Characterization of Potential Adverse Outcome Pathways Related to Metabolic Outcomes and Exposure to Per- and Polyfluoroalkyl Substances Using Artificial Intelligence
Abstract
:1. Introduction
2. Materials and Methods
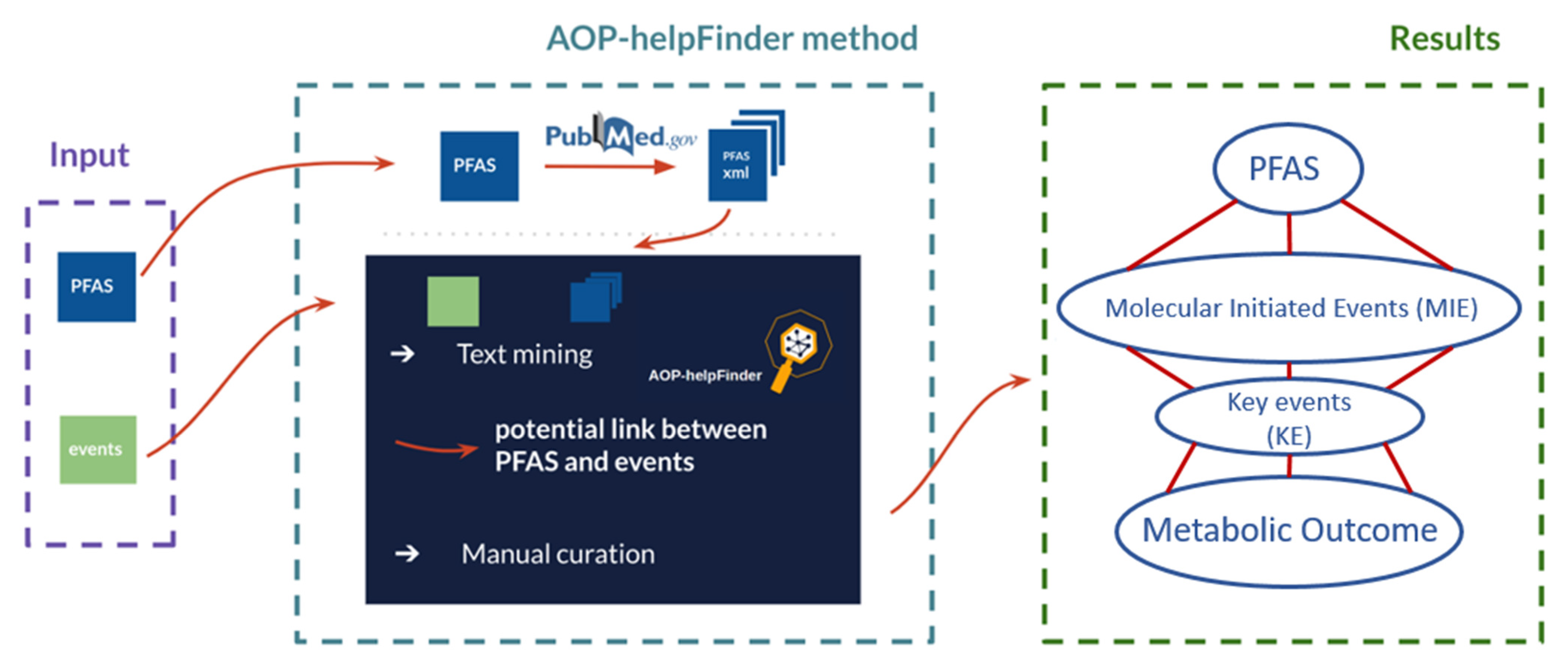
2.1. Application of the AOP-helpFinder
2.1.1. Development of the Dictionaries of Stressors and Events to Be Screened
2.1.2. Automatic Screening of the Literature Using the AOP-helpFinder Tool—Multistep Procedure
3. Results
3.1. Linking PFAS to Adverse Effects
3.1.1. Collected Data from In Vivo Studies Using the AOP-helpFinder
3.1.2. Collected Data from In Vitro Studies Using the AOP-helpFinder
3.1.3. Collected Data from Epidemiological Studies Using the AOP-helpFinder
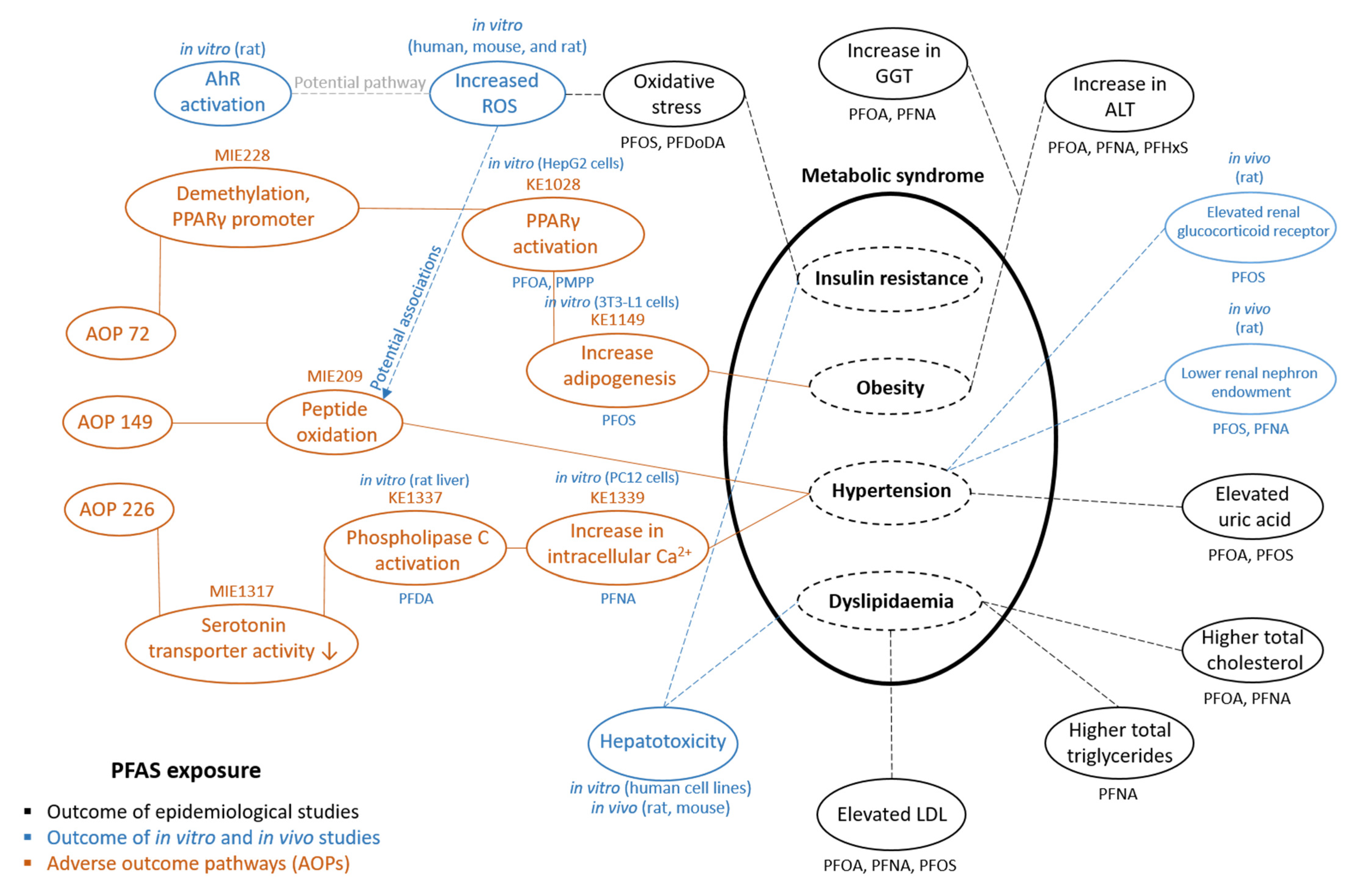
3.2. Linking PFAS to AOPs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vinken, M. The adverse outcome pathway concept: A pragmatic tool in toxicology. Toxicology 2013, 312, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Ankley, G.T.; Bennett, R.S.; Erickson, R.J.; Hoff, D.J.; Hornung, M.W.; Johnson, R.D.; Mount, D.R.; Nichols, J.W.; Russom, C.L.; Schmieder, P.K.; et al. Adverse outcome pathways: A conceptual framework to support ecotoxicology research and risk assessment. Environ. Toxicol. Chem. 2010, 29, 730–741. [Google Scholar] [CrossRef] [PubMed]
- Organisation for Economic Co-operation and Development (OECD). Proposal for a template, and guidance on developing and assessing the completeness of adverse outcome pathways. In OECD Environment, Health and Safety Publications Series on Testing and Assessment; 2012; Available online: https://www.oecd.org/env/ehs/testing/49963554.pdf (accessed on 8 January 2022).
