Cytotoxicity Assessment of Nanoplastics and Plasticizers Exposure in In Vitro Lung Cell Culture Systems—A Systematic Review
Abstract
:1. Introduction
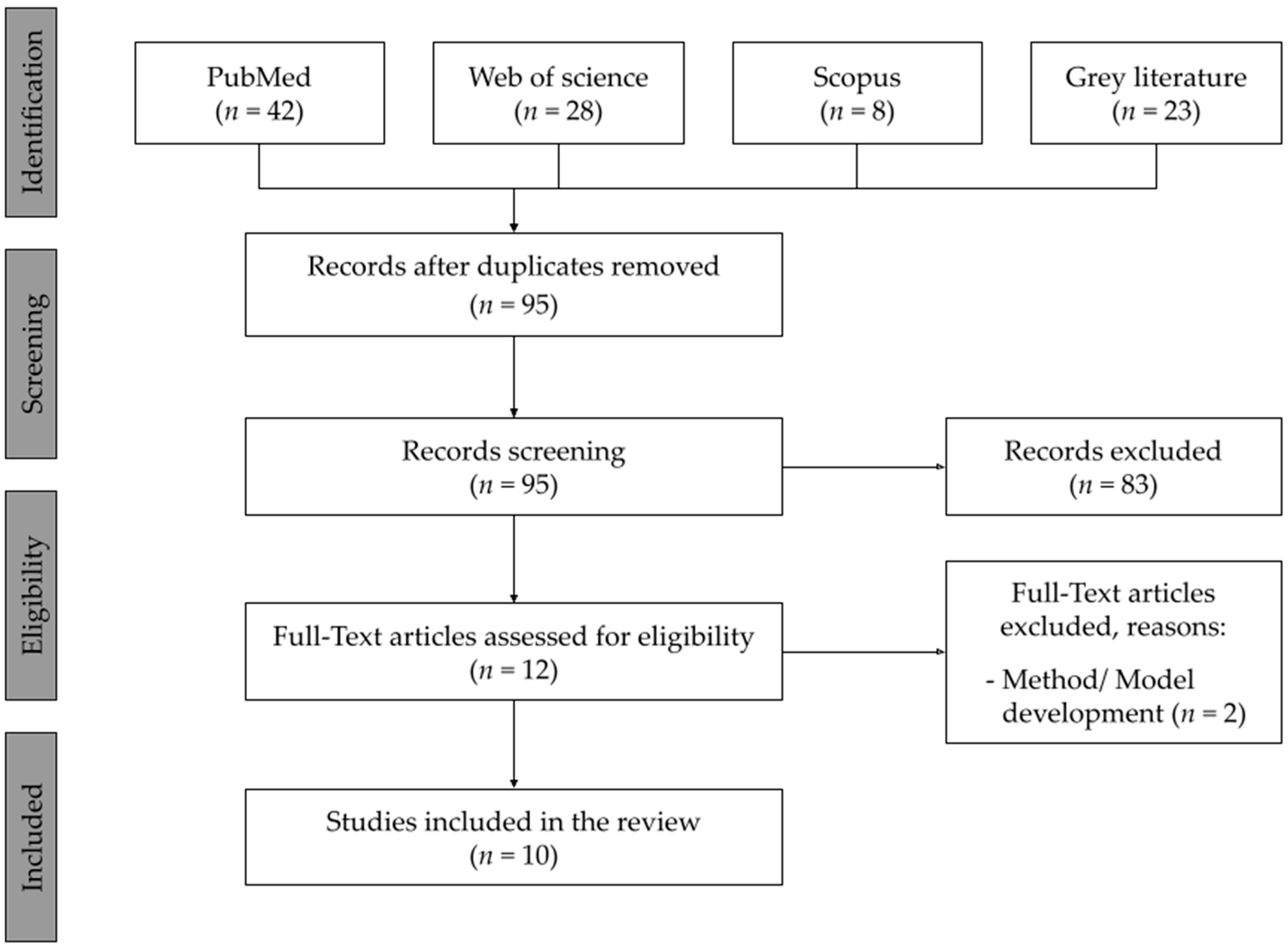
2. Materials and Methods
2.1. Search Strategy
2.2. Screening and Eligibility Criteria
2.3. Study Selection
2.4. Data Extraction
2.5. Synthesis of the Evidence
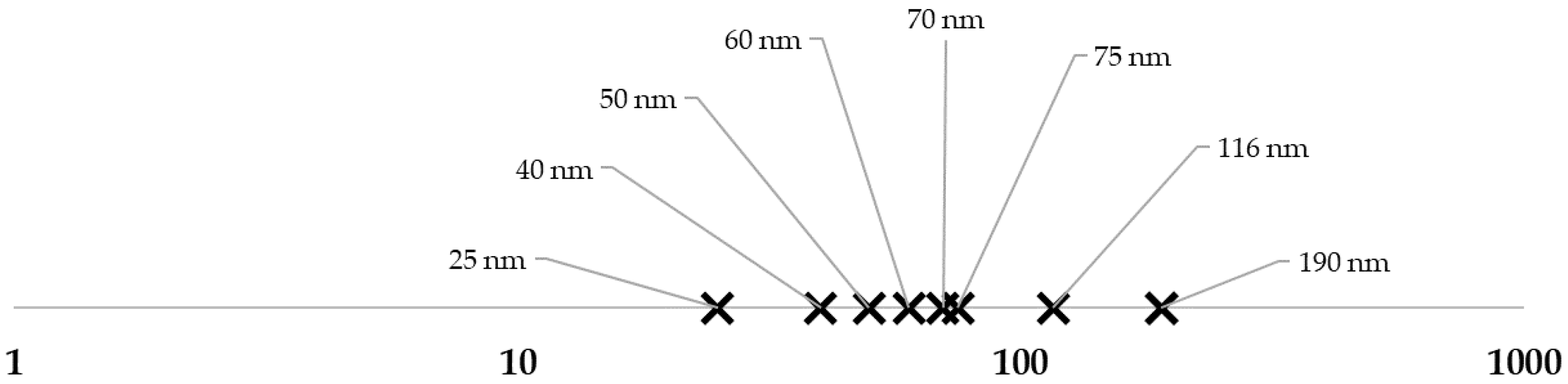
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Andrady, A.L.; Neal, M.A. Applications and Societal Benefits of Plastics. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1977–1984. [Google Scholar] [CrossRef] [PubMed]
- Ebewele, R.O. Polymer Science and Technology; CRC Press: Boca Raton, FL, USA, 2000; ISBN 9780429127922. [Google Scholar]
- Banerjee, A.; Shelver, W.L. Micro- and Nanoplastic Induced Cellular Toxicity in Mammals: A Review. Sci. Total Environ. 2021, 755, 142518. [Google Scholar] [CrossRef] [PubMed]
- Duis, K.; Coors, A. Microplastics in the Aquatic and Terrestrial Environment: Sources (with a Specific Focus on Personal Care Products), Fate and Effects. Environ. Sci. Eur. 2016, 28, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, D.; Shen, M.; Zhang, Y.; Li, H.; Zeng, G. Microplastics and Nanoplastics: Would They Affect Global Biodiversity Change? Environ. Sci. Pollut. Res. 2019, 26, 19997–20002. [Google Scholar] [CrossRef]
- Akhbarizadeh, R.; Dobaradaran, S.; Amouei Torkmahalleh, M.; Saeedi, R.; Aibaghi, R.; Faraji Ghasemi, F. Suspended Fine Particulate Matter (PM2.5), Microplastics (MPs), and Polycyclic Aromatic Hydrocarbons (PAHs) in Air: Their Possible Relationships and Health Implications. Environ. Res. 2021, 192, 110339. [Google Scholar] [CrossRef]
- Sierra, I.; Chialanza, M.R.; Faccio, R.; Carrizo, D.; Fornaro, L.; Pérez-Parada, A. Identification of Microplastics in Wastewater Samples by Means of Polarized Light Optical Microscopy. Environ. Sci. Pollut. Res. 2020, 27, 7409–7419. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on Nanoparticles and Nanostructured Materials: History, Sources, Toxicity and Regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef] [Green Version]
- Luengo, C.; Allen, N.S.; Edge, M.; Wilkinson, A.; Parellada, M.D.; Barrio, J.A.; Santa, V.R. Photo-Oxidative Degradation Mechanisms in Styrene–Ethylene–Butadiene–Styrene (SEBS) Triblock Copolymer. Polym. Degrad. Stab. 2006, 91, 947–956. [Google Scholar] [CrossRef]
