Ecotoxicological Risk Assessment of Actellic 50 EC Insecticide on Non-Target Organisms in Parallel with the Application of Standardized Tests
Abstract
1. Introduction
- -
- The germination and growth of seedlings, their biomass, and their assimilation of pigments for Lepidium sativum, Sinapis alba, and Sorghum saccharatum;
- -
- The plant growth and biomass of Triticum aestivum;
- -
- The germination of spores and the differentiation of gametophytes for Athyrium filix-femina and Asplenium scolopendrium;
- -
- The survival rate, behavioral changes, respiratory rate, oxygen consumption, number of figurative elements, and blood glucose of Carassius gibelio;
- -
- The successful hatching of Pelophylax ridibundus tadpoles, their survival rate, and changes in the morphology of the body.
2. Materials and Methods
2.1. Experiments on Plants
2.1.1. Application of the Phytotoxkit Microbiotest
2.1.2. Application of the Triticum Test
2.1.3. Contamination Tests on Fern Spores
2.2. Experiments on Animals
2.2.1. Experiments on Fish
2.2.2. Experiments on Marsh Frogs
- -
- Control I, consisting of 30 eggs kept in dechlorinated, permanently aerated water under controlled climatic conditions for 9 days.
- -
- Lot I.1, consisting of 30 eggs kept in a 0.1 mg L−1 Actellic 50 EC solution for 9 days.
- -
- Lot I.2, consisting of 30 eggs kept in a 0.01 mg L−1 Actellic 50 EC solution for 9 days.
- -
- Lot I.3, consisting of 30 eggs held in a 0.001 mg L−1 Actellic 50 EC solution for 9 days.
- -
- Control II, consisting of 15 tadpoles kept in dechlorinated, permanently aerated and climate-controlled water for 5 days.
- -
- Lot II.1, consisting of 15 tadpoles kept in a 0.1 mg L−1 Actellic 50 EC solution for 5 days.
- -
- Lot II.2, consisting of 15 tadpoles in a 0.01 mg L−1 Actellic 50 EC solution for 5 days.
- -
- Lot II.3, consisting of 15 tadpoles in a 0.001 mg L−1 Actellic 50 EC solution for 5 days.
2.2.3. Statistical Interpretation
3. Results and Discussion
3.1. Phytotoxkit Microbiotest Application Results
3.2. Influence of Actellic 50 EC on - Growth of Triticum aestivum Seedlings
3.3. Influence of Actellic 50 EC on Fern Spore Germination and Gametophyte Differentiation
3.4. The Action of Actellic 50 EC on Fish
3.4.1. Influence of Actellic 50 EC on Survival
3.4.2. Influence of Actellic 50 EC on Behavior
3.4.3. Influence of Actellic 50 EC on Respiratory Rate and Average Oxygen Consumption
3.4.4. Influence of Actellic 50 EC on Hematological Parameters: Blood Figure Elements and Glucose
3.5. Results for Marsh Frogs
3.6. Evaluation of the Effects of Organophosphorus Insecticides on Genotoxicity and Reproductive Function, Related to the Period of Exposure (Acute, Sub-Chronic, and Chronic Toxicity)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harte, J.; Holdren, C.; Schneider, R.; Shirley, C. Toxics A to Z: A Guide to Everyday Pollution Hazards; University of California Press: Berkeley, CA, USA, 1991; pp. 112–126. [Google Scholar]
- Integrated Pest Management Framework (IPMF), Ministry of Agriculture, Animal Industry and Fisheries, Republic of Uganda, January, 2014—(PDF) Republic of Uganda-. Available online: https://dokumen.tips/documents/republic-of-uganda-documents-reports-all-republic-of-uganda-ministry-of.html?page=4 (accessed on 18 October 2022).
- Jensen, B.H.; Petersen, A.; Petersen, P.B.; Christensen, T.; Fagt, S.; Trolle, E.; Poulsen, M.E.; Andersen, J.H. Cumulative dietary risk assessment of pesticides in food for the Danish population for the period 2012–2017. Food Chem. Toxicol. 2022, 168, 113359. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. Conclusion on the peer review of pirimiphos-methyl, Conclusion regarding the peer review of the pesticide risk assessment of the active substance Pirimiphos-methyl|EFSA (europa.eu). EFSA Sci. Rep. 2005, 44, 1–53. [Google Scholar]
- Actellic 50 EC—Safety Data Sheet. Available online: https://www.orionagriscience.co.nz/storage/products/March2021/Actellic-50EC-SDS-Apr-2020.pdf (accessed on 18 October 2022).
