Application of an Ecotoxicological Battery Test to the Paddy Field Soils of the Albufera Natural Park
Abstract
:1. Introduction
2. Materials and Methods
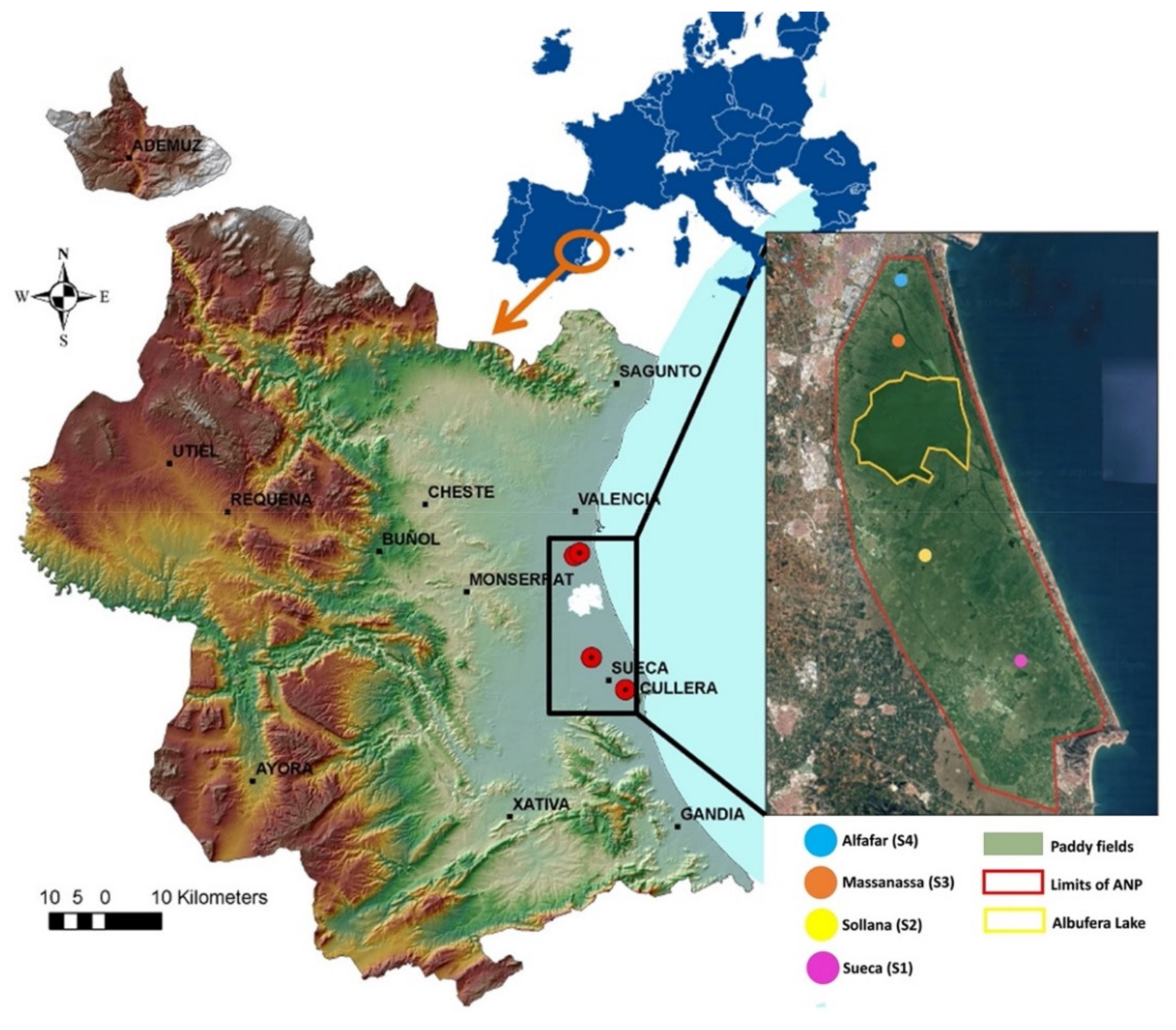
2.1. Study Area and Sampling
2.2. Soil Analyses
2.3. Terrestrial Ecotoxicity Tests
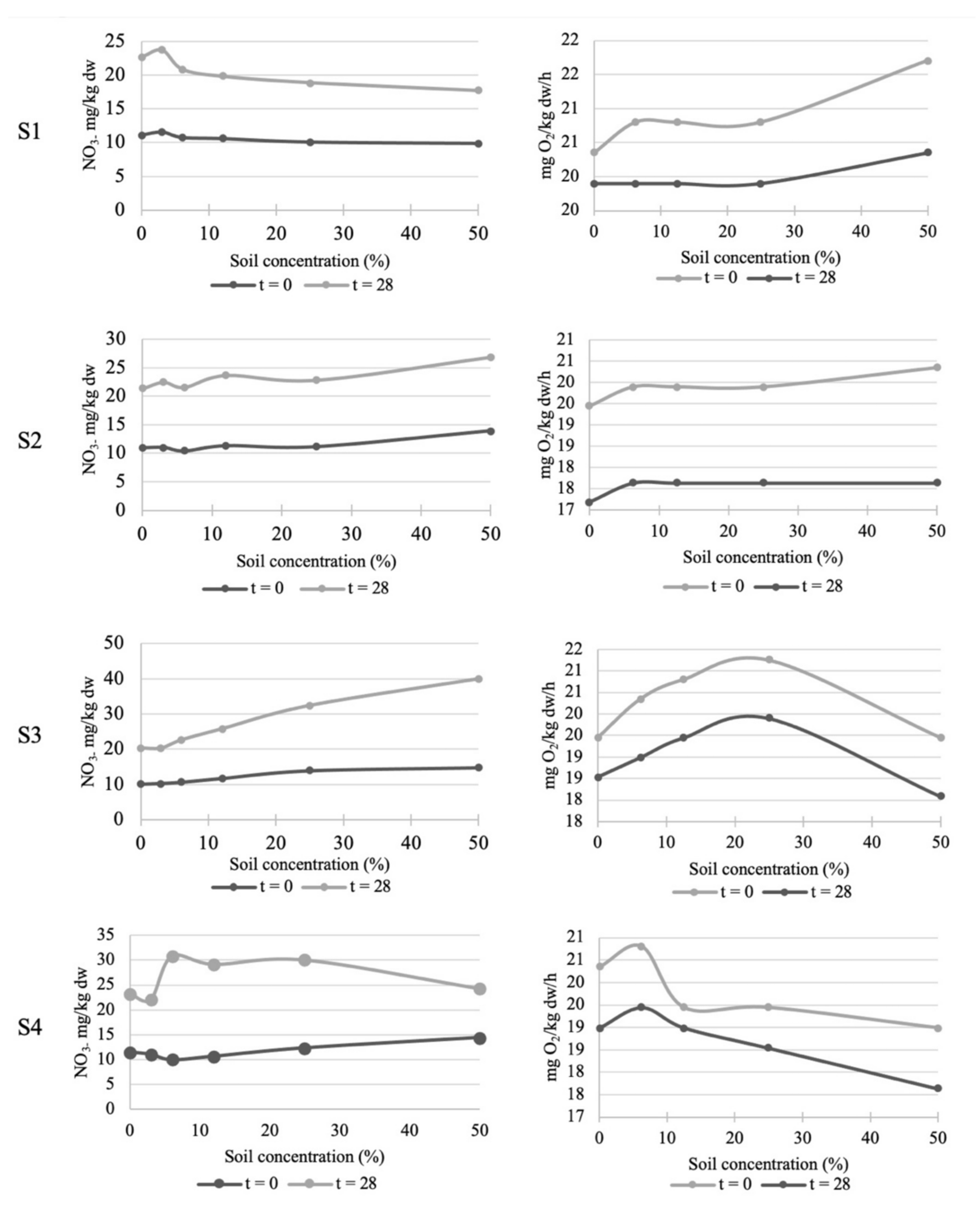
2.3.1. Carbon Mineralization Test
2.3.2. Nitrogen Mineralization Test
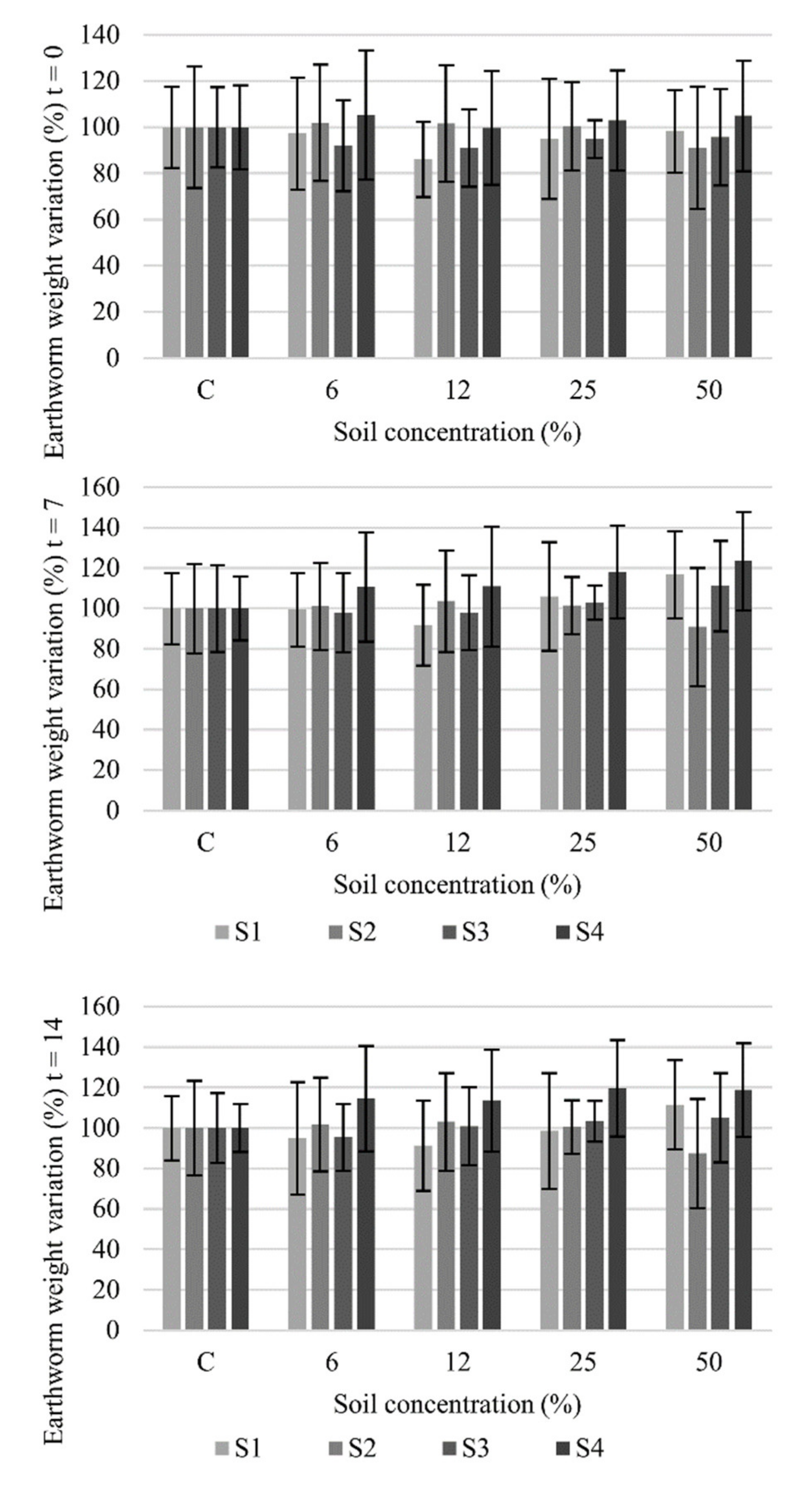
2.3.3. Acute Toxicity Test with Earthworms
2.4. Aquatic Ecotoxicity Tests
2.4.1. Algal Growth Inhibition Test
2.4.2. Daphnia Magna Acute Immobilization Test
2.5. Statistical Analysis
3. Results
3.1. Soil Properties and PTEs Contents
3.2. Soil Ecotoxicity Assessment
3.2.1. Terrestrial Ecotoxicological Bioassays
3.2.2. Aquatic Ecotoxicological Bioassays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.-Q. Heavy Metals and Pesticides Toxicity in Agricultural Soil and Plants: Ecological Risks and Human Health Implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, V.; Sarkar, A.; Singh, S.; Singh, P.; de Araujo, A.S.F.; Singh, R.P. Agroecological Responses of Heavy Metal Pollution with Special Emphasis on Soil Health and Plant Performances. Front. Environ. Sci. 2017, 5, 64. [Google Scholar] [CrossRef] [Green Version]
- Szara-Bąk, M.; Baran, A.; Klimkowicz-Pawlas, A.; Tkaczewska, J.; Wojtasik, B. Mobility, ecotoxicity, bioaccumulation and sources of trace elements in the bottom sediments of the Rożnów reservoir. Environ. Geochem. Health 2021, 43, 4701–4718. [Google Scholar] [CrossRef] [PubMed]
- Bori, J.; Vallès, B.; Navarro, A.M.; Riva, C. Ecotoxicological risks of the abandoned F–Ba–Pb–Zn mining area of Osor (Spain). Environ. Geochem. Health 2017, 39, 665–679. [Google Scholar] [CrossRef]
- Khan, Y.K.; Toqeer, M.; Shah, M.H. Mobility, bioaccessibility, pollution assessment and risk characterization of potentially toxic metals in the urban soil of Lahore, Pakistan. Environ. Geochem. Health 2022, 2022, 1–22. [Google Scholar] [CrossRef]
