Abstract
The Mongolian economy is supported by rich deposits of natural resources, such as copper, coal, and gold. However, the risk of heavy metal pollution to livestock and human have been recently discussed. This research collected various samples from soil and animal (sheep, goat, horse, cow, and camel), blood and organs (kidney and liver) in the Mongolian countryside. These samples were processed, and the concentration of metals was quantified using inductively coupled plasma-mass spectrometry (ICP/MS). As previously reported, arsenic was found at high levels of accumulation in soil. Selenium is another concern, as median concentration in one area exceeded the maximum allowable level. Cadmium and selenium were found to be highly accumulated in animal kidney. This research revealed the current pollution level in Mongolia based on evaluation of soil and animals. The concentration in animals could not indicate that animals had severe effects because of heavy metal exposure. However, kidney is eaten in Mongolia, and so there is a direct connection to human health, and this research suggested the possible risks posed by each edible animal. In particular, evaluation of metals in livestock is rare in Mongolia. This result can contribute to animal and human health in Mongolian communities.
1. Introduction
Mongolia is a landlocked country located between Russian and China, known for its rich diversity of natural resources, including gold, silver, copper, coal, fluoride, iron, petroleum oil, and uranium [1]. Traditionally, the main economic activity in Mongolia is based on agriculture, livestock, and the mineral industry. Mongolia’s economy has been expanding through exploitation of vast mineral resources during the past 20 years. The mining sector has supported economic growth and development in Mongolia, as mining recently contributed approximately 20% of gross domestic product (GDP) and about 80% of total exports in 2017 [2,3].
However, metal contamination derived from mining and industrial activity has been observed by many groups and has been reported worldwide [4,5,6,7]. For example, the area near mining site in Zambia recorded high concentration of metals including lead and cadmium, and geographic analysis revealed that the mining activity was possible source of pollution [4]. Heavy metals represent one of the main environmental pollutants that cause adverse effects to human and animal health. These effects, such as neurotoxicity, hepatoxicity, carcinogenicity, teratogenicity, and nephrotoxicity, have also been reported [8,9]. The International Agency for Research on Cancer (IARC) has classified heavy metals such as arsenic, cadmium and nickel as Group1 (carcinogenic), and lead and cobalt as Group2B (possibly carcinogenic). While metals affect human health, many reports have also indicated heavy metal toxicity in animals. For instance, cadmium is highly toxic, affecting nearly all animal systems. Arsenic shows various toxicities with gastrointestinal and nervous symptoms in cattle and induced weight loss, reduction of milk yield, anemia, and liver and kidney damage by chronic exposure [10]. Another consideration is that heavy metals cannot be degraded through physical processes. Therefore, they persist in the animal body and environment for a long period [11].
Environmental contamination and exposure of humans to metals are practical concerns in Mongolia. Contamination with metals usually results from many different sources, such as industrial waste, chemicals used in agriculture, construction, vehicular exhaust, coal and fossil fuel combustion, and mining [8,12,13]. In Ulaanbaatar, Mongolia, survey sampling in 2007 showed high arsenic contamination in soil. The possible source of this arsenic could be coal combustion and vehicles in the city [12]. Another study of samples collected in 2019 also showed high arsenic contamination in soil in Ulaanbaatar, and arsenic and chromium have been evaluated as metals with high ecological risk [14].
In recent years, environmental problems have gained attention in Mongolia. Economic losses because of metals through toxic effects on livestock and humans have also been found in other countries [15,16]. Concerning human health, various pathways through food, air, and water were considered as intake routes for metals, and animal products are one possible source of metals to humans [8,17]. Traditionally, Mongolians eat a lot of meats, and sheep meat is one of the main protein sources. The major livestock are sheep and cows for meat and milk in Mongolia. Mongolians eat not only muscle, but also liver, kidney, heart, etc. This unique food culture may increase the risk of metal contamination of animal products. As toxicity of metals in humans has been a concern in several countries, monitoring metals in grazing livestock in Mongolia is necessary in order to protect both animal health and human food safety. However, this kind of scientific research has not been pursued in detail in Mongolia at present. Therefore, monitoring environmental pollution that can cause toxicity to animals is desirable in Mongolia. Thus, this research targeted various livestock and soils in Mongolia, analyzing metal levels in collected samples.
2. Materials and Methods
2.1. Study Area
This study was conducted on animal farms in two areas: Dornogovi (Ulaanbadrakh: UB, Zuun-bayan: ZB and Airag: AE soum) and Tuv-Aimag (Zaamar soum). Dornogovi (44°53′N 110°09′E) is one of the 21 aimags (provinces) of Mongolia, located about 456 km southeast of the capital city, Ulaanbaatar. Dornogovi is known for its harsh weather conditions; it is located in the Gobi Desert, where frequent sand- and snowstorms occur. Tuv-aimag is another province of Mongolia, located about 45 km from the capital city. Many animal products, including milk and meat, are produced in both areas and distributed in Ulaanbaatar. Another notable product in Tuv-Aimag is gold from the mining located on the Tuul River. This area is still used for mining, and new mines are continuously being developed. Dornogovi also has mining areas, for fluorspar, uranium, etc. The soil and tissue samples were collected near mining areas. Details are shown in Figure 1.
Figure 1.
Sampling location. Sampling areas in Tuv-Aimag Zaamar, Dornogovi Airag, Zuun-bayan and Ulaanbadrakh were described by map using ArcGIS 10.7.1 (ESRI Co., Redlands, CA, USA).
