A Comprehensive Review for Removal of Non-Steroidal Anti-Inflammatory Drugs Attained from Wastewater Observations Using Carbon-Based Anodic Oxidation Process
Abstract
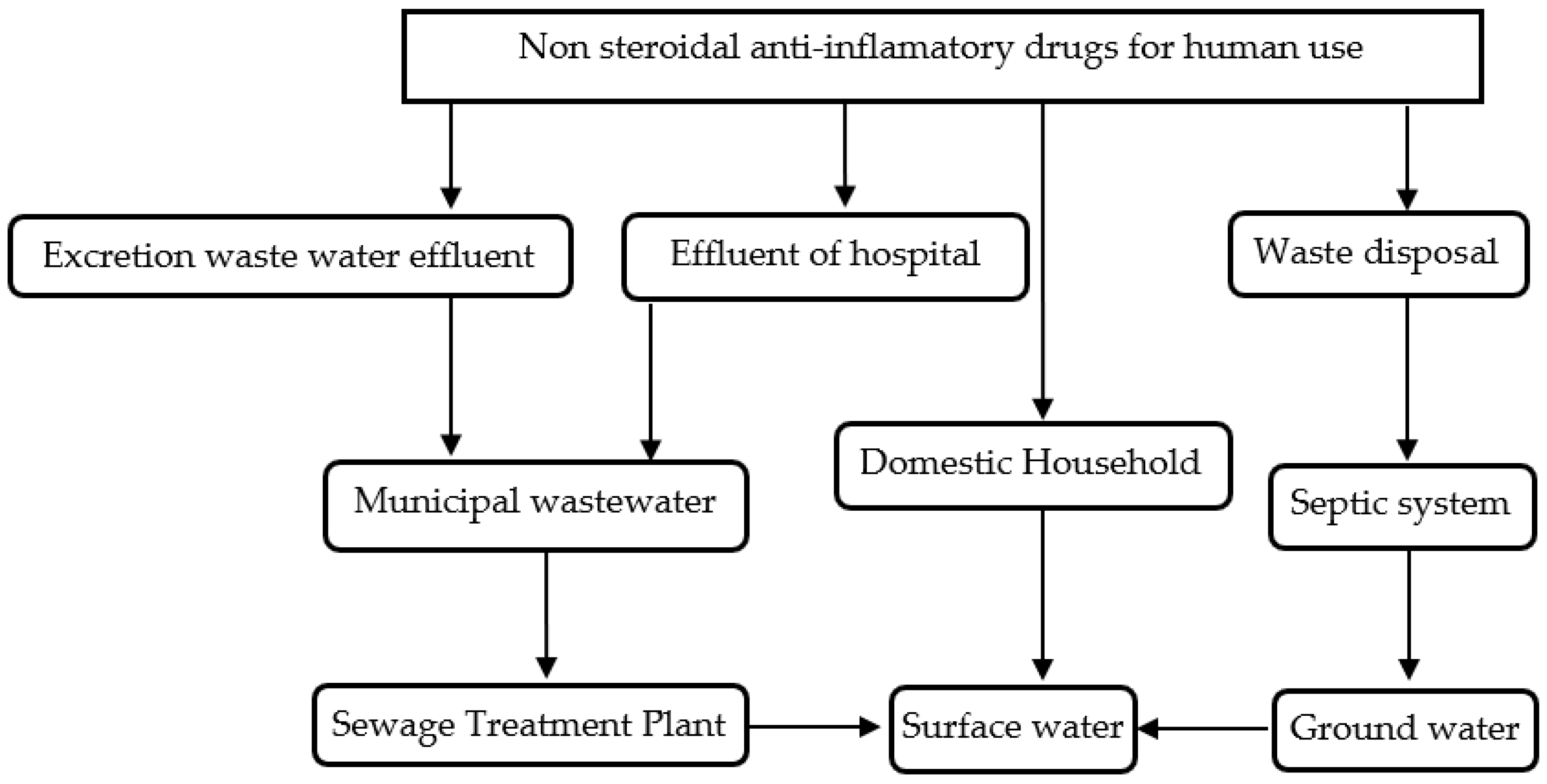
1. Introduction
2. The Exhibited Literature Review on NSAIDs
2.1. NSAIDs Discussed in This Review
2.2. Occurrence of NSAIDs in Surface Water, Influent, and Effluent Wastewater Samples
2.3. Evaluation of Wastewater Treatment Plants on Removal Efficacy of NSAIDs
2.4. Electrochemical Advanced Oxidation Processes
2.5. Application of Anodic Electrochemical Oxidation Processes
| Compound | Anode Material | Removal% | Main Byproducts | Observations | References |
|---|---|---|---|---|---|
| Diclofenac | BDD | 100% (TOC) 100% (DIC) |
|
| [98] |
| Graphite felt | 88% | NS |
| [102] | |
| BDD disk | 100% (DIC) 100% (TOC) | NS |
| [117] | |
| Graphite-PVC | 100% (DIC) | Several byproducts were generated in both positive and negative ionization modes, so we presented the main byproducts only: C6H5OCl2; C11H8O4NCl3; C14H11O3NCl2; C13H8ONCl5 |
| [103] | |
| BDD thin-film | 100% (DIC) | Several byproducts were generated, so we presented the main byproducts only: C14H11O3NCl2; C13H9O3NCl2; C13H9O2NCl2 |
| [110] | |
| Activated carbon fiber | 100% | Several byproducts were generated, so we presented the main byproducts only: C14H11O3NCl2; C13H11O2NCl2 C13H11ONCl2, C13H9ONCl2 |
| [111] | |
| ND-BDD | 72% (DIC) | Several byproducts were generated, so we presented the main byproducts only: C14H9O3NCl2; C15H13O2NCl2 C14H10O2NCl3 |
| [118] | |
| BDD | 72% (TOC) | Several chlorinated and non-chlorinated byproducts were formed, such as: 2,6 -dichlorobenzeneamine; 1-(2,6-Dichlorocyclohexa-2,4-dienyl)indolin-2-one; 2,5-Dihydroxyl-benzeneacetic acid; 2.5-Dihydroxybenzyl alcohol; Oxalic acid; benzoic acid |
| [112] | |
| Ketoprofen | BDD | 100% (KTP) 36% (COD) | NS |
| [105] |
| Thin-film BDD | 45–100% (KTP) >90% (TOC) | Aromatic compounds: 3-acetylbenzophenone (C15H12O2) 3-hydroxyethyl benzophenone (C15H14O2) Benzophenone (C13H10O) 3-ethyl benzophenone (C15H14O) Carboxylic acid: Ten compounds, most of them are formic acid, acetic acid, malic acid, oxalic acid, and so on. |
| [104] | |
| Thin-film BDD | 100% (TOC) | NS |
| [100] | |
| Ibuprofen | Black carbon | 60% | NS |
| [119] |
| BDD | 70–90% (TOC) | -different organic compounds were produced (data not shown). -carboxylic compounds: Maleic acid; oxamic acid; acetic acid; formic acid |
| [120] | |
| BDD | 60–95% (COD) 48–92% (TOC) | One compound has been reported: [2-(4-carboxycarbonyl)phenyl] propanoic acid (C11H10O5) |
| [112] | |
| BDD CNT GC | BDD = 50% CNT = 75 GC = 45 | NS |
| [113] | |
| Thin-film BDD | >95% 91–96% (TOC) | Aromatic compounds: P-benzoquinone 4-isobutyhlphenol, 4-isobuthylacetophenone Carboxylic acid: Oxalic acid, glyoxylic acid, formic acid, acetic acid, and pyruvic acid |
| [106] | |
| Naproxen | MWCNTs-GCE | >80% (NPX) >60% (TOC) | Several byproducts were generated, so we presented the main byproducts only: C13H12O2; C13H14O2; C12H10O2; C12H10O3 |
| [121] |
3. Possible Future Research Work
- -
- Application the electrochemical oxidation process in waste treatment plants rather than conventional oxidation process.
- -
- Application electrochemical oxidation process on other therapeutic classes such as beta-blockers, lipid modifying agents, diabetics, and so on which is ready for human consumption.
- -
- More environmental monitoring studies are required to give clear picture on the occurrence and the fate of NSAIDs in influent and effluent hospital, and leachate.