- Mgbenka, B.; Oluah, N.; Arungwa, A. Erythropoietic response and hematological parameters in the catfish Clarias albopunctatus exposed to sublethal concentrations of actellic. Ecotoxicol. Environ. Saf. 2005, 62, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Mensink, B.J.W.G. Environmental Riskm Limits for Pirimiphos-methyl, RIVM Letter Report 601716011. 2008. Available online: https://www.rivm.nl/bibliotheek/rapporten/601716011.pdf (accessed on 18 October 2022).
- World Health Report—2002 Reducing Risks, Promoting Healthy Life. 2022. Available online: https://www.who.int/publications/i/item/9241562072 (accessed on 18 October 2022).
- Lawal, M.O.; Samuel, O.B. Investigation of Acute Toxicity of Pirimiphos-Methyl (Actellic®, 25% EC) on Guppy (Poecilia reticulata, Peters, 1859). Pak. J. Biol. Sci. 2010, 13, 405–408. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ansari, A.M.; Mittal, P.K.; Razdan, R.K.; Dhiman, R.C.; Kumar, A.K. Evaluation of pirimiphos-methyl (50% EC) against the immatures of Anopheles stephensi/A.culicifacies (malaria vectors) and Culex quinquefaciatus (vector of bancroftian filariasis). J. Vect. Borne. Dis. 2004, 41, 10–16. [Google Scholar]
- Sabra, F.S.; Mehana, E.-S.E.-D. Pesticides toxicity in fish with particular reference to insecticides. Asian J. Agricult. Food Sci. 2015, 3, 40–60. Available online: https://www.researchgate.net/publication/358734155_Effects_of_Pesticides_on_Haematological_Parameters_of_Fish_Recent_Updates (accessed on 18 October 2022).
- Cox, C.; Surgan, M. Unidentified inert ingredients in pesticides: Implications for human and environmental health. Environ. Health Perspect. 2006, 114, 1803–1806. [Google Scholar] [CrossRef]
- Kayhan, F.E.; Kaymak, G.; Yön, N.D. Insecticide Groups and Their Effects in Aquatic Environment. Marmara Fen Bilim. Derg. 2013, 25, 167–183. Available online: https://www.researchgate.net/publication/273379099_Insecticide_Groups_and_Their_Effects_in_Aquatic_Environment (accessed on 18 October 2022). [CrossRef]
- Fisher, S.W. Changes in the toxicity of three pesticides as a function of environmental pH and temperature. Bull. Environ. Contam. Toxicol. 1991, 46, 197–202. [Google Scholar] [CrossRef]
- Fulton, M.H.; Key, P.R.; Delorenzo, M.E. Inseticide toxicity in fish. In Organic Chemical Toxicology of Fishes; Tierny, K.B., Farrell, A.P., Brauner, C.J., Eds.; Academic Press: Amsterdam, The Netherlands, 2014; pp. 309–368. [Google Scholar]
- Shafiei, T.M.; Costa, H.H. The susceptibility and resistance of fry and fingerlings of Oreochromis mossambicus Peters to some pesticides commonly used in Sri Lanka. J. Appl. Ichthyol. 1990, 6, 73–80. [Google Scholar] [CrossRef]
- Shutler, D.; Marcogliese, D.J. Leukocyte Profiles of Northern Leopard Frogs, Lithobates pipiens, Exposed to Pesticides and Hematozoa in Agricultural Wetlands. Copeia 2011, 2011, 301–307. [Google Scholar] [CrossRef]
- Aryal, N.; Wood, J.; Rijal, I.; Deng, D.; Jha, M.K.; Ofori-Boadu, A. Fate of environmental pollutants: A review. Water Environ. Res. 2020, 92, 1587–1594. [Google Scholar] [CrossRef] [PubMed]
- Dyck, A.; Robinson, S.; Young, S.; Renaud, J.; Sabourin, L.; Lapen, D.; Pick, F. The Effects of Ditch Management in Agroecosystems on Embryonic and Tadpole Survival, Growth, and Development of Northern Leopard Frogs (Lithobates pipiens). Arch. Environ. Contam. Toxicol. 2021, 81, 107–122. [Google Scholar] [CrossRef] [PubMed]
- Moutinho, M.; de Almeida, E.; Espíndola, E.; Daam, M.; Schiesari, L. Herbicides employed in sugarcane plantations have lethal and sublethal effects to larval Boana pardalis (Amphibia, Hylidae). Ecotoxicology 2020, 29, 1043–1051. [Google Scholar] [CrossRef] [PubMed]
- Persoone, G.; Marsalah, B.; Blinova, I.; Torokne, A.; Zarina, D.; Manusodzianas, L.; Nalecz-Jawecki, G.; Tofan, L.; Stepanova, N.; Tothova, L.; et al. A practical and User-Friendly Toxicity Classification System with Microbiotests for Natural waters and Wastewaters. Environ. Toxicol. 2003, 18, 395–402. [Google Scholar] [CrossRef] [PubMed]
- MicroBioTests. Available online: https://www.microbiotests.com/toxkit/ (accessed on 18 October 2022).