2.2. Sample Collection
All samplings were done in June, 2016, in Mongolia. In total, 53 tissue samples (liver and kidney) were collected from various deceased animals (horse, goat, sheep, and cow) in Dornogovi province for metal analysis. In addition, 55 soil samples were collected from the same area. Approximately 50 g of soil from a depth of 0–5 cm was collected for each sample and kept in a plastic tube. At least three composite soil samples were collected from each sampling point. Approximately 150 blood samples were randomly collected from various animals (horse, goat, sheep, cow, and camel) in Dornogovi and Tuv-Aimag. Collected tissue and blood samples were stored in cooler boxes with dry ice during the sampling on-site, and all samples were transported and kept at 4 °C for soil and at −20 °C for blood and organs at the School of Veterinary Medicine, Mongolian University of Life Sciences, before being transported and analyzed for metal concentration at the School of Veterinary Medicine, Hokkaido University, Japan around 20 days after sample collection.
2.3. Sample Preparation and Metal Extraction
All laboratory materials and instruments used in metal extraction were washed in 2% nitric acid (HNO3) and oven-dried (50 °C). Metals were extracted from the samples (tissue, soil, and blood) by acid digestion with the method used in previous reports for tissue samples [18] and environment samples [19], with minor modifications.
Tissue samples (liver and kidney) were weighed (1 g) and dried for 48 h in an oven at 60 °C. Then, the dried tissue samples were digested using 5 mL 30% nitric acid (HNO3) and 1 mL 30% hydrogen peroxide (H2O2) (analytical grade, Kanto Chemical Corp. Japan) in a speed wave MWS-2 microwave oven system. Digestion of blood samples (1 mL) followed the same process as the tissue samples. The microwave system was programmed to ramp in five min to 160 °C (held for five min), followed by a ramp time of three min increased to 200 °C (held for 20 min), and then hold for 10 min at 75 °C. After digestion, samples were cooled and transferred to a 15 mL polypropylene tube followed by dilution to 10 mL with Milli Q water.
In the case of soil samples, extraction was performed using about 0.5 g of sample and 5 mL 60% HNO3, and 1 mL 30% H2O2. The microwave was programmed at an initial temperature of 150 °C (held for 5 min), then with a ramp time of 2 min increased to 175 °C (held for 10 min) and with a ramp time of three min increased to 190 °C (held for 30 min). After digestion, samples were filtered through ADVANTEC 5C filter paper into 15 mL polypropylene tubes and made up to 10 mL using Milli Q water. Analytical blanks were run for all samples.
Tissues, blood, and soil were assessed for concentrations of ten metals (Cr, Mn, Co, Ni, Cu, Zn, As, Se, Cd, and Pb) using an inductively coupled plasma –mass spectrometer (ICP-MS; 7700 series, Agilent Technologies, Tokyo, Japan) using 9Be, 115In, and 205Tl as internal standards.
2.4. Quality Control and Quality Assurance
All chemicals and standard stock solutions were analytical reagent grade (Wako Pure Chemicals, Osaka, Japan). For quality control, blanks were analyzed after every 10 samples, and a calibration curve for each element was constructed with an R2 value greater than 0.995. Analytical quality control was performed using certified reference materials: DOLT-4 (dogfish liver, National Research Council of Canada), DORM (fish protein, National Research Council of Canada, Ottawa, Canada), SRM-1944 (New/York/New Jersey Waterway Sediment, National Institute of Standards and Technology, New York, USA), and BCR-320 (channel sediment, Community Bureau of Reference of European Commission, Brussels, Belgium). Replicate analysis of these reference materials showed good recoveries, ranging from 90–110%.
2.5. Statistical Analysis
JMP 16 (SAS Institute, NC, USA) software was used for all statistical analyses, with a significance threshold of p < 0.05. The regional difference in soils was tested using Steel-Dwass tests. Outliers were detected assessing quantile range outliers (tail quantile 0.05, Q 10). Four metal concentrations in animal blood (from two individual outlier animals) were extracted. These two animals were also defined as the first and second biggest neighborhood distance calculated from K nearest neighbor outliers (K: 2–8, threshold was not decided). Thus, these two animals were excluded from the sample dataset. There was no outlier among soil samples, which were handled in the same way as animal samples.
3. Results
The metal concentration in the soil is shown in Table 1 and Supplementary Table S1. The Tuv-Aimag Zaamar region had generally higher concentrations of most metals; copper and chromium concentrations were significantly higher than in other areas (p < 0.05) (Supplementary Table S1). Table 1 shows the median concentration of metals in the highest-concentration area, the highest concentration in this study, and the regulations (such as the maximum allowable level in Mongolia, maximum permissible level summarized in [20] and Ecological Soil Screening Levels (Eco-SSLs) [21,22,23,24,25,26,27,28]). Eco-SSLs were provided from the U.S. Environmental Protection Agency (EPA). The Eco-SSLs suggest screening the level of contaminants in soil to indicate ecological risks. Contaminants exceeding their permissible concentration may require further evaluation for protection against ecological risk. The Eco-SSLs mention four different levels of concentration (plants, soil invertebrates, birds, and mammals); this study used its levels for mammals, as shown in Table 1. Chiroma T. M et al. (2014) summarized the maximum allowable limit using World Health Organization (WHO), Food and Agricultural Organization (FAO), and Ewers, U. (1991), Standard Guidelines in Europe. Some of the highest concentrations of target metals exceeded these maximum levels. In particular, arsenic and selenium concentrations were high, and some soil samples have exceeded these levels (Figure 2). For example, more than 50% of samples in Dornogovi AE exceeded the maximum permissible level of selenium (10 mg/kg) in Mongolia.