- Wilkes, R.A.; Aristilde, L. Degradation and Metabolism of Synthetic Plastics and Associated Products by Pseudomonas Sp.: Capabilities and Challenges. J. Appl. Microbiol. 2017, 123, 582–593. [Google Scholar] [CrossRef] [Green Version]
- Campanale, C.; Massarelli, C.; Savino, I.; Locaputo, V.; Uricchio, V.F. A Detailed Review Study on Potential Effects of Microplastics and Additives of Concern on Human Health. Int. J. Environ. Res. Public Health 2020, 17, 1212. [Google Scholar] [CrossRef] [Green Version]
- Facciolà, A.; Visalli, G.; Pruiti Ciarello, M.; di Pietro, A. Newly Emerging Airborne Pollutants: Current Knowledge of Health Impact of Micro and Nanoplastics. Int. J. Environ. Res. Public Health 2021, 18, 2997. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, S.M.; Lawler, J. Knowledge Gaps on Micro and Nanoplastics and Human Health: A Critical Review. Case Stud. Chem. Environ. Eng. 2021, 3, 100091. [Google Scholar] [CrossRef]
- Ganesan, S.; Comstock, A.T.; Sajjan, U.S. Barrier Function of Airway Tract Epithelium. Tissue Barriers 2013, 1, e24997. [Google Scholar] [CrossRef]
- Gasperi, J.; Wright, S.L.; Dris, R.; Collard, F.; Mandin, C.; Guerrouache, M.; Langlois, V.; Kelly, F.J.; Tassin, B. Microplastics in Air: Are We Breathing It In? Curr. Opin. Environ. Sci. Health 2018, 1, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Hiemstra, P.S.; Grootaers, G.; van der Does, A.M.; Krul, C.A.M.; Kooter, I.M. Human Lung Epithelial Cell Cultures for Analysis of Inhaled Toxicants: Lessons Learned and Future Directions. Toxicol. Vitr. 2018, 47, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Cheng, Y.; Chen, Z.; Liu, T.; Yin, L.; Pu, Y.; Liang, G. In Vitro Evaluation of Nanoplastics Using Human Lung Epithelial Cells, Microarray Analysis and Co-Culture Model. Ecotoxicol. Environ. Saf. 2021, 226, 112837. [Google Scholar] [CrossRef] [PubMed]
- González-Acedo, A.; García-Recio, E.; Illescas-Montes, R.; Ramos-Torrecillas, J.; Melguizo-Rodríguez, L.; Costela-Ruiz, V.J. Evidence from in Vitro and in Vivo Studies on the Potential Health Repercussions of Micro- and Nanoplastics. Chemosphere 2021, 280, 130826. [Google Scholar] [CrossRef]
- Dong, C.-D.; Chen, C.-W.; Chen, Y.-C.; Chen, H.-H.; Lee, J.-S.; Lin, C.-H. Polystyrene Microplastic Particles: In Vitro Pulmonary Toxicity Assessment. J. Hazard. Mater. 2020, 385, 121575. [Google Scholar] [CrossRef]
