Polycyclic Aromatic Hydrocarbons (PAHs) in the Dissolved Phase, Particulate Matter, and Sediment of the Sele River, Southern Italy: A Focus on Distribution, Risk Assessment, and Sources
Abstract
:1. Introduction
2. Materials and Methods
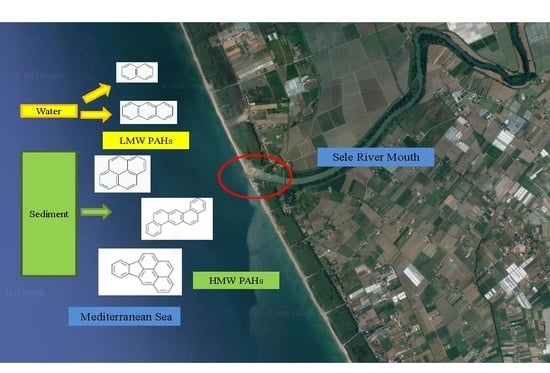
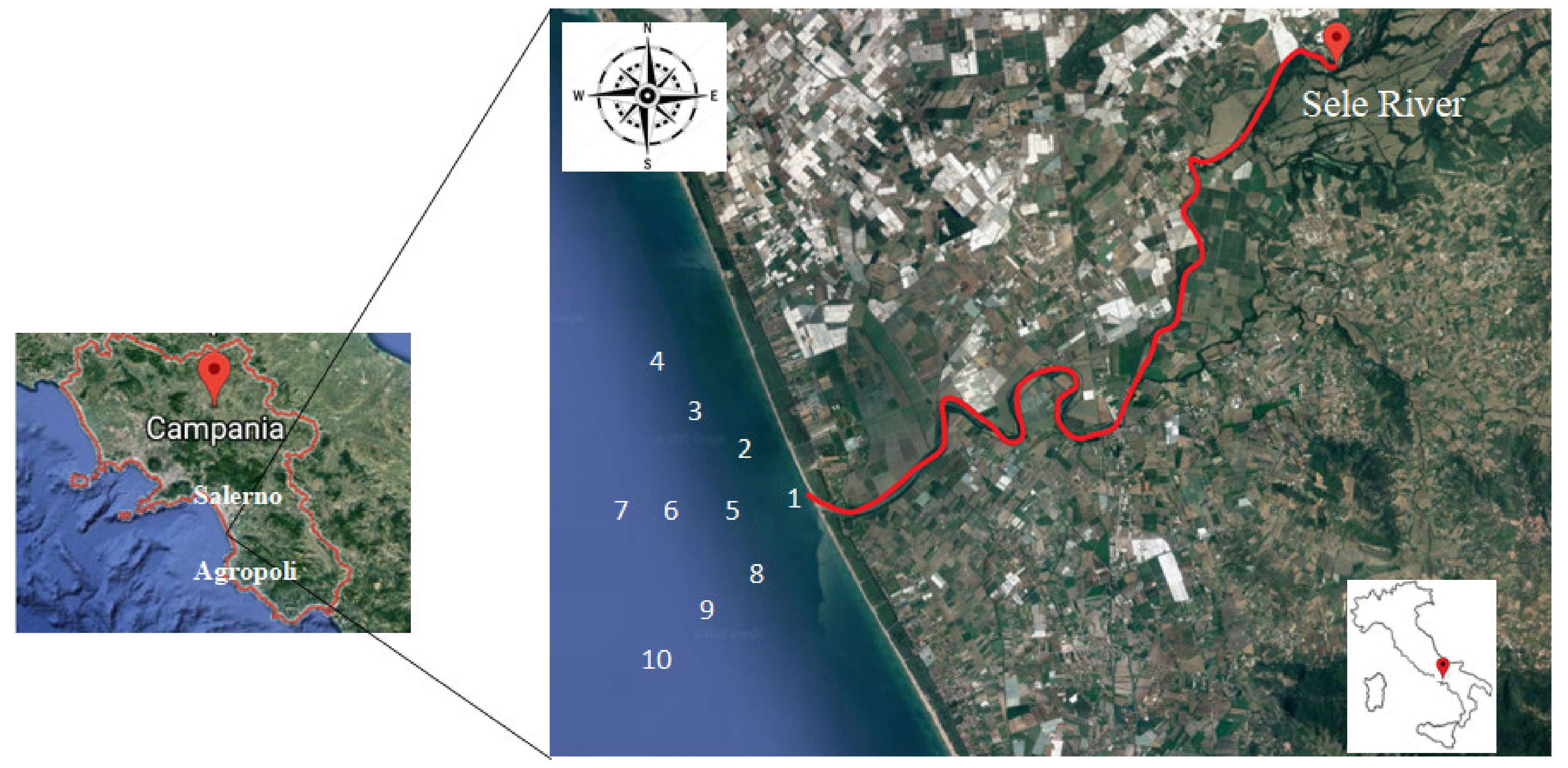
2.1. Study Area
2.2. Sampling
2.3. Extraction and Analysis
2.4. Instrumental Analysis
2.5. Water-Sediment Partitioning
2.6. Risk Assessment and Determination of Toxicity
2.6.1. Biological Adverse Effects
2.6.2. Toxicity Determination
2.7. Identifying the Source of PAHs
3. Results and Discussion
3.1. PAH Distribution in Water, SPM, and Sediment
3.2. PAH Fugacity in the Aquatic System
3.3. Risk Assessment of PAHs
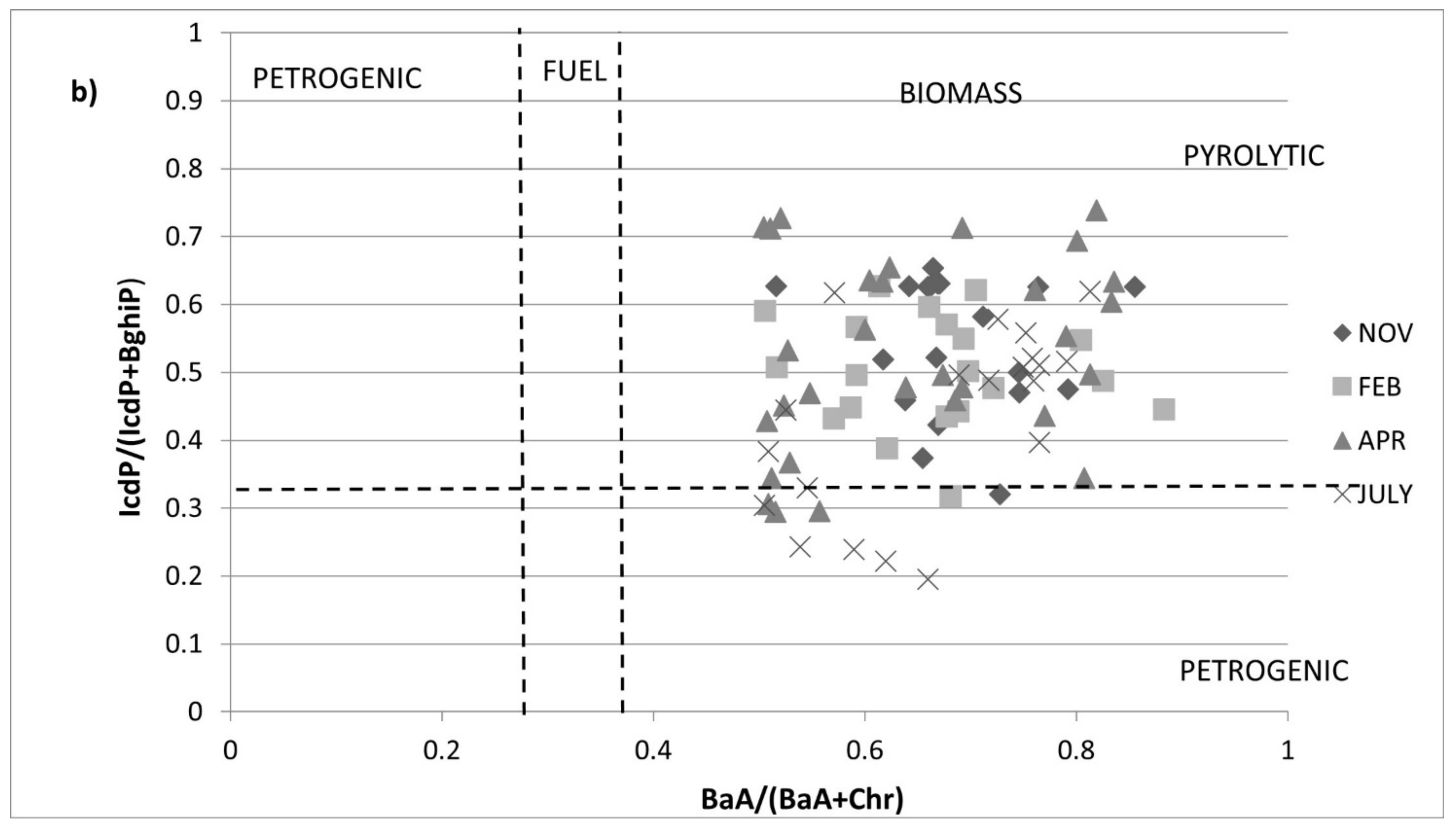
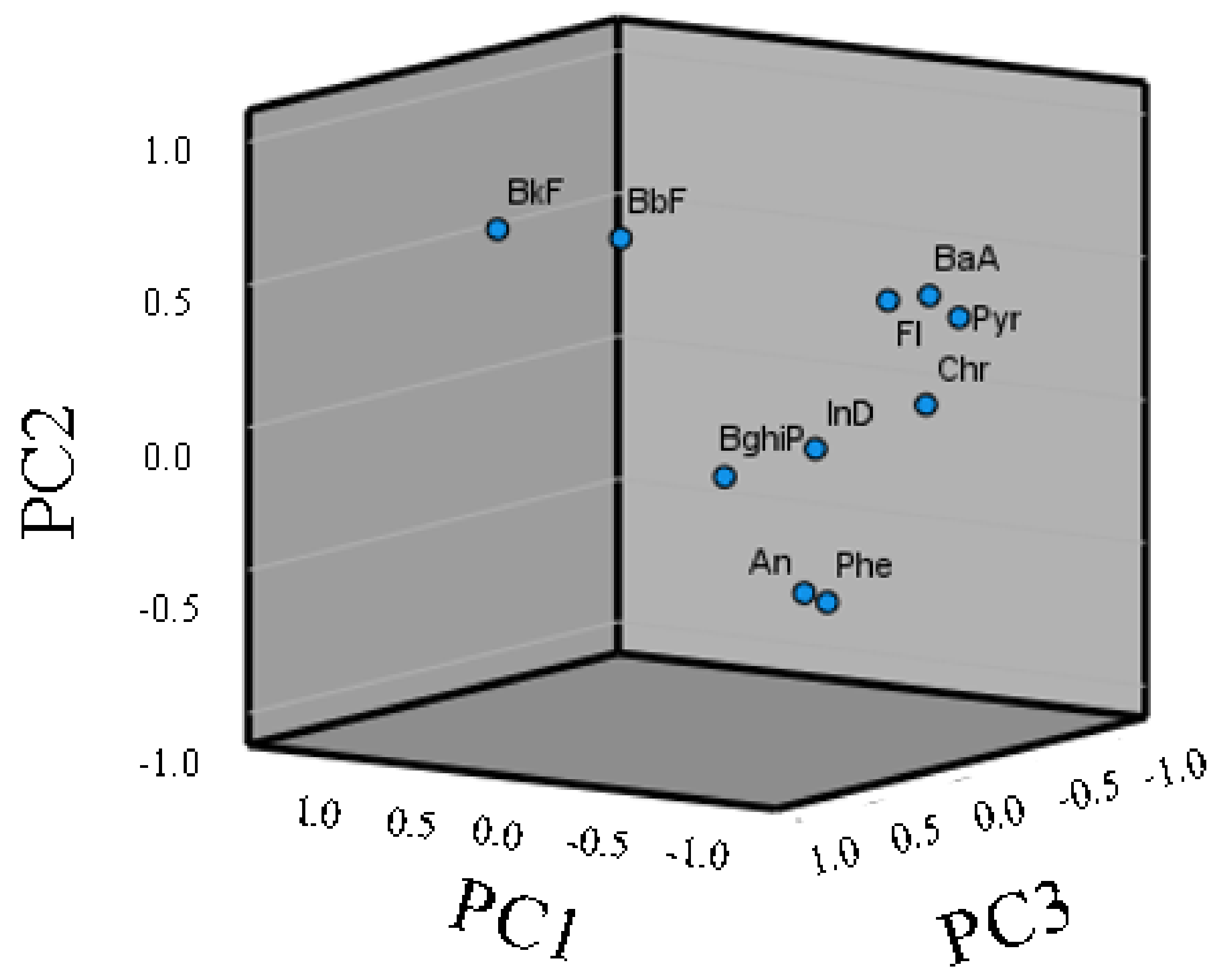
3.4. Source Identification by PAH Diagnostic Ratios
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, J.; Liu, G.; Wang, R.; Huang, H. Polycyclic aromatic hydrocarbons in the water-SPM-sediment system from the middle reaches of Huai River, China: Distribution, partitioning, origin tracing and ecological risk assessment. Environ. Pollut. 2017, 230, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-F.; Ju, Y.-R.; Su, Y.-C.; Lim, Y.C.; Kao, C.-M.; Chen, C.-W.; Dong, C.-D. Distribution, sources, and behavior of PAHs in estuarine water systems exemplified by Salt River, Taiwan. Mar. Pollut. Bull. 2020, 154, 111029. [Google Scholar] [CrossRef] [PubMed]
- USEPA (US Environmental Protection Agency). Regional Screening Levels for Chemical Contaminants at Superfund Sites. Regional Screening Table. User’s Guide. 2012. Available online: https://www.epa.gov/risk/regional-screening-levels-rsls-generic-tables (accessed on 4 May 2022).
- An, N.; Liu, S.; Yin, Y.; Cheng, F.; Dong, S.; Wu, X. Spatial distribution and sources of polycyclic aromatic hydrocarbons (PAHs) in the reservoir sediments after impoundment of Manwan Dam in the middle of Lancang River, China. Ecotoxicology 2016, 25, 1072–1081. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wu, J.; Zhao, Z. Spatial and temporal distribution of Polycyclic Aromatic Hydrocarbons (PAHs) in sediments from Poyang Lake, China. PLoS ONE 2018, 13, e0205484. [Google Scholar] [CrossRef] [PubMed]