- European Commission (EC). Adverse Outcome Pathways—Have your say and shape the future of Chemical Risk Assessment. 2019. Available online: https://ec.europe.eu/jrc/en/science-update/adverse-outcome-pathways-have-your-say-and-shape-future-chemical-risk-assessment (accessed on 3 February 2022).
- Society for Advancement of AOPs. AOP-Wiki. 2022. Available online: https://aopwiki.org/aops (accessed on 28 February 2022).
- Glüge, J.; Scheringer, M.; Cousins, I.T.; DeWitt, J.C.; Goldenman, G.; Herzke, D.; Lohmann, R.; Ng, C.A.; Trier, X.; Wang, Z. An overview of the uses of per- and polyfluoroalkyl substances (PFAS). Environ. Sci. Processes Impacts 2020, 22, 2345–2373. [Google Scholar] [CrossRef] [PubMed]
- Sunderland, E.M.; Hu, X.C.; Dassuncao, C.; Tokranov, A.K.; Wagner, C.C.; Allen, J.G. A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 131–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosato, I.; Zare Jeddi, M.; Ledda, C.; Gallo, E.; Fletcher, T.; Pitter, G.; Batzella, E.; Canova, C. How to investigate human health effects related to exposure to mixtures of per- and polyfluoroalkyl substances: A systematic review of statistical methods. Environ. Res. 2022, 205, 112565. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Risk to human health related to the presence of perfluoroalkyl substances in food: EFSA Panel on Contaminants in the Food Chain (EFSA CONTAM Panel). Scientific Opinion. EFSA J. 2020, 18, e06223. [Google Scholar]
- Fenton, S.E.; Ducatman, A.; Boobis, A.; DeWitt, J.C.; Lau, C.; Ng, C.; Smith, J.S.; Roberts, S.M. Per- and Polyfluoroalkyl Substance Toxicity and Human Health Review: Current State of Knowledge and Strategies for Informing Future Research. Environ. Toxicol. Chem. 2021, 40, 606–630. [Google Scholar] [CrossRef] [PubMed]
- Haverinen, E.; Fernandez, M.F.; Mustieles, V.; Tolonen, H. Metabolic Syndrome and Endocrine Disrupting Chemicals: An Overview of Exposure and Health Effects. Int. J. Environ. Res. Public Health 2021, 18, 13047. [Google Scholar] [CrossRef]
- Zare Jeddi, M.; Soltanmohammadi, R.; Barbieri, G.; Fabricio, A.S.C.; Pitter, G.; Dalla Zuanna, T.; Canova, C. To which extent are per-and poly-fluorinated substances associated to metabolic syndrome? Rev. Environ. Health 2021, 37, 211–228. [Google Scholar] [CrossRef]
- Huang, P.L. A comprehensive definition for metabolic syndrome. Dis. Model. Mech. 2009, 2, 231–237. [Google Scholar] [CrossRef] [Green Version]
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.-C.; James, W.P.T.; Loria, C.M.; Smith, S.C. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention, National Heart, Lung, and Blood Institute, American Heart Association, World Heart Federation, International Atherosclerosis Society, and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef] [Green Version]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [Green Version]
- Hoyeck, M.P.; Matteo, G.; MacFarlane, E.M.; Perera, I.; Bruin, J.E. Persistent organic pollutants and β-cell toxicity: A comprehensive review. Am. J. Physiol. Endocrinol. Metab. 2022, 322, E383–E413. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Furnary, T.; Vasiliou, V.; Yan, Q.; Nyhan, K.; Jones, D.P.; Johnson, C.H.; Liew, Z. Non-targeted metabolomics and associations with per- and polyfluoroalkyl substances (PFAS) exposure in humans: A scoping review. Environ. Int. 2022, 162, 107159. [Google Scholar] [CrossRef]
- Society for Advancement of AOPs. Perfluorooctanoic acid-Stressor: 175. 2022. Available online: https://aopwiki.org/stressors/175 (accessed on 8 January 2022).