- Shi, Q.; Tang, J.; Wang, L.; Liu, R.; Giesy, J.P. Combined Cytotoxicity of Polystyrene Nanoplastics and Phthalate Esters on Human Lung Epithelial A549 Cells and Its Mechanism. Ecotoxicol. Environ. Saf. 2021, 213, 112041. [Google Scholar] [CrossRef]
- Heudorf, U.; Mersch-Sundermann, V.; Angerer, J. Phthalates: Toxicology and Exposure. Int. J. Hyg. Environ. Health 2007, 210, 623–634. [Google Scholar] [CrossRef]
- Oehlmann, J.; Schulte-Oehlmann, U.; Kloas, W.; Jagnytsch, O.; Lutz, I.; Kusk, K.O.; Wollenberger, L.; Santos, E.M.; Paull, G.C.; van Look, K.J.W.; et al. A Critical Analysis of the Biological Impacts of Plasticizers on Wildlife. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2047–2062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, P.-H.; Chang, Y.-Z.; Chang, H.-P.; Wang, S.-L.; Haung, H.-I.; Huang, P.-C.; Chen, J.-Y. Exposure to Di(2-Ethylhexyl) Phthalate in Premature Neonates in a Neonatal Intensive Care Unit in Taiwan. Pediatr. Crit. Care Med. 2012, 13, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Fréry, N.; Santonen, T.; Porras, S.P.; Fucic, A.; Leso, V.; Bousoumah, R.; Duca, R.C.; el Yamani, M.; Kolossa-Gehring, M.; Ndaw, S.; et al. Biomonitoring of Occupational Exposure to Phthalates: A Systematic Review. Int. J. Hyg. Environ. Health 2020, 229, 113548. [Google Scholar] [CrossRef]
- Kolena, B.; Petrovicova, I.; Pilka, T.; Pucherova, Z.; Munk, M.; Matula, B.; Vankova, V.; Petlus, P.; Jenisova, Z.; Rozova, Z.; et al. Phthalate Exposure and Health-Related Outcomes in Specific Types of Work Environment. Int. J. Environ. Res. Public Health 2014, 11, 5628–5639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, S.-Y.; Lao, J.-Y.; Wu, C.-C.; Bao, L.-J.; Zeng, E.Y. In Vitro Inhalation Bioaccessibility for Particle-Bound Hydrophobic Organic Chemicals: Method Development, Effects of Particle Size and Hydrophobicity, and Risk Assessment. Environ. Int. 2018, 120, 295–303. [Google Scholar] [CrossRef]
- Sayes, C.M.; Singal, M. The Link between Delivered Aerosol Dose and Inflammatory Responses: Exposing a Lung Cell Co-Culture System to Selected Allergens and Irritants. J. Aerosol Sci. 2021, 151, 105677. [Google Scholar] [CrossRef]
- Tang, H.; Xue, G. Major Physiological Signaling Pathways in the Regulation of Cell Proliferation and Survival. In Mechanisms of Drug Resistance in Cancer Therapy; Springer: Cham, Switzerland, 2017; pp. 13–30. [Google Scholar]