- -
- Study the ecotoxicological risks for algae, crustaceans and fish based on detected concentrations of NSAIDs contaminants in surface water and other wastewater samples.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feng, L.; van Hullebusch, E.D.; Rodrigo, M.A.; Esposito, G.; Oturan, M.A. Removal of Residual Anti-Inflammatory and Analgesic Pharmaceuticals from Aqueous Systems by Electrochemical Advanced Oxidation Processes: A Review. Chem. Eng. J. 2013, 228, 944–964. [Google Scholar] [CrossRef]
- Nikolaou, A.; Meric, S.; Fatta, D. Occurrence Patterns of Pharmaceuticals in Water and Wastewater Environments. Anal. Bioanal. Chem. 2007, 387, 1225–1234. [Google Scholar] [CrossRef] [PubMed]
- Hashim, N.; Yuzir, A.; Al-Qaim, F.F.; Yahaya, N.K.E.M. Occurrence and Distribution of 17 Targeted Human Pharmaceuticals in Various Aquatic Environmental Matrices in Southeast Asia with Particular Reference to Malaysia: A Comprehensive Review. J. Mex. Chem. Soc. 2021, 65, 434–456. [Google Scholar] [CrossRef]
- Rastogi, A.; Tiwari, M.K.; Ghangrekar, M.M. A Review on Environmental Occurrence, Toxicity and Microbial Degradation of Non-Steroidal Anti-Inflammatory Drugs (NSAIDs). J. Environ. Manag. 2021, 300, 113694. [Google Scholar] [CrossRef]
- Korashy, M.A.R.; Gawad, S.A.A.; Hassan, N.Y.; AbdelKawy, M. Solid Phase Extraction and Simultaneous Chromatographic Quantification of Some Non-Steroidal Anti-Inflammatory Drug Residues; an Application in Pharmaceutical Industrial Wastewater Effluent. Braz. J. Pharm. Sci. 2022, 58. [Google Scholar] [CrossRef]
- Hanafiah, Z.M.; Mohtar, W.H.M.W.; Abd Manan, T.S.B.; Bachi, N.A.; Abdullah, N.A.; Abd Hamid, H.H.; Beddu, S.; Kamal, N.L.M.; Ahmad, A.; Rasdi, N.W. The Occurrence of Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) in Malaysian Urban Domestic Wastewater. Chemosphere 2022, 287, 132134. [Google Scholar] [CrossRef]
- Al-Qaim, F.F.; Abdullah, M.P.; Othman, M.R.; Latip, J.; Afiq, W. A Validation Method Development for Simultaneous LC-ESI-TOF/MS Analysis of Some Pharmaceuticals in Tangkas River-Malaysia. J. Braz. Chem. Soc. 2014, 25, 271–281. [Google Scholar] [CrossRef]
- Al-Qaim, F.F.; Abdullah, M.P.; Othman, M.R.; Latip, J.; Zakaria, Z. Multi-Residue Analytical Methodology-Based Liquid Chromatography-Time-of-Flight-Mass Spectrometry for the Analysis of Pharmaceutical Residues in Surface Water and Effluents from Sewage Treatment Plants and Hospitals. J. Chromatogr. A 2014, 1345, 139–153. [Google Scholar] [CrossRef]
- Scheytt, T.J.; Mersmann, P.; Heberer, T. Mobility of Pharmaceuticals Carbamazepine, Diclofenac, Ibuprofen, and Propyphenazone in Miscible-Displacement Experiments. J. Contam. Hydrol. 2006, 83, 53–69. [Google Scholar] [CrossRef]
- Ziylan, A.; Ince, N.H. The Occurrence and Fate of Anti-Inflammatory and Analgesic Pharmaceuticals in Sewage and Fresh Water: Treatability by Conventional and Non-Conventional Processes. J. Hazard. Mater. 2011, 187, 24–36. [Google Scholar] [CrossRef]
- Al-Qaim, F.F.; Mussa, Z.H.; Yuzir, A. Development and Validation of a Comprehensive Solid-Phase Extraction Method Followed by LC-TOF/MS for the Analysis of Eighteen Pharmaceuticals in Influent and Effluent of Sewage Treatment Plants. Anal. Bioanal. Chem. 2018, 410, 4829–4846. [Google Scholar] [CrossRef]
- Yan, J.; Lin, W.; Gao, Z.; Ren, Y. Use of Selected NSAIDs in Guangzhou and Other Cities in the World as Identified by Wastewater Analysis. Chemosphere 2021, 279, 130529. [Google Scholar] [CrossRef]
- Kosjek, T.; Heath, E.; Kompare, B. Removal of Pharmaceutical Residues in a Pilot Wastewater Treatment Plant. Anal. Bioanal. Chem. 2007, 387, 1379–1387. [Google Scholar] [CrossRef]
- Mompelat, S.; Le Bot, B.; Thomas, O. Occurrence and Fate of Pharmaceutical Products and By-Products, from Resource to Drinking Water. Environ. Int. 2009, 35, 803–814. [Google Scholar] [CrossRef]
- Fatta, D.; Achilleos, A.; Nikolaou, A.; Meric, S. Analytical Methods for Tracing Pharmaceutical Residues in Water and Wastewater. TrAC Trends Anal. Chem. 2007, 26, 515–533. [Google Scholar] [CrossRef]
- Heberer, T. Occurrence, Fate, and Removal of Pharmaceutical Residues in the Aquatic Environment: A Review of Recent Research Data. Toxicol. Lett. 2002, 131, 5–17. [Google Scholar] [CrossRef]
- Zhang, Y.; Geißen, S.-U.; Gal, C. Carbamazepine and Diclofenac: Removal in Wastewater Treatment Plants and Occurrence in Water Bodies. Chemosphere 2008, 73, 1151–1161. [Google Scholar] [CrossRef]
- Hua, W.; Bennett, E.R.; Letcher, R.J. Ozone Treatment and the Depletion of Detectable Pharmaceuticals and Atrazine Herbicide in Drinking Water Sourced from the Upper Detroit River, Ontario, Canada. Water Res. 2006, 40, 2259–2266. [Google Scholar] [CrossRef]
- Daughton, C.G.; Ternes, T.A. Pharmaceuticals and Personal Care Products in the Environment: Agents of Subtle Change? Environ. Health Perspect. 1999, 107, 907–938. [Google Scholar] [CrossRef]
- Jones, O.A.; Lester, J.N.; Voulvoulis, N. Pharmaceuticals: A Threat to Drinking Water? TRENDS Biotechnol. 2005, 23, 163–167. [Google Scholar] [CrossRef]
- Dietrich, D.R.; Webb, S.F.; Petry, T. Hot Spot Pollutants: Pharmaceuticals in the Environment. Toxicol. Lett. 2002, 131, 1–3. [Google Scholar] [CrossRef]
- Sebastine, I.M.; Wakeman, R.J. Consumption and Environmental Hazards of Pharmaceutical Substances in the UK. Process Saf. Environ. Prot. 2003, 81, 229–235. [Google Scholar] [CrossRef]
