White-Tailed Eagles’ (Haliaeetus albicilla) Exposure to Anticoagulant Rodenticides and Causes of Poisoning in Poland (2018–2020)
Abstract
:1. Introduction
2. Materials and Methods
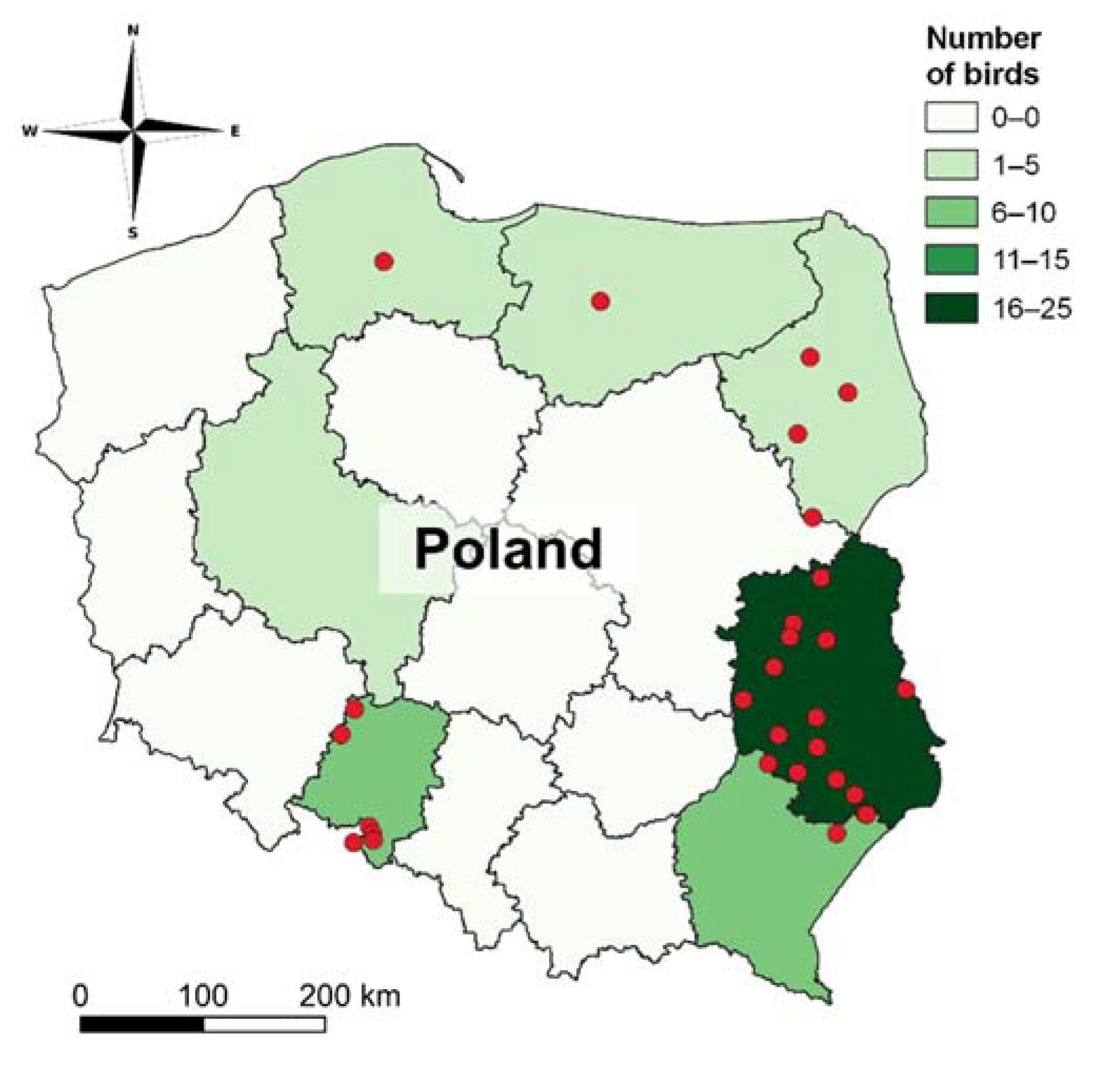
2.1. Samples Collection
2.2. Analytical Method
2.2.1. Sample Preparation
2.2.2. Liquid Chromatography–Mass Spectrometry Analysis of AR
2.2.3. Calibration Range, Recovery, and Limit of Quantification
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Stone, W.B.; Okoniewski, J.C.; Stedelin, J.R. Poisoning of Wildlife with Anticoagulant Rodenticides in New York. J. Wildl. Dis. 1999, 35, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Vandenbrouck, V.; Bousquet-Melou, A.; De Backer, P.; Croubels, S. Pharmacokinetics of Eight Anticoagulant Rodenticides in Mice after Single Oral Administration. J. Vet. Pharmacol. 2008, 31, 437. [Google Scholar] [CrossRef] [PubMed]
- Erickson, W.; Urban, D. Potential Risks of Nine Rodenticides to Birds and Nontarget Mammals: A Comparative Approach. United States Environmental Protection Agency Report; Office of Pesticides Programs Environmental Fate and Effects Division: Washington, DC, USA, 2004; pp. 1–230. [Google Scholar]
- Nakayama, S.M.M.; Morita, A.; Ikenaka, Y.; Mizukawa, H.; Ishizuka, M. A Review: Poisoning by Anticoagulant Rodenticides in Non-Target Animals Globally. J. Vet. Med. Sci. 2019, 81, 298–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herring, G.; Eagles-Smith, C.A.; Buck, J. Characterizing Golden Eagle Risk to Lead and Anticoagulant Rodenticide Exposure: A Review. J. Raptor Res. 2017, 51, 273–292. [Google Scholar] [CrossRef] [Green Version]
- Koivisto, S.; Laakso, S.; Suomalainen, K. Literature Review on Residues of Anticoagulant Rodenticides in Non-Target Animals; TemaNord; Nordic Council of Ministers: Copenhagen, Denmark, 2010; ISBN 978-92-893-3155-5. [Google Scholar]
- Thomas, P.J.; Mineau, P.; Shore, R.F.; Champoux, L.; Martin, P.A.; Wilson, L.K.; Fitzgerald, G.; Elliott, J.E. Second Generation Anticoagulant Rodenticides in Predatory Birds: Probabilistic Characterisation of Toxic Liver Concentrations and Implications for Predatory Bird Populations in Canada. Environ. Int. 2011, 37, 914–920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Barbudo, I.S.; Camarero, P.R.; Mateo, R. Primary and Secondary Poisoning by Anticoagulant Rodenticides of Non-Target Animals in Spain. Sci. Total Environ. 