Development of an LC-MS/MS Method for Non-Invasive Biomonitoring of Neonicotinoid and Systemic Herbicide Pesticide Residues in Bat Hair
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reference Substances, Chemicals, Solvents
2.2. County Pesticide Application Estimate—Selection of Pesticides
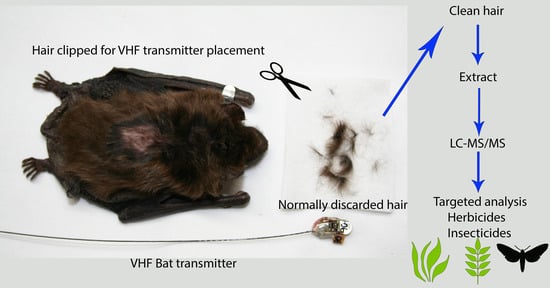
2.3. Sample Collection
2.4. Hair Sample Preparation
2.5. Hair Sample Cleanup
2.6. Optimization of MRM Transition Parameters
2.7. Pesticide Quantification by Liquid Chromatography Tandem Mass Spectrometry (LC-MS/MS)
2.8. Limit of Detection and Limit of Quantification
2.9. Recovery Assay
2.10. Matrix Effect
3. Results
3.1. Pesticide Agricultural Application in Missouri
3.2. Extraction and Clean-Up Assessment
3.3. Absolute Matrix Effects
3.4. Determination of Pesticides in Bat Hair
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Conenna, I.; Rocha, R.; Russo, D.; Cabeza, M. Insular bats and research effort: A review of global patterns and priorities. Mammal Rev. 2017, 47, 169–182. [Google Scholar] [CrossRef] [Green Version]
- Hutson, A.M.; Mickleburgh, S.P.; Racey, P.A. Microchiropteran Bats: Global Status Survey and Conservation Action Plan; IUCN: Cambridge, UK, 2001. [Google Scholar]
- O’Shea, T.J.; Cryan, P.M.; Hayman, D.T.S.; Plowright, R.K.; Streicker, D.G. Multiple mortality events in bats: A global review. Mammal Rev. 2016, 46, 175–190. [Google Scholar] [CrossRef]
- Kurta, A.; Hayes, J.P.; Lacki, M.J. Bats in Forests: Conservation and Management; John Hopkins University Press: Baltimore, MD, USA, 2001. [Google Scholar]
- Ghanem, S.J.; Voigt, C.C. Increasing awareness of ecosystem services provided by bats. In Advances in the Study of Behavior; Elsevier: Amsterdam, The Netherlands, 2012; Volume 44, pp. 279–302. [Google Scholar]
- Kunz, T.H.; Braun de Torrez, E.; Bauer, D.; Lobova, T.; Fleming, T.H. Ecosystem services provided by bats. Ann. N. Y. Acad. Sci. 2011, 1223, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Frick, W.F.; Kingston, T.; Flanders, J. A review of the major threats and challenges to global bat conservation. Ann. N. Y. Acad. Sci. 2020, 1469, 5–25. [Google Scholar] [CrossRef]
- Bayat, S.; Geiser, F.; Kristiansen, P.; Wilson, S.C. Organic contaminants in bats: Trends and new issues. Environ. Int. 2014, 63, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Williams-Guillén, K.; Olimpi, E.; Maas, B.; Taylor, P.J.; Arlettaz, R. Bats in the Anthropogenic Matrix: Challenges and Opportunities for the Conservation of Chiroptera and Their Ecosystem Services in Agricultural Landscapes. In Bats in the Anthropocene: Conservation of Bats in a Changing World; Voigt, C.C., Kingston, T., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 151–186. [Google Scholar] [CrossRef] [Green Version]
- United States Environmental Protection Agency. DDT-A Brief History and Status. Available online: https://www.epa.gov/ingredients-used-pesticide-products/ddt-brief-history-and-status: (accessed on 29 July 2021).