Table 1.
Metal concentration in soil in this study, previous study in Mongolia, and maximum allowable limits.
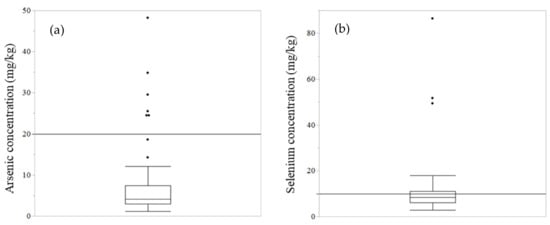
Figure 2.
Arsenic and selenium concentration in soil (all samples in this study). Arsenic (a) and selenium (b) concentration in all soil samples are shown in a box plot. The black line shows the maximum allowable limit, as regulated in Mongolia (arsenic: 20 mg/kg soil, selenium: 10 mg/kg soil).
Blood metal concentration is described in Table 2. Strong species differences were not observed for many metals in the same area. However, the camel accumulated a slightly higher concentration of zinc than the other animals in all areas. The median concentration of zinc in Dornogovi UB was 50.4 mg/kg (dry weight) in camels’ blood, while goats and sheep had blood levels of only 29.0 and 20.6 mg/kg, respectively. In the case of copper, the concentration in small ruminants, such as goat and sheep, was a bit higher than in other animals. The order of copper median concentration in Dornogovi ZB was goat > sheep > horse > camel > cow (Table 2). Comparing the regional difference showed that the Tuv-Aimag Zaamar region had a higher concentration of arsenic than other areas, as the soil concentration had already suggested. However, in the case of zinc, selenium, and lead, the median concentration in soil in Dornogovi UB was the lowest, but comparatively high concentrations of metals were detected in blood in that area, as the median zinc and lead concentration (29.0 and 0.1 mg/kg dry weight, respectively) was the highest in goat among all areas.

Table 2.
Metal concentration in blood.
To give a better understanding of the absolute concentrations of metals in livestock, Supplementary Table S2 summarizes the normal range of metals in animal organs, referring to published values [29]. The concentration in liver and kidney in sheep, goat, and cattle is shown in Table 3. The sample number of organs was not enough to determine regional difference. Thus, Table 3 shows mixed results from all areas (Dornogovi UB, ZB, AE). We also extracted the results of copper, zinc, and selenium in liver and kidney, as shown in Figure 3, with toxic and deficient levels described in a previous report [30]. The relatively high concentration of copper was also seen in sheep liver (median concentrations were 235.1, 92.4, and 148.3 mg/kg dry weight in sheep, goat, and cow, respectively). Arsenic concentration in organs was not as high compared with other high toxic metals, such as cadmium and lead (Table 3).

Table 3.
Metal concentration in liver (3a) and kidney (3b).

Figure 3.
Copper, zinc and selenium concentration in liver. Copper (a), zinc (b) and selenium (c) concentration in liver samples are shown in a box plot. The black line shows toxic and deficient level in (a) and (b), and marginally deficient level in (c). Deficient lines in (b) and (c) indicated 40 and 1.25 mg/kg although they had range 20—40 and 0.6—1.25 mg/kg respectively.
We additionally collected only two horse kidney samples, but concentrations of cadmium (122.65 and 352.79 mg/kg) in kidney were quite high in both individuals, compared with other animals, as median concentration values in sheep, goat, and cattle were 0.28, 1.02, and 3.78 mg/kg respectively (data not shown).
To summarize our results, each metal concentration was normalized using its sum and standard deviation of all the samples. Then, the mean of each normalized concentration, as shown in Supplementary Figure S1, was used to evaluate the accumulation pattern of each metal.
4. Discussion
4.1. Regulations Related to Heavy Metal and Overview of Results
Table 1 shows two previous studies conducted in Mongolia. The sampling location was different from this study. This study targeted the countryside, while previous reports focused on the city [12,14]. In the current study, notable accumulation was seen in arsenic and selenium, as their concentration in some samples exceeded both Mongolian regulations and Eco-SSLs. The Mongolian regulation level was almost the same as the maximum permissible level summarized in Chiroma T. M et al. (2014), except in the cases of chromium (manganese was not evaluated in the Mongolian regulation). Thus, we used Mongolian regulations to discuss the concentration of metals in soil, as these correspond better to local people in Mongolia. Firstly, the concentration of arsenic and selenium in soil was higher than Mongolian regulation in some samples, and these metals are highly toxic. Thus, Section 4.2 and Section 4.3 mentions arsenic and selenium concentrations in Mongolia and compared with previous studies. Section 4.4 focuses on copper and zinc, which are essential metals in animal body. The concentrations of cadmium and lead were not noticeable in the soil sample. However, these metals were known as one of the most toxic metals to animal body. Thus, we mentioned the toxicities of these metals in Section 4.5 with the current results. At the end, we discuss the possible sources of heavy metal contamination in livestock and humans in Section 4.6.