2012, 420, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Valverde, I.; Espín, S.; Gómez-Ramírez, P.; Navas, I.; María-Mojica, P.; Sánchez-Virosta, P.; Jiménez, P.; Torres-Chaparro, M.Y.; García-Fernández, A.J. Wildlife Poisoning: A Novel Scoring System and Review of Analytical Methods for Anticoagulant Rodenticide Determination. Ecotoxicology 2021, 30, 767–782. [Google Scholar] [CrossRef] [PubMed]
- Fisher, P. Persistence of Four Anticoagulant Rodenticides in the Livers of Laboratory Rats. DOC Sci. Intern. Ser. 2003, 139, 18. [Google Scholar]
- Koivisto, E.; Santangeli, A.; Koivisto, P.; Korkolainen, T.; Vuorisalo, T.; Hanski, I.K.; Loivamaa, I.; Koivisto, S. The Prevalence and Correlates of Anticoagulant Rodenticide Exposure in Non-Target Predators and Scavengers in Finland. Sci. Total Environ. 2018, 642, 701–707. [Google Scholar] [CrossRef]
- Vyas, N.B.; Kuncir, F.; Clinton, C.C. Influence of Poisoned Prey on Foraging Behavior of Ferruginous Hawks. Am. Midl. Nat. 2017, 177, 75–83. [Google Scholar] [CrossRef] [Green Version]
- Christensen, T.K.; Lassen, P.; Elmeros, M. High Exposure Rates of Anticoagulant Rodenticides in Predatory Bird Species in Intensively Managed Landscapes in Denmark. Arch Env. Contam Toxicol 2012, 63, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Walther, B.; Ennen, H.; Geduhn, A.; Schlötelburg, A.; Klemann, N.; Endepols, S.; Schenke, D.; Jacob, J. Effects of Anticoagulant Rodenticide Poisoning on Spatial Behavior of Farm Dwelling Norway Rats. Sci. Total Environ. 2021, 787, 147520. [Google Scholar] [CrossRef] [PubMed]
- Kelly, T.R.; Poppenga, R.H.; Woods, L.A.; Hernandez, Y.Z.; Boyce, W.M.; Samaniego, F.J.; Torres, S.G.; Johnson, C.K. Causes of Mortality and Unintentional Poisoning in Predatory and Scavenging Birds in California. Vet. Rec. Open 2014, 1, e000028. [Google Scholar] [CrossRef] [PubMed]
- Joermann, G. A Review of Secondary-Poisoning Studies with Rodenticides. EPPO Bull. 1998, 28, 157–176. [Google Scholar] [CrossRef]