- Pollock, C.G. Silent Spring Revisited: A 21st-Century Look at the Effect of Pesticides on Wildlife. J. Avian Med. Surg. 2001, 15, 50–53. [Google Scholar] [CrossRef]
- Carson, R. Silent Spring, 40th ed.; Houghton Mifflin: Boston, MA, USA, 2002. [Google Scholar]
- Banaszkiewicz, T. Evolution of pesticide use. In Contemporary Problems of Management and Environmental Protection Influence of the Pesticide Dump on the Environment; Skibniewska, K.A., Ed.; University of Warmia and Mazury: Olszty, Poland, 2010; Volume 5, pp. 7–18. [Google Scholar]
- Sharma, A.; Kumar, V.; Shahzad, B.; Tanveer, M.; Sidhu, G.P.S.; Handa, N.; Kohli, S.K.; Yadav, P.; Bali, A.S.; Parihar, R.D.; et al. Worldwide pesticide usage and its impacts on ecosystem. SN Appl. Sci. 2019, 1, 1446. [Google Scholar] [CrossRef] [Green Version]
- Richmond, M.E. Glyphosate: A review of its global use, environmental impact, and potential health effects on humans and other species. J. Environ. Stud. Sci. 2018, 8, 416–434. [Google Scholar] [CrossRef]
- Hernout, B.V.; McClean, C.J.; Arnold, K.E.; Walls, M.; Baxter, M.; Boxall, A.B.A. Fur: A non-invasive approach to monitor metal exposure in bats. Chemosphere 2016, 147, 376–381. [Google Scholar] [CrossRef] [Green Version]
- Rashed, M.N.; Soltan, M.E. Animal Hair as Biological Indicator for Heavy Metal Pollution in Urban and Rural Areas. Environ. Monit. Assess. 2005, 110, 41–53. [Google Scholar] [CrossRef]
- O’Mara, M.T.; Wikelski, M.; Dechmann, D.K.N. 50 years of bat tracking: Device attachment and future directions. Methods Ecol. Evol. 2014, 5, 311–319. [Google Scholar] [CrossRef] [Green Version]
- Amelon, S.; Godsey, L. A Regional, Interactive, Economic Model for Assessing Ecosystem Services Provided by Bats. Bat Res. News 2012, 53, 62. [Google Scholar]
- Cooper, G.A.A.; Kronstrand, R.; Kintz, P. Society of Hair Testing guidelines for drug testing in hair. Forensic Sci. Int. 2012, 218, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Bishop, C.A.; Moran, A.J.; Toshack, M.C.; Elle, E.; Maisonneuve, F.; Elliott, J.E. Hummingbirds and bumble bees exposed to neonicotinoid and organophosphate insecticides in the Fraser Valley, British Columbia, Canada: Hummingbirds, bees, and neonicotinoids in Canada. Environ. Toxicol. Chem. 2018, 37, 2143–2152. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. ICH Q2 (R1) Validation of Analytical Procedures: Text and Methodology. Available online: https://www.ema.europa.eu/en/ich-q2-r1-validation-analytical-procedures-text-methodology (accessed on 1 August 2021).
- Matuszewski, B.K.; Constanzer, M.L.; Chavez-Eng, C.M. Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MS/MS. Anal. Chem. 2003, 75, 3019–3030. [Google Scholar] [CrossRef] [PubMed]
- Panuwet, P.; Hunter, R.E.; D’Souza, P.E.; Chen, X.; Radford, S.A.; Cohen, J.R.; Marder, M.E.; Kartavenka, K.; Ryan, P.B.; Barr, D.B. Biological Matrix Effects in Quantitative Tandem Mass Spectrometry-Based Analytical Methods: Advancing Biomonitoring. Crit. Rev. Anal. Chem. 2016, 46, 93–105. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Jerez, A.; Adriaanse, P.; Aldrich, A.; Berny, P.; Coja, T.; Duquesne, S.; Gimsing, A.L.; Marina, M.; Millet, M.; Pelkonen, O.; et al. Scientific statement on the coverage of bats by the current pesticide risk assessment for birds and mammals. EFSA J. 2019, 17, e05758. [Google Scholar] [CrossRef] [PubMed]
- Rodhouse, T.J.; Rodriguez, R.M.; Banner, K.M.; Ormsbee, P.C.; Barnett, J.; Irvine, K.M. Evidence of region-wide bat population decline from long-term monitoring and Bayesian occupancy models with empirically informed priors. Ecol. Evol. 2019, 9, 11078–11088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammerson, G.A.; Kling, M.; Harkness, M.; Ormes, M.; Young, B.E. Strong geographic and temporal patterns in conservation status of North American bats. Biol. Conserv. 2017, 212, 144–152. [Google Scholar] [CrossRef]
- World Health Organization; United Nations Environmental Programme. Public Health Impact of Pesticides Used in Agriculture. 1990. Available online: https://apps.who.int/iris/handle/10665/39772 (accessed on 1 August 2021).