- Carvaillo, J.-C.; Barouki, R.; Coumoul, X.; Audouze, K. Linking Bisphenol S to Adverse Outcome Pathways Using a Combined Text Mining and Systems Biology Approach. Environ. Health Perspect. 2019, 127, 47005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rugard, M.; Coumoul, X.; Carvaillo, J.-C.; Barouki, R.; Audouze, K. Deciphering Adverse Outcome Pathway Network Linked to Bisphenol F Using Text Mining and Systems Toxicology Approaches. Toxicol. Sci. 2020, 173, 32–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jornod, F.; Rugard, M.; Tamisier, L.; Coumoul, X.; Andersen, H.R.; Barouki, R.; Audouze, K. AOP4EUpest: Mapping of pesticides in adverse outcome pathways using a text mining tool. Bioinformatics 2020, 36, 4379–4381. [Google Scholar] [CrossRef]
- Zgheib, E.; Kim, M.J.; Jornod, F.; Bernal, K.; Tomkiewicz, C.; Bortoli, S.; Coumoul, X.; Barouki, R.; de Jesus, K.; Grignard, E.; et al. Identification of non-validated endocrine disrupting chemical characterization methods by screening of the literature using artificial intelligence and by database exploration. Environ. Int. 2021, 154, 106574. [Google Scholar] [CrossRef] [PubMed]
- Audouze, K.; Sarigiannis, D.; Alonso-Magdalena, P.; Brochot, C.; Casas, M.; Vrijheid, M.; Babin, P.J.; Karakitsios, S.; Coumoul, X.; Barouki, R. Integrative Strategy of Testing Systems for Identification of Endocrine Disruptors Inducing Metabolic Disorders-An Introduction to the OBERON Project. Int. J. Mol. Sci. 2020, 21, 2988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jornod, F.; Jaylet, T.; Blaha, L.; Sarigiannis, D.; Tamisier, L.; Audouze, K. AOP-helpFinder webserver: A tool for comprehensive analysis of the literature to support adverse outcome pathways development. Bioinformatics 2021, 38, 1173–1175. [Google Scholar] [CrossRef] [PubMed]
- HBM4EU. Scoping Documents for 2021 for the First and Second Second round HBM4EU Priority Substances: Deliverable Report D4.9 WP4—Prioritisation and Input to the Annual Work Plan Deadline: September 2020 Upload by Coordinator: 24 November 2020. 2020. Available online: https://www.hbm4eu.eu/wp-content/uploads/2021/03/HBM4EU_D4.9_Scoping_Documents_HBM4EU_priority_substances_v1.0.pdf (accessed on 8 June 2022).
- Steenland, K.; Tinker, S.; Shankar, A.; Ducatman, A. Association of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) with uric acid among adults with elevated community exposure to PFOA. Environ. Health Perspect. 2010, 118, 229–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shankar, A.; Xiao, J.; Ducatman, A. Perfluoroalkyl chemicals and chronic kidney disease in US adults. Am. J. Epidemiol. 2011, 174, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Shankar, A.; Xiao, J.; Ducatman, A. Perfluoroalkyl chemicals and elevated serum uric acid in US adults. Clin. Epidemiol. 2011, 3, 251–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geiger, S.D.; Xiao, J.; Shankar, A. Positive association between perfluoroalkyl chemicals and hyperuricemia in children. Am. J. Epidemiol. 2013, 177, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Guo, X.; Sun, P.; Chen, Y.; Zhang, W.; Gao, A. Association of serum levels of perfluoroalkyl substances (PFASs) with the metabolic syndrome (MetS) in Chinese male adults: A cross-sectional study. Sci. Total Environ. 2018, 621, 1542–1549. [Google Scholar] [CrossRef]