- ISO 11269-1; 2nd Edition, Soil Quality—Determination of the Effects of Pollutants on Soil Flora—Part 1: Method for the Measurement of Inhibition of Root Growth, ISO 11269-1: Soil quality—Determination of the effects of pollutants on soil flora—Part 1: Method for the measurement of inhibition of root growth. Available online: ihs.com (accessed on 1 March 2012).
- ISO 11269-2; 3rd Edition, Soil Quality—Determination of the Effects of Pollutants on Soil Flora—Part 2: Effects of Contaminated Soil on the Emergence and Early Growth of Higher Plants, ISO 11269-2: Soil Quality—Determination of the Effects of Pollutants on Soil Flora—Part 2: Effects of Contaminated Soil on the Emergence and Early Growth of Higher Plants. Available online: ihs.com (accessed on 15 January 2012).
- Ferreira, T.; Rasband, W.; Image, J. User Guide. Available online: https://imagej.nih.gov/ij/docs/guide/user-guide.pdf (accessed on 18 October 2022).
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectroscopy. In Current Protocols in Food Analytical Chemistry; Wrolstad, R.E., Ed.; John Wiley & Sons Inc: Hoboken, NJ, USA, 2001; pp. 1–8. [Google Scholar]
- Drăghiceanu, O.A.; Soare, L.C.; Șuțan, A.N.; Fierascu, I.; Fierascu, R.C.; Dobrescu, C.M. The use of Triticum test in the evaluation of the materials’ phytotoxicity. In Development of Plant Extracts and İnnovative Phytosynthesized Nanostructures Mixtures with Phytotherapeutic Applications, İn Order to Reduce Biocenotic Stress in Horticultural Crops; Fierăscu, R.C., Fierăscu, I., Soare, L.C., Eds.; Ruse Press: Ruse, Bulgaria, 2021; pp. 87–116. [Google Scholar]
- OECD. Guideline for Testing Chemicals 208. Terrestrial Plant Test: Seedling emergence and Seedling Growth Test. Available online: https://www.oecd-ilibrary.org/docserver/9789264070066-en.pdf?expires=1665428888&id=id&accname=guest&checksum=44BCC57332A86CC960F601B352F708BA (accessed on 10 October 2022).
- U.S. EPA. Pirimiphos-Methyl IRED Facts. 2013. Available online: https://archive.epa.gov/pesticides/reregistration/web/html/index-220.html (accessed on 10 October 2022).