- Pinto, E.; Aguiar, A.; Ferreira, I. Influence of Soil Chemistry and Plant Physiology in the Phytoremediation of Cu, Mn, and Zn. Crit. Rev. Plant Sci. 2014, 33, 351–373. [Google Scholar] [CrossRef] [Green Version]
- Ramos-Miras, J.J.; Roca-Perez, L.; Guzmán-Palomino, M.; Boluda, R.; Gil, C. Background levels and baseline values of available heavy metals in Mediterranean greenhouse soils (Spain). J. Geochem. Explor. 2011, 110, 186–192. [Google Scholar] [CrossRef]
- Abd Aziz, A.; Lee, B.T.; Han, H.J.; Kim, K.W. Assessment of the stabilization of heavy metal contaminants in soils using chemical leaching and an earthworm bioassay. Environ. Geochem. Health 2019, 41, 447–460. [Google Scholar] [CrossRef]
- Alvarenga, P.; Palma, P.; De Varennes, A.; Cunha-Queda, A.C. A contribution towards the risk assessment of soils from the São Domingos Mine (Portugal): Chemical, microbial and ecotoxicological indicators. Environ. Pollut. 2012, 161, 50–56. [Google Scholar] [CrossRef]
- Madrid, F.; Reinoso, R.; Florido, M.C.; Barrientos, E.D.; Ajmone-Marsan, F.; Davidson, C.M.; Madrid, L. Estimating the extractability of potentially toxic metals in urban soils: A comparison of several extracting solutions. Environ. Pollut. 2007, 147, 713–722. [Google Scholar] [CrossRef]
- Peris, M.; Recatalá, L.; Micó, C.; Sánchez, R.; Sánchez, J. Heavy metal contents in horticultural crops of a representative area of the European Mediterranean region. Sci. Total Environ. 2007, 378, 42–48. [Google Scholar] [CrossRef]
- Roca-Perez, L.; Boluda, R.; Perez-Bermúdez, P. Soil-plant relationships, micronutrient contents, and cardenolide production in natural populations of Digitalis obscura. J. Plant Nutr. Soil Sci. 2004, 167, 79–84. [Google Scholar] [CrossRef]
- Royal Decree 9/2005, January 14, Which Establishes the List of Potentially Soil Contaminating Activities and the Criteria and Standards for the Declaration of Contaminated Soils. Available online: https://www.boe.es/eli/es/rd/2005/01/14/9 (accessed on 10 March 2022). (In Spanish).