- Fent, K.; Weston, A.A.; Caminada, D. Erratum to “Ecotoxicology of Human Pharmaceuticals” [Aquatic Toxicology 76 (2006) 122–159]. Aquat. Toxicol. 2006, 2, 207. [Google Scholar] [CrossRef]
- Taggart, M.A.; Senacha, K.R.; Green, R.E.; Jhala, Y.V.; Raghavan, B.; Rahmani, A.R.; Cuthbert, R.; Pain, D.J.; Meharg, A.A. Diclofenac Residues in Carcasses of Domestic Ungulates Available to Vultures in India. Environ. Int. 2007, 33, 759–765. [Google Scholar] [CrossRef]
- Oaks, J.L.; Gilbert, M.; Virani, M.Z.; Watson, R.T.; Meteyer, C.U.; Rideout, B.A.; Shivaprasad, H.L.; Ahmed, S.; Iqbal Chaudhry, M.J.; Arshad, M. Diclofenac Residues as the Cause of Vulture Population Decline in Pakistan. Nature 2004, 427, 630–633. [Google Scholar] [CrossRef]
- Halling-Sørensen, B.; Nielsen, S.N.; Lanzky, P.F.; Ingerslev, F.; Lützhøft, H.C.H.; Jørgensen, S.E. Occurrence, Fate and Effects of Pharmaceutical Substances in the Environment-A Review. Chemosphere 1998, 36, 357–393. [Google Scholar] [CrossRef]
- Cleuvers, M. Mixture Toxicity of the Anti-Inflammatory Drugs Diclofenac, Ibuprofen, Naproxen, and Acetylsalicylic Acid. Ecotoxicol. Environ. Saf. 2004, 59, 309–315. [Google Scholar] [CrossRef]
- Schwaiger, J.; Ferling, H.; Mallow, U.; Wintermayr, H.; Negele, R.D. Toxic Effects of the Non-Steroidal Anti-Inflammatory Drug Diclofenac: Part I: Histopathological Alterations and Bioaccumulation in Rainbow Trout. Aquat. Toxicol. 2004, 68, 141–150. [Google Scholar] [CrossRef]
- Brozinski, J.-M.; Lahti, M.; Meierjohann, A.; Oikari, A.; Kronberg, L. The Anti-Inflammatory Drugs Diclofenac, Naproxen and Ibuprofen Are Found in the Bile of Wild Fish Caught Downstream of a Wastewater Treatment Plant. Environ. Sci. Technol. 2013, 47, 342–348. [Google Scholar] [CrossRef]
- Aissaoui, S.; Sifour, M.; Ouled-Haddar, H.; Benguedouar, L.; Lahouel, M. Toxicity Assessment of Diclofenac and Its Biodegradation Metabolites toward Mice. Toxicol. Environ. Health Sci. 2017, 9, 284–290. [Google Scholar] [CrossRef]
- Makuch, E.; Ossowicz-Rupniewska, P.; Klebeko, J.; Janus, E. Biodegradation of L-Valine Alkyl Ester Ibuprofenates by Bacterial Cultures. Materials 2021, 14, 3180. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Utrilla, J.; Sánchez-Polo, M.; Ferro-García, M.Á.; Prados-Joya, G.; Ocampo-Pérez, R. Pharmaceuticals as Emerging Contaminants and Their Removal from Water. A Review. Chemosphere 2013, 93, 1268–1287. [Google Scholar] [CrossRef] [PubMed]
- Al-Qaim, F.F.; Abdullah, M.P.; Othman, M.R.; Mussa, Z.H.; Zakaria, Z.; Latip, J.; Afiq, W.M. Investigation of the Environmental Transport of Human Pharmaceuticals to Surface Water: A Case Study of Persistence of Pharmaceuticals in Effluent of Sewage Treatment Plants and Hospitals in Malaysia. J. Braz. Chem. Soc. 2015, 26. [Google Scholar] [CrossRef]
- Petrie, B.; Camacho-Muñoz, D. Analysis, Fate and Toxicity of Chiral Non-Steroidal Anti-Inflammatory Drugs in Wastewaters and the Environment: A Review. Environ. Chem. Lett. 2021, 19, 43–75. [Google Scholar] [CrossRef]
- Madhavan, J.; Grieser, F.; Ashokkumar, M. Combined Advanced Oxidation Processes for the Synergistic Degradation of Ibuprofen in Aqueous Environments. J. Hazard. Mater. 2010, 178, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Loraine, G.A.; Pettigrove, M.E. Seasonal Variations in Concentrations of Pharmaceuticals and Personal Care Products in Drinking Water and Reclaimed Wastewater in Southern California. Environ. Sci. Technol. 2006, 40, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Roberts, P.H.; Thomas, K.V. The Occurrence of Selected Pharmaceuticals in Wastewater Effluent and Surface Waters of the Lower Tyne Catchment. Sci. Total Environ. 2006, 356, 143–153. [Google Scholar] [CrossRef]
- Yu, Z.; Peldszus, S.; Huck, P.M. Adsorption Characteristics of Selected Pharmaceuticals and an Endocrine Disrupting Compound—Naproxen, Carbamazepine and Nonylphenol—On Activated Carbon. Water Res. 2008, 42, 2873–2882. [Google Scholar] [CrossRef]
- Tauxe-Wuersch, A.; de Alencastro, L.F.; Grandjean, D.; Tarradellas, J. Occurrence of Several Acidic Drugs in Sewage Treatment Plants in Switzerland and Risk Assessment. Water Res. 2005, 39, 1761–1772. [Google Scholar] [CrossRef]
- Mussa, Z.H.; Othman, M.R.; Abdullah, M.P.; Nordin, N. Decolorization of Landfill Leachate Using Electrochemical Technique. Int. J. Chem. Sci. 2013, 11, 1636–1646. [Google Scholar]
- Sim, W.-J.; Lee, J.-W.; Lee, E.-S.; Shin, S.-K.; Hwang, S.-R.; Oh, J.-E. Occurrence and Distribution of Pharmaceuticals in Wastewater from Households, Livestock Farms, Hospitals and Pharmaceutical Manufactures. Chemosphere 2011, 82, 179–186. [Google Scholar] [CrossRef]
- Kot-Wasik, A.; Jakimska, A.; Śliwka-Kaszyńska, M. Occurrence and Seasonal Variations of 25 Pharmaceutical Residues in Wastewater and Drinking Water Treatment Plants. Environ. Monit. Assess. 2016, 188, 661. [Google Scholar] [CrossRef]
- Stülten, D.; Zühlke, S.; Lamshöft, M.; Spiteller, M. Occurrence of Diclofenac and Selected Metabolites in Sewage Effluents. Sci. Total Environ. 2008, 405, 310–316. [Google Scholar] [CrossRef]
- Scheurell, M.; Franke, S.; Shah, R.M.; Hühnerfuss, H. Occurrence of Diclofenac and Its Metabolites in Surface Water and Effluent Samples from Karachi, Pakistan. Chemosphere 2009, 77, 870–876. [Google Scholar] [CrossRef]
- Kallio, J.-M.; Lahti, M.; Oikari, A.; Kronberg, L. Metabolites of the Aquatic Pollutant Diclofenac in Fish Bile. Environ. Sci. Technol. 2010, 44, 7213–7219. [Google Scholar] [CrossRef]