- Val, M.M.; Mendes, L.A.; Alarcão, A.; Carvalho, L.; Carreira, I.; Rodrigues, C.F.D.; Alpoim, M.C. Senescent Bronchial Fibroblasts Induced to Senescence by Cr(VI) Promote Epithelial–Mesenchymal Transition When Co-Cultured with Bronchial Epithelial Cells in the Presence of Cr(VI). Mutagenesis 2015, 30, 277–286. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Xu, M.; Halimu, G.; Zhang, Q.; Song, Y.; Fu, X.; Li, Y.; Li, Y.; Zhang, H. Internalization and Toxicity: A Preliminary Study of Effects of Nanoplastic Particles on Human Lung Epithelial Cell. Sci. Total Environ. 2019, 694, 133794. [Google Scholar] [CrossRef]
- Roshanzadeh, A.; Park, S.; Ganjbakhsh, S.E.; Park, J.; Lee, D.H.; Lee, S.; Kim, E.S. Surface Charge-Dependent Cytotoxicity of Plastic Nanoparticles in Alveolar Cells under Cyclic Stretches. Nano Lett. 2020, 20, 7168–7176. [Google Scholar] [CrossRef]
- Ji, Y.; Wang, Y.; Shen, D.; Kang, Q.; Ma, J.; Chen, L. Revisiting the Cellular Toxicity of Benzo[: A] Pyrene from the View of Nanoclusters: Size- And Nanoplastic Adsorption-Dependent Bioavailability. Nanoscale 2021, 13, 1016–1028. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Wang, Y.; Shen, D.; Kang, Q.; Chen, L. Mucin Corona Delays Intracellular Trafficking and Alleviates Cytotoxicity of Nanoplastic-Benzopyrene Combined Contaminant. J. Hazard. Mater. 2021, 406, 124306. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.L.; Ng, C.T.; Zou, L.; Lu, Y.; Chen, J.; Bay, B.H.; Shen, H.M.; Ong, C.N. Targeted Metabolomics Reveals Differential Biological Effects of Nanoplastics and NanoZnO in Human Lung Cells. Nanotoxicology 2019, 13, 1117–1132. [Google Scholar] [CrossRef] [PubMed]
- Rafael-Vázquez, L.; García-Trejo, S.; Aztatzi-Aguilar, O.G.; Bazán-Perkins, B.; Quintanilla-Vega, B. Exposure to Diethylhexyl Phthalate (DEHP) and Monoethylhexyl Phthalate (MEHP) Promotes the Loss of Alveolar Epithelial Phenotype of A549 Cells. Toxicol. Lett. 2018, 294, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Binder, S.; Cao, X.; Bauer, S.; Rastak, N.; Kuhn, E.; Dragan, G.C.; Monsé, C.; Ferron, G.; Breuer, D.; Oeder, S.; et al. In Vitro Genotoxicity of Dibutyl Phthalate on A549 Lung Cells at Air–Liquid Interface in Exposure Concentrations Relevant at Workplaces. Environ. Mol. Mutagenesis 2021, 62, 490–501. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.D.; Grant, M.H.; Blass, C.R.; Courtney, J.M.; Barbenel, J.C. Poly(Vinyl Chloride) Formulations: Acute Toxicity to Cultured Human Cell Lines. J. Biomater. Sci. Polym. Ed. 1995, 7, 453–459. [Google Scholar] [CrossRef]