- DIN 38414-S4; German Standard Methods for the Examination of Water, Waste Water and Sludge; Sludge and Sediments (Group S); Determination of Leachability by Water (S4). DIN: Berlin, Germany, 1984.
- García-Lorenzo, M.L.; Martinez-Sanchez, M.J.; Perez-Sirvent, C.; Molina, J. Ecotoxicological evaluation for the screening of areas polluted by mining activities. Ecotoxicology 2009, 18, 1077–1086. [Google Scholar] [CrossRef]
- Palma, P.; López-Orozco, R.; Mourinha, C.; Oropesa, A.L.; Novais, M.H.; Alvarenga, P. Assessment of the environmental impact of an abandoned mine using an integrative approach: A case-study of the “Las Musas” mine (Extremadura, Spain). Sci. Total Environ. 2019, 659, 84–94. [Google Scholar] [CrossRef]
- Afzal, M.; Yu, M.; Tang, C.; Zhang, L.; Muhammad, N.; Zhao, H.; Feng, J.; Yu, L.; Xu, J. The negative impact of cadmium on nitrogen transformation processes in a paddy soil is greater under non-flooding than flooding conditions. Environ. Int. 2019, 129, 451–460. [Google Scholar] [CrossRef]
- Hamsa, N.; Yogesh, G.S.; Koushik, U.; Patil, L. Nitrogen Transformation in Soil: Effect of Heavy Metals. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 816–832. [Google Scholar] [CrossRef] [Green Version]
- Carabassa, V.; Domene, X.; Ortiz, O.; Marks, E.A.N.; Alcañiz, J.M. Determination of EC50 Values for Cu, Zn, and Cr on Microorganisms Activity in a Mediterranean Sandy Soil. Clean Soil Air Water 2019, 47, 1700617. [Google Scholar] [CrossRef] [Green Version]
- Boluda, R.; Roca-Pérez, L.; Marimón, L. Soil plate bioassay: An effective method to determine ecotoxicological risks. Chemosphere 2011, 84, 1–8. [Google Scholar] [CrossRef]
- Lorenzo, M.; Campo, J.; Morales Suárez-Varela, M.; Picó, Y. Occurrence, Distribution and Behavior of Emerging Persistent Organic Pollutants (POPs) in a Mediterranean Wetland Protected Area. Sci. Total Environ. 2019, 646, 1009–1020. [Google Scholar] [CrossRef]
- Sadutto, D.; Andreu, V.; Ilo, T.; Akkanen, J.; Pico, Y. Pharmaceuticals and personal care products in a Mediterranean coastal wetland: Impact of anthropogenic and spatial factors and environmental risk assessment. Environ. Pollut. 2021, 271, 116353. [Google Scholar] [CrossRef]