- COMMISSION REGULATION (EU) No 835/2011 of 19 August 2011 amending Regulation (EC) No 1881/2006 as Regards Maximum Levels for Polycyclic Aromatic Hydrocarbons in Foodstuffs. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2011:215:0004:0008:En:PDF (accessed on 18 May 2022).
- Liu, Y.; Zarfl, C.; Basu, N.B.; Cirpka, O.A. Turnover and legacy of sediment-associated PAH in a baseflow-dominated river. Sci. Total Environ. 2019, 671, 754–764. [Google Scholar] [CrossRef]
- Zanardi-Lamardo, E.; Mitra, S.; Vieira-Campos, A.A.; Cabral, C.B.; Yogui, G.; Sarkar, S.K.; Biswas, J.K.; Godhantaraman, N. Distribution and sources of organic contaminants in surface sediments of Hooghly river estuary and Sundarban mangrove, eastern coast of India. Mar. Pollut. Bull. 2019, 146, 39–49. [Google Scholar] [CrossRef]
- Castro-Jiménez, J.; Berrojalbiz, N.; Wollgast, J.; Dachs, J. Polycyclic aromatic hydrocarbons (PAHs) in the Mediterranean Sea: Atmospheric occurrence, deposition and decoupling with settling fluxes in the water column. Environ. Pollut. 2012, 166, 40–47. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.; Zhang, J.; Ma, Q.; Chen, Y.; Ju, H. Polycyclic aromatic hydrocarbons (PAHs) in water and sediment from a river basin: Sediment-water partitioning, source identification and environmental health risk assessment. Environ. Geochem. Health 2016, 39, 63–74. [Google Scholar] [CrossRef]
- Montuori, P.; Aurino, S.; Garzonio, F.; Sarnacchiaro, P.; Nardone, A.; Triassi, M. Distribution, sources and ecological risk assessment of polycyclic aromatic hydrocarbons in water and sediments from Tiber River and estuary, Italy. Sci. Total Environ. 2016, 566–567, 1254–1267. [Google Scholar] [CrossRef] [Green Version]
- Montuori, P.; De Rosa, E.; Di Duca, F.; Provvisiero, D.P.; Sarnacchiaro, P.; Nardone, A.; Triassi, M. Estima-tion of Polycyclic Aromatic Hydrocarbons Pollution in Mediterranean Sea from Volturno River, Southern It-aly: Distribution, Risk Assessment and Loads. Int. J. Environ. Res. Public Health 2021, 18, 1383. [Google Scholar] [CrossRef]