- Kataria, A.; Trachtman, H.; Malaga-Dieguez, L.; Trasande, L. Association between perfluoroalkyl acids and kidney function in a cross-sectional study of adolescents. Environ. Health 2015, 14, 89. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.-Y.; Lin, L.-Y.; Chiang, C.-K.; Wang, W.-J.; Su, Y.-N.; Hung, K.-Y.; Chen, P.-C. Investigation of the associations between low-dose serum perfluorinated chemicals and liver enzymes in US adults. Am. J. Gastroenterol. 2010, 105, 1354–1363. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.B.; Ducatman, A. Selective Associations of Recent Low Concentrations of Perfluoroalkyl Substances With Liver Function Biomarkers: NHANES 2011 to 2014 Data on US Adults Aged ≥20 Years. J. Occup. Environ. Med. 2019, 61, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.B. Concentration of selected liver enzymes across the stages of glomerular function: The associations with PFOA and PFOS. Heliyon 2019, 5, e02168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, R.; McConnell, R.; Catherine, C.; Xu, S.; Walker, D.I.; Stratakis, N.; Jones, D.P.; Miller, G.W.; Peng, C.; Conti, D.V.; et al. Perfluoroalkyl substances and severity of nonalcoholic fatty liver in Children: An untargeted metabolomics approach. Environ. Int. 2020, 134, 105220. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Jiao, J.; Zhuang, P.; Chen, X.; Wang, J.; Zhang, Y. Serum polyfluoroalkyl chemicals are associated with risk of cardiovascular diseases in national US population. Environ. Int. 2018, 119, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Koshy, T.T.; Attina, T.M.; Ghassabian, A.; Gilbert, J.; Burdine, L.K.; Marmor, M.; Honda, M.; Chu, D.B.; Han, X.; Shao, Y.; et al. Serum perfluoroalkyl substances and cardiometabolic consequences in adolescents exposed to the World Trade Center disaster and a matched comparison group. Environ. Int. 2017, 109, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Khalil, N.; Ebert, J.R.; Honda, M.; Lee, M.; Nahhas, R.W.; Koskela, A.; Hangartner, T.; Kannan, K. Perfluoroalkyl substances, bone density, and cardio-metabolic risk factors in obese 8–12 year old children: A pilot study. Environ. Res. 2018, 160, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.B.; Ducatman, A. Roles of gender and obesity in defining correlations between perfluoroalkyl substances and lipid/lipoproteins. Sci. Total Environ. 2019, 653, 74–81. [Google Scholar] [CrossRef]
- Sun, Q.; Zong, G.; Valvi, D.; Nielsen, F.; Coull, B.; Grandjean, P. Plasma Concentrations of Perfluoroalkyl Substances and Risk of Type 2 Diabetes: A Prospective Investigation among U.S. Women. Environ. Health Perspect. 2018, 126, 37001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mancini, F.R.; Rajaobelina, K.; Praud, D.; Dow, C.; Antignac, J.P.; Kvaskoff, M.; Severi, G.; Bonnet, F.; Boutron-Ruault, M.-C.; Fagherazzi, G. Nonlinear associations between dietary exposures to perfluorooctanoic acid (PFOA) or perfluorooctane sulfonate (PFOS) and type 2 diabetes risk in women: Findings from the E3N cohort study. Int. J. Hyg. Environ. Health 2018, 221, 1054–1060. [Google Scholar] [CrossRef]
- Liao, Y.; Wang, J.; Huang, Q.-S.; Fang, C.; Kiyama, R.; Shen, H.; Dong, S. Evaluation of cellular response to perfluorooctane sulfonate in human umbilical vein endothelial cells. Toxicol. Vitr. 2012, 26, 421–428. [Google Scholar] [CrossRef]
- Watkins, A.M.; Wood, C.R.; Lin, M.T.; Abbott, B.D. The effects of perfluorinated chemicals on adipocyte differentiation in vitro. Mol. Cell. Endocrinol. 2015, 400, 90–101. [Google Scholar] [CrossRef]
- Yamamoto, J.; Yamane, T.; Oishi, Y.; Kobayashi-Hattori, K. Perfluorooctanoic acid binds to peroxisome proliferator-activated receptor γ and promotes adipocyte differentiation in 3T3-L1 adipocytes. Biosci. Biotechnol. Biochem. 2015, 79, 636–639. [Google Scholar] [CrossRef] [PubMed]