- Li, R.; He, J.; Xie, H.; Wang, W.; Bose, S.-K.; Sun, Y.; Hu, J.; Yin, H. Effects of chitosan nanoparticles on seed germination and seedling growth of wheat (Triticum aestivum L.). Int. J. Biol. Macromolec. 2018, 126, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Catalá, M.; Esteban, M.; Quintanilla, L.G. Mitochondrial Activity Of Fern Spores For The Evaluation Of Acute Toxicity in Higher Plant Development. In Working With Ferns, Issues An Applications; Springer: New York, NY, USA, 2011. [Google Scholar]
- Dyer, A.F. The experimental biology of ferns. Trans. Bot. Soc. Edinb. 1979, 43, 75–90. [Google Scholar] [CrossRef]
- Official Journal of the European Union. Directive 2010/63/EU of the European Parliament and of the Council on the protection of animals used for scientific purposes. Off. J. Eur. Union 2010, 276, 33–80. [Google Scholar]
- Report—Synthesis of water quality (2018–2020), 2018-2020—Administrația Bazinală de Apă Argeș-Vedea (Rowater.ro—The folder Ape de suprafata (in Surface waters—pdf, pg. 120). Available online: https://arges-vedea.rowater.ro/despre-noi/descrierea-activitatii/managementul-european-integrat-resurse-de-apa/gospodarirea-apelor/sinteza-calitatii-apelor-in-sh-bh/ (accessed on 18 October 2022).
- Patakioutas, G.I.; Karras, G.; Hela, D.; Albanis, T.A. Pirimiphos-methyl and benalaxyl losses in surface runoff from plots cultivated with potatoes. Pest Manag. Sci. 2002, 58, 1194–1204. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Halim, K.; Salama, A.; El-Khateeb, E.; Bakry, N. Organophosphorus pollutants (OPP) in aquatic environment at Damietta Governorate, Egypt: Implications for monitoring and biomarker responses. Chemosphere 2006, 63, 1491–1498. [Google Scholar] [CrossRef]
- Montuori, P.; De Rosa, E.; Di Duca, F.; De Simone, B.; Scippa, S.; Russo, I.; Sorrentino, M.; Sarnacchiaro, P.; Triassi, M. Occurrence, Distribution, and Risk Assessment of Organophosphorus Pesticides in the Aquatic Environment of the Sele River Estuary, Southern Italy. Toxics 2022, 10, 377. [Google Scholar] [CrossRef]
- Picoş, C.A.; Năstăsescu, G.H. Lucrări Practice de Fiziologie Animală; Tipografia Universităţii din Bucureşti: Bucureşti, Romania, 1988. [Google Scholar]
- Zheng, R.; Liu, R.; Wu, M.; Wang, H.; Xie, L. Effects of sodium perchlorate and exogenous L-thyroxine on growth, development and leptin signaling pathway of Bufo gargarizans tadpoles during metamorphosis. Ecotoxicol. Environ. Saf. 2020, 206, 111410. [Google Scholar] [CrossRef] [PubMed]
- Gosner, K.L. A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 1960, 16, 183–190. [Google Scholar]
- Sparling, D.W.; Linder, G.; Bishop, C.; Krest, S. Ecotoxicology of Amphibians and Reptiles; SETAC/Taylor & Francis: Boca Raton, FL, USA, 2010. [Google Scholar]
- Bantle, J.A.; Dumont, J.N.; Finch, R.; Linder, G. Atlas of Abnormalities: A Guide for the Performance of FETAX; Oklahoma State University: Stillwater, OK, USA, 1991. [Google Scholar]
- Dumitriu, I.; Fierascu, R.C.; Bunghez, I.R.; Ion, R.M. Application of inductively coupled plasma—atomic emission spectroscopy (ICP-AES) based analysis for water quality control. Environ. Eng. Manag. J. 2009, 8, 347–351. [Google Scholar] [CrossRef]
- Relyea, R.A. Morphological and behavioral plasticity of larval anurans in response to different predators. Ecology 2001, 82, 523–540. [Google Scholar] [CrossRef]
- Czerniawska-Kusza, I.; Ciesielczuk, T.; Kusza, G.; Cichoń, A. Comparison of the Phytotoxkit microbiotest and chemical variables for toxicity evaluation of sediments. Environ. Toxicol. 2006, 21, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Czerniawska-Kusza, I.; Kusza, G. The potential of the Phytotoxkit microbiotest for hazard evaluation of sediments in eutrophic freshwater ecosystems. Environ. Monit. Assess. 2011, 179, 113–121. [Google Scholar] [CrossRef]