- Montuori, P.; Triassi, M. Polycyclic aromatic hydrocarbons loads into the Mediterranean Sea: Estimate of Sarno River inputs. Mar. Pollut. Bull. 2012, 64, 512–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qu, C.; Li, J.; Albanese, S.; Lima, A.; Wang, M.; Sacchi, M.; Molisso, F.; De Vivo, B. Polycyclic aromatic hydrocarbons in the sediments of the Gulfs of Naples and Salerno, Southern Italy: Status, sources and ecological risk. Ecotoxicol. Environ. Saf. 2018, 161, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Qu, C.; Albanese, S.; Lima, A.; Li, J.; Doherty, A.L.; Qi, S.; De Vivo, B. Residues of hexachlorobenzene and chlorinated cyclodiene pesticides in the soils of the Campanian Plain, southern Italy. Environ. Pollut. 2017, 231, 1497–1506. [Google Scholar] [CrossRef] [PubMed]
- Arienzo, M.; Albanese, S.; Lima, A.; Cannatelli, C.; Aliberti, F.; Cicotti, F.; Qi, S.; De Vivo, B. Assessment of the concentrations of polycyclic aromatic hydrocarbons and organochlorine pesticides in soils from the Sarno River basin, Italy, and ecotoxicological survey by Daphnia magna. Environ. Monit. Assess. 2015, 187, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diodato, N.; Fagnano, M.; Alberico, I. Geospatial and visual modeling for exploring sediment source areas across the Sele river landscape, Italy. Ital. J. Agron. 2011, 6, e14. [Google Scholar] [CrossRef]
- Albanese, S.; De Vivo, B.; Lima, A.; Cicchella, D.; Civitillo, D.; Cosenza, A. Geochemical baselines and risk assessment of the Bagnoli brownfield site coastal sea sediments (Naples, Italy). J. Geochem. Explor. 2010, 105, 19–33. [Google Scholar] [CrossRef]
- Liu, Q.; Xu, X.; Wang, L.; Lin, L.; Wang, D. Simultaneous determination of forty-two parent and halogenated polycyclic aromatic hydrocarbons using solid-phase extraction combined with gas chromatography-mass spectrometry in drinking water. Ecotoxicol. Environ. Saf. 2019, 181, 241–247. [Google Scholar] [CrossRef]
- Kafilzadeh, F.; Shiva, A.H.; Malekpour, R. Determination of polycyclic aromatic hydrocarbons (PAHs) in water and sediments of the Kor River, Iran. Middle-East J. Sci. Res. 2011, 10, 1–7. [Google Scholar]
- Lin, L.; Dong, L.; Meng, X.; Li, Q.; Huang, Z.; Li, C.; Li, R.; Yang, W.; Crittenden, J. Distribution and sources of polycyclic aromatic hydrocarbons and phthalic acid esters in water and surface sediment from the Three Gorges Reservoir. J. Environ. Sci. 2018, 69, 271–280. [Google Scholar] [CrossRef]