4.2. Arsenic Contamination in Mongolia
This study showed the same trend of high arsenic concentration in soil as the results reported in other studies [12,14]. Thus, arsenic pollution could be a problem all around Mongolia. Arsenic was one of main hazardous inorganic chemical in farm animals, and many toxicities in animals were reported. Daily administration of 20% of the acute lethal dose 50 (ALD50) of arsenic for 12 weeks to goats caused body weight loss, increased respiratory and heart rate, and animal death [31]. A study targeting farm animals in an arsenic-polluted area in India showed significantly higher liver markers, such as aspartate aminotransaminase (AST) and alanine aminotransaminase (ALT), lipid peroxides, and lower superoxide dismutase (SOD), which is one of difference mechanisms against oxidative damage, compared with a control area [32]. Oxidative stress derived from arsenic has been demonstrated using experimental rodents [33]. In this study, in spite of the high concentration of arsenic in soil, its contamination in blood and organs was less than that of other toxic metals. This result followed previous research conducted in Galicia, in which the majority of sheep samples did not accumulate arsenic, even in an arsenic-contaminated area [34]. The monitoring of only one environment (soil) and animals (blood and organs) can mask the risk to animals, as the accumulation patterns of metals and metalloids can be different, depending on the metals (Supplementary Figure S1). The lack of usefulness of blood arsenic concentration as a biomarker for long-term exposure to arsenic has already been reported; this is due to its earlier excretion from the blood compared to other metals and metalloids [35]. Thus, investigating both animals and environments would be beneficial to understand the effects of metals on livestock.
4.3. Selenium Contamination in Mongolia
This study revealed similar concentrations of metals to those described by a previous study conducted in Mongolia examining many metals; however, selenium concentration was high in soil in this study (Table 1). Selenium is distributed variably in the world, depending on geological conditions. Selenium is an essential element for both human and animal bodies, but there is a narrow margin between toxic and deficient levels [36]. For instance, high doses of selenium in lambs were associated with myocardial necrosis, pulmonary edema, and hemorrhage in one histopathological study [37]. An exposure experiment conducted using buffalo calves found that adverse effects and mortality were observed when blood concentrations of selenium became more than 2 and 3.4 µg/mL, respectively. That study investigated relatively acute toxicity during a 30-day observation [38]. Extrapolation to the current study should be done carefully, but the maximum selenium concentration found in this study was around 0.44 µg/mL. A previous review suggested that a selenium concentration between 1.25 and 2.5 mg/kg in the liver is adequate level for cattle, and between 2.3 and 8.0 mg/kg dry weight for newborn calves [30]. The median concentration in sheep and goat was around 2.0 mg/kg, which is an adequate level in liver in this study (Figure 3). Another review mentioned that selenium levels of more than 2 ppm in liver caused acute toxicity, and in many cases reported that sheep died due to acute/subacute intoxication [36]. We did not use the wet weight for calculation because we collected liver samples from the dead body, and conditions such as water content can vary depending on when and where animals have died. However, considering the normal water content in liver (around 3.5—4 times higher concentration in dry weight than that of wet weight [29]), most liver samples would have levels of selenium less than 2 ppm. Thus, even though the selenium concentration in soil exceeded Mongolian regulation levels, we cannot conclude that animals showed an intolerable toxic effect from selenium in Mongolia. However, in cows, the median concentration was higher than in small ruminants. The concentration in three out of four cows exceeded adequate levels. Monitoring selenium in cows is thus required.
4.4. Copper and Zinc Contamination in Mongolia
Copper and zinc are essential elements for the animal body [39]. Thus, the high absolute concentrations of these metals in animal blood, liver, and kidney in this study were reasonable. Susceptibility to copper is higher in sheep > cattle > monogastric animals [40]. The results indicate that susceptible animals were exposed to copper more frequently. The season and hemoglobin type differentiate copper concentrations in sheep. Thus, simple comparison was difficult, but the copper concentration in blood in the current study was relatively low compared with other previous studies [41]. In this area, copper deficiency might be a risk to animals, rather than intoxication. When focusing on liver concentration, previous review report summarized that the cutoff for deficient and adequate copper concentration in liver would be less than 33 and more than 125 mg/kg dry weight, respectively [30]. Most individuals collected in this study demonstrated more than a deficient level (Figure 3). The same report mentioned that copper at a level more than 1250 mg/kg is toxic in cattle liver, but no animal exceeded this value in this study. In the case of zinc, most samples ranged between adequate and high accumulation level. The toxic level (more than 1000 mg/kg in cattle) was not seen in the current dataset (Figure 3) [30] and the risk related to zinc would be relatively low in the current sampling area. Both copper and zinc had broad range of concentration from the lowest to the highest in samples. We did not have their ages data, and this is our limitation. For more proper diagnosis of deficiency, the sampling of liver and kidney from not only dead body but also from healthy slaughter animals with their enough information such as age are needed. Overall, considering the concentration of copper and zinc in blood and animal organs, the risk of intoxication was relatively low compared with arsenic and selenium.
However, zinc concentration in camel blood was high in two areas where we took camel samples. An exposure study in cows showed significant induction of the enzymes which have protective effects against oxidative stress such as SOD at around 2.5—3.0 ug/mL blood zinc concentration [42]. The current study revealed that some camels had more than 5.0 ug/mL of zinc in their blood (data not shown, Table 2 shows only dry weight). Camels are a unique animal living in a severe desert environment. The camel has a unique antioxidant defense system that uses copper; zinc superoxide dismutase was also found [43]. This mechanism allows a high concentration of zinc in camels. However, a previous report also showed that camels maintain a low plasma zinc concentration compared with cows, even when the two species ate similar feed containing multiple metals [44], contrary to the current study. Reports describing camels are fewer in number than those describing other livestock. Further study and measurement of blood parameters, including oxidative stress, in camel are needed to evaluate the effect of metals on camels in this area.