- Shams-El-Din, A.M.; Abo-El-Seoud, M.A.; Danial, L.N.; Ahmed, S.M. Biochemical impacts of some organophosphorus insecticides on cabbage plants. J. Plant Dis. Prot. 1995, 102, 655–662. [Google Scholar]
- Abo-El-Seoud, M.A.; Frost, M. Biochemical changes in wheat plants as affected by residues of dimethoate and pirimicarb. Environ. Manag. Health 1998, 9, 188–193. [Google Scholar] [CrossRef]
- Shiboob, M.H.M. Chlorophyll and carotenoid contents in tomato and cucumber plants in relation to dimethoate and profenofos application. J. Agric. Sci. Mansoura Univ. 2002, 27, 1853–1861. [Google Scholar]
- Radwan, M.A.; Shiboob, M.H.; Abu-Elamayem, M.M.; Abdel-Aal, A. Residues of pirimiphos-methyl and profenofos on green pepper and eggplant fruits and their effect on some quality properties. Emir. J. Agric. Sci. 2004, 16, 32–42. [Google Scholar] [CrossRef]
- Salem, H.A.I. Effects of pesticide treatments and sowing dates on growth parameters and yield of onion plant (Allium cepa L.). Alex. Sci. Exch. J. 2012, 33, 89–98. [Google Scholar]
- Abla, D.E. Agro-İnput Use in Peri-Urban okra Production in the Greater Accra Region. Master’s Thesis, University of Ghana, Legon, Ghana, 2015. [Google Scholar]
- Kandil, A.A.; Abd-El-Monaem, M.A.; Mohamed, M.A.A. Impact of Pre-Harvesting Spraying Pesticides and Post-Harvesting with Phosphine Fumigation on Germination and Seedling Parameters of Bread Wheat. J. Plant Prod. 2022, 13, 33–38. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Thukral, A.K.; Bhardwaj, R. Responses of plants to pesticide toxicity: An overview. Planta Daninha 2019, 37, e019184291. [Google Scholar] [CrossRef]
- Soare, L.C.; Păunescu, A.; Ponepal, M.C. The Morphophysiological, Histological, and Biochemical Response of Some Nontarget Organisms to the Stress Induced by the Pesticides in the Environment. In Pesticides Use and Misuse and Teir Impact in the Environment; Larramendy, M., Soloneski, S., Eds.; IntechOpen: London, UK, 2019; pp. 1–25. [Google Scholar] [CrossRef]
- Soare, L.C.; Dobrescu, C.M.; Popescu, M.; Boeru, A.G. The Effects of Some Pesticides on Spore Germination and Gametophyte Differentiation in Athyrium filix-femina (L.) Roth. and Polypodium vulgare L. Not. Bot. Horti Agrobot. 2013, 41, 458–462. [Google Scholar] [CrossRef][Green Version]
- Omoregie, E.; Ufodike, E. Acute roxieity of gammalin 20 and actellic25 EC to Oreochromis niloticus niloticus. Acta Hydrobiol. 1990, 32, 447–455. Available online: https://www.researchgate.net/publication/237099818_Acute_roxieity_of_gammalin_20_and_actellic25_EC_to_Oreochromis_niloticus_niloticus#fullTextFileContent (accessed on 15 September 2022).
- Banaee, M. Adverse Effect of İnsecticides on Various Aspects of Fish’s Biology and Physiology: Insecticides- Basic and Other Applications Book; Sonia, S., Marcelo, L., Eds.; InTech: London, UK, 2012; Chapter 6101126. [Google Scholar]
- Wang, C.; Lu, G.; Cui, J.; Wang, P. Sublethal effects of pesticide mixtures on selected biomarkers of Carassius auratus. Environ. Toxicol. Pharmacol. 2009, 28, 414–419. [Google Scholar] [CrossRef]
- Kumar, B.K.; Vineela, D.; Reddy, S.J. Impact of heptachlor on haematological and histopathological indices of fish Catla catla. Asian J. Pharm. Pharmacol. 2019, 5, 1152–1161. [Google Scholar] [CrossRef]
- Ferrari, A.; Venturino, A.; de D’Angelo, A.M.P. Time course of brain cholinesterase inhibition and recovery following acute and subacute azinphosmethyl, parathion and carbaryl exposure in the goldfish (Carassius auratus). Ecotoxicol. Environ. Saf. 2004, 57, 420–425. [Google Scholar] [CrossRef]