- USA-Environmental Protection Agency (US EPA). Method 3540C: Soxhlet Extraction; USA Environmental Protection Agency: Washington, DC, USA, 1996.
- Ashayeri, N.Y.; Keshavarzi, B. Geochemical characteristics, partitioning, quantitative source apportionment, and ecological and health risk of heavy metals in sediments and water: A case study in Shadegan Wetland, Iran. Mar. Pollut. Bull. 2019, 149, 110495. [Google Scholar] [CrossRef]
- Gou, Y.; Zhao, Q.; Yang, S.; Wang, H.; Qiao, P.; Song, Y.; Cheng, Y.; Li, P. Removal of polycyclic aromatic hydrocarbons (PAHs) and the response of indigenous bacteria in highly contaminated aged soil after persulfate oxidation. Ecotoxicol. Environ. Saf. 2019, 190, 110092. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yang, C.; He, W.; Xu, F. Nationwide health risk assessment of juvenile exposure to polycyclic aromatic hydrocarbons (PAHs) in the water body of Chinese lakes. Sci. Total Environ. 2020, 723, 138099. [Google Scholar] [CrossRef] [PubMed]
- Fakhradini, S.S.; Moore, F.; Keshavarzi, B.; Lahijanzadeh, A. Polycyclic aromatic hydrocarbons (PAHs) in water and sediment of Hoor Al-Azim wetland, Iran: A focus on source apportionment, environmental risk assessment, and sediment-water partitioning. Environ. Monit. Assess. 2019, 191, 233. [Google Scholar] [CrossRef]
- Zhao, Z.; Gong, X.; Zhang, L.; Jin, M.; Cai, Y.; Wang, X. Riverine transport and water-sediment exchange of polycyclic aromatic hydrocarbons (PAHs) along the middle-lower Yangtze River, China. J. Hazard. Mater. 2020, 403, 123973. [Google Scholar] [CrossRef] [PubMed]
- Ashayeri, N.Y.; Keshavarzi, B.; Moore, F.; Kersten, M.; Yazdi, M.; Lahijanzadeh, A.R. Presence of polycyclic aromatic hydrocarbons in sediments and surface water from Shadegan wetland-Iran: A focus on source apportionment, human and ecological risk assessment and Sediment-Water Exchange. Ecotoxicol. Environ. Saf. 2018, 148, 1054–1066. [Google Scholar] [CrossRef]
- Wenning, R.J.; Ingersoll, C.G. Summary of the SETAC Pellston Workshop on Use of Sediment Quality Guide-Lines and Related Tools for the Assessment of Contaminated Sediments; Society of Environmental Toxicology and Chemistry (SETAC): Pensacola, FL, USA, 2002; Available online: http://www.setac.org/files/SQGSummary.pdf (accessed on 18 May 2022).