- Alimi, O.S.; Farner Budarz, J.; Hernandez, L.M.; Tufenkji, N. Microplastics and Nanoplastics in Aquatic Environments: Aggregation, Deposition, and Enhanced Contaminant Transport. Environ. Sci. Technol. 2018, 52, 1704–1724. [Google Scholar] [CrossRef]
- Shen, M.; Zhang, Y.; Zhu, Y.; Song, B.; Zeng, G.; Hu, D.; Wen, X.; Ren, X. Recent Advances in Toxicological Research of Nanoplastics in the Environment: A Review. Environ. Pollut. 2019, 252, 511–521. [Google Scholar] [CrossRef]
- Bianco, A.; Sordello, F.; Ehn, M.; Vione, D.; Passananti, M. Degradation of Nanoplastics in the Environment: Reactivity and Impact on Atmospheric and Surface Waters. Sci. Total Environ. 2020, 742, 140413. [Google Scholar] [CrossRef]
- Yuan, S.Y.; Liu, C.; Liao, C.S.; Chang, B.V. Occurrence and Microbial Degradation of Phthalate Esters in Taiwan River Sediments. Chemosphere 2002, 49, 1295–1299. [Google Scholar] [CrossRef]
- Mayer, F.L.; Stalling, D.L.; Johnson, J.L. Phthalate Esters as Environmental Contaminants. Nature 1972, 238, 411–413. [Google Scholar] [CrossRef] [PubMed]
- Zolfaghari, M.; Drogui, P.; Seyhi, B.; Brar, S.K.; Buelna, G.; Dubé, R. Occurrence, Fate and Effects of Di (2-Ethylhexyl) Phthalate in Wastewater Treatment Plants: A Review. Environ. Pollut. 2014, 194, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Rudel, R.A.; Perovich, L.J. Endocrine Disrupting Chemicals in Indoor and Outdoor Air. Atmos. Environ. 2009, 43, 170–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lunderberg, D.M.; Kristensen, K.; Liu, Y.; Misztal, P.K.; Tian, Y.; Arata, C.; Wernis, R.; Kreisberg, N.; Nazaroff, W.W.; Goldstein, A.H. Characterizing Airborne Phthalate Concentrations and Dynamics in a Normally Occupied Residence. Environ. Sci. Technol. 2019, 53, 7337–7346. [Google Scholar] [CrossRef] [Green Version]
- Card, J.W.; Zeldin, D.C.; Bonner, J.C.; Nestmann, E.R. Pulmonary Applications and Toxicity of Engineered Nanoparticles. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2008, 295, L400–L411. [Google Scholar] [CrossRef] [Green Version]
- Bonner, J.C. Nanoparticles as a Potential Cause of Pleural and Interstitial Lung Disease. Proc. Am. Thorac. Soc. 2010, 7, 138–141. [Google Scholar] [CrossRef] [Green Version]
- Xi, W.; Tang, H.; Liu, Y.; Liu, C.; Gao, Y.; Cao, A.; Liu, Y.; Chen, Z.; Wang, H. Cytotoxicity of Vanadium Oxide Nanoparticles and Titanium Dioxide-coated Vanadium Oxide Nanoparticles to Human Lung Cells. J. Appl. Toxicol. 2020, 40, 567–577. [Google Scholar] [CrossRef]