- Hydock, K.L.; DeClementi, C.; Fish, P.H. Second-Generation Anticoagulant Rodenticide Poisoning in a Captive Andean Condor (Vultur Gryphus). J. Avian Med. Surg. 2017, 31, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Lohr, M.T. Anticoagulant Rodenticide Exposure in an Australian Predatory Bird Increases with Proximity to Developed Habitat. Sci. Total Environ. 2018, 643, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Ramírez, P.; Shore, R.F.; van den Brink, N.W.; van Hattum, B.; Bustnes, J.O.; Duke, G.; Fritsch, C.; García-Fernández, A.J.; Helander, B.O.; Jaspers, V.; et al. An Overview of Existing Raptor Contaminant Monitoring Activities in Europe. Environ. Int. 2014, 67, 12–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eason, C.T.; Murphy, E.C.; Wright, G.R.G.; Spurr, E.B. Assessment of Risks of Brodifacoum to Non-Target Birds and Mammals in New Zealand. Ecotoxicology 2002, 11, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Berny, P.J.; Buronfosse, T.; Buronfosse, F.; Lamarque, F.; Lorgue, G. Field Evidence of Secondary Poisoning of Foxes (Vulpes Vulpes) and Buzzards (Buteo Buteo) by Bromadiolone, a 4-Year Survey. Chemosphere 1997, 35, 1817–1829. [Google Scholar] [CrossRef]
- Howald, G.R.; Mineau, P.; Elliott, J.E.; Cheng, K.M. Brodifacoum Poisoning of Avian Scavengers During Rat Control on a Seabird Colony. Ecotoxicology 1999, 8, 431–447. [Google Scholar] [CrossRef]
- Stone, W.B.; Okoniewski, J.C.; Stedelin, J.R. Anticoagulant Rodenticides and Raptors: Recent Findings from New York, 1998–2001. Bull. Environ. Contam. Toxicol. 2003, 70, 0034–0040. [Google Scholar] [CrossRef] [PubMed]
- Dulsat-Masvidal, M.; Lourenço, R.; Lacorte, S.; D’Amico, M.; Albayrak, T.; Andevski, J.; Aradis, A.; Baltag, E.; Berger-Tal, O.; Berny, P.; et al. A Review of Constraints and Solutions for Collecting Raptor Samples and Contextual Data for a European Raptor Biomonitoring Facility. Sci. Total Environ. 2021, 793, 148599. [Google Scholar] [CrossRef] [PubMed]
- Rial-Berriel, C.; Acosta-Dacal, A.; Zumbado, M.; Henríquez-Hernández, L.A.; Rodríguez-Hernández, Á.; Macías-Montes, A.; Boada, L.D.; Travieso-Aja, M.d.M.; Martin-Cruz, B.; Suárez-Pérez, A.; et al. Epidemiology of Animal Poisonings in the Canary Islands (Spain) during the Period 2014–2021. Toxics 2021, 9, 267. [Google Scholar] [CrossRef] [PubMed]
