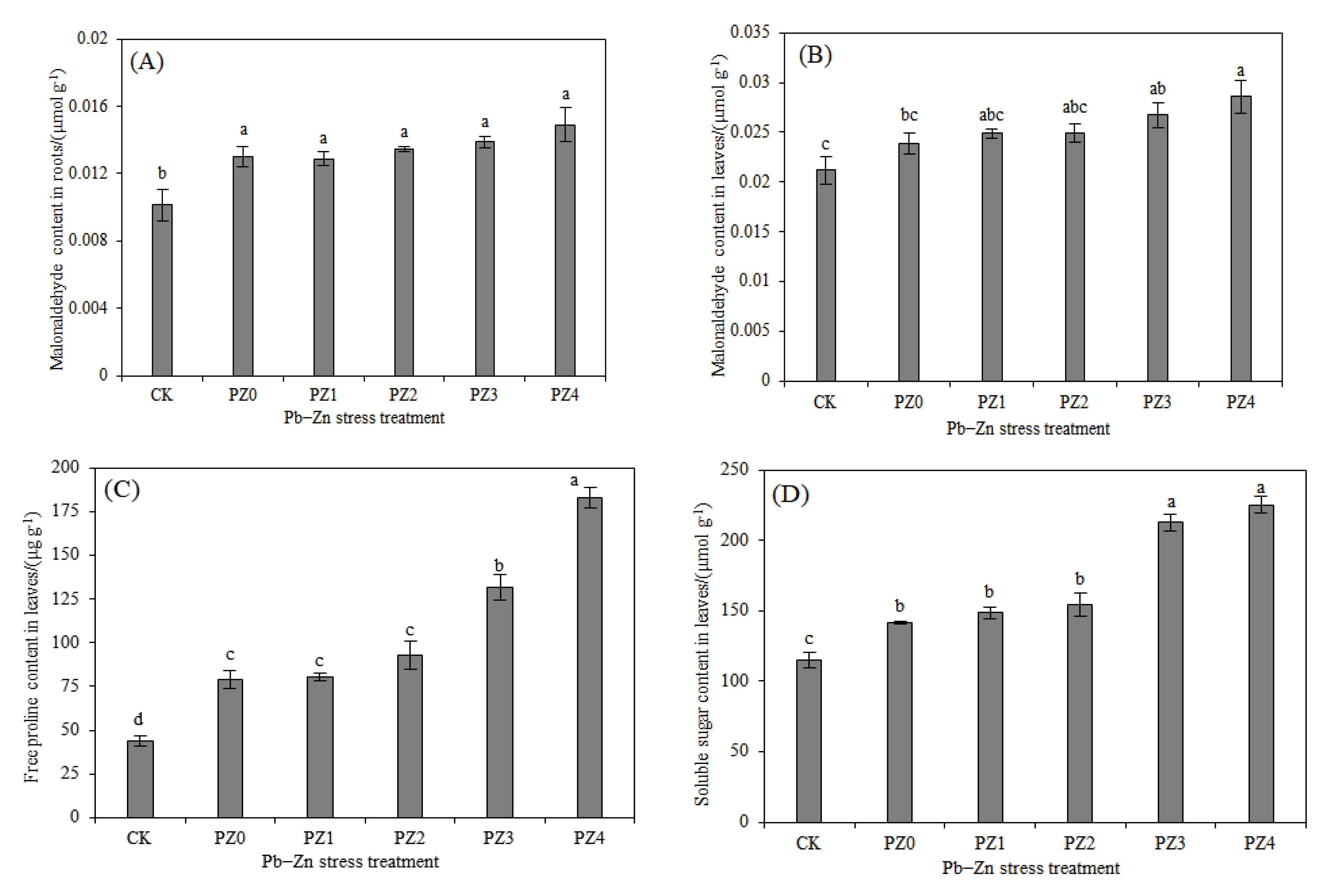
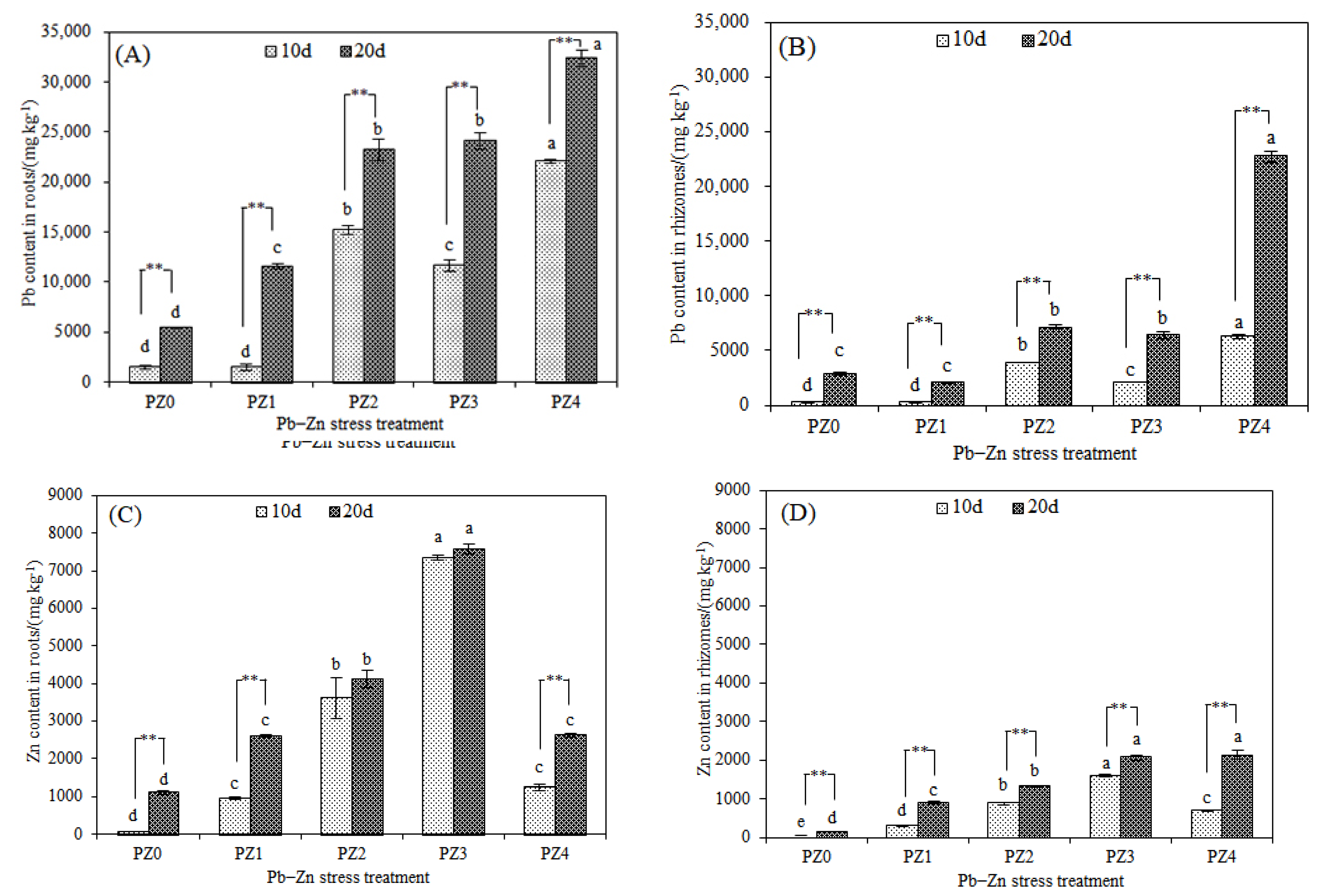

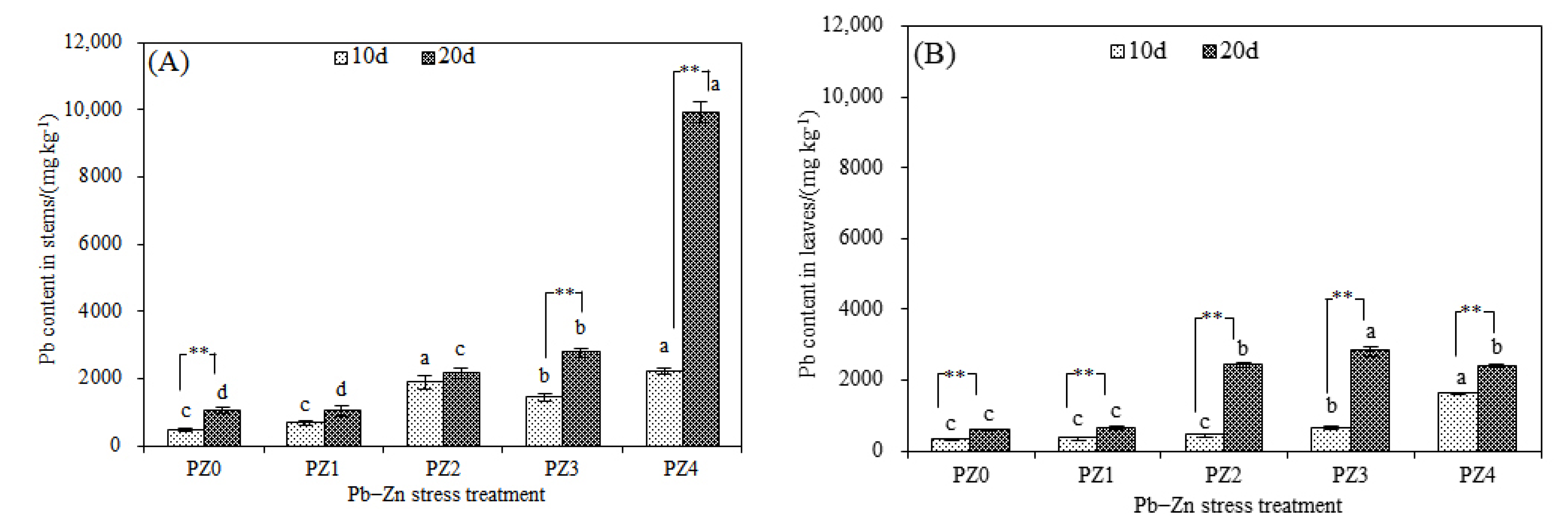
| Treatment Time | Zn Stress Treatment | Bioconcentration Factors | |||
|---|---|---|---|---|---|
| Root | Rhizome | Stem | Leaf | ||
| 15d | Z1 | 85.2 | 34.9 | 19.4 | 5.6 |
| Z2 | 56.3 | 20.4 | 16.8 | 6.9 | |
| Z3 | 35.0 | 10.8 | 8.4 | 4.7 | |
| Z4 | 20.8 | 7.3 | 7.0 | 4.4 | |
| Z5 | 11.2 | 4.7 | 4.1 | 4.1 | |
| 45d | Z1 | 137.1 | 46.2 | 21.5 | 8.2 |
| Z2 | 70.2 | 16.2 | 17.6 | 9.9 | |
| Z3 | 32.3 | 10.9 | 12.7 | 8.8 | |
| Z4 | 29.8 | 9.4 | 7.1 | 6.7 | |
| Z5 | 8.3 | 4.4 | 5.0 | 5.5 | |
| Organ Species | Pb–Zn Stress Treatment | Bioconcentration Factors | ||
|---|---|---|---|---|
| Pb | Zn | Total | ||
| Root | PZ0 | 18.0 | - | - |
| PZ1 | 38.7 | 34.8 | 37.9 | |
| PZ2 | 77.5 | 27.4 | 60.8 | |
| PZ3 | 80.3 | 25.3 | 52.8 | |