- Appenzeller, B.M.R.; Tsatsakis, A.M. Hair analysis for biomonitoring of environmental and occupational exposure to organic pollutants: State of the art, critical review and future needs. Toxicol. Lett. 2012, 210, 119–140. [Google Scholar] [CrossRef]
- Lehmann, E.; Oltramare, C.; Dibié, J.-J.N.; Konaté, Y.; Alencastro, L.F.d. Assessment of human exposure to pesticides by hair analysis: The case of vegetable-producing areas in Burkina Faso. Environ. Int. 2018, 111, 317–331. [Google Scholar] [CrossRef]
- US Fish and Wildlife Service Range-Wide Indiana Bat Survey Guidelines. 2020. Available online: https://www.fws.gov/midwest/endangered/mammals/inba/surveys/pdf/FINAL%20Range-wide%20IBat%20Survey%20Guidelines%203.23.20.pdf (accessed on 5 March 2021).
- The Habitats Directive-Environment-European Commission. Available online: https://ec.europa.eu/environment/nature/legislation/habitatsdirective/index_en.htm (accessed on 6 September 2021).
- United States. The Endangered Species Act as Amended by Public Law 97-304 (the Endangered Species Act amendments of 1982); Congress GOV: Washington, DC, USA, 1983.
- United States Geologic Service. Pesticide National Synthesis Project Estimated Annual Agricultural Pesticide Use How the Estimates and Maps are Made. Available online: https://water.usgs.gov/nawqa/pnsp/usage/maps/about.php (accessed on 6 July 2021).
- United States Environmental Protection Agency. Search for Registered Pesticide Products. Available online: https://www.epa.gov/safepestcontrol/search-registered-pesticide-products (accessed on 1 September 2021).
- Alford, A.; Krupke, C.H. Translocation of the neonicotinoid seed treatment clothianidin in maize. PLoS ONE 2017, 12, e0173836. [Google Scholar] [CrossRef] [Green Version]
- Thompson, D.A.; Lehmler, H.-J.; Kolpin, D.W.; Hladik, M.L.; Vargo, J.D.; Schilling, K.E.; LeFevre, G.H.; Peeples, T.L.; Poch, M.C.; LaDuca, L.E.; et al. A critical review on the potential impacts of neonicotinoid insecticide use: Current knowledge of environmental fate, toxicity, and implications for human health. Environ. Sci. Process. Impacts 2020, 22, 1315–1346. [Google Scholar] [CrossRef]
- Hitaj, C.; Smith, D.J.; Code, A.; Wechsler, S.; Esker, P.D.; Douglas, M.R. Sowing Uncertainty: What We Do and Don’t Know about the Planting of Pesticide-Treated Seed. BioScience 2020, 70, 390–403. [Google Scholar] [CrossRef] [Green Version]
- Jeschke, P.; Nauen, R.; Schindler, M.; Elbert, A. Overview of the Status and Global Strategy for Neonicotinoids. J. Agric. Food Chem. 2011, 59, 2897–2908. [Google Scholar] [CrossRef] [PubMed]
- Bonmatin, J.-M.; Giorio, C.; Girolami, V.; Goulson, D.; Kreutzweiser, D.P.; Krupke, C.; Liess, M.; Long, E.; Marzaro, M.; Mitchell, E.A.D.; et al. Environmental fate and exposure; neonicotinoids and fipronil. Environ. Sci. Pollut. Res. Int. 2015, 22, 35–67. [Google Scholar] [CrossRef] [PubMed]
- Kanissery, R.; Gairhe, B.; Kadyampakeni, D.; Batuman, O.; Alferez, F. Glyphosate: Its Environmental Persistence and Impact on Crop Health and Nutrition. Plants 2019, 8, 499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Pesticide | Category | Class | Estimated Applied Amount (kg per km2) | Total National Estimated Use (million kgs) | ||
|---|---|---|---|---|---|---|
| Audrain | Platte | St. Louis | ||||
| 2,4-dichlorophenoxyacetic acid (2,4-D) | Herbicides | Systemic herbicide | 7.7–30.4 | 167.5–168.1 | 25.2–27.8 | 19.5–20.4 |
| Atrazine | Herbicides | Systemic herbicide | 272.3 | 326.3–27.1 | 54.9 | 32.7–33.6 |
| 3,6-Dichloro-2-methoxybenzoic acid (Dicamba) | Herbicides | Selective herbicide | 64.1–66.2 | 41.9–42.3 | 12.6–12.7 | 7.7–9.1 |