4.5. Cadmium and Lead Contamination in Mongolia
The median concentration of lead and cadmium in soil was less than levels allowed by Mongolian regulation. However, values exceeded Eco-SSLs in some samples. These metals are well known to have high toxicity to humans. The high concentration of cadmium in horses was derived from its high accumulative ability in these animals [45]. The previous report showed much higher concentration of cadmium in horses than in other farm animals, even from the same area [46]. The concentration in horse kidney in this study also incommensurably high compared with other animals in same area. This study has only two kidney samples in horse, but the result might provide hints for communities in Mongolia to carefully consider the use of horse kidneys as food. Itai-itai disease was a notorious incident resulting from cadmium pollution, causing severe symptoms in humans in Japan [47]. The cadmium concentration in soil was quite low in Mongolia, and most of samples had less than 0.1 mg/kg in this study. However, cadmium is highly accumulated in cow kidneys (median concentration: 3.8 mg/kg). Lead also induces severe symptoms in mammals. For instance, previous in vivo Pb exposure study on mice and environmental Pb exposure effect on human showed inhibited activity of d-aminolevulinic acid dehydratase (d-ALAD), a porphobilinogen synthase enzyme which is responsible for heme synthesis [48,49]. The activity of ALAD was estimated to be inhibited by lead with less than around 0.55 mg/kg dry weight in cattle [50]. When focusing on ALAD activity, the cattle in the current sampling area would not get a significant effect of lead as the highest median concentration in cattle was 0.15 mg/kg in blood in Tuv-Aimag Zaamar. However, in particular, cadmium and lead induce severe toxicity to mammals at low concentrations [51]. Thus, even if the detected concentration was less than allowed by Mongolian regulation, we should continue to monitor these metals in order to secure good animal health.
4.6. Source of Heavy Metal Contamination into Livestock and Human
When comparing regional differences in selenium accumulation, the Tuv-Aimag Zaamar area was noticeably lower than other areas, as median concentration in goat and sheep was 0.27 and 0.26 mg/kg in blood, respectively (Table 2). However, the soil accumulated selenium equally between Dornogovi and Tuv-Aimag Zaamar (Supplementary Table S1). As a result, other pathways for heavy metals to reach livestock must also be considered. One of the important sources of heavy metal in livestock is water [10]. For example, a report from India revealed that livestock drinking water containing mercury and manganese at levels higher than their permissible limits. The relationship of metals in livestock to metals in their feed has been also frequently reported, as feed is a major pathway of toxic metal intake for livestock [15,52]. By free grazing, which is the usual form in the Mongolian countryside, sheep and goats eat wild plants. The regional difference in selenium in animal blood could be derived from the local plants. Unusual contamination patterns were also found, such as an animal living in a low-contamination area (Dornogovi UB) accumulating a relatively high concentration of zinc and lead in its blood. As a result of these contaminations, the animals may suffer from effects of the accumulation of heavy metals in their feed (wild plants) and drinking water. Species differences can cause different accumulation patterns of metals in plant [53]. Thus, further investigation of vegetation at sampling sites and metal accumulation in livestock drinking water and plants would be needed to identify the source of contaminants in animals.
Both soil and animal products (liver and kidney) can be sources of metal contamination in human. Previous research monitored the heavy metal concentration in soil in Mongolia and alerted the community to the possible risk on human health [14]. In addition, this research will contribute to evaluation of the impact of metals on human health, particularly involving animal products. The results in this study clearly showed the difference of accumulation pattern of metals among animal and soil samples. For example, arsenic and chromium were much higher in soil than animal samples (Supplementary Figure S1). Even between liver and kidney, for example, selenium and cadmium preferably accumulated in kidney. This might help the identification of possible sources of metals in human. However, for the evaluation of possible risk in human, the daily intake of kidney and liver in Mongolia is necessary, but unfortunately these kinds of information were not found. Thus, further investigation of liver and kidney intake is desirable.
5. Conclusions
The international scientific report targeting metals in Mongolia was limited to the veterinary field. This paper revealed that arsenic and selenium were highly accumulated in Mongolian soil. They should be considered carefully as a risk, but the current concentration results in animal organs were not enough to conclude that animals suffer severe adverse effects because of exposure to these metals. This research also indicates that screening of all of soils, blood, and organs can be effective to monitor metals and to understand the inclusive polluted levels in Mongolia, as arsenic highly accumulated in soil but not in animals. For a better understanding of sources of heavy metal contamination, the monitoring of heavy metals in wild plant and livestock drinking water is desirable. We also focused on animal organs (liver and kidney), which are eaten in the countryside in Mongolia. Metals can be transferred to humans via animal products. Our results contribute to the protection of not only animal health, but also human food safety. This research will be beneficial to protect animal health, inform future studies targeting humans, and help reveal the source of metal contamination.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/toxics10120773/s1, Table S1: Metal concentration in this study in four areas (mg/kg dry weight); Table S2: The normal range of metals in livestock; Figure S1: Metal concentration in all the samples.
Author Contributions
Conceptualization, B.B.; methodology, B.B., D.D., T.-A.S., L.G., B.P., Y.B.Y. and Y.I.; investigation, B.B., D.D., T.-A.S., L.G., B.P. and S.G.; formal analysis, B.B. and K.M.; funding acquisition, L.G., S.M.M.N., M.I. and Y.I.; supervision, L.G., B.P., S.M.M.N., M.I. and Y.I.; writing—original draft, B.B., K.M. and D.D.; writing—review & editing, K.M., T.S., Y.B.Y. and Y.I. All authors have read and agreed to the published version of the manuscript.