- Requena-Riera, M.S. Caracterización y Aprovechamiento de los Sedimentos del lago de la Albufera de Valencia. Doctoral Dissertation, Universitat Politècnica de València, Valencia, Spain, 2002. [Google Scholar]
- Peris, E.; Requena, S.; de la Guardia, M.; Pastor, A.; Carrasco, J.M. Organochlorinated pesticides in sediments from the lake Albufera of Valencia (Spain). Chemosphere 2005, 60, 1542–1549. [Google Scholar] [CrossRef]
- Romo, S.; Villena, M.J.; Sahuquillo, M.; Soria, J.M.; Giménez, M.; Alfonso, T.; Vicente, E.; Miracle, M.R. Response of a Shallow Mediterranean Lake to Nutrient Diversion: Does It Follow Similar Patterns as in Northern Shallow Lakes? Freshw. Biol. 2005, 50, 1706–1717. [Google Scholar] [CrossRef]
- Vera-Herrera, L.; Romo, S.; Soria, J. How Agriculture, Connectivity and Water Management Can Affect Water Quality of a Mediterranean Coastal Wetland. Agronomy 2022, 12, 486. [Google Scholar] [CrossRef]
- Boluda, R.; Andreu, V.; Gilabert, M.A.; Sobrino, P. Relation between reflectance of rice crop and indices of pollution by heavy metals in soils of Albufera Natural Park (Valencia, Spain). Soil Technol. 1993, 6, 351–363. [Google Scholar] [CrossRef]
- Boluda, R.; Quintanilla, J.F.; Bonilla, J.A.; Sáez, E.; Gamón, M. Application of the Microtox test and pollution indices to the study of water toxicity in the Albufera Natural Park (Valencia, Spain). Chemosphere 2002, 46, 355–369. [Google Scholar] [CrossRef]
- Gamón, M.; Sáez, E.; Boluda, R. Direct and indirect exogenous contamination by pesticides of rice-farming soils in a Mediterranean wetland. Environ. Contam. Toxicol. 2003, 44, 141–151. [Google Scholar] [CrossRef]
- Gimeno-García, E.; Andreu, V.; Boluda, R. Distribution of heavy metals in rice farming soils. Environ. Contam. Toxicol. 1995, 29, 476–483. [Google Scholar] [CrossRef]
- Gimeno-García, E.; Andreu, V.; Boluda, R. Heavy metals incidence in the application of inorganic fertilizers and pesticides to rice farming soils. Environ. Pollut. 1996, 92, 19–25. [Google Scholar] [CrossRef]