| Glyphosate | Herbicides | Systemic herbicide | 796.5–801.2 | 792.8–793.2 | 244.9–245.1 | 122.5–127.0 |
| Carbaryl | Insecticides | Carbamates | 0.2 | 0.4 | 0.08 | 0.3–0.7 |
| Clothianidin | Insecticides | Neonicotinoid | No estimated use | No estimated use | No estimated use | 0.05–0.09 |
| Imidacloprid | Insecticides | Neonicotinoid | 0–1.0 | 0.02 | 0.65 | 0.5–0.6 |
| Thiamethoxam | Insecticides | Neonicotinoid | 0–0.9 * | 0.002–0.91 * | 0.002–0.91 * | 0.09 to 0.11 |
| Category and Class of Analyte | Analyte | Mode ESI | Retention Time (min) | Precursor Ion (m/z) | Product Ions (1/2) | Cone (V) | EC (ev) | LOD (pg/mg) | LOQ (pg/mg) | Recovery Rate | Calibration Equation | R2 | CV | MEionization (%) (100 ppb) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Systemic herbicide | 2,4-dichlorophenoxyacetic acid (2,4-D) | Negative | 8.66 | 218.79 | 160.80 | 30 | 14 | 14.7 | 44.6 | 85.1% | y = 899,916x + 311.78 | 1.00 | 11.7% | 83.6% |
| Systemic herbicide | Atrazine | Positive | 9.54 | 216.04 | 216.10/173.90 | 55 | 18 | 2.1 | 6.4 | 101.5% | y = 8962.7x + 2265.8 | 0.999 | 4.3% | 101.1% |
| Systemic herbicide | 3,6-Dichloro-2-methoxybenzoic acid (Dicamba) | Negative | 7.08 | 219.00 | 175.00/145.00 | 20 | 10 | 17.5 | 53.1 | 76.8% | y = 177.04x + 630.89 | 0.999 | 13.2% | 69.9% |
| Systemic herbicide | Glyphosate | Positive | 7.37 | 171.23 | 125.14/111.02 | 30 | Tune | 5.7 | 17.4 | 70.8% | y = 2967.9ln(x) + 56,967 | 0.993 | 9.8% | 87.0% |
| Carbonate insecticide | Carbaryl | Positive | 9.34 | 202.04 | 144.9/127.09 | 25 | 12 | 0.12 | 0.36 | 121.0% | y = 14,260x + 1502.3 | 0.999 | 3.4% | 98.9% |
| Neonicotinoid insecticide | Clothianidin | Positive | 7.43 | 249.96 | 168.70/132.07 | 25 | 12 | 27.7 | 84.0 | 103.3% | y = 533.3x − 3515.4 | 0.993 | 6.9% | 96.9% |
| Neonicotinoid insecticide | Imidacloprid | Positive | 7.42 | 255.96 | 208.9/175.16 | 35 | 16 | 1.2 | 4.0 | 84.3% | y = 6 × 106x + 2562.3 | 1.00 | 1.1% | 96.7% |
| Neonicotinoid insecticide | Thiamethoxam | Positive | 6.96 | 291.96 | 210.8/181.13 | 30 | 12 | 0.5 | 1.6 | 120.5% | y = 5138x + 12.222 | 0.999 | 5.7% | 98.2% |
| Pesticide | Audrain Hair Pool (pg/mg) | Platte Hair Pool (pg/mg) | St. Louis Hair Pool (pg/mg) |
|---|---|---|---|
| 2,4-D | <LOQ | <LOQ | 431.9 |
| Atrazine | 83.3 | 40.5 | ND |
| Carbaryl | 41.4 | 216.7 | ND |
| Clothianidin | 1949.8 | ND | 841.2 |
| Dicamba | 1574.8 | <LOQ | ND |
| Glyphosate | 3580.8 | 4505.2 | <LOQ |
| Imidacloprid | 10.6 | 13.57 | <LOQ |
| Thiamethoxam | 45.5 | 46.28 | ND |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hooper, S.E.; Amelon, S.K.; Lin, C.-H. Development of an LC-MS/MS Method for Non-Invasive Biomonitoring of Neonicotinoid and Systemic Herbicide Pesticide Residues in Bat Hair. Toxics 2022, 10, 73. https://doi.org/10.3390/toxics10020073
Hooper SE, Amelon SK, Lin C-H. Development of an LC-MS/MS Method for Non-Invasive Biomonitoring of Neonicotinoid and Systemic Herbicide Pesticide Residues in Bat Hair. Toxics. 2022; 10(2):73. https://doi.org/10.3390/toxics10020073
Chicago/Turabian StyleHooper, Sarah E., Sybill K. Amelon, and Chung-Ho Lin. 2022. "Development of an LC-MS/MS Method for Non-Invasive Biomonitoring of Neonicotinoid and Systemic Herbicide Pesticide Residues in Bat Hair" Toxics 10, no. 2: 73. https://doi.org/10.3390/toxics10020073
APA StyleHooper, S. E., Amelon, S. K., & Lin, C.-H. (2022). Development of an LC-MS/MS Method for Non-Invasive Biomonitoring of Neonicotinoid and Systemic Herbicide Pesticide Residues in Bat Hair. Toxics, 10(2), 73. https://doi.org/10.3390/toxics10020073