- González, L.M.; Margalida, A.; Mañosa, S.; Sánchez, R.; Oria, J.; Molina, J.I.; Caldera, J.; Aranda, A.; Prada, L. Causes and Spatio-Temporal Variations of Non-Natural Mortality in the Vulnerable Spanish Imperial Eagle Aquila Adalberti during a Recovery Period. Oryx 2007, 41, 495–502. [Google Scholar] [CrossRef] [Green Version]
- Wobeser, G.; Bollinger, T.; Leighton, F.A.; Blakley, B.; Mineau, P. Secondary Poisoning of Eagles Following Intentional Poisoning of Coyotes with Anticholinesterase Pesticides in Western Canada. J. Wildl. Dis. 2004, 40, 163–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitowski, I.; Łopucki, R.; Stachniuk, A.; Fornal, E. A Pesticide Banned in the European Union over a Decade Ago Is Still Present in Raptors in Poland. Environ. Conserv. 2020, 47, 310–314. [Google Scholar] [CrossRef]
- Sell, B.; Sniegocki, T.; Zmudzki, J.; Posyniak, A. Development of an Analytical Procedure for the Determination of Multiclass Compounds for Forensic Veterinary Toxicology. J. Anal. Toxicol. 2018, 42, 183–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olszewski, A.; Matusiak, J.; Sea-eagle, W. Breeding biology and ecology of the White-tailed Sea-eagle Haliaeetus albicilla in the Kampinos National Park. Kulon 2020, 25, 69–91. [Google Scholar]
- Wardecki, Ł.; Chodkiewicz, T.; Beuch, S.; Smyk, B.; Sikora, A.; Neubauer, G.; Meissner, W.; Marchowski, D.; Wylegała, P.; Chylarecki, P. Monitoring Ptaków Polski w latach 2018–2021. Biul. Monit. Przyr. 2021, 22, 1–80. [Google Scholar]
- Zawadzki, G.; Zawadzka, D.; Sołtys, A.; Drozdowski, S. Nest-Site Selection by the White-Tailed Eagle and Black Stork—Implications for Conservation Practice. For. Ecosyst. 2020, 7, 59. [Google Scholar] [CrossRef]
- Ekblad, C.; Tikkanen, H.; Sulkava, S.; Laaksonen, T. Diet and Breeding Habitat Preferences of White-Tailed Eagles in a Northern Inland Environment. Polar Biol 2020, 43, 2071–2084. [Google Scholar] [CrossRef]
- Rached, A.; Moriceau, M.-A.; Serfaty, X.; Lefebvre, S.; Lattard, V. Biomarkers Potency to Monitor Non-Target Fauna Poisoning by Anticoagulant Rodenticides. Front. Vet. Sci. 2020, 7, 616276. [Google Scholar] [CrossRef] [PubMed]
- Badry, A.; Schenke, D.; Treu, G.; Krone, O. Linking Landscape Composition and Biological Factors with Exposure Levels of Rodenticides and Agrochemicals in Avian Apex Predators from Germany. Environ. Res. 2021, 193, 110602. [Google Scholar] [CrossRef] [PubMed]