Funding
This work was carried out with the financial support of JICA, with the “The Project for Strengthening the Capacity for Human Resource Development in the Field of Veterinary and Animal Husbandry” in Mongolia. This work was supported by the Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan awarded to M. Ishizuka (Nos. 16H0177906, 18K1984708, 18KK028708, 21H02351), Y. Ikenaka (18H0413208, 22K1845), and S.M.M. Nakayama (Nos. 17KK0009 and 20K20633). This work was also supported by the foundation of JSPS Bilateral Open Partnership Joint Research Projects (JPJSBP120209902; SMMN). This work was also supported by JSPS CORE to CORE program (MI), The Japan Prize Foundation; Hokkaido University’s SOUSEI Support Program for Young Researchers in FY2020 (SMMN); and Hokkaido University Specific Research Projects (MI). We also acknowledge the financial support from the Sumitomo Foundation and the Nihon Seimei Foundation. This research was also supported by NRF/JST’s AJ-CORE program, JST/JICA, SATREPS (Science and Technology Research Partnership for Sustainable Development; No. JPMJSA1501) and aXis (Accelerating Social Implementation for SDGs Achievement; No. JPMJAS2001) funded by JST as well as Program for supporting introduction of the new sharing system (JPMXS0420100619).
Institutional Review Board Statement
This study did not sacrifice any animals. Organs were collected from dead animals. Experienced veterinarians collected blood from grazing livestock managed by herdsmen. Thus, animals did not endure undue stress from blood collection. We always explained and received permission from owners and herdsmen when obtaining blood from animals. We also obtained permission from the Mongolian government for transporting samples from Mongolia to Japan. We did not use any human sample or private individual information in this research.
Data Availability Statement
The datasets used in the study are available in the Supplementary Materials at https://www.mdpi.com/article/10.3390/toxics10120773/s1 and from the corresponding author on reasonable request.
Acknowledgments
We are grateful to JICA for not only their financial support (“The Project for Strengthening the Capacity for Human Resource Development in the Field of Veterinary and Animal Husbandry”), but also support in producing a better environment for our activity in the university, as well as language translation. We are sincerely grateful to all veterinarians and herdsmen in Dornogovi and Tuv provinces who cooperated in this research. We wish to thank our colleagues in the Toxicology Laboratory of Hokkaido University of Japan, as well as those in the Department of Pharmacology and Internal Medicine, School of Veterinary Medicine, MULS. We would also like to thank Editage for English-language editing.
Conflicts of Interest
The authors declare no conflict of interest.
References
- AZOMINING. Available online: https://www.azomining.com/ (accessed on 17 August 2022).
- The Observatory of Economic Complexity (OEC). Available online: https://oec.world/ (accessed on 17 August 2022).
- The Organisation for Economic Co-Operation and Development (OECD) ILibrary. Available online: https://www.oecd-ilibrary.org/ (accessed on 17 August 2022).
- Nakayama, S.M.M.; Ikenaka, Y.; Hamada, K.; Muzandu, K.; Choongo, K.; Teraoka, H.; Mizuno, N.; Ishizuka, M. Metal and Metalloid Contamination in Roadside Soil and Wild Rats around a Pb-Zn Mine in Kabwe, Zambia. Environ. Pollut. 2011, 159, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Swarup, D.; Naresh, R.; Varshney, V.P.; Balagangatharathilagar, M.; Kumar, P.; Nandi, D.; Patra, R.C. Changes in Plasma Hormones Profile and Liver Function in Cows Naturally Exposed to Lead and Cadmium around Different Industrial Areas. Res. Vet. Sci. 2007, 82, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Angelovičová, L.; Fazekašová, D. Contamination of the Soil and Water Environment by Heavy Metals in the Former Mining Area of Rudňany (Slovakia). Soil Water Res. 2014, 9, 18–24. [Google Scholar] [CrossRef]
- Orisakwe, O.E.; Oladipo, O.O.; Ajaezi, G.C.; Udowelle, N.A. Horizontal and Vertical Distribution of Heavy Metals in Farm Produce and Livestock around Lead-Contaminated Goldmine in Dareta and Abare, Zamfara State, Northern Nigeria. J. Environ. Public Health 2017, 2017, 3506949. [Google Scholar] [CrossRef] [PubMed]
- Duruibe, J.O.; Ogwuegbu, M.O.C.; Egwurugwu, J.N. Heavy Metal Pollution and Human Biotoxic Effects. Int. J. Phys. Sci. 2007, 2, 112–118. [Google Scholar]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy Metal Pollution in the Environment and Their Toxicological Effects on Humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Mukesh, K.R.; Puneet, K.; Manoj, S.; Anand, S. Toxic Effect of Heavy Metals in Livestock Health. Vet. World 2008, 1, 28–30. [Google Scholar]