| PZ4 | 108.0 | 4.4 | 38.9 | |
| Rhizome | PZ0 | 9.7 | - | - |
| PZ1 | 7.0 | 12.0 | 8.0 | |
| PZ2 | 23.7 | 8.8 | 18.8 | |
| PZ3 | 21.4 | 6.9 | 14.2 | |
| PZ4 | 75.9 | 3.5 | 27.6 | |
| Stem | PZ0 | 3.5 | - | - |
| PZ1 | 3.4 | 6.7 | 4.1 | |
| PZ2 | 7.2 | 7.2 | 7.2 | |
| PZ3 | 9.2 | 6.8 | 8.0 | |
| PZ4 | 33.1 | 4.9 | 14.3 | |
| Leaf | PZ0 | 1.9 | - | - |
| PZ1 | 2.2 | 2.6 | 2.3 | |
| PZ2 | 8.0 | 2.3 | 6.1 | |
| PZ3 | 9.4 | 2.8 | 6.1 | |
| PZ4 | 8.0 | 4.1 | 5.4 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, J.; Li, N.; Yang, Y.; Yang, J.; Tian, Y.; Luo, Z.; Jiang, M. Tolerance and Heavy Metal Accumulation Characteristics of Sasa argenteostriata (Regel) E.G. Camus under Zinc Single Stress and Combined Lead–Zinc Stress. Toxics 2022, 10, 450. https://doi.org/10.3390/toxics10080450
Liao J, Li N, Yang Y, Yang J, Tian Y, Luo Z, Jiang M. Tolerance and Heavy Metal Accumulation Characteristics of Sasa argenteostriata (Regel) E.G. Camus under Zinc Single Stress and Combined Lead–Zinc Stress. Toxics. 2022; 10(8):450. https://doi.org/10.3390/toxics10080450
Chicago/Turabian StyleLiao, Jiarong, Ningfeng Li, Yixiong Yang, Jing Yang, Yuan Tian, Zhenghua Luo, and Mingyan Jiang. 2022. "Tolerance and Heavy Metal Accumulation Characteristics of Sasa argenteostriata (Regel) E.G. Camus under Zinc Single Stress and Combined Lead–Zinc Stress" Toxics 10, no. 8: 450. https://doi.org/10.3390/toxics10080450
APA StyleLiao, J., Li, N., Yang, Y., Yang, J., Tian, Y., Luo, Z., & Jiang, M. (2022). Tolerance and Heavy Metal Accumulation Characteristics of Sasa argenteostriata (Regel) E.G. Camus under Zinc Single Stress and Combined Lead–Zinc Stress. Toxics, 10(8), 450. https://doi.org/10.3390/toxics10080450