- Ajibola, A.S.; Fawole, S.T.; Ajibola, F.O.; Adewuyi, G.O. Diclofenac and Ibuprofen Determination in Sewage Sludge Using a QuEChERS Approach: Occurrence and Ecological Risk Assessment in Three Nigerian Wastewater Treatment Plants. Bull. Environ. Contam. Toxicol. 2021, 106, 690–699. [Google Scholar] [CrossRef]
- Thalla, A.K.; Vannarath, A.S. Occurrence and Environmental Risks of Nonsteroidal Anti-Inflammatory Drugs in Urban Wastewater in the Southwest Monsoon Region of India. Environ. Monit. Assess. 2020, 192, 193. [Google Scholar] [CrossRef]
- Madikizela, L.M.; Ncube, S. Occurrence and Ecotoxicological Risk Assessment of Non-Steroidal Anti-Inflammatory Drugs in South African Aquatic Environment: What Is Known and the Missing Information? Chemosphere 2021, 280, 130688. [Google Scholar] [CrossRef]
- Eslami, A.; Amini, M.M.; Yazdanbakhsh, A.R.; Rastkari, N.; Mohseni-Bandpei, A.; Nasseri, S.; Piroti, E.; Asadi, A. Occurrence of Non-Steroidal Anti-Inflammatory Drugs in Tehran Source Water, Municipal and Hospital Wastewaters, and Their Ecotoxicological Risk Assessment. Environ. Monit. Assess. 2015, 187, 734. [Google Scholar] [CrossRef]
- Rodrigues, J.A.; Silva, S.; Cardoso, V.V.; Benoliel, M.J.; Cardoso, E.; Coelho, M.R.; Martins, A.; Almeida, C.M.M. Screening and Seasonal Behavior of Analgesics, Non-Steroidal Anti-Inflammatory Drugs, and Antibiotics in Two Urban Wastewater Treatment Plants. Environ. Manag. 2021, 68, 411–425. [Google Scholar] [CrossRef]
- Kermia, A.E.B.; Fouial-Djebbar, D.; Trari, M. Occurrence, Fate and Removal Efficiencies of Pharmaceuticals in Wastewater Treatment Plants (WWTPs) Discharging in the Coastal Environment of Algiers. Comptes Rendus Chim. 2016, 19, 963–970. [Google Scholar] [CrossRef]
- Vymazal, J.; Březinová, T.D.; Koželuh, M.; Kule, L. Occurrence and Removal of Pharmaceuticals in Four Full-Scale Constructed Wetlands in the Czech Republic—The First Year of Monitoring. Ecol. Eng. 2017, 98, 354–364. [Google Scholar] [CrossRef]
- Fang, T.-H.; Nan, F.-H.; Chin, T.-S.; Feng, H.-M. The Occurrence and Distribution of Pharmaceutical Compounds in the Effluents of a Major Sewage Treatment Plant in Northern Taiwan and the Receiving Coastal Waters. Mar. Pollut. Bull. 2012, 64, 1435–1444. [Google Scholar] [CrossRef]
- Perkons, I.; Rusko, J.; Zacs, D.; Bartkevics, V. Rapid Determination of Pharmaceuticals in Wastewater by Direct Infusion HRMS Using Target and Suspect Screening Analysis. Sci. Total Environ. 2021, 755, 142688. [Google Scholar] [CrossRef]
- Neale, P.A.; Branch, A.; Khan, S.J.; Leusch, F.D.L. Evaluating the Enantiospecific Differences of Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) Using an Ecotoxicity Bioassay Test Battery. Sci. Total Environ. 2019, 694, 133659. [Google Scholar] [CrossRef]
- Kwak, K.; Ji, K.; Kho, Y.; Kim, P.; Lee, J.; Ryu, J.; Choi, K. Chronic Toxicity and Endocrine Disruption of Naproxen in Freshwater Waterfleas and Fish, and Steroidogenic Alteration Using H295R Cell Assay. Chemosphere 2018, 204, 156–162. [Google Scholar] [CrossRef]
- Camacho-Muñoz, D.; Kasprzyk-Hordern, B. Multi-Residue Enantiomeric Analysis of Human and Veterinary Pharmaceuticals and Their Metabolites in Environmental Samples by Chiral Liquid Chromatography Coupled with Tandem Mass Spectrometry Detection. Anal. Bioanal. Chem. 2015, 407, 9085–9104. [Google Scholar] [CrossRef]
- Wang, F.; Wang, B.; Qu, H.; Zhao, W.; Duan, L.; Zhang, Y.; Zhou, Y.; Yu, G. The Influence of Nanoplastics on the Toxic Effects, Bioaccumulation, Biodegradation and Enantioselectivity of Ibuprofen in Freshwater Algae Chlorella Pyrenoidosa. Environ. Pollut. 2020, 263, 114593. [Google Scholar] [CrossRef]
- Suzuki, T.; Kosugi, Y.; Hosaka, M.; Nishimura, T.; Nakae, D. Occurrence and Behavior of the Chiral Anti-inflammatory Drug Naproxen in an Aquatic Environment. Environ. Toxicol. Chem. 2014, 33, 2671–2678. [Google Scholar] [CrossRef]
- Hashim, N.H.; Nghiem, L.D.; Stuetz, R.M.; Khan, S.J. Enantiospecific Fate of Ibuprofen, Ketoprofen and Naproxen in a Laboratory-Scale Membrane Bioreactor. Water Res. 2011, 45, 6249–6258. [Google Scholar] [CrossRef]
- Li, Q.; Wang, P.; Chen, L.; Gao, H.; Wu, L. Acute Toxicity and Histopathological Effects of Naproxen in Zebrafish (Danio rerio) Early Life Stages. Environ. Sci. Pollut. Res. 2016, 23, 18832–18841. [Google Scholar] [CrossRef] [PubMed]
- Chopra, S.; Kumar, D. Ibuprofen as an Emerging Organic Contaminant in Environment, Distribution and Remediation. Heliyon 2020, 6, e04087. [Google Scholar] [CrossRef] [PubMed]
- Ferrando-Climent, L.; Collado, N.; Buttiglieri, G.; Gros, M.; Rodriguez-Roda, I.; Rodriguez-Mozaz, S.; Barceló, D. Comprehensive Study of Ibuprofen and Its Metabolites in Activated Sludge Batch Experiments and Aquatic Environment. Sci. Total Environ. 2012, 438, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Selke, S.; Scheurell, M.; Shah, M.R.; Hühnerfuss, H. Identification and Enantioselective Gas Chromatographic Mass-Spectrometric Separation of O-Desmethylnaproxen, the Main Metabolite of the Drug Naproxen, as a New Environmental Contaminant. J. Chromatogr. A 2010, 1217, 419–423. [Google Scholar] [CrossRef]
- Zhu, S.; Chen, H.; Li, J. Sources, Distribution and Potential Risks of Pharmaceuticals and Personal Care Products in Qingshan Lake Basin, Eastern China. Ecotoxicol. Environ. Saf. 2013, 96, 154–159. [Google Scholar] [CrossRef]