- Klein, S.G.; Hennen, J.; Serchi, T.; Blömeke, B.; Gutleb, A.C. Potential of Coculture in Vitro Models to Study Inflammatory and Sensitizing Effects of Particles on the Lung. Toxicol. Vitr. 2011, 25, 1516–1534. [Google Scholar] [CrossRef]
- Mittal, S.; Pandey, A.K. Cerium Oxide Nanoparticles Induced Toxicity in Human Lung Cells: Role of ROS Mediated DNA Damage and Apoptosis. BioMed Res. Int. 2014, 2014, 891934. [Google Scholar] [CrossRef]
- Banerjee, A.; Billey, L.O.; Shelver, W.L. Uptake and Toxicity of Polystyrene Micro/Nanoplastics in Gastric Cells: Effects of Particle Size and Surface Functionalization. PLoS ONE 2021, 16, e0260803. [Google Scholar] [CrossRef]
- Materić, D.; Ludewig, E.; Brunner, D.; Röckmann, T.; Holzinger, R. Nanoplastics Transport to the Remote, High-Altitude Alps. Environ. Pollut. 2021, 288, 117697. [Google Scholar] [CrossRef] [PubMed]
- Petithory, T.; Pieuchot, L.; Josien, L.; Ponche, A.; Anselme, K.; Vonna, L. Size-Dependent Internalization Efficiency of Macrophages from Adsorbed Nanoparticle-Based Monolayers. Nanomaterials 2021, 11, 1963. [Google Scholar] [CrossRef]
- Wahl, A.; le Juge, C.; Davranche, M.; el Hadri, H.; Grassl, B.; Reynaud, S.; Gigault, J. Nanoplastic Occurrence in a Soil Amended with Plastic Debris. Chemosphere 2021, 262, 127784. [Google Scholar] [CrossRef] [PubMed]
- Helmberger, M.S.; Tiemann, L.K.; Grieshop, M.J. Towards an Ecology of Soil Microplastics. Funct. Ecol. 2020, 34, 550–560. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Xu, K.; Zhang, B.; Ye, Y.; Zhang, Q.; Jiang, W. Cellular Internalization and Release of Polystyrene Microplastics and Nanoplastics. Sci. Total Environ. 2021, 779, 146523. [Google Scholar] [CrossRef]
- Mendes, L.A.; Amorim, M.J.B.; Scott-Fordsmand, J.J. Assessing the Toxicity of Safer by Design CuO Surface-Modifications Using Terrestrial Multispecies Assays. Sci. Total Environ. 2019, 678, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ma, Y.; Zhu, D.; Xia, T.; Qi, Y.; Yao, Y.; Guo, X.; Ji, R.; Chen, W. Polystyrene Nanoplastics-Enhanced Contaminant Transport: Role of Irreversible Adsorption in Glassy Polymeric Domain. Environ. Sci. Technol. 2018, 52, 2677–2685. [Google Scholar] [CrossRef] [PubMed]
- Cimmino, I.; Fiory, F.; Perruolo, G.; Miele, C.; Beguinot, F.; Formisano, P.; Oriente, F. Potential Mechanisms of Bisphenol A (BPA) Contributing to Human Disease. Int. J. Mol. Sci. 2020, 21, 5761. [Google Scholar] [CrossRef]