- Ali, H.; Khan, E.; Ilahi, I. Environmental Chemistry and Ecotoxicology of Hazardous Heavy Metals: Environmental Persistence, Toxicity, and Bioaccumulation. J. Chem. 2019, 2019, 6730305. [Google Scholar] [CrossRef]
- Batjargal, T.; Otgonjargal, E.; Baek, K.; Yang, J.S. Assessment of Metals Contamination of Soils in Ulaanbaatar, Mongolia. J. Hazard Mater. 2010, 184, 872–876. [Google Scholar] [CrossRef]
- Vardhan, K.H.; Kumar, P.S.; Panda, R.C. A Review on Heavy Metal Pollution, Toxicity and Remedial Measures: Current Trends and Future Perspectives. J. Mol. Liq. 2019, 290, 111197. [Google Scholar] [CrossRef]
- Battsengel, E.; Murayama, T.; Fukushi, K.; Nishikizawa, S.; Chonokhuu, S.; Ochir, A.; Tsetsgee, S.; Davaasuren, D. Ecological and Human Health Risk Assessment of Heavy Metal Pollution in the Soil of the Ger District in Ulaanbaatar, Mongolia. Int. J. Environ. Res. Public Health 2020, 17, 4668. [Google Scholar] [CrossRef] [PubMed]
- Rajaganapathy, V.; Xavier, F.; Sreekumar, D.; Mandal, P.K. Heavy Metal Contamination in Soil, Water and Fodder and Their Presence in Livestock and Products: A Review. J. Environ. Sci. Technol. 2011, 4, 234–249. [Google Scholar] [CrossRef]
- Anees, M.; Jamal, Q.; Durani, P.; Khan, K.; Munir, S.; Hussain, S.; Munir, K. Heavy Metals Accumulation and Their Toxic Effects: Review. J. Bio-Mol. Sci. (JBMS) 2013, 1, 27–36. [Google Scholar]
- Pareja-Carrera, J.; Mateo, R.; Rodríguez-Estival, J. Lead (Pb) in Sheep Exposed to Mining Pollution: Implications for Animal and Human Health. Ecotoxicol. Environ. Saf. 2014, 108, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Yohannes, Y.B.; Ikenaka, Y.; Nakayama, S.M.; Mizukawa, H.; Ishizuka, M. Trace Element Contamination in Tissues of Four Bird Species from the Rift Valley Region. Bull. Environ. Contam. Toxicol. 2017, 98, 172–177. [Google Scholar] [CrossRef]
- Yohannes, Y.B.; Ikenaka, Y.; Saengtienchai, A.; Watanabe, K.P.; Nakayama, S.M.M.; Ishizuka, M. Occurrence, Distribution, and Ecological Risk Assessment of DDTs and Heavy Metals in Surface Sediments from Lake Awassa-Ethiopian Rift Valley Lake. Environ. Sci. Pollut. Res. 2013, 20, 8663–8671. [Google Scholar] [CrossRef]
- Chiroma, T.M.; Ebewele, R.O.; Hymore, F.K. Comparative Assessment of Heavy Metal Levels in Soil, Vegetables and Urban Grey Waste Water Used for Irrigation in Yola and Kano. Int. Refereed J. Eng. Sci. 2014, 3, 1–9. [Google Scholar]
- U.S. Environmental Protection Agency. Ecological Soil Screening Levels for Zinc; U.S. Environmental Protection Agency: Washington, DC, USA, 2007. [Google Scholar]
- U.S. Environmental Protection Agency. Ecological Soil Screening Levels for Cadmium; U.S. Environmental Protection Agency: Washington, DC, USA, 2005. [Google Scholar]
- U.S. Environmental Protection Agency. Ecological Soil Screening Levels for Arsenic; U.S. Environmental Protection Agency: Washington, DC, USA, 2005. [Google Scholar]
- U.S. Environmental Protection Agency. Ecological Soil Screening Levels for Manganese; U.S. Environmental Protection Agency: Washington, DC, USA, 2007. [Google Scholar]
- U.S. Environmental Protection Agency. Ecological Soil Screening Levels for Selenium; U.S. Environmental Protection Agency: Washington, DC, USA, 2007. [Google Scholar]
- U.S. Environmental Protection Agency. Ecological Soil Screening Levels for Copper; U.S. Environmental Protection Agency: Washington, DC, USA, 2006. [Google Scholar]
- U.S. Environmental Protection Agency. Ecological Soil Screening Levels for Lead; U.S. Environmental Protection Agency: Washington, DC, USA, 2005. [Google Scholar]
- U.S. Environmental Protection Agency. Ecological Soil Screening Levels for Chromium; U.S. Environmental Protection Agency: Washington, DC, USA, 2005. [Google Scholar]
- Puls, R. Mineral Levels in Animal Health: Diagnostic Data; Sherpa International: Aldergrove, BC, Canada, 1994. [Google Scholar]
- Kincaid, R.L. Assessment of Trace Mineral Status of Ruminants: A Review. J. Anim. Sci. 1999, 77, 1–10. [Google Scholar] [CrossRef]
- Biswas, U.; Sarkar, S.; Bhowmik, M.K.; Samanta, A.K.; Biswas, S. Chronic Toxicity of Arsenic in Goats: Clinicobiochemical Changes, Pathomorphology and Tissue Residues. Small Rumin. Res. 2000, 38, 229–235. [Google Scholar] [CrossRef]
- Rana, T.; Bera, A.K.; Das, S.; Bhattacharya, D.; Bandyopadhyay, S.; Pan, D.; Kumar Das, S. Effect of Chronic Intake of Arsenic-Contaminated Water on Blood Oxidative Stress Indices in Cattle in an Arsenic-Affected Zone. Ecotoxicol. Environ. Saf. 2010, 73, 1327–1332. [Google Scholar] [CrossRef]