- Padhye, L.P.; Yao, H.; Kung’u, F.T.; Huang, C.-H. Year-Long Evaluation on the Occurrence and Fate of Pharmaceuticals, Personal Care Products, and Endocrine Disrupting Chemicals in an Urban Drinking Water Treatment Plant. Water Res. 2014, 51, 266–276. [Google Scholar] [CrossRef]
- Dai, G.; Wang, B.; Huang, J.; Dong, R.; Deng, S.; Yu, G. Occurrence and Source Apportionment of Pharmaceuticals and Personal Care Products in the Beiyun River of Beijing, China. Chemosphere 2015, 119, 1033–1039. [Google Scholar] [CrossRef]
- Praveena, S.M.; Shaifuddin, S.N.M.; Sukiman, S.; Nasir, F.A.M.; Hanafi, Z.; Kamarudin, N.; Ismail, T.H.T.; Aris, A.Z. Pharmaceuticals Residues in Selected Tropical Surface Water Bodies from Selangor (Malaysia): Occurrence and Potential Risk Assessments. Sci. Total Environ. 2018, 642, 230–240. [Google Scholar] [CrossRef]
- Salerno, F.; Gaetano, V.; Gianni, T. Urbanization and Climate Change Impacts on Surface Water Quality: Enhancing the Resilience by Reducing Impervious Surfaces. Water Res. 2018, 144, 491–502. [Google Scholar] [CrossRef]
- Kummerová, M.; Zezulka, Š.; Babula, P.; Tříska, J. Possible Ecological Risk of Two Pharmaceuticals Diclofenac and Paracetamol Demonstrated on a Model Plant Lemna Minor. J. Hazard. Mater. 2016, 302, 351–361. [Google Scholar] [CrossRef]
- Lindqvist, N.; Tuhkanen, T.; Kronberg, L. Occurrence of Acidic Pharmaceuticals in Raw and Treated Sewages and in Receiving Waters. Water Res. 2005, 39, 2219–2228. [Google Scholar] [CrossRef]
- Santos, J.L.; Aparicio, I.; Alonso, E. Occurrence and Risk Assessment of Pharmaceutically Active Compounds in Wastewater Treatment Plants. A Case Study: Seville City (Spain). Environ. Int. 2007, 33, 596–601. [Google Scholar] [CrossRef]
- Rutere, C.; Knoop, K.; Posselt, M.; Ho, A.; Horn, M.A. Ibuprofen Degradation and Associated Bacterial Communities in Hyporheic Zone Sediments. Microorganisms 2020, 8, 1245. [Google Scholar] [CrossRef]
- Fernández-López, C.; Guillén-Navarro, J.M.; Padilla, J.J.; Parsons, J.R. Comparison of the Removal Efficiencies of Selected Pharmaceuticals in Wastewater Treatment Plants in the Region of Murcia, Spain. Ecol. Eng. 2016, 95, 811–816. [Google Scholar] [CrossRef]
- Madikizela, L.M.; Chimuka, L. Occurrence of Naproxen, Ibuprofen, and Diclofenac Residues in Wastewater and River Water of KwaZulu-Natal Province in South Africa. Environ. Monit. Assess. 2017, 189, 348. [Google Scholar] [CrossRef]
- Kanama, K.M.; Daso, A.P.; Mpenyana-Monyatsi, L.; Coetzee, M.A.A. Assessment of Pharmaceuticals, Personal Care Products, and Hormones in Wastewater Treatment Plants Receiving Inflows from Health Facilities in North West Province, South Africa. J. Toxicol. 2018, 2018, 3751930. [Google Scholar] [CrossRef]
- Aydin, S.; Aydin, M.E.; Ulvi, A. Monitoring the Release of Anti-Inflammatory and Analgesic Pharmaceuticals in the Receiving Environment. Environ. Sci. Pollut. Res. 2019, 26, 36887–36902. [Google Scholar] [CrossRef]
- Gómez, M.J.; Bueno, M.J.M.; Lacorte, S.; Fernández-Alba, A.R.; Agüera, A. Pilot Survey Monitoring Pharmaceuticals and Related Compounds in a Sewage Treatment Plant Located on the Mediterranean Coast. Chemosphere 2007, 66, 993–1002. [Google Scholar] [CrossRef]
- Sun, Q.; Li, M.; Ma, C.; Chen, X.; Xie, X.; Yu, C.-P. Seasonal and Spatial Variations of PPCP Occurrence, Removal and Mass Loading in Three Wastewater Treatment Plants Located in Different Urbanization Areas in Xiamen, China. Environ. Pollut. 2016, 208, 371–381. [Google Scholar] [CrossRef]
- Patrolecco, L.; Capri, S.; Ademollo, N. Occurrence of Selected Pharmaceuticals in the Principal Sewage Treatment Plants in Rome (Italy) and in the Receiving Surface Waters. Environ. Sci. Pollut. Res. 2015, 22, 5864–5876. [Google Scholar] [CrossRef]
- Martín, J.; Camacho-Muñoz, D.; Santos, J.L.; Aparicio, I.; Alonso, E. Occurrence of Pharmaceutical Compounds in Wastewater and Sludge from Wastewater Treatment Plants: Removal and Ecotoxicological Impact of Wastewater Discharges and Sludge Disposal. J. Hazard. Mater. 2012, 239, 40–47. [Google Scholar] [CrossRef]
- Paiga, P.; Santos, L.H.; Ramos, S.; Jorge, S.; Silva, J.G.; Delerue-Matos, C. Presence of Pharmaceuticals in the Lis River (Portugal): Sources, Fate and Seasonal Variation. Sci. Total Environ. 2016, 573, 164–177. [Google Scholar] [CrossRef]
- Hoque, M.E.; Cloutier, F.; Arcieri, C.; McInnes, M.; Sultana, T.; Murray, C.; Vanrolleghem, P.A.; Metcalfe, C.D. Removal of Selected Pharmaceuticals, Personal Care Products and Artificial Sweetener in an Aerated Sewage Lagoon. Sci. Total Environ. 2014, 487, 801–812. [Google Scholar] [CrossRef]
- Zunngu, S.S.; Madikizela, L.M.; Chimuka, L.; Mdluli, P.S. Synthesis and Application of a Molecularly Imprinted Polymer in the Solid-Phase Extraction of Ketoprofen from Wastewater. Comptes Rendus Chim. 2017, 20, 585–591. [Google Scholar] [CrossRef]
- Peng, J.; Wang, X.; Yin, F.; Xu, G. Characterizing the Removal Routes of Seven Pharmaceuticals in the Activated Sludge Process. Sci. Total Environ. 2019, 650, 2437–2445. [Google Scholar] [CrossRef] [PubMed]
- Jewell, K.S.; Falås, P.; Wick, A.; Joss, A.; Ternes, T.A. Transformation of Diclofenac in Hybrid Biofilm–Activated Sludge Processes. Water Res. 2016, 105, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Zwiener, C.; Frimmel, F.H. Short-Term Tests with a Pilot Sewage Plant and Biofilm Reactors for the Biological Degradation of the Pharmaceutical Compounds Clofibric Acid, Ibuprofen, and Diclofenac. Sci. Total Environ. 2003, 309, 201–211. [Google Scholar] [CrossRef]