- ISO 10390; Soil Quality—Determination of PH. ISO: Geneva, Switzerland, 1994.
- Walkley, A.; Black, I.A. An examination of Degtjareff method. For determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Day, P.R. Particle fractionation and particle-size analysis. In Methods of Soil Analysis: Part 1 Physical and Mineralogical Properties, Including Statistics of Measurement and Sampling; Black, C.A., Ed.; American society of Agronomy Inc.: Madison, WI, USA, 1965; pp. 545–567. [Google Scholar]
- ISO 11265; Soil Quality—Determination Specific Electrical Conductivity. ISO: Geneva, Switzerland, 1994.
- ISO 10693; Soil Quality—Determination of Carbonate Content. Volumetric Method. ISO: Geneva, Switzerland, 1995.
- ISO 13878; Soil Quality—Determination of Total Nitrogen Content by Dry Combustion (“Elemental Analysis”). ISO: Geneva, Switzerland, 1998.
- Gil, C.; Boluda, R.; Rodríguez Martín, J.A.; Guzmán, M.; del Moral, F.; Ramos--Miras, J. Assessing soil contamination and temporal trends of heavy metal contents in greenhouses on semiarid land. Land Degrad. Dev. 2018, 29, 3344–3354. [Google Scholar] [CrossRef]
- OECD. Test Guideline 217. OECD Guidelines for the Testing of Chemicals, Soil Microorganisms: Carbon Transformation Test; OECD: Paris, France, 2000. [Google Scholar] [CrossRef]
- OECD. Test Guideline 216. OECD Guidelines for the Testing of Chemicals, Soil Microorganisms: Nitrogen Transformation Test; OECD: Paris, France, 2000. [Google Scholar] [CrossRef]
- OECD. Test Guideline 207. OECD Guidelines for the Testing of Chemicals, Earthworm, Acute Toxicity Tests; OECD: Paris, France, 1984. [Google Scholar] [CrossRef]
- OECD. Test Guideline 201. OECD Guidelines for the Testing of Chemicals, Freshwater Alga and Cyanobacteria, Growth Inhibition Test; OECD: Paris, France, 2011. [Google Scholar] [CrossRef] [Green Version]
- ISO 8692:2012; Water Quality-Fresh Water Algal Growth Inhibition Test with Unicellular Green Algae. ISO: Geneva, Switzerland, 2012.