- Rao, J.V. Effects of monocrotophos and its analogs in acetylcholinesterase activity’s inhibition and its pattern of recovery on euryhaline fish, Oreochromis mossambicus. Ecotoxicol. Environ. Saf. 2004, 59, 217–222. [Google Scholar] [CrossRef]
- Berntssen, M.H.; Rosenlund, G.; Garlito, B.; Amlund, H.; Sissener, N.H.; Bernhard, A.; Sanden, M. Sensitivity of Atlantic salmon to the pesticide pirimiphos-methyl, present in plant-based feeds. Aquaculture 2021, 531, 735825. [Google Scholar] [CrossRef]
- Ba-Omar, T.; Al-Jardani, S.; Victor, R. Effects of pesticide temephos on the gills of Aphanius dispar (Pisces: Cyprinodontidae). Tissue Cell 2011, 43, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghanbousi, R.; Ba-Omar, T.; Victor, R. Effect of deltamethrin on the gills of Aphanius dispar: A microscopic study. Tissue Cell 2012, 44, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Tilak, K.S.; Kumari, R.S. Acute toxicity of Nuvan, an organophosphate to freshwater fish Ctenopharyngodon idella and its effect on oxygen consumption. J. Environ. Biol. 2009, 30, 1031–1033. [Google Scholar]
- Mishra, A.; Poddar, A.N. Acute toxicity and behavioural response of the food fish Channa punctatus (Bloch) to an insecticide dichlorvos. J. Indus. Poll. Cont. 2014, 30, 219–222. Available online: https://www.icontrolpollution.com/articles/acute-toxicity-and-behavioral-response-of-the-food-fish-channa-punctatus-blochto-an-insecticide-dichlorvos-219-222.pdf (accessed on 18 October 2022).
- Verma, S.R.; Rani, S.; Tonk, I.P.; Dalela, R.C. Effects of pesticides and their combination of three serum phosphotases of Mystus vittatus. Water Air Soil Poll. 1984, 21, 9–14. Available online: https://www.researchgate.net/publication/318572328_A_review_on_the_toxicity_and_other_effects_of_Dichlorvos_an_organophosphate_pesticide_to_the_freshwater_fish (accessed on 12 September 2022). [CrossRef]
- Rath, S.; Misra, B. Sub-lethal effects of dichlorvos (DDVP) on respiratory metabolism of Tilapia mossambica, peters of 3 age groups. Exp. Gerontol. 1979, 14, 37–41. [Google Scholar] [CrossRef]
- Sinha, B.K.; Gour, J.K.; Singh, M.K.; Nigam, A.K. Effects of Pesticides on Haematological Parameters of Fish: Recent Updates. J. Sci. Res. 2022, 66, 269–283. [Google Scholar] [CrossRef]
- Oluah, N.S.; Mgbenka, B.O. Effect of ACTELLIC 25 EC on the differential leucocyte counts of the catfish Clarias albopunctatus (NICHOLE & LAMONTE, 1953). Anim. Res. Int. 2004, 1, 52–56. Available online: https://www.unn.edu.ng/wp-content/uploads/2016/06/10.-OLUAH-and-MGBENKA.pdf (accessed on 12 September 2022).
- Nannu, M.; Mostakim, G.; Khatun, M.; Rahman, M.; Sadiqul, M. Hematological and histo-architectural damages in the kidney and liver of Nile tilapia on exposure to kinalux. Progress. Agric. 2015, 26, 173–178. [Google Scholar] [CrossRef]
- Olsvik, P.A.; Berntssen, M.H.; Søfteland, L. In vitro toxicity of pirimiphos-methyl in Atlantic salmon hepatocytes. Toxicol. Vitr. 2017, 39, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Gautam, R.K.; Shakya, S.; Shamim, I.; Khajuria, V. Toxic effect of Nuvan (Organophosphate) on blood biochemistry of freshwater fish Clarias batrachus. Int. J. Interdiscip. Res. 2014, 1, 1–7. [Google Scholar]
- Srinivas, A.; Venugopal, G.; Pisca, R.S.; Waghray, S. Some aspects of haemato-biochemistry of Indian major carp Catla catla influenced by malathion and dichlorvos (DDVP). J. Aqua. Biol. 2001, 16, 53–56. [Google Scholar]