- Ingabire, A.S. Post-Treatment of Municipal Wastewater Effluent: Effect on Organic Matter and Micropollutant Removal. Master’s Thesis, Ghent University, Ghent, Belgium, 2014. [Google Scholar]
- Metcalfe, C.D.; Koenig, B.G.; Bennie, D.T.; Servos, M.; Ternes, T.A.; Hirsch, R. Occurrence of Neutral and Acidic Drugs in the Effluents of Canadian Sewage Treatment Plants. Environ. Toxicol. Chem. Int. J. 2003, 22, 2872–2880. [Google Scholar] [CrossRef] [PubMed]
- Barrera-Díaz, C.; Linares-Hernández, I.; Roa-Morales, G.; Bilyeu, B.; Balderas-Hernández, P. Removal of Biorefractory Compounds in Industrial Wastewater by Chemical and Electrochemical Pretreatments. Ind. Eng. Chem. Res. 2009, 48, 1253–1258. [Google Scholar] [CrossRef]
- Sirés, I.; Brillas, E. Remediation of Water Pollution Caused by Pharmaceutical Residues Based on Electrochemical Separation and Degradation Technologies: A Review. Environ. Int. 2012, 40, 212–229. [Google Scholar] [CrossRef] [PubMed]
- Panizza, M.; Cerisola, G. Direct and Mediated Anodic Oxidation of Organic Pollutants. Chem. Rev. 2009, 109, 6541–6569. [Google Scholar] [CrossRef]
- Marselli, B.; Garcia-Gomez, J.; Michaud, P.-A.; Rodrigo, M.A.; Comninellis, C. Electrogeneration of Hydroxyl Radicals on Boron-Doped Diamond Electrodes. J. Electrochem. Soc. 2003, 150, D79. [Google Scholar] [CrossRef]
- Kapałka, A.; Fóti, G.; Comninellis, C. The Importance of Electrode Material in Environmental Electrochemistry: Formation and Reactivity of Free Hydroxyl Radicals on Boron-Doped Diamond Electrodes. Electrochim. Acta 2009, 54, 2018–2023. [Google Scholar] [CrossRef]
- Kapałka, A.; Fóti, G.; Comninellis, C. Investigations of Electrochemical Oxygen Transfer Reaction on Boron-Doped Diamond Electrodes. Electrochim. Acta 2007, 53, 1954–1961. [Google Scholar] [CrossRef]
- Waterston, K.; Wang, J.W.; Bejan, D.; Bunce, N.J. Electrochemical Waste Water Treatment: Electrooxidation of Acetaminophen. J. Appl. Electrochem. 2006, 36, 227–232. [Google Scholar] [CrossRef]
- Brillas, E.; Sirés, I.; Arias, C.; Cabot, P.L.; Centellas, F.; Rodríguez, R.M.; Garrido, J.A. Mineralization of Paracetamol in Aqueous Medium by Anodic Oxidation with a Boron-Doped Diamond Electrode. Chemosphere 2005, 58, 399–406. [Google Scholar] [CrossRef]
- Brillas, E.; Garcia-Segura, S.; Skoumal, M.; Arias, C. Electrochemical Incineration of Diclofenac in Neutral Aqueous Medium by Anodic Oxidation Using Pt and Boron-Doped Diamond Anodes. Chemosphere 2010, 79, 605–612. [Google Scholar] [CrossRef]
- Zhao, X.; Hou, Y.; Liu, H.; Qiang, Z.; Qu, J. Electro-Oxidation of Diclofenac at Boron Doped Diamond: Kinetics and Mechanism. Electrochim. Acta 2009, 54, 4172–4179. [Google Scholar] [CrossRef]
- Murugananthan, M.; Latha, S.S.; Raju, G.B.; Yoshihara, S. Anodic Oxidation of Ketoprofen—An Anti-Inflammatory Drug Using Boron Doped Diamond and Platinum Electrodes. J. Hazard. Mater. 2010, 180, 753–758. [Google Scholar] [CrossRef]
- Sánchez-Carretero, A.; Sáez, C.; Cañizares, P.; Rodrigo, M.A. Electrochemical Production of Perchlorates Using Conductive Diamond Electrolyses. Chem. Eng. J. 2011, 166, 710–714. [Google Scholar] [CrossRef]
- Pourzamani, H.; Mengelizadeh, N.; Hajizadeh, Y.; Mohammadi, H. Electrochemical Degradation of Diclofenac Using Three-Dimensional Electrode Reactor with Multi-Walled Carbon Nanotubes. Environ. Sci. Pollut. Res. 2018, 25, 24746–24763. [Google Scholar] [CrossRef]
- Mussa, Z.H.; Al-Qaim, F.F.; Othman, M.R.; Abdullah, M.P.; Latip, J.; Zakria, Z. Pseudo First Order Kinetics and Proposed Transformation Products Pathway for the Degradation of Diclofenac Using Graphite–PVC Composite as Anode. J. Taiwan Inst. Chem. Eng. 2017, 72, 37–44. [Google Scholar] [CrossRef]
- Feng, L.; Oturan, N.; van Hullebusch, E.D.; Esposito, G.; Oturan, M.A. Degradation of Anti-Inflammatory Drug Ketoprofen by Electro-Oxidation: Comparison of Electro-Fenton and Anodic Oxidation Processes. Environ. Sci. Pollut. Res. 2014, 21, 8406–8416. [Google Scholar] [CrossRef]
- Domínguez, J.R.; González, T.; Palo, P.; Sánchez-Martín, J. Anodic Oxidation of Ketoprofen on Boron-Doped Diamond (BDD) Electrodes. Role of Operative Parameters. Chem. Eng. J. 2010, 162, 1012–1018. [Google Scholar] [CrossRef]
- Ambuludi, S.L.; Panizza, M.; Oturan, N.; Özcan, A.; Oturan, M.A. Kinetic Behavior of Anti-Inflammatory Drug Ibuprofen in Aqueous Medium during Its Degradation by Electrochemical Advanced Oxidation. Environ. Sci. Pollut. Res. 2013, 20, 2381–2389. [Google Scholar] [CrossRef]
- Srinivasan, R.; Nambi, I.M. An Electro-Peroxone-Based Multi-Pronged Strategy for the Treatment of Ibuprofen and an Emerging Pharmaceutical Wastewater Using a Novel Graphene-Coated Nickel Foam Electrode. Chem. Eng. J. 2022, 450, 137618. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Yuan, S.; Li, Z.; Wang, B.; Huang, J.; Deng, S.; Yu, G. Degradation of the Anti-Inflammatory Drug Ibuprofen by Electro-Peroxone Process. Water Res. 2014, 63, 81–93. [Google Scholar] [CrossRef]
- Feng, L.; Song, W.; Oturan, N.; Karbasi, M.; van Hullebusch, E.D.; Esposito, G.; Giannakis, S.; Oturan, M.A. Electrochemical Oxidation of Naproxen in Aqueous Matrices: Elucidating the Intermediates’ Eco-Toxicity, by Assessing Its Degradation Pathways via Experimental and Density Functional Theory (DFT) Approaches. Chem. Eng. J. 2022, 451, 138483. [Google Scholar] [CrossRef]