- Tylkowska, A.; Pilarczyk, B.; Tomza-Marciniak, A.; Pilarczyk, R. The Prevalence of Intestinal Nematodes among Red Foxes (Vulpes Vulpes) in North-Western Poland. Acta Vet. Scand. 2021, 63, 19. [Google Scholar] [CrossRef] [PubMed]
- Berny, P. Pesticides and the Intoxication of Wild Animals. J. Vet. Pharmacol. Ther. 2007, 30, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Guitart, R.; Sachana, M.; Caloni, F.; Croubels, S.; Vandenbroucke, V.; Berny, P. Animal Poisoning in Europe. Part 3: Wildlife. Vet. J. 2010, 183, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.C. Carbofuran Toxicity. J. Toxicol. Environ. Health 1994, 43, 383–418. [Google Scholar] [CrossRef]
| Analyte | Precursor Ion (Da) | Product Ions (Da) | Declustering Potential (V) | Collision Energy (CE) |
|---|---|---|---|---|
| Bromadiolone | 525.0 | 181.0 | −262 | −47 |
| 250.0 | −47 | |||
| Brodifacoum | 521.1 | 135.1 | −120 | −48 |
| 143.1 | −80 | |||
| Chlorophacinone | 373.0 | 145.1 | −225 | −33 |
| 201.2 | −30 | |||
| Coumachlor | 340.9 | 160.9 | −120 | −30 |
| 284.0 | −34 | |||
| Coumatetralyl | 291.7 | 248.0 | −270 | −30 |
| 142.0 | −40 | |||
| Difenacoum | 443.0 | 135.0 | −254 | −45 |
| 143.0 | −75 | |||
| Difethialone | 539.1 | 151 | −90 | −50 |
| 143 | −99 | |||
| Diphacinone | 339.1 | 116.1 | −254 | −59 |
| 167.2 | −34 | |||
| Flocoumafen | 541.2 | 161.1 | −205 | −47 |
| 382.3 | −35 | |||
| Warfarin | 307.1 | 161.1 | −250 | −28 |
| 250.2 | −29 |
| Year | ID | Concentration (µg/kg, w.w.) | ||||
|---|---|---|---|---|---|---|
| Bromadiolone | Brodifacoum | Difenacoum | Flocoumafen | Sum of AR | ||
| 2018 | #1 | 11.1 | 1.5 | 9.2 | 1.1 | 22.9 |
| #2 1 | 358.4 | 35.4 | 393.8 | |||
| #3 | 79.5 | 14.2 | 93.7 | |||
| #4 1 | 53.0 | 199.3 | 252.3 | |||
| #5 | 2.5 | 2.5 | ||||
| #6 | 2.5 | 2.5 | ||||
| #7 | 143.2 | 35.8 | 179.0 | |||
| #8 | 78.1 | 16.4 | 94.5 | |||
| #9 | 10.0 | 21.4 | 31.4 | |||
| #10 | 6.4 | 12.7 | 19.1 | |||
| #11 | 18.2 | 20.1 | 1.8 | 40.1 | ||
| #12 | 69.0 | 172.0 | 241.0 | |||
| #13 1 | 170.0 | 59.6 | 7.6 | 7.6 | 244.8 | |
| 2019 | #14 | 19.4 | 61.0 | 80.4 | ||
| #15 1,2 | 362.0 | 53.6 | 415.6 | |||
| #16 | 57.2 | 90.6 | 147.8 | |||
| #17 | 36.7 | 68.6 | 105.3 | |||
| #18 | 83.1 | 89.1 | 172.2 | |||