- Nandi, D.; Patra, R.C.; Swarup, D. Effect of Cysteine, Methionine, Ascorbic Acid and Thiamine on Arsenic-Induced Oxidative Stress and Biochemical Alterations in Rats. Toxicology 2005, 211, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Alonso, M.L.; Benedito, J.L.; Miranda, M.; Castillo, C.; Hernández, J.; Shore, R.F. Cattle as Biomonitors of Soil Arsenic, Copper, and Zinc Concentrations in Galicia (NW Spain). Arch. Environ. Contam. Toxicol. 2002, 43, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Jenisova, Z.; Feszterova, M.; Baros, S.; Liska, J.; Hudecova, D.; Rhodes, C.J.; Valko, M. Arsenic: Toxicity, Oxidative Stress and Human Disease. J. Appl. Toxicol. 2011, 31, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Raj Khanal, D.; Knight, A.P. Selenium: Its Role in Livestock Health and Productivity. J. Agric. Environ. 2010, 11, 101–106. [Google Scholar] [CrossRef]
- Tiwary, A.K.; Stegelmeier, B.L.; Panter, K.E.; James, L.F.; Hall, J.O. Comparative Toxicosis of Sodium Selenite and Selenomethionine in Lambs. J. Vet. Diagn. Investig. 2006, 18, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Deore, M.D.; Srivastava, A.K.; Sharma, S.K. Effect of Reduced Glutathione Treatment on Selenosis, Blood Selenium Concentration and Glutathione Peroxidase Activity after Repeated Short-Term Selenium Exposure in Buffalo Calves. Toxicology 2005, 213, 169–174. [Google Scholar] [CrossRef]
- Osredkar, J.; Sustar, N. Copper and Zinc, Biological Role and Significance of Copper/Zinc Imbalance. Clin. Toxicol. 2011, s3, 1. [Google Scholar] [CrossRef]
- Todd, J.R. Chronic Copper Toxicity of Ruminants. Proc. Nutr. Soc. 1969, 28, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Wiener, G.; Hall, J.G.; Hayter, S. An Association between the Concentration of Copper in Whole Blood and Haemoglobin Type in Sheep. Anim. Prod. 1973, 17, 1–7. [Google Scholar] [CrossRef]
- Sobhanirad, S.; Naserian, A.A. Effects of High Dietary Zinc Concentration and Zinc Sources on Hematology and Biochemistry of Blood Serum in Holstein Dairy Cows. Anim. Feed Sci. Technol. 2012, 177, 242–246. [Google Scholar] [CrossRef]
- Chafik, A.; Essamadi, A.; Çelik, S.Y.; Mavi, A. Purification and Biochemical Characterization of a Novel Copper, Zinc Superoxide Dismutase from Liver of Camel (Camelus Dromedarius): An Antioxidant Enzyme with Unique Properties. Bioorg. Chem. 2019, 86, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Bengoumi, M.; Essamadi, A.K.; Tressol, J.C.; Faye, A.B. Comparative Study of Copper and Zinc Metabolism in Cattle and Camel. Biol. Trace Elem. Res. 1998, 63, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Farmer, A.A.; Farmer, A.M.; Stan, T. Concentrations of Cadmium, Lead and Zinc in Livestock Feed and Organs around a Metal Production Centre in Eastern Kazakhstan. Sci. Total Environ. 2000, 257, 53–60. [Google Scholar] [CrossRef]
- Jorhem, L. Lead and Cadmium in Tissues from Horse, Sheep, Lamb and Reindeer in Sweden. Z Lebensm. Unters Forsch. 1999, 208, 106–109. [Google Scholar] [CrossRef]
- Janette, O. Itai-Itai Disease Caused by Cadmium Poisoning. J. Heavy Met. Toxic. Dis. 2021, 6, 455–463. [Google Scholar]
- Nakata, H.; Nakayama, S.M.M.; Kataba, A.; Yohannes, Y.B.; Ikenaka, Y.; Ishizuka, M. Evaluation of the Ameliorative Effect of Spirulina (Arthrospira Platensis) Supplementation on Parameters Relating to Lead Poisoning and Obesity in C57BL/6J Mice. J. Funct. Foods 2021, 77, 104344. [Google Scholar] [CrossRef]
- Nakata, H.; Nakayama, S.M.M.; Yabe, J.; Muzandu, K.; Toyomaki, H.; Yohannes, Y.B.; Kataba, A.; Zyambo, G.; Ikenaka, Y.; Choongo, K.; et al. Clinical Biochemical Parameters Associated with the Exposure to Multiple Environmental Metals in Residents from Kabwe, Zambia. Chemosphere 2021, 262, 127788. [Google Scholar] [CrossRef]
- Nakata, H.; Nakayama, S.M.M.; Ikenaka, Y.; Mizukawa, H.; Ishii, C.; Yohannes, Y.B.; Konnai, S.; Darwish, W.S.; Ishizuka, M. Metal Extent in Blood of Livestock from Dandora Dumping Site, Kenya: Source Identification of Pb Exposure by Stable Isotope Analysis. Environ. Pollut. 2015, 205, 8–15. [Google Scholar] [CrossRef]
- Pandey, G.; Madhuri, S. Heavy Metals Causing Toxicity in Animals and Fishes. Res. J. Anim. Vet. Fish. Sci. 2014, 2, 17–23. [Google Scholar]
- Reglero, M.M.; Monsalve-González, L.; Taggart, M.A.; Mateo, R. Transfer of Metals to Plants and Red Deer in an Old Lead Mining Area in Spain. Sci. Total Environ. 2008, 406, 287–297. [Google Scholar] [CrossRef]
- Filipović-Trajković, R.; Ilić, Z.S.; Šunić, L.; Andjelković, S. The Potential of Different Plant Species for Heavy Metals Accumulation and Distribution. J. Food Agric. Environ. 2005, 10, 959–964. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).