- Heim, C.; Rajab, M.; Greco, G.; Grosse, S.; Drewes, J.E.; Letzel, T.; Helmreich, B. Fate of Diclofenac and Its Transformation and Inorganic By-Products in Different Water Matrices during Electrochemical Advanced Oxidation Process Using a Boron-Doped Diamond Electrode. Water 2020, 12, 1686. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhao, C.; Liang, J.; Shang, R.; Zhu, X.; Ding, L.; Deng, H.; Zheng, H.; Strathmann, T.J. Rapid Removal of Diclofenac in Aqueous Solution by Soluble Mn (III)(Aq) Generated in a Novel Electro-Activated Carbon Fiber-Permanganate (E-ACF-PM) Process. Water Res. 2019, 165, 114975. [Google Scholar] [CrossRef] [PubMed]
- Ciríaco, L.; Anjo, C.; Correia, J.; Pacheco, M.J.; Lopes, A. Electrochemical Degradation of Ibuprofen on Ti/Pt/PbO2 and Si/BDD Electrodes. Electrochim. Acta 2009, 54, 1464–1472. [Google Scholar] [CrossRef]
- Motoc, S.; Manea, F.; Pop, A.; Pode, R.; Teodosiu, C. Electrochemical Degradation of Pharmaceutical Effluents on Carbon-Based Electrodes. Environ. Eng. Manag. J. 2012, 11, 627–634. [Google Scholar]
- Mandal, P.; Dubey, B.K.; Gupta, A.K. Review on Landfill Leachate Treatment by Electrochemical Oxidation: Drawbacks, Challenges and Future Scope. Waste Manag. 2017, 69, 250–273. [Google Scholar] [CrossRef]
- Anglada, A.; Ortiz, D.; Urtiaga, A.M.; Ortiz, I. Electrochemical Oxidation of Landfill Leachates at Pilot Scale: Evaluation of Energy Needs. Water Sci. Technol. 2010, 61, 2211–2217. [Google Scholar] [CrossRef]
- Li, W.; Zhou, Q.; Hua, T. Removal of Organic Matter from Landfill Leachate by Advanced Oxidation Processes: A Review. Int. J. Chem. Eng. 2010, 2010, 270532. [Google Scholar] [CrossRef]
- Coria, G.; Nava, J.L.; Carreño, G. Electrooxidation of Diclofenac in Synthetic Pharmaceutical Wastewater Using an Electrochemical Reactor Equipped with a Boron Doped Diamond Electrode. J. Mex. Chem. Soc. 2014, 58, 303–308. [Google Scholar] [CrossRef]
- Qiu, H.; Fan, P.; Li, X.; Hou, G. Electrochemical Degradation of DCF by Boron-Doped Diamond Anode: Degradation Mechanism, Pathways and Influencing Factors. Water Sci. Technol. 2021, 84, 431–444. [Google Scholar] [CrossRef]
- Soltani, R.D.C.; Mashayekhi, M. Decomposition of Ibuprofen in Water via an Electrochemical Process with Nano-Sized Carbon Black-Coated Carbon Cloth as Oxygen-Permeable Cathode Integrated with Ultrasound. Chemosphere 2018, 194, 471–480. [Google Scholar] [CrossRef]
- Calzadilla, W.; Espinoza, L.C.; Diaz-Cruz, M.S.; Sunyer, A.; Aranda, M.; Peña-Farfal, C.; Salazar, R. Simultaneous Degradation of 30 Pharmaceuticals by Anodic Oxidation: Main Intermediaries and by-Products. Chemosphere 2021, 269, 128753. [Google Scholar] [CrossRef]
- Díaz, E.; Stożek, S.; Patiño, Y.; Ordóñez, S. Electrochemical Degradation of Naproxen from Water by Anodic Oxidation with Multiwall Carbon Nanotubes Glassy Carbon Electrode. Water Sci. Technol. 2019, 79, 480–488. [Google Scholar] [CrossRef]
- Fernandes, A.; Pacheco, M.J.; Ciríaco, L.; Lopes, A. Review on the Electrochemical Processes for the Treatment of Sanitary Landfill Leachates: Present and Future. Appl. Catal. B Environ. 2015, 176, 183–200. [Google Scholar] [CrossRef]
- Mollah, M.Y.A.; Schennach, R.; Parga, J.R.; Cocke, D.L. Electrocoagulation (EC)—Science and Applications. J. Hazard. Mater. 2001, 84, 29–41. [Google Scholar] [CrossRef]
- Aloui, F.; Fki, F.; Loukil, S.; Sayadi, S. Application of Combined Membrane Biological Reactor and Electro-Oxidation Processes for the Treatment of Landfill Leachates. Water Sci. Technol. 2009, 60, 605–614. [Google Scholar] [CrossRef]
- Feki, F.; Aloui, F.; Feki, M.; Sayadi, S. Electrochemical Oxidation Post-Treatment of Landfill Leachates Treated with Membrane Bioreactor. Chemosphere 2009, 75, 256–260. [Google Scholar] [CrossRef]
- Del Moro, G.; Prieto-Rodríguez, L.; de Sanctis, M.; Di Iaconi, C.; Malato, S.; Mascolo, G. Landfill Leachate Treatment: Comparison of Standalone Electrochemical Degradation and Combined with a Novel Biofilter. Chem. Eng. J. 2016, 288, 87–98. [Google Scholar] [CrossRef]
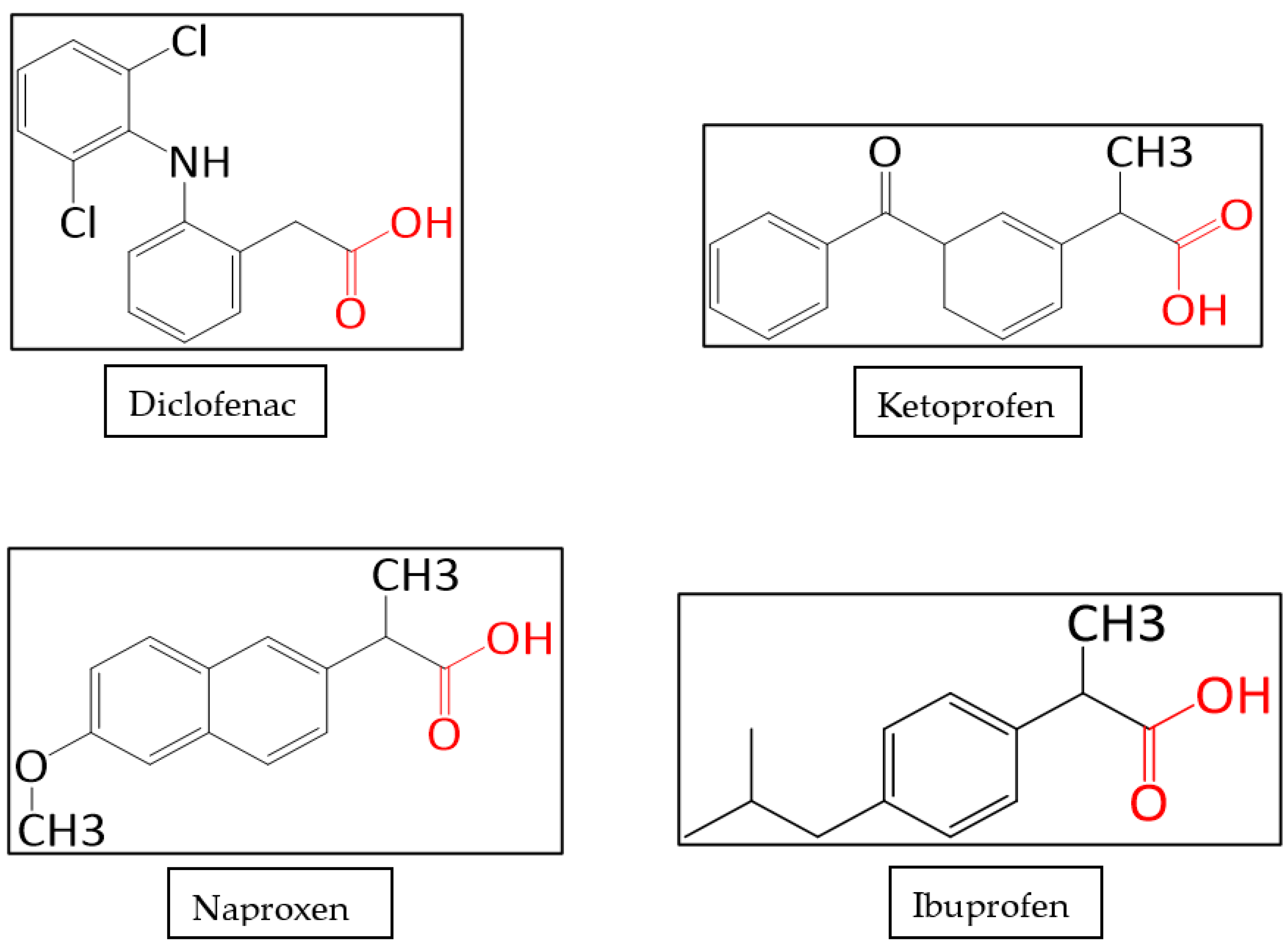
| Compound Name | Molecular Formula | pKa | LogKow | Molar Mass (g mol−1) | Solubility (mg L−1) | Excretion% | References |
|---|---|---|---|---|---|---|---|
| Diclofenac | C14H11Cl2NO2 | 4.18 | 4.02 | 296.1 | 4.82 | 40%-bile 60%-urine | [4] |
| Ketoprofen | C16H14O3 | 3.88 | 3.12 | 254.28 | 120.4 | NA | [34] |
| Naproxen | C14H14O3 | 4.19 | 3.10 | 230.26 | 144.9 | 95%-urine | [34] |
| Ibuprofen | C13H18O2 | 4.85 | 3.79 | 206.28 | 41.04 | 95%-urine | [34] |
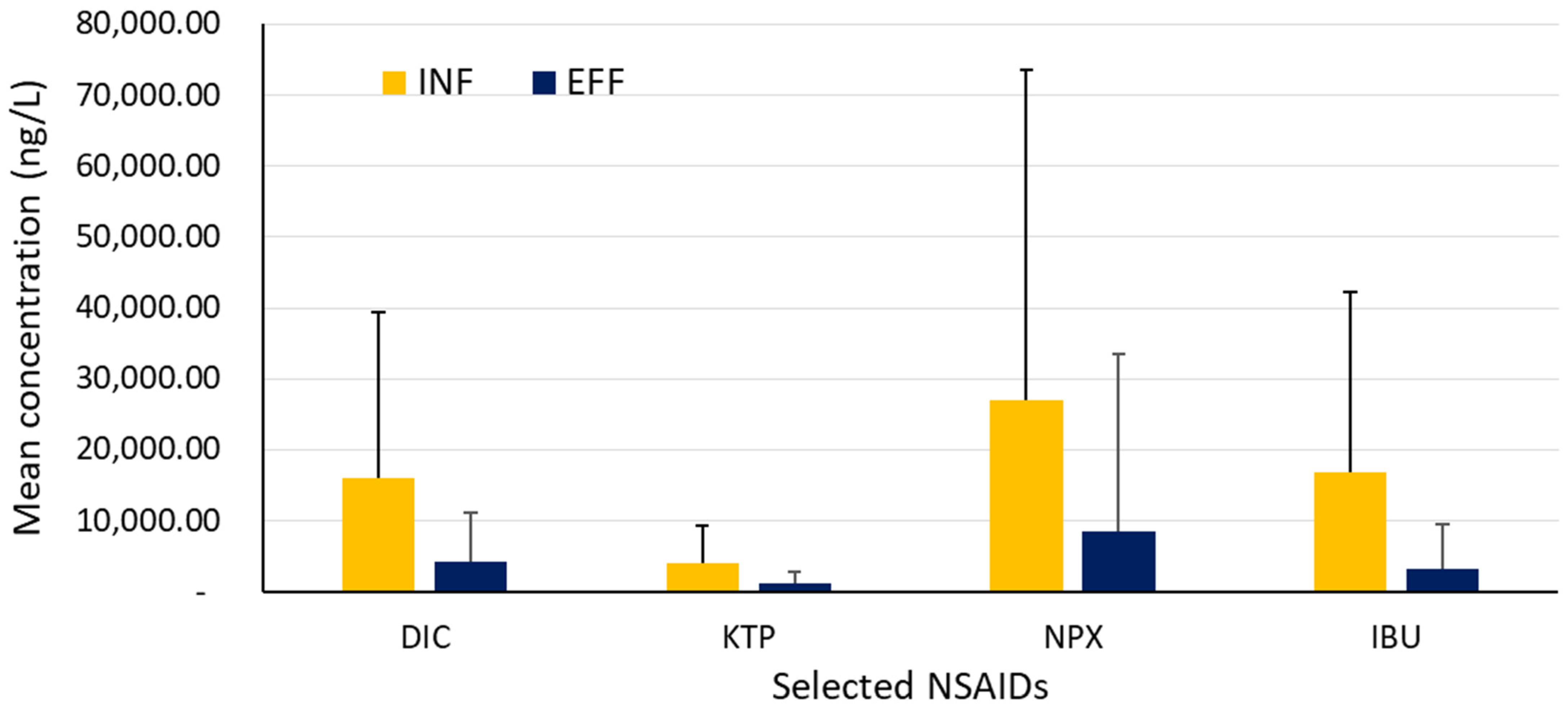
| Name of Compound | Drinking Water/Tap Water/Ground Water (ng L−1) | Surface Water (ngL−1) | Influent WWTP (ng L−1) | Effluent WWTP (ng L−1) | Country | References |
|---|---|---|---|---|---|---|
| Diclofenac | 0.184–380 a | NS | NS | NS | Spain | [4] |
| NS | NS | 250 | 215 | Spain | [32] | |
| NS | NS | NS | 500–1020 | Nigeria | [46] | |
| NS | NS | 1240–53,000 | <LOQ(1000)-15,000 | South Africa | [47] | |
| NS NS | ND-10,000 | 22,300 | 19,000 | South Africa | [48] | |
| NS | NS | 44–230 | 22–33 | Iran | [49] | |
| NS | NS | 2120–62,722 | 1720–1997 | Portugal | [50] | |
| NS | NS | 990–2319 | 1616–2711 | Algeria | [51] | |
| NS | NS | 12,000 | 2000 | Czech Republic | [52] | |
| NS | NS | 152–185 | 100–131 | Taiwan | [53] | |
| NS | NS | 27–3160 | 23–570 | Latvia | [54] | |
| 114 b | NS | 556–4001 | 743–5402 | Poland | [42] | |