- Buhrke, T.; Krüger, E.; Pevny, S.; Rößler, M.; Bitter, K.; Lampen, A. Perfluorooctanoic acid (PFOA) affects distinct molecular signalling pathways in human primary hepatocytes. Toxicology 2015, 333, 53–62. [Google Scholar] [CrossRef]
- Ma, Y.; Yang, J.; Wan, Y.; Peng, Y.; Ding, S.; Li, Y.; Xu, B.; Chen, X.; Xia, W.; Ke, Y.; et al. Low-level perfluorooctanoic acid enhances 3 T3-L1 preadipocyte differentiation via altering peroxisome proliferator activated receptor gamma expression and its promoter DNA methylation. J. Appl. Toxicol. 2018, 38, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Behr, A.-C.; Plinsch, C.; Braeuning, A.; Buhrke, T. Activation of human nuclear receptors by perfluoroalkylated substances (PFAS). Toxicol. Vitr. 2020, 62, 104700. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Shimpi, P.; Armstrong, L.; Salter, D.; Slitt, A.L. PFOS induces adipogenesis and glucose uptake in association with activation of Nrf2 signaling pathway. Toxicol. Appl. Pharmacol. 2016, 290, 21–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, X.; Wu, C.; Li, H.; Yuan, W.; Wang, X. Elevation of intracellular calcium and oxidative stress is involved in perfluorononanoic acid-induced neurotoxicity. Toxicol. Ind. Health 2018, 34, 139–145. [Google Scholar] [CrossRef] [PubMed]
- REO, N. Perfluorodecanoic acid, a peroxisome proliferator, activates phospholipase C, inhibits CTP:phosphocholine cytidylyltransferase, and elevates diacylglycerol in rat liver. Toxicol. Lett. 1996, 86, 1–11. [Google Scholar] [CrossRef]
- Zhao, W.; Cui, R.; Wang, J.; Dai, J. Inhibition effects of perfluoroalkyl acids on progesterone production in mLTC-1. J. Environ. Sci. 2017, 56, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Fleisch, A.F.; Rifas-Shiman, S.L.; Mora, A.M.; Calafat, A.M.; Ye, X.; Luttmann-Gibson, H.; Gillman, M.W.; Oken, E.; Sagiv, S.K. Early-Life Exposure to Perfluoroalkyl Substances and Childhood Metabolic Function. Environ. Health Perspect. 2017, 125, 481–487. [Google Scholar] [CrossRef] [Green Version]
- Fassler, C.S.; Pinney, S.E.; Xie, C.; Biro, F.M.; Pinney, S.M. Complex relationships between perfluorooctanoate, body mass index, insulin resistance and serum lipids in young girls. Environ. Res. 2019, 176, 108558. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, H.Y.; Jeon, J.D.; Kho, Y.; Kim, S.-K.; Park, M.-S.; Hong, Y.-C. The modifying effect of vitamin C on the association between perfluorinated compounds and insulin resistance in the Korean elderly: A double-blind, randomized, placebo-controlled crossover trial. Eur. J. Nutr. 2016, 55, 1011–1020. [Google Scholar] [CrossRef]
- Cardenas, A.; Gold, D.R.; Hauser, R.; Kleinman, K.P.; Hivert, M.-F.; Calafat, A.M.; Ye, X.; Webster, T.F.; Horton, E.S.; Oken, E. Plasma Concentrations of Per- and Polyfluoroalkyl Substances at Baseline and Associations with Glycemic Indicators and Diabetes Incidence among High-Risk Adults in the Diabetes Prevention Program Trial. Environ. Health Perspect. 2017, 125, 107001. [Google Scholar] [CrossRef] [Green Version]
- Grønnestad, R.; Johanson, S.M.; Müller, M.H.B.; Schlenk, D.; Tanabe, P.; Krøkje, Å.; Jaspers, V.L.B.; Jenssen, B.M.; Ræder, E.M.; Lyche, J.L.; et al. Effects of an environmentally relevant PFAS mixture on dopamine and steroid hormone levels in exposed mice. Toxicol. Appl. Pharmacol. 2021, 428, 115670. [Google Scholar] [CrossRef] [PubMed]