- Mogashane, T.M.; Mujuru, M.; McCrindle, R.I.; Ambushe, A.A. Quantification, source apportionment and risk assessment of polycyclic aromatic hydrocarbons in sediments from Mokolo and Blood Rivers in Limpopo Province, South Africa. J. Environ. Sci. Health Part A 2019, 55, 71–81. [Google Scholar] [CrossRef]
- Zaghden, H.; Tedetti, M.; Sayadi, S.; Serbaji, M.M.; Elleuch, B.; Saliot, A. Origin and distribution of hydrocarbons and organic matter in the surficial sediments of the Sfax-Kerkennah channel (Tunisia, Southern Mediterranean Sea). Mar. Pollut. Bull. 2017, 117, 414–428. [Google Scholar] [CrossRef]
- Yuan, H.M.; Li, T.G.; Ding, X.G.; Zhao, G.M.; Ye, S.Y. Distribution, Sources Analysis and Eco-Toxicological Risk Assessment of Polycyclic Aromatic Hydrocarbons (PAHs) in Surface Soils in the Northern Yellow River Delta, China. Adv. Mater. Res. 2013, 726–731, 750–756. [Google Scholar] [CrossRef]
- Liu, X.; Chen, Z.; Xia, C.; Wu, J.; Ding, Y. Characteristics, distribution, source and ecological risk of polycyclic aromatic hydrocarbons (PAHs) in sediments along the Yangtze River Estuary Deepwater Channel. Mar. Pollut. Bull. 2019, 150, 110765. [Google Scholar] [CrossRef]
- Pozo, K.; Perra, G.; Menchi, V.; Urrutia, R.; Parra, O.; Rudolph, A.; Focardi, S. Levels and spatial distribution of polycyclic aromatic hydrocarbons (PAHs) in sediments from Lenga Estuary, central Chile. Mar. Pollut. Bull. 2011, 62, 1572–1576. [Google Scholar] [CrossRef]
- Křůmal, K.; Mikuška, P. Mass concentrations and lung cancer risk assessment of PAHs bound to PM1 aerosol in six industrial, urban and rural areas in the Czech Republic, Central Europe. Atmos. Pollut. Res. 2019, 11, 401–408. [Google Scholar] [CrossRef]
- Gupte, A.; Tripathi, A.; Patel, H.; Rudakiya, D.; Gupte, S. Bioremediation of Polycyclic Aromatic Hydrocarbon (PAHs): A Perspective. Open Biotechnol. J. 2016, 10, 363–378. [Google Scholar] [CrossRef] [Green Version]
- Mojiri, A.; Zhou, J.L.; Ohashi, A.; Ozaki, N.; Kindaichi, T. Comprehensive review of polycyclic aromatic hydrocarbons in water sources, their effects and treatments. Sci. Total Environ. 2019, 696, 133971. [Google Scholar] [CrossRef] [PubMed]
- Tongo, I.; Ezemonye, L.; Akpeh, K. Levels, distribution and characterization of Polycyclic Aromatic Hydrocarbons (PAHs) in Ovia river, Southern Nigeria. J. Environ. Chem. Eng. 2017, 5, 504–512. [Google Scholar] [CrossRef]
- Gdara, I.; Zrafi, I.; Balducci, C.; Cecinato, A.; Ghrabi, A. Seasonal occurrence, source evaluation and ecological risk assessment of polycyclic aromatic hydrocarbons in industrial and agricultural effluents discharged in Wadi El Bey (Tunisia). Environ. Geochem. Health 2018, 40, 1609–1627. [Google Scholar] [CrossRef]
- Haiba, N.S.A. Polycyclic Aromatic Hydrocarbons (PAHs) in the River Nile, Egypt: Occurrence and Distribution. Polycycl. Aromat. Compd. 2017, 39, 425–433. [Google Scholar] [CrossRef]
- Lima, E.A.R.; Neves, P.A.; Patchineelam, S.R.; da Silva, J.F.B.R.; Takiyama, L.R.; Martins, C.C.; Lourenço, R.A.; Taniguchi, S.; Elias, V.O.; Bícego, M.C. Anthropogenic and natural inputs of polycyclic aromatic hydrocarbons in the sediment of three coastal systems of the Brazilian Amazon. Environ. Sci. Pollut. Res. 2021, 28, 19485–19496. [Google Scholar] [CrossRef]
- Triassi, M.; Nardone, A.; Giovinetti, M.C.; De Rosa, E.; Canzanella, S.; Sarnacchiaro, P.; Montuori, P. Ecological risk and estimates of organophosphate pesticides loads into the Central Mediterranean Sea from Volturno River, the river of the “Land of Fires” area, southern Italy. Sci. Total Environ. 2019, 678, 741–754. [Google Scholar] [CrossRef]
- Pearson, K. On lines and planes of closest fit to systems of points in space. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1901, 2, 559–572. [Google Scholar] [CrossRef] [Green Version]
- Alberico, I.; Amato, V.; Aucelli, P.P.C.; Di Paola, G.; Pappone, G.; Rosskopf, C.M. Historical and recent changes of the Sele River coastal plain (Southern Italy): Natural variations and human pressures. Rend. Lince 2011, 23, 3–12. [Google Scholar] [CrossRef]