| #19 1,2 | 88.2 | 128.2 | 3.3 | 219.7 | ||
| #20 1 | 132.7 | 33.9 | 5.4 | 172.0 | ||
| #21 | 60.7 | 2.2 | 62.9 | |||
| #22 | 11.6 | 145.0 | 156.6 | |||
| 2020 | #23 | 43.4 | 7.6 | 51.0 | ||
| #24 | 25.1 | 12.9 | 38.0 | |||
| #25 | 45.5 | 16.1 | 61.6 | |||
| #26 | 38.1 | 38.1 | ||||
| #27 | 78.1 | 56.3 | 134.4 | |||
| #28 | 12.7 | 44.8 | 57.5 | |||
| #29 1 | 260.0 | 27.1 | 287.1 | |||
| #30 1,2 | 903.0 | 19.1 | 922.1 | |||
| #31 | 77.3 | 71.3 | 148.6 | |||
| #32 | 72.7 | 63.3 | 136.0 | |||
| #33 | 24.6 | 42.5 | 67.1 | |||
| #34 1,2 | 802.0 | 423.0 | 1225.0 | |||
| #35 | 84.3 | 12.9 | 97.2 | |||
| #36 1 | 219.0 | 74.3 | 293.3 | |||
| #37 | 95.4 | 37.5 | 10.2 | 143.1 | ||
| #38 | 31.5 | 13.6 | 45.1 | |||
| #39 | 26.0 | 14.1 | 40.1 | |||
| #40 | 34.0 | 7.6 | 41.6 | |||
| Bromadiolone | Brodifacoum | Difenacoum | Flocoumafen | Sum of AR | |
|---|---|---|---|---|---|
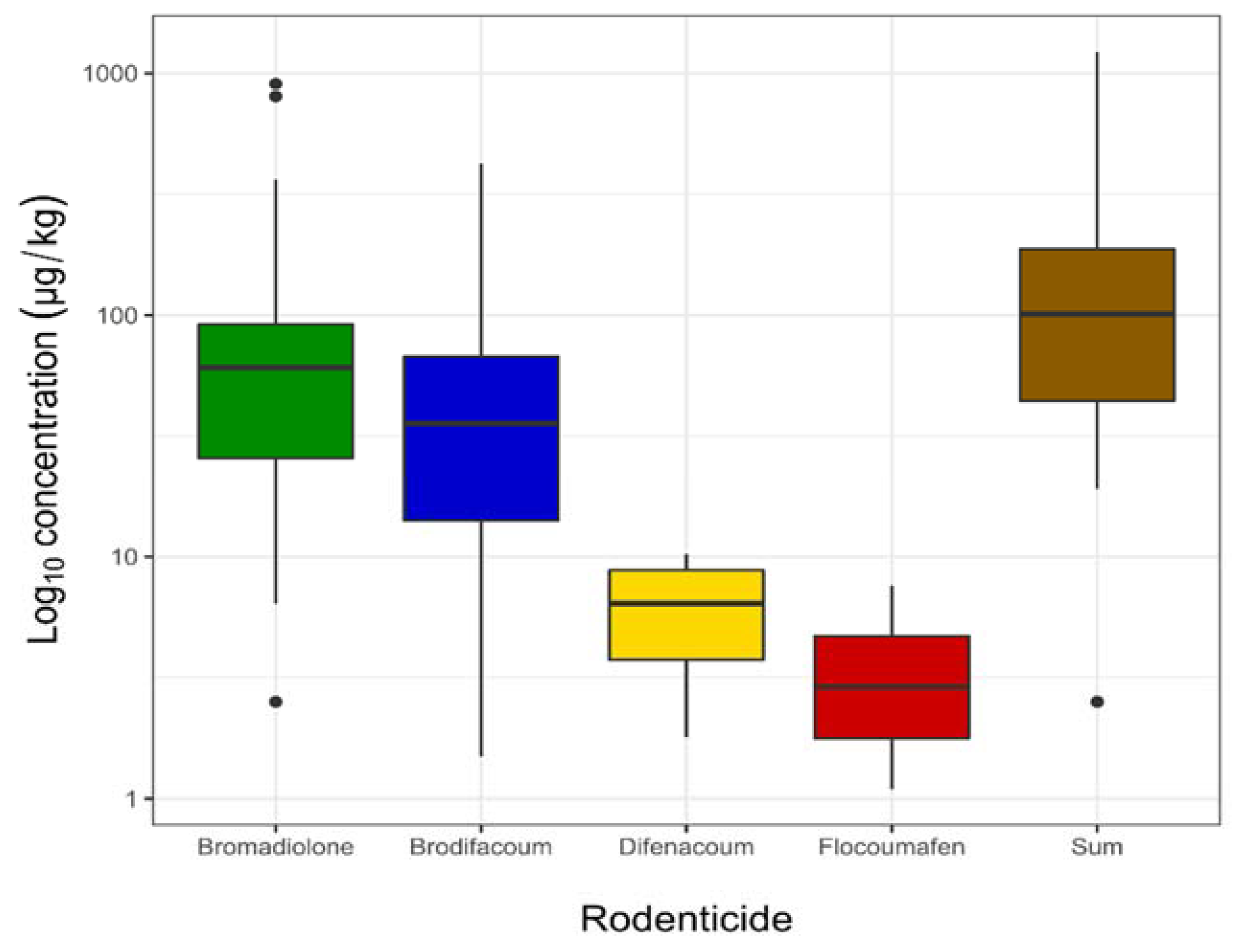
| Number of Cases | 39/40 | 38/40 | 6/40 | 2/40 | 40/40 |
| Concentration (µg/kg, w.w.) | |||||
| Maximum | 903.0 | 423.0 | 10.2 | 7.6 | 1225.0 |
| Minimum | 2.5 | 1.5 | 1.8 | 1.1 | 2.5 |
| Median | 60.7 | 35.6 | 6.5 | 4.4 | 101.3 |
| Mean | 121.1 | 58.1 | 6.2 | 4.4 | 174.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sell, B.; Śniegocki, T.; Giergiel, M.; Posyniak, A. White-Tailed Eagles’ (Haliaeetus albicilla) Exposure to Anticoagulant Rodenticides and Causes of Poisoning in Poland (2018–2020). Toxics 2022, 10, 63. https://doi.org/10.3390/toxics10020063
Sell B, Śniegocki T, Giergiel M, Posyniak A. White-Tailed Eagles’ (Haliaeetus albicilla) Exposure to Anticoagulant Rodenticides and Causes of Poisoning in Poland (2018–2020). Toxics. 2022; 10(2):63. https://doi.org/10.3390/toxics10020063
Chicago/Turabian StyleSell, Bartosz, Tomasz Śniegocki, Marta Giergiel, and Andrzej Posyniak. 2022. "White-Tailed Eagles’ (Haliaeetus albicilla) Exposure to Anticoagulant Rodenticides and Causes of Poisoning in Poland (2018–2020)" Toxics 10, no. 2: 63. https://doi.org/10.3390/toxics10020063
APA StyleSell, B., Śniegocki, T., Giergiel, M., & Posyniak, A. (2022). White-Tailed Eagles’ (Haliaeetus albicilla) Exposure to Anticoagulant Rodenticides and Causes of Poisoning in Poland (2018–2020). Toxics, 10(2), 63. https://doi.org/10.3390/toxics10020063