- OECD. Test Guideline 202. OECD Guidelines for the Testing of Chemicals, Daphnia sp. Acute Immobilisation Test; OECD: Paris, France, 2004. [Google Scholar] [CrossRef]
- ISO 6341:2012.; Water quality-Determination of the Inhibition of the Mobility of Daphnia Magna Straus (Cladocera, Crustacea)-Acute toxicity test. ISO: Geneva, Switzerland, 2012.
- Daphtoxkit F™. Standard Operational Procedure, Daphnia magna Toxicity Test; Microbiotests Inc.: Gent, Belgium, 2000. [Google Scholar]
- Galán, E.; Romero, A. Contaminación de suelos por metales pesados. Rev. De La Soc. Española De Mineral 2008, 10, 48–60. [Google Scholar]
- Gemeda, F.T.; Guta, D.D.; Wakjira, F.S.; Gebresenbet, G. Occurrence of heavy metal in water, soil, and plants in fields irrigated with industrial wastewater in Sabata town, Ethiopia. Environ. Sci. Pollut. Res. 2021, 28, 12382–12396. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Cortijo, J.; Ruiz-Canales, A. Effect of heavy metals on rice irrigated fields with waste water in high pH Mediterranean soils: The particular case of the Valencia area in Spain. Agric. Water Manag. 2018, 210, 108–123. [Google Scholar] [CrossRef]
- Hammer, D.; Keller, C. Changes in the rhizosphere of metal accumulating plants evidenced by chemical extractants. J. Environ. Qual. 2002, 31, 1561–1569. [Google Scholar] [CrossRef]
- Alvarez, J.M.; Lopez-Valdivia, L.M.; Novillo, J.; Obrador, A.; Rico, M.I. Comparison of EDTA and sequential extraction tests for phytoavailability prediction of manganese and zinc in agricultural alkaline soils. Geoderma 2006, 132, 450–463. [Google Scholar] [CrossRef]
- Jalali, M.; Hurseresht, Z.; Ranjbar, F. Comparison of different chemical agents in the single extraction of some potentially toxic elements (PTEs) from contaminated soils. Environ. Earth Sci. 2022, 81, 282. [Google Scholar] [CrossRef]
- Pascual-Aguilar, J.; Andreu, V.; Picó, Y. An environmental forensic procedure to analyse anthropogenic pressures of urban origin on surface water of protected coastal agro-environmental wetlands (L’Albufera de Valencia Natural Park, Spain). J. Hazard. Mater. 2013, 263, 214–223. [Google Scholar] [CrossRef]
- Iranzo, M.; Gamón, M.; Boluda, R.; Mormeneo, S. Analysis of pharmaceutical biodegradation of WWTP sludge using composting and identification of certain microorganisms involved in the process. Sci. Total Environ. 2018, 640–641, 840–848. [Google Scholar] [CrossRef]
- Madrid, F.; Diaz-Barrientos, E.; Madrid, L. Availability and bio-accessibility of metals in the clay fraction of urban soils of Sevilla. Environ. Pollut. 2008, 156, 605–610. [Google Scholar] [CrossRef]
- Errecalde, M.F.; Boluda, R.; Lagarda, M.J.; Farre, R. Índices de contaminación por metales pesados en el suelo de cultivo intensivo: Aplicación de la comarca de l’Horta (Valencia). Suelo Planta 1991, 1, 483–494. [Google Scholar]
- Alvarenga, P.; Palma, P.; Gonçalves, A.P.; Fernandes, R.M.; de Varennes, A.; Vallini, G.; Duarte, E.; Cunha-Queda, A.C. Evaluation of tests to assess the quality of mine-contaminated soils. Environ. Geochem. Health 2008, 30, 95–99. [Google Scholar] [CrossRef] [Green Version]
- Domínguez-Rodríguez, V.I.; Adams, R.H.; Sánchez-Madrigal, F.; Pascual-Chablé, J.D.L.S.; Gómez-Cruz, R. Soil contact bioassay for rapid determination of acute toxicity with Eisenia foetida. Heliyon 2020, 6, e03131. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Wang, M.; Jiang, R.; Zheng, L.; Chen, W. Evaluation of joint toxicity of heavy metals and herbicide mixtures in soils to earthworms (Eisenia foetida). J. Environ. Sci. 2020, 94, 137–146. [Google Scholar] [CrossRef]