- Giordano, L.; Alberico, I.; Ferraro, L.; Marsella, E.; Lirer, F.; Di Fiore, V. A new tool to promote sustainability of coastal zones. The case of Sele plain, southern Italy. Rend. Lince 2013, 24, 113–126. [Google Scholar] [CrossRef]
- Yu, H.; Liu, Y.; Han, C.; Fang, H.; Weng, J.; Shu, X.; Pan, Y.; Ma, L. Polycyclic aromatic hydrocarbons in surface waters from the seven main river basins of China: Spatial distribution, source apportionment, and potential risk assessment. Sci. Total Environ. 2020, 752, 141764. [Google Scholar] [CrossRef] [PubMed]
- Kim, L.; Jeon, H.-J.; Kim, Y.-C.; Yang, S.-H.; Choi, H.; Kim, T.-O.; Lee, S.-E. Monitoring polycyclic aromatic hydrocarbon concentrations and distributions in rice paddy soils from Gyeonggi-do, Ulsan, and Pohang. Appl. Biol. Chem. 2019, 62, 18. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.-H.; Lai, S.-O.; Hsieh, L.-T.; Hsueh, H.-J.; Chi, T.-W. Profiles of PAH emission from steel and iron industries. Chemosphere 2002, 48, 1061–1074. [Google Scholar] [CrossRef]
- Guo, W.; He, M.; Yang, Z.; Lin, C.; Quan, X.; Wang, H. Distribution of polycyclic aromatic hydrocarbons in water, suspended particulate matter and sediment from Daliao River watershed, China. Chemosphere 2007, 68, 93–104. [Google Scholar] [CrossRef]
- Liu, X.; Wang, H.; Wei, L.; Liu, J.; Reitz, R.D.; Yao, M. Development of a reduced toluene reference fuel (TRF)-2,5-dimethylfuran-polycyclic aromatic hydrocarbon (PAH) mechanism for engine applications. Combust. Flame 2016, 165, 453–465. [Google Scholar] [CrossRef]
- Gong, G.; Zhao, X.; Wu, S. Effect of natural antioxidants on inhibition of parent and oxygenated polycyclic aromatic hydrocarbons in Chinese fried bread youtiao. Food Control 2018, 87, 117–125. [Google Scholar] [CrossRef]
- Kumar, A.; Schimmelmann, A.; Sauer, P.E.; Brassell, S.C. Distribution and sources of polycyclic aromatic hydrocarbons (PAHs) in laminated Santa Barbara Basin sediments. Org. Geochem. 2017, 113, 303–314. [Google Scholar] [CrossRef]
- Ferraro, A.; Massini, G.; Miritana, V.M.; Panico, A.; Pontoni, L.; Race, M.; Rosa, S.; Signorini, A.; Fabbricino, M.; Pirozzi, F. Bioaugmentation strategy to enhance polycyclic aromatic hydrocarbons anaerobic biodegradation in contaminated soils. Chemosphere 2021, 275, 130091. [Google Scholar] [CrossRef]
- Liu, Z.; He, L.; Lu, Y.; Su, J.; Song, H.; Zeng, X.; Yu, Z. Distribution, source, and ecological risk assessment of polycyclic aromatic hydrocarbons (PAHs) in surface sediments from the Hun River, northeast China. Environ. Monit. Assess. 2015, 187, 290. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Shafy, H.I.; Mansour, M.S.M. A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation. Egypt. J. Pet. 2016, 25, 107–123. [Google Scholar] [CrossRef] [Green Version]
- Tzoraki, O.; Karaouzas, I.; Patrolecco, L.; Skoulikidis, N.; Nikolaidis, N.P. Polycyclic Aromatic Hydrocarbons (PAHs) and Heavy Metal Occurrence in Bed Sediments of a Temporary River. Water Air Soil Pollut. 2015, 226, 421. [Google Scholar] [CrossRef]
- Chizhova, T.; Koudryashova, Y.; Prokuda, N.; Tishchenko, P.; Hayakawa, K. Polycyclic Aromatic Hydro-carbons in the Estuaries of Two Rivers of the Sea of Japan. Int. J. Environ. Res. Public Health 2020, 17, 6019. [Google Scholar] [CrossRef] [PubMed]
- Sicre, M.A.; Fernandes, M.B.; Pont, D. Poly-aromatic hydrocarbon (PAH) inputs from the Rhône River to the Mediterranean Sea in relation with the hydrological cycle: Impact of floods. Mar. Pollut. Bull. 2008, 56, 1935–1942. [Google Scholar] [CrossRef]
- Cao, Y.; Xin, M.; Wang, B.; Lin, C.; Liu, X.; He, M.; Lu, S. Spatiotemporal distribution, source, and ecological risk of polycyclic aromatic hydrocarbons (PAHs) in the urbanized semi-enclosed Jiaozhou Bay, China. Sci. Total Environ. 2020, 717, 137224. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, L.; Sun, T.; Li, H.; Luo, Q. Spatial distribution and seasonal variation of polycyclic aromatic hydrocarbons (PAHs) contaminations in surface water from the Hun River, Northeast China. Environ. Monit. Assess. 2013, 185, 1451–1462. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; He, M.; Yang, Z.; Lin, C.; Quan, X.; Men, B. Distribution, partitioning and sources of polycyclic aromatic hydrocarbons in Daliao River water system in dry season, China. J. Hazard. Mater. 2009, 164, 1379–1385. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, C.; Zhang, J.; Zhang, F. Assessing 16 Polycyclic Aromatic Hydrocarbons (PAHs) in River Basin Water and Sediment Regarding Spatial-Temporal Distribution, Partitioning, and Ecological Risks. Pol. J. Environ. Stud. 2018, 27, 579–589. [Google Scholar] [CrossRef]