- Batel, A.; Linti, F.; Scherer, M.; Erdinger, L.; Braunbeck, T. Transfer of Benzo[ a ]Pyrene from Microplastics to Artemia Nauplii and Further to Zebrafish via a Trophic Food Web Experiment: CYP1A Induction and Visual Tracking of Persistent Organic Pollutants. Environ. Toxicol. Chem. 2016, 35, 1656–1666. [Google Scholar] [CrossRef]
- Lindner, K.; Ströbele, M.; Schlick, S.; Webering, S.; Jenckel, A.; Kopf, J.; Danov, O.; Sewald, K.; Buj, C.; Creutzenberg, O.; et al. Biological Effects of Carbon Black Nanoparticles Are Changed by Surface Coating with Polycyclic Aromatic Hydrocarbons. Part. Fibre Toxicol. 2017, 14, 8. [Google Scholar] [CrossRef]
- Trevisan, R.; Voy, C.; Chen, S.; di Giulio, R.T. Nanoplastics Decrease the Toxicity of a Complex PAH Mixture but Impair Mitochondrial Energy Production in Developing Zebrafish. Environ. Sci. Technol. 2019, 53, 8405–8415. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.; Liu, X.; Qu, M. Nanoplastics and Human Health: Hazard Identification and Biointerface. Nanomaterials 2022, 12, 1298. [Google Scholar] [CrossRef] [PubMed]
- Mendes, L.A.; Barreto, A.; Santos, J.; Amorim, M.J.B.; Maria, V.L. Co-Exposure of Nanopolystyrene and Other Environmental Contaminants—Their Toxic Effects on the Survival and Reproduction of Enchytraeus Crypticus. Toxics 2022, 10, 193. [Google Scholar] [CrossRef] [PubMed]
- Stock, V.; Böhmert, L.; Dönmez, M.H.; Lampen, A.; Sieg, H. An Inverse Cell Culture Model for Floating Plastic Particles. Anal. Biochem. 2020, 591, 113545. [Google Scholar] [CrossRef] [PubMed]
| PEO | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Population | In vitro respiratory system cells | Cohorts, cross-sectional and in vivo studies, in vitro studies on other cell systems |
| Exposure | Nanoplastics (up to 1000 nm in diameter), plasticizers | Microplastics (>1000 nm) |
| Outcome | Cell viability, cell morphology, cell fate, DNA damage and protein expression |
| Exposure | Main Findings | References | |||||
|---|---|---|---|---|---|---|---|
| Contaminant Type | Cell Type | Cell Viability | Cell Death | DNA Damage | Protein Expression | ||
| NPs | PS | A549 | ✔ | ✔ | ✔ | ✔ | [31] |
| ✔ | ✔ | ✔ | [32] | ||||
| ✔ | [33] | ||||||
| ✔ | [34] | ||||||
| BEAS-2B | ✔ | ✔ | ✔ | [35] | |||
| ✔ | ✔ | [17] | |||||
| HPAEpiC | ✔ | ✔ | [17] | ||||
| Plasticizers | MEHP, DEHP | A549 | ✔ | ✔ | ✔ | [36] | |
| DBP | A549 | ✔ | ✔ | [37] | |||
| NPs + Plasticizers | PS + DEHP, DBP | A549 | ✔ | [20] | |||