- Ritz, K.B.; Griffiths, S.; Wheathley, R.E. Soil Microbial Biomass and Activity under a Potato Crop Fertilized with N with and without C. Biol. Fertil. Soils 1992, 12, 265–271. [Google Scholar] [CrossRef]
- Adachi, M.; Bekku, Y.S.; Rashidah, W.; Okuda, T.; Koizumi, H. Differences in soil respiration between different tropical ecosystems. Appl. Soil Ecology 2006, 34, 258–265. [Google Scholar] [CrossRef]
- Fan, D.; Sun, J.; Liu, C.; Wang, S.; Han, J.; Agathokleous, E.; Zhu, Y. Measurement and modeling of hormesis in soil bacteria and fungi under single and combined treatments of Cd and Pb. Sci. Total Environ. 2021, 783, 147494. [Google Scholar] [CrossRef]
- Wang, S.; Huang, B.; Fan, D.; Agathokleous, E.; Guo, Y.; Zhu, Y.; Han, J. Hormetic responses of soil microbiota to exogenous Cd: A step toward linking community-level hormesis to ecological risk assessment. J. Hazard. Mater. 2021, 416, 125760. [Google Scholar] [CrossRef]
- Choi, W.S.; Hong, Y.K.; Min, K.J.; Kim, K.J.; Kim, S.C. Evaluating soil respiration as indicator of heavy metal pollution in agricultural field. Korean J. Soil Sci. Fertil. 2017, 50, 472–481. [Google Scholar] [CrossRef]
- Maisto, G.; Manzo, S.; De Nicola, F.; Carotenuto, R.; Rocco, A.; Alfani, A. Assessment of the effects of Cr, Cu, Ni and Pb soil contamination by ecotoxicological tests. J. Environ. Monit. 2011, 13, 3049–3056. [Google Scholar] [CrossRef]
- Cui, R.; Kwak, J.I.; An, Y.J. Comparative study of the sensitivity of Daphnia galeata and Daphnia magna to heavy metals. Ecotoxicol. Environ. Saf. 2018, 162, 63–70. [Google Scholar] [CrossRef]
- Agathokleous, E.; Feng, Z.; Iavicoli, I.; Calabrese, E.J. The two faces of nanomaterials: A quantification of hormesis in algae and plants. Environ. Int. 2019, 131, 105044. [Google Scholar] [CrossRef] [PubMed]
- Aruoja, V.; Kurvet, I.; Dubourguier, H.C.; Kahru, A. Toxicity testing of heavy--metal--polluted soils with algae Selenastrum capricornutum: A soil suspension assay. Environ. Toxicol. Int. J. 2004, 19, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Iavicoli, I.; Leso, V.; Fontana, L.; Calabrese, E. Nanoparticle Exposure and Hormetic Dose–Responses: An Update. Int. J. Mol. Sci. 2018, 19, 805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mykhaylenko, N.F.; Zolotareva, E.K. The effect of copper and selenium nanocarboxylates on biomass accumulation and photosynthetic energy transduction efficiency of the green algae chlorella vulgaris. Nanoscale Res. Lett. 2017, 12, 147. [Google Scholar] [CrossRef] [Green Version]
- De Schamphelaere, K.A.; Janssen, C. Bioavailability models for predicting copper toxicity to freshwater green microalgae as a function of water chemistry. Environ. Sci. Technol. 2006, 40, 4514–4522. [Google Scholar] [CrossRef]
- De Schamphelaere, K.A.; Heijerick, D.G.; Janssen, C.R. Refinement and field validation of a biotic ligand model predicting acute copper toxicity to Daphnia magna. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2002, 133, 243–258. [Google Scholar] [CrossRef]
- ECHA 2022, Manganese Registration Dossier. Available online: https://www.echa.europa.eu/registration-dossier/-/registered-dossier/15553/6/2/6 (accessed on 3 March 2022).
| Soil | Leachates | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Samples | Sand | Silt | Clay | pH | EC | CaCO3 | SOM | N | CEC | pH | EC | NO3− |
| % | dS/m | % | % | % | cmolc/kg | dS/m | mg/L | |||||
| S1 | 6 ± 2 b | 53 ± 1 a | 41 ± 2 a | 7.92 ± 0.12 a | 0.78 ± 0.07 a | 35.96 ± 1.93 a | 5.29 ± 0.09 bc | 0.32 ± 0.01 c | 23.69 ± 2.01 ab | 8.10 | 0.72 | 9.60 |