- Akhbarizadeh, R.; Moore, F.; Keshavarzi, B.; Moeinpour, A. Aliphatic and polycyclic aromatic hydrocarbons risk assessment in coastal water and sediments of Khark Island, SW Iran. Mar. Pollut. Bull. 2016, 108, 33–45. [Google Scholar] [CrossRef]
- Long, E.R.; Macdonald, D.D.; Smith, S.L.; Calder, F.D. Incidence of adverse biological effects within ranges of chemical concentrations in marine and estuarine sediments. Environ. Manag. 1995, 19, 81–97. [Google Scholar] [CrossRef]
- Macdonald, D.D.; Carr, R.S.; Calder, F.D.; Long, E.R.; Ingersoll, C.G. Development and evaluation of sedi-ment quality guidelines for Florida coastal waters. Ecotoxicology 1996, 5, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Yunker, M.B.; Macdonald, R.W.; Vingarzan, R.; Mitchell, R.H.; Goyette, D.; Sylvestre, S. PAHs in the Fraser River basin: A critical appraisal of PAH ratios as indicators of PAH source and composition. Org. Geochem. 2002, 33, 489–515. [Google Scholar] [CrossRef]
- Ekpo, B.O.; Oyo-Ita, O.E.; Oros, D.R.; Simoneit, B.R.T. Distributions and sources of polycyclic aromatic hydrocarbons in surface sediments from the Cross River estuary, S.E. Niger Delta, Nigeria. Environ. Monit. Assess. 2011, 184, 1037–1047. [Google Scholar] [CrossRef]
- Tobiszewski, M.; Namieśnik, J. PAH diagnostic ratios for the identification of pollution emission sources. Environ. Pollut. 2012, 162, 110–119. [Google Scholar] [CrossRef]
- Lin, B.-S.; Brimblecombe, P.; Lee, C.-L.; Liu, J.T. Tracing typhoon effects on particulate transport in a submarine canyon using polycyclic aromatic hydrocarbons. Mar. Chem. 2013, 157, 1–11. [Google Scholar] [CrossRef]
- Pérez-Fernández, B.; Viñas, L.; Franco, M.; Bargiela, J. PAHs in the Ría de Arousa (NW Spain): A consideration of PAHs sources and abundance. Mar. Pollut. Bull. 2015, 95, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Tavakoly Sany, S.; Hashim, R.; Salleh, A.; Rezayi, M.; Mehdinia, A.; Safari, O. Polycyclic aromatic hydrocar-bons in coastal sediment of Klang Strait, Malaysia: Distribution pattern, risk assessment and sources. PLoS ONE 2014, 9, e9490. [Google Scholar] [CrossRef]
- Jiang, Y.F.; Wang, X.T.; Wang, F.; Jia, Y.; Wu, M.H.; Sheng, G.Y.; Fu, J.M. Levels, composition profiles and sources of polycyclic aromatic hydrocarbons in urban soil of Shanghai, China. Chemosphere 2009, 75, 1112–1118. [Google Scholar] [CrossRef]
- Khabouchi, I.; Khadhar, S.; Driouich Chaouachi, R.; Chekirbene, A.; Asia, L.; Doumenq, P. Study of organic pollution in superficial sediments of Meliane river catchment area: Aliphatic and polycyclic aromatic hydro-carbons. Environ. Monit. Assess. 2020, 192, 283. [Google Scholar] [CrossRef]



| Sampling Location | ΣPAHs | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Site Number Identification | Site Characteristics | Site Location | Dissolved Phase (ng L−1) | Particulate Phase (ng L−1) (ng g−1 Dry wt) | Sediment (ng g−1 Dry wt) | ||||||
| Apr | Jul | Nov | Feb | Apr | Jul | Nov | Feb | Apr | |||
| 1 (river water) | Sele river source | 40°28′55″ N 14°56′33″ E | 419.3 | 567.2 | 487.3 | 309.9 | 520.1 (41,364.1) | 276.1 (28,122.3) | 234.8 (19,865.6) | 654.3 (23,487.2) | 871.1 |
| 2 (sea water) | River mouth at 500 mt north | 40°29′04″ N 14°56′14″ E | 204.2 | 387.3 | 471.2 | 200.0 | 332.3 (30,542.6) | 144.8 (18,657.1) | 138.3 (6068.5) | 381.0 (26,589.1) | 712.4 |
| 3 (sea water) | River mouth at 500 mt central | 40°29′12″ N 14°55′56″ E | 226.5 | 552.3 | 408.2 | 331.8 | 233.9 (74,510.7) | 182.3 (26,789.8) | 128.3 (58,745.8) | 277.7 (16,895.9) | 724.3 |
| 4 (sea water) | River mouth at 500 mt south | 40°29′20″ N 14°55′38″ E | 487.3 | 560.2 | 509.1 | 334.1 | 504.2 (41,263.6) | 261.2 (48,756.3) | 181.2 (5986.8) | 507.2 (47,596.2) | 852.2 |