| Naproxen | <3–17 a | NS | NS | NS | Nigeria | [4] |
| NS | NS | 99 | 108 | Spain | [32] | |
| NS | 23,800 | 159,000 | 91,100 | South Africa | [48] | |
| NS | NS | <LOQ(770)-37,000 | <LOQ(770)-4900 | South Africa | [47] | |
| NS | NS | 88–340 | 33–54 | Iran | [49] | |
| NS | NS | 11,044–13,093 | 25–41 | Portugal | [50] | |
| NS | NS | 1220–9585 | ND-334 | Algeria | [51] | |
| NS | 35 | 32,900 | 4120 | U.K. | [55] | |
| NS | 43 | NS | NS | China | [56] | |
| NS | <7 | 370 | <13 | U.K. | [57] | |
| NS | 136 | NS | 1330 | U.K. | [58] | |
| NS | 80 | 430 | 110 | Japan | [59] | |
| NS | NS | 67,600 | 161 | Australia | [60] | |
| NS | NS | 350–4280 | 306–961 | Latvia | [54] | |
| Ibuprofen | 0.16–988 a | NS | NS | NS | Spain | [4] |
| NS | NS | 516 | 266 | Spain | [32] | |
| NS | NS | NS | 600–6600 | Nigeria | [46] | |
| NS | 445–689 | 1060 | 1380 | South Africa | [48] | |
| NS | NS | <LOQ(3400)-72,000 | <LOQ(3400)-21,000 | South Africa | [47] | |
| NS | NS | 233–1051 | 31–45 | Iran | [49] | |
| NS | NS | 11,044–13,093 | 25–41 | Portugal | [50] | |
| NS | NS | 1607–8612 | 341–431 | Algeria | [51] | |
| NS | <9 | ND | 572 | U.K. | [55] | |
| NS | 325 | NS | NS | China | [56] | |
| NS | 75 | 390 | 75 | China | [61] | |
| NS | NS | 1643 | 210 | Spain | [27] | |
| NS | <236 | NS | 460 | U.K. | [58] | |
| NS | 121 | 179 | 7 | Australia | [60] | |
| NS | NS | 64,000 | 15,000 | Czech Republic | [52] | |
| NS | NS | 108–28,500 | 152–1070 | Latvia | [54] | |
| 5.7–224 b | NS | 4198–10,864 | 24–644 | Poland | [42] | |
| Ketoprofen | NS | NS | 451 | 318 | Spain | [32] |
| NS | Nd-437 | 3150 | 380 | South Africa | [48] | |
| NS | NS | 565 | 1035 | Algeria | [51] | |
| NS | NS | 39 | 200 | U.K. | [55] | |
| NS | NS | 510 | 177 | Spain | [27] | |
| NS | NS | 15,300 | 6 | Australia | [60] | |
| NS | NS | 6500 | 1000 | Czech Republic | [52] | |
| NS | NS | 377–9090 | 511–3730 | Latvia | [54] | |
| 13–167 b | NS | 73–322 | 1225–4030 | Poland | [42] |
| Compound | Type of Treatment | Removal% | Country | Observations | References |
|---|---|---|---|---|---|
| Diclofenac | CAS + UV/chlorine | 18–45 | Spain |
| [74] |
| (HSF CW) | 42 | Czech Republic |
| [52] | |
| NA | 4–88 | South Africa |
| [75] | |
| (GC) + (AT) + (SST) + (MP-SDU) | 80 | South Africa |
| [76] | |
| Chlorination | 81 | South Africa |
| [75] | |
| NA | 30 and (−174) | Algeria |
| [51] | |
| Primary treatment: (SGR + SD) Secondary treatment: (CAS + SD) Disinfection system: (UR) | 13–89 | Turkey |
| [77] | |
| (SR) + (CST) + (ASBT) | 39 | Spain |
| [78] | |
| WWTP1: (SD + BAF + UD) WWTP2: (SD + SOOD + UD) WWTP3: (SD + AA/O + SD + CD) | 18 | China |
| [79] | |
| (CMBT) | −41 | Poland |
| [42] | |
| (AST) | 38 | Italy |
| [80] | |
| (PS + AST) | −55 | Spain |
| [81] | |
| (CAS) | 83 | Portugal |
| [82] | |
| Ketoprofen | (SR) + Chlorination | 88–90 | South Africa |
| [83] |
| (MBR) | 100 |
| [74] | ||
| (HSF CW) | 52 | Czech Republic |
| [52] | |
| NA | −83 | Algeria |
| [51] | |
| 74–81 | Turkey |
| [77] | |
| (GC) + (AT) + (SST) + (MP-SDU) | 63 | South Africa |
| [76] | |
| (CMBT) | 94 | Poland |
| [42] | |
| 8 | China |
| [79] | |
| (PS + AST) | 88 | Spain |
| [81] | |
| (CAS) | −59 | Portugal |
| [82] | |
| Naproxen | (CAS + UV/chlorine) | 28–55 | Spain |
| [74] |
| (MBR) | 100 |
| [74] | ||
| NA | 16–87 | South Africa |
| [75] | |
| Chlorination | 95 | South Africa |
| [75] | |
| NA | 73 | Algeria |
| [51] | |
| 99–88 | Turkey |
| [77] | |
| (AST) | 47 | Italy |
| [80] | |
| (PS + AST) | 57 | Spain |
| [81] | |
| (CAS) | 86 | Portugal |
| [82] | |
| Ibuprofen | (HSF CW) | 56 | Czech Republic |
| [52] |
| NA | 25–83 | South Africa |
| [75] | |
| Chlorination | 95 | South Africa |
| [75] | |
| NA | 88 | Algeria |
| [51] | |
| 97–80 | Turkey |
| [77] | |
| (SR) + (CST) + (ASBT) | 83 | Spain |
| [78] | |
| (CMBT) | 98 | Poland |
| [42] | |
| WWTP1: (SD + BAF + UD) WWTP2: (SD + SOOD + UD) WWTP3: (SD + AA/O + SD + CD) | 83 | China |
| [79] | |
| (PS + AST) | 87 | Spain |
| [81] | |
| (CAS) | 90 | Portugal |
| [82] | |
| (AS) + (UD) | 17 | Canada |
| [84] | |
| (GC) + (AT) + (SST) + (MP-SDU) | 85 | South Africa |
| [76] | |
| (AST) | 63 | Italy |
| [80] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mussa, Z.H.; Al-Qaim, F.F.; Jawad, A.H.; Scholz, M.; Yaseen, Z.M. A Comprehensive Review for Removal of Non-Steroidal Anti-Inflammatory Drugs Attained from Wastewater Observations Using Carbon-Based Anodic Oxidation Process. Toxics 2022, 10, 598. https://doi.org/10.3390/toxics10100598
Mussa ZH, Al-Qaim FF, Jawad AH, Scholz M, Yaseen ZM. A Comprehensive Review for Removal of Non-Steroidal Anti-Inflammatory Drugs Attained from Wastewater Observations Using Carbon-Based Anodic Oxidation Process. Toxics. 2022; 10(10):598. https://doi.org/10.3390/toxics10100598
Chicago/Turabian StyleMussa, Zainab Haider, Fouad Fadhil Al-Qaim, Ali H. Jawad, Miklas Scholz, and Zaher Mundher Yaseen. 2022. "A Comprehensive Review for Removal of Non-Steroidal Anti-Inflammatory Drugs Attained from Wastewater Observations Using Carbon-Based Anodic Oxidation Process" Toxics 10, no. 10: 598. https://doi.org/10.3390/toxics10100598
APA StyleMussa, Z. H., Al-Qaim, F. F., Jawad, A. H., Scholz, M., & Yaseen, Z. M. (2022). A Comprehensive Review for Removal of Non-Steroidal Anti-Inflammatory Drugs Attained from Wastewater Observations Using Carbon-Based Anodic Oxidation Process. Toxics, 10(10), 598. https://doi.org/10.3390/toxics10100598