- Al-Ghanim, K.A. Acute toxicity and effects of sub-lethal malathion exposure on biochemical and haematological parameters of Oreochromis niloticus. Sci. Res. Essays 2012, 7, 1674–1680. [Google Scholar] [CrossRef]
- Koundinya, P.R.; Ramamurthi, R. Effect of organophosphate pesticide Sumithion (Fenitrothion) on some aspects of carbohydrate metabolism in a freshwater fish, Sarotherodon (Tilapia) mossambicus (Peters). Experientia 1979, 35, 1632–1633. [Google Scholar] [CrossRef]
- Ritu, R.F.; Majharul Islam, S.M.; Rashid, H.; Haque, S.M.; Zulfahmi, I.; Sumon, K.A. Application of fenitrothion on Heteropneustes fossilis causes alteration in morphology of erythrocytes via modifying hematological parameters. Toxicology 2003, 9, 895–904. [Google Scholar] [CrossRef]
- Begum, G.; Vijayaraghavan, S. Carbohydrate metabolism in hepatic tissue of freshwater catfish Clarias batrachus L. during dimethoate exposure. Food Chem. Toxicol. 1995, 33, 423–426. [Google Scholar] [CrossRef]
- Begum, G.; Vijayaraghavan, S. Effect of Acute Exposure of the Organophosphate Insecticide Rogor on Some Biochemical Aspects of Clarias batrachus (Linnaeus). Environ. Res. 1999, 80, 80–83. [Google Scholar] [CrossRef]
- Rao, J.V. Sublethal effects of an organophosphorus insecticide (RPR-II) on biochemical parameters of tilapia, Oreochromis mossambicus. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2006, 143, 492–498. [Google Scholar] [CrossRef]
- Pimpão, C.; Zampronio, A.; de Assis, H.S. Effects of deltamethrin on hematological parameters and enzymatic activity in Ancistrus multispinis (Pisces, Teleostei). Pestic. Biochem. Physiol. 2007, 88, 122–127. [Google Scholar] [CrossRef]
- Ali, H.A.J.; Rani, V.J. Effect of phosalone on haematological indices in the tilapia, Oreochromis mossambicus. Turk. J. Veter-Anim. Sci. 2009, 33, 407–411. [Google Scholar] [CrossRef]
- Burraco, P.; Valdés, A.E.; Orizaola, G. Metabolic costs of altered growth trajectories across life transitions in amphibians. J. Anim. Ecol. 2019, 89, 855–866. [Google Scholar] [CrossRef] [PubMed]
- Lanctôt, C.; Navarro-Martín, L.; Robertson, C.; Park, B.; Jackman, P.; Pauli, B.; Trudeau, V. Effects of glyphosate-based herbicides on survival, development, growth and sex ratios of wood frog (Lithobates sylvaticus) tadpoles. II: Agriculturally relevant exposures to Roundup WeatherMax® and Vision® under laboratory conditions. Aquat. Toxicol. 2014, 154, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Merola, C.; Fabrello, J.; Matozzo, V.; Faggio, C.; Iannetta, A.; Tinelli, A.; Perugini, M. Dinitroaniline herbicide pendimethalin afects development and induces biochemical and histological alterations in zebrafsh early-life stages. Sci. Total. Environ. 2022, 828, 154414. [Google Scholar] [CrossRef]
- Park, H.; Yun, B.H.; Lim, W.; Song, G. Dinitramine induces cardiotoxicity and morphological alterations on zebrafsh embryo development. Aquat. Toxicol. 2021, 240, 105982. [Google Scholar] [CrossRef]
- da Silva, M.B.; Fraga, R.E.; Nishiyama, P.B.; da Silva, I.S.; Badaró Costa, N.L.; Alves de Oliveira, L.A.; Rocha, M.A.; Juncá, F.A. Leukocyte Profiles in Odontophrynus carvalhoi (Amphibia: Odontophrynidae) Tadpoles Exposed to Organophosphate Chlorpyrifos Pesticides. Water Air Soil Pollut. 2020, 231, 372. [Google Scholar] [CrossRef]
- Amniattalab, A.; Razi, M. Effect of phosalone on testicular tissue and in vitro fertilizing potential. Int. J. Fertil. Steril. 2015, 9, 93. [Google Scholar]
- Khodabandeh, Z.; Etebari, M.; Aliomrani, M. Study of the probable genotoxic effects of Zolone (Phosalone) exposure in mice bone marrow derived cells. Genes Environ. 2021, 43, 1–9. [Google Scholar] [CrossRef]