| 5 (sea water) | River mouth at 1000 mt north | 40°28′55″ N 14°56′12″ E | 309.5 | 497.3 | 424.4 | 121.9 | 370.3 (29,865.2) | 125.1 (11,587.3) | 204.5 (13,501.6) | 589.9 (2843.2) | 649.5 |
| 6 (sea water) | River mouth at 1000 mt central | 40°28′55″ N 14°55′50″ E | 227.3 | 498.3 | 529.3 | 249.7 | 328.7 (10,859.8) | 214.7 (65,741.0) | 190.2 (18,459.2) | 461.1 (14,896.2) | 708.1 |
| 7 (sea water) | River mouth at 1000 mt south | 40°28′55″ N 14°55′28″ E | 302.1 | 499.2 | 502.6 | 262.3 | 467.6 (36,587.2) | 294.9 (24,189.2) | 188.2 (10,453.2) | 369.1 (4875.2) | 744.3 |
| 8 (sea water) | River mouth at 1500 mt north | 40°28′47″ N 14°56′16″ E | 300.2 | 112.3 | 289.7 | 10.1 | 367.9 (19,845.5) | 121.9 (10,354.3) | 219.0 (16,181.1) | 192.3 (5489.5) | 331.7 |
| 9 (sea water) | River mouth at 1500 mt central | 40°28′39″ N 14°55′56″ E | 361.7 | 331.2 | 424.8 | 175.9 | 482.1 (86,412.3) | 240.2 (66,587.4) | 185.7 (58,476.5) | 277.9 (13,489.2) | 602.1 |
| 10 (sea water) | River mouth at 1500 mt south | 40°28′30″ N 14°55′38″ E | 471.0 | 489.3 | 509.1 | 207.1 | 545.8 (85,647.1) | 387.3 (29,875.1) | 173.2 (39,485.2) | 451.7 (8746.2) | 683.2 |
| PAHs | log Koc a | log K′oc (Mean) | ff |
|---|---|---|---|
| Nap | 3.11 | 3.25 | 0.05 |
| Any | 3.51 | 3.78 | 0.10 |
| Ace | 3.43 | 4.15 | 0.06 |
| Flu | 3.70 | 3.58 | 0.04 |
| Phe | 3.87 | 4.22 | 0.06 |
| Ant | 3.40 | 4.00 | 0.06 |
| Fla | 3.70 | 4.79 | 0.09 |
| Pyr | 4.66 | 3.88 | 0.08 |
| BaA | 5.30 | 4.29 | 0.12 |
| Chr | 5.43 | 4.05 | 0.18 |
| Bbf | 5.36 | 1.21 | 0.27 |
| Bkf | 5.57 | 1.18 | 0.23 |
| BaP | 5.61 | 2.22 | 0.12 |
| IcdP | 6.64 | 0.41 | 0.28 |
| DahA | 6.22 | 2.10 | 0.10 |
| Bghip | 6.90 | 0.83 | 0.05 |
| PAHs | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nap | Acy | Ace | Flu | Phe | Ant | Fla | Pyr | BaA | Chr | BbF | BkF | BaP | DahA | BghiP | IcdP | ∑PAHs | |
| TEL a | 34.6 | 5.87 | 6.71 | 21.2 | 86.7 | 46.9 | 113 | 153 | 74.8 | 108 | - | - | 88.8 | 6.22 | - | - | 1684 |
| Percentage of samples over the TEL | 20 | 100 | 100 | 40 | 0 | 0 | 0 | 0 | 0 | 0 | 70 | 100 | 0 | ||||
| PEL a | 391 | 128 | 88.9 | 144 | 544 | 245 | 1494 | 1398 | 693 | 846 | - | - | 763 | 135 | - | - | 16770 |
| Percentage of samples over the PEL | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 10 | 0 | ||||
| ERL b | 160 | 44 | 16 | 19 | 240 | 85 | 600 | 665 | 261 | 384 | - | - | 430 | 63.4 | - | - | 4022 |
| Percentage of samples over the ERL | 0 | 0 | 50 | 20 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 0 | ||||
| ERM b | 2100 | 640 | 500 | 540 | 1500 | 1100 | 5100 | 2600 | 1600 | 2800 | - | - | 1600 | 260 | - | - | 44792 |
| Percentage of samples over the ERM | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montuori, P.; De Rosa, E.; Di Duca, F.; De Simone, B.; Scippa, S.; Russo, I.; Sarnacchiaro, P.; Triassi, M. Polycyclic Aromatic Hydrocarbons (PAHs) in the Dissolved Phase, Particulate Matter, and Sediment of the Sele River, Southern Italy: A Focus on Distribution, Risk Assessment, and Sources. Toxics 2022, 10, 401. https://doi.org/10.3390/toxics10070401
Montuori P, De Rosa E, Di Duca F, De Simone B, Scippa S, Russo I, Sarnacchiaro P, Triassi M. Polycyclic Aromatic Hydrocarbons (PAHs) in the Dissolved Phase, Particulate Matter, and Sediment of the Sele River, Southern Italy: A Focus on Distribution, Risk Assessment, and Sources. Toxics. 2022; 10(7):401. https://doi.org/10.3390/toxics10070401
Chicago/Turabian StyleMontuori, Paolo, Elvira De Rosa, Fabiana Di Duca, Bruna De Simone, Stefano Scippa, Immacolata Russo, Pasquale Sarnacchiaro, and Maria Triassi. 2022. "Polycyclic Aromatic Hydrocarbons (PAHs) in the Dissolved Phase, Particulate Matter, and Sediment of the Sele River, Southern Italy: A Focus on Distribution, Risk Assessment, and Sources" Toxics 10, no. 7: 401. https://doi.org/10.3390/toxics10070401
APA StyleMontuori, P., De Rosa, E., Di Duca, F., De Simone, B., Scippa, S., Russo, I., Sarnacchiaro, P., & Triassi, M. (2022). Polycyclic Aromatic Hydrocarbons (PAHs) in the Dissolved Phase, Particulate Matter, and Sediment of the Sele River, Southern Italy: A Focus on Distribution, Risk Assessment, and Sources. Toxics, 10(7), 401. https://doi.org/10.3390/toxics10070401