| S2 | 7 ± 1 b | 50 ± 1 a | 44 ± 1 a | 8.01 ± 0.10 a | 0.50 ± 0.06 a | 35.52 ± 1.25 a | 4.39 ± 0.05 c | 0.25 ± 0.01 c | 18.90 ± 1.20 b | 7.96 | 0.53 | 8.83 |
| S3 | 23 ± 1 a | 49 ± 1 a | 28 ± 1 b | 7.70 ± 0.08 b | 1.22 ± 0.11 bc | 34.11 ± 1.02 a | 7.01 ± 0.09 b | 0.41 ± 0.01 b | 27.4 ± 1.82 ab | 8.01 | 0.73 | 10.26 |
| S4 | 26 ± 1 a | 53 ± 1 a | 21 ± 1 b | 7.55 ± 0.15 c | 1.68 ± 0.18 c | 30.09 ± 2.01 a | 8.89 ± 0.11 a | 0.52 ± 0.02 a | 30.8 ± 2.56 a | 7.75 | 0.60 | 10.16 |
| Samples and Reference Soils | Soil | ||||||
|---|---|---|---|---|---|---|---|
| Co | Cr | Cu | Ni | Pb | Zn | Eq. Zn | |
| S1 | 0.10 ± 0.02 b | 0.18 ± 0.01 b | 5.14 ± 0.40 c | 0.32 ± 0.02 b | 4.75 ± 0.51 b | 1.55 ± 0.23 b | 14.36 |
| S2 | 0.27 ± 0.03 ab | 0.22 ± 0.01 b | 5.01 ± 0.62 c | 0.51 ± 0.04 b | 2.74 ± 0.35 b | 2.06 ± 0.31 b | 16.18 |
| S3 | 0.40 ± 0.03 a | 0.60 ± 0.04 a | 42.20 ± 3.50 b | 8.40 ± 0.70 a | 47.50 ± 5.04 a | 57.03 ± 5.71 b | 208.60 |
| S4 | 0.30 ± 0.04 a | 0.80 ± 0.08 a | 71.30 ± 8.10 a | 8.90 ± 0.94 a | 61.80 ± 7.20 a | 179.15 ± 18.90 a | 392.95 |
| Gil et al. [38] | 1.6 | - | 6.8 | 1.7 | 24.3 | 11.5 | - |
| Ramos-Miras et al. [7] | 1.7 | - | 9.5 | 1.7 | 26.3 | 11.8 | 44.6 |
| Madrid et al. [55] | - | 3.73 | 18.4 | 0.57 | 65.4 | 35.5 | - |
| Peris et al. [11] | 0.23 | 0.07 | 6.5 | 0.54 | 20 | 18.3 | - |
| Leachates | |||||||
| Ca/Mg | K/P | Na/S | Si/Fe | Mn | B | Cu | |
| S1 | 112.93/20.64 | 6.37/0.22 | 44.19/82.5 | 3.99/0.02 | 20.64 | 0.12 | 0.04 |
| S2 | 75.25/12.46 | 2.40/0.11 | 36.86/38.30 | 2.02/0.02 | 12.46 | 0.04 | 0.03 |
| S3 | 94.96/15.80 | 8.48/0.91 | 53.84/75.75 | 3.68/0.02 | 15.80 | 0.29 | 0.09 |
| S4 | 76.38/12.97 | 6.94/0.32 | 43.31/50.83 | 3.21/0.02 | 12.97 | 0.13 | 0.04 |
| Contact | Organism | Endpoint | Ecotoxicolog-ical Parameter | Soil Sample | |||
|---|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S4 | ||||
| Direct (whole soil) | Eisenia foetida | Mortality | 14 d EC50 (%) | >50% | >50% | >50% | >50% |
| Soil microorganisms (N2) | Nitrogen transformation | 28 d EC50 (%) | >50% | >50% | >50% | 98% | |
| Soil microorganisms (O2) | Carbon transformation | 28 d EC50 (%) | >50% | >50% | >50% | >50% | |
| Indirect (soil leachate) | Daphnia magna | Immobilization | 48 h EC50 (%) | NT | NT | NT | NT |
| Raphidocelis subcapitata | Growth rate | 72 h ErC50 (%) | >50% (102 *) | >50% (105 *) | >50% (110 *) | 68.6% | |
| Soil classification | NC | NC | NC | NC | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andreu-Sánchez, O.; Moratalla-López, J.; Rodríguez-Martín, J.A.; Roca-Pérez, L. Application of an Ecotoxicological Battery Test to the Paddy Field Soils of the Albufera Natural Park. Toxics 2022, 10, 375. https://doi.org/10.3390/toxics10070375
Andreu-Sánchez O, Moratalla-López J, Rodríguez-Martín JA, Roca-Pérez L. Application of an Ecotoxicological Battery Test to the Paddy Field Soils of the Albufera Natural Park. Toxics. 2022; 10(7):375. https://doi.org/10.3390/toxics10070375
Chicago/Turabian StyleAndreu-Sánchez, Oscar, Jesús Moratalla-López, José Antonio Rodríguez-Martín, and Luis Roca-Pérez. 2022. "Application of an Ecotoxicological Battery Test to the Paddy Field Soils of the Albufera Natural Park" Toxics 10, no. 7: 375. https://doi.org/10.3390/toxics10070375
APA StyleAndreu-Sánchez, O., Moratalla-López, J., Rodríguez-Martín, J. A., & Roca-Pérez, L. (2022). Application of an Ecotoxicological Battery Test to the Paddy Field Soils of the Albufera Natural Park. Toxics, 10(7), 375. https://doi.org/10.3390/toxics10070375