| PVC + DEHP, DIOP | WI38va13 | ✔ | [38] | ||||
| Contaminant Type | Cell Type | Exposure Time | Concentration Range | Cell Viability | Reference |
|---|---|---|---|---|---|
| NPs | A549 | 24 h | 2.5–30 μg/mL | No significant changes at <5 μg/mL Significantly increase at 10 and 15 μg/mL Significant toxic effects at >25 μg/mL | [31] |
| 10–300 μg/mL | Significant effects at >160 μg/mL | [31] | |||
| 500 μg/mL | No significant effects | [33,34] | |||
| BEAS-2B | 24 h | 0–100 μg/mL | Significantly inhibition at >10 μg/mL | [35] | |
| 0–40 μg/cm2 | Significantly inhibition at >10 μg/cm2 | [17] | |||
| HPAEpiC | 24 h | 0–40 μg/cm2 | Significantly inhibition at >15μg/cm2 | [17] | |
| Plasticizers | A549 | 4 h–24 h | 0–1000 µM MEHP | 70% reduction at 100 and 1000 µM for MEHP and significant difference at 10–1000 μM | [36] |
| 24 h–48 h | 0–1000 µM MEHP 0–1000 µM DEHP | Significant difference at 5–1000 μM for MEHP 70% reduction at 50 μM for DEHP and a significant difference with 100–1000 μM | [36] | ||
| 0–20 ng/cm2 DBP | No significant changes observed | [37] | |||
| NPs + Plasticizers | A549 | 24 h | 0–1000 µg/mL NPs + 5 µg/mL Plasticizers | Significant effects at >200 μg/mL for NPs and at 5 μg/mL for plasticizers | [20] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clérigo, F.; Ferreira, S.; Ladeira, C.; Marques-Ramos, A.; Almeida-Silva, M.; Mendes, L.A. Cytotoxicity Assessment of Nanoplastics and Plasticizers Exposure in In Vitro Lung Cell Culture Systems—A Systematic Review. Toxics 2022, 10, 402. https://doi.org/10.3390/toxics10070402
Clérigo F, Ferreira S, Ladeira C, Marques-Ramos A, Almeida-Silva M, Mendes LA. Cytotoxicity Assessment of Nanoplastics and Plasticizers Exposure in In Vitro Lung Cell Culture Systems—A Systematic Review. Toxics. 2022; 10(7):402. https://doi.org/10.3390/toxics10070402
Chicago/Turabian StyleClérigo, Fabiana, Sandra Ferreira, Carina Ladeira, Ana Marques-Ramos, Marina Almeida-Silva, and Luís André Mendes. 2022. "Cytotoxicity Assessment of Nanoplastics and Plasticizers Exposure in In Vitro Lung Cell Culture Systems—A Systematic Review" Toxics 10, no. 7: 402. https://doi.org/10.3390/toxics10070402
APA StyleClérigo, F., Ferreira, S., Ladeira, C., Marques-Ramos, A., Almeida-Silva, M., & Mendes, L. A. (2022). Cytotoxicity Assessment of Nanoplastics and Plasticizers Exposure in In Vitro Lung Cell Culture Systems—A Systematic Review. Toxics, 10(7), 402. https://doi.org/10.3390/toxics10070402