- Foguth, R.; Sepúlveda, M.S.; Cannon, J. Per- and Polyfluoroalkyl Substances (PFAS) Neurotoxicity in Sentinel and Non-Traditional Laboratory Model Systems: Potential Utility in Predicting Adverse Outcomes in Human Health. Toxics 2020, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, E.; Stratakis, N.; Basagaña, X.; Brantsæter, A.L.; Casas, M.; Fossati, S.; Gražulevičienė, R.; Småstuen Haug, L.; Heude, B.; Maitre, L.; et al. Prenatal and postnatal exposure to PFAS and cardiometabolic factors and inflammation status in children from six European cohorts. Environ. Int. 2021, 157, 106853. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Liu, Y.; Papandonatos, G.D.; Calafat, A.M.; Eaton, C.B.; Kelsey, K.T.; Cecil, K.M.; Kalkwarf, H.J.; Yolton, K.; Lanphear, B.P.; et al. Gestational and childhood exposure to per- and polyfluoroalkyl substances and cardiometabolic risk at age 12 years. Environ. Int. 2021, 147, 106344. [Google Scholar] [CrossRef]
- Ding, N.; Karvonen-Gutierrez, C.A.; Herman, W.H.; Calafat, A.M.; Mukherjee, B.; Park, S.K. Associations of perfluoroalkyl and polyfluoroalkyl substances (PFAS) and PFAS mixtures with adipokines in midlife women. Int. J. Hyg. Environ. Health 2021, 235, 113777. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, T.; Galloway, T.S.; Melzer, D.; Holcroft, P.; Cipelli, R.; Pilling, L.C.; Mondal, D.; Luster, M.; Harries, L.W. Associations between PFOA, PFOS and changes in the expression of genes involved in cholesterol metabolism in humans. Environ. Int. 2013, 57–58, 2–10. [Google Scholar] [CrossRef]
- Szilagyi, J.T.; Avula, V.; Fry, R.C. Perfluoroalkyl Substances (PFAS) and Their Effects on the Placenta, Pregnancy, and Child Development: A Potential Mechanistic Role for Placental Peroxisome Proliferator-Activated Receptors (PPARs). Curr. Environ. Health Rep. 2020, 7, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Itoh, S.; Yuasa, M.; Baba, T.; Miyashita, C.; Sasaki, S.; Nakajima, S.; Uno, A.; Nakazawa, H.; Iwasaki, Y.; et al. Association of perfluorinated chemical exposure in utero with maternal and infant thyroid hormone levels in the Sapporo cohort of Hokkaido Study on the Environment and Children’s Health. Environ. Health Prev. Med. 2016, 21, 334–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kishi, R.; Nakajima, T.; Goudarzi, H.; Kobayashi, S.; Sasaki, S.; Okada, E.; Miyashita, C.; Itoh, S.; Araki, A.; Ikeno, T.; et al. The Association of Prenatal Exposure to Perfluorinated Chemicals with Maternal Essential and Long-Chain Polyunsaturated Fatty Acids during Pregnancy and the Birth Weight of Their Offspring: The Hokkaido Study. Environ. Health Perspect. 2015, 123, 1038–1045. [Google Scholar] [CrossRef] [Green Version]

| Study Type (Number of Publ. Associated with Event) | Organism or Organism From Which Cells Were Originally Derived | Frequency (%) | Main Event Identified (Number of Publ. Associated with Event) | Substances Associated with Event |
|---|---|---|---|---|
| In vivo (114) | Rat | 40 | Hepatotoxicity (16) | PFCAs (C8-C12), PFOS |
| Decreased cholesterol (8) | PFDA, PFOS | |||
| PPARα activation (6) | PFOA, PFOS | |||
| Mouse | 53 | Hepatotoxicity (13) | PFOA, PFOS, 6:2 FTSA, 6:2 FTCA, 6:2 Cl-PFESA, HFPO-TA, PFO2HxA, PFO3OA, PFO4DA | |
| PPARα activation (13) | PFCAs (C8-C10), PFHxS, PFOS | |||
| Decreased body weight (7) | PFCAs (C8-C10), PFOS | |||
| Others, e.g., hamster, guinea pig, monkey, zebrafish, carp | 7 | Hepatotoxicity, decreased body weight | PFOA, PFOS, PFDoDA |
| Study Type (Number of Publ. Associated with Event) | Organism or Organism From Which Cells Were Originally Derived | Frequency (%) | Main Event Identified (Number of Publ. Associated with Event) | Substances Associated with Event |
|---|---|---|---|---|
| In vitro (75) | Rat | 20 | Increased ROS (6) | PFOA, PFOS, 8:2 FTOH, PFOSA, PFDoDA |
| Cell death (3) | 8:2 FTOH, PFOS, PFOA, PFOSA | |||
| Activation of AhR (1) | PFCAs (C8-C12), PFHxS, PFOS | |||
| Mouse | 17 | PPARα activation (4) | PFCAs (C5-C9, C11-C12), PFHxS, PFOS | |
| Increased ROS (4) | PFOA, PFDA, PFOS | |||
| Human | 60 | Increased ROS (12) | PFCAs (C8-C11), PFHxS, PFOS | |
| Hepatotoxicity (11) | PFOA, PFOS | |||
| PPARα activity (3) | PFOA, PFOS, PFOSA | |||
| Others, e.g., baikal seal, hamster | 4 | Increased ROS, PPARα activity | PFOA, PFOS, PFAAs (C4-C12) |
| Event | Identified Publications via AOP-helpFinder | Identified Associations with PFAS (Number of Publ.) | No Associations Found (Number of Publ.) | |
|---|---|---|---|---|
| Hypertension | Total number of publications: | 20 | ||
| Years: | 2012–2020 | |||
| Addressed hypertension or preeclampsia: | 15 | PFBA (1), PFOA (9), PFNA (4), PFDA (1), PFHxS (2), PFHpS (1), PFOS (3), br-PFOS (2), PFDS (1), MeFOSAA (1), total 12-PFAS (1), | PFOA (3), PFNA (1), PFHxS (2), PFOS (4), EtFOSAA (1) | |
| Did not address hypertension: | 5 | e.g., higher uric acid levels | ||
| Overweight and obesity | Total number of publications: | 15 | ||
| Years: | 1996–2019 | |||
| Addressed overweight or obesity: | 7 | PFOA (3), PFNA (2), PFOS (1) | PFOA (1), PFNA (1), PFHxS (1), PFOS (2), PFOSA (1) | |
| Did not address overweight or obesity: | 8 | e.g., lower birth weight, elevated LDL or TC, study participants were obese in general | ||
| Insulin resistance | Total number of publications: | 11 | ||
| Years: | 2010–2019 | |||
| Addressed insulin resistance: | 11 | ↓ HOMA-IR↓: PFHxS (4), PFOS (1), PFOA (1), PFNA (2), PFDA (1), PFAA4 (1) ↑ HOMA-IR↑: PFHxS (1), PFOS (2), PFOA (1), PFDoDA (1) GDM: PFHxS (1), PFHpA (1), PFOA (1), PFNA (2), PFDoDA (1) | PFOA (2), PFNA (2), PFHxS (1), PFOS (2), | |
| Did not address insulin resistance: | 0 | |||
| Type 2 diabetes mellitus | Total number of publications: | 4 | ||
| Years: | 2018–2020 | |||
| Addressed insulin resistance: | 4 | PFOS (1), PFOA (2) | PFOS (1) | |
| Did not address insulin resistance: | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaiser, A.-M.; Zare Jeddi, M.; Uhl, M.; Jornod, F.; Fernandez, M.F.; Audouze, K. Characterization of Potential Adverse Outcome Pathways Related to Metabolic Outcomes and Exposure to Per- and Polyfluoroalkyl Substances Using Artificial Intelligence. Toxics 2022, 10, 449. https://doi.org/10.3390/toxics10080449
Kaiser A-M, Zare Jeddi M, Uhl M, Jornod F, Fernandez MF, Audouze K. Characterization of Potential Adverse Outcome Pathways Related to Metabolic Outcomes and Exposure to Per- and Polyfluoroalkyl Substances Using Artificial Intelligence. Toxics. 2022; 10(8):449. https://doi.org/10.3390/toxics10080449
Chicago/Turabian StyleKaiser, Andreas-Marius, Maryam Zare Jeddi, Maria Uhl, Florence Jornod, Mariana F. Fernandez, and Karine Audouze. 2022. "Characterization of Potential Adverse Outcome Pathways Related to Metabolic Outcomes and Exposure to Per- and Polyfluoroalkyl Substances Using Artificial Intelligence" Toxics 10, no. 8: 449. https://doi.org/10.3390/toxics10080449
APA StyleKaiser, A.-M., Zare Jeddi, M., Uhl, M., Jornod, F., Fernandez, M. F., & Audouze, K. (2022). Characterization of Potential Adverse Outcome Pathways Related to Metabolic Outcomes and Exposure to Per- and Polyfluoroalkyl Substances Using Artificial Intelligence. Toxics, 10(8), 449. https://doi.org/10.3390/toxics10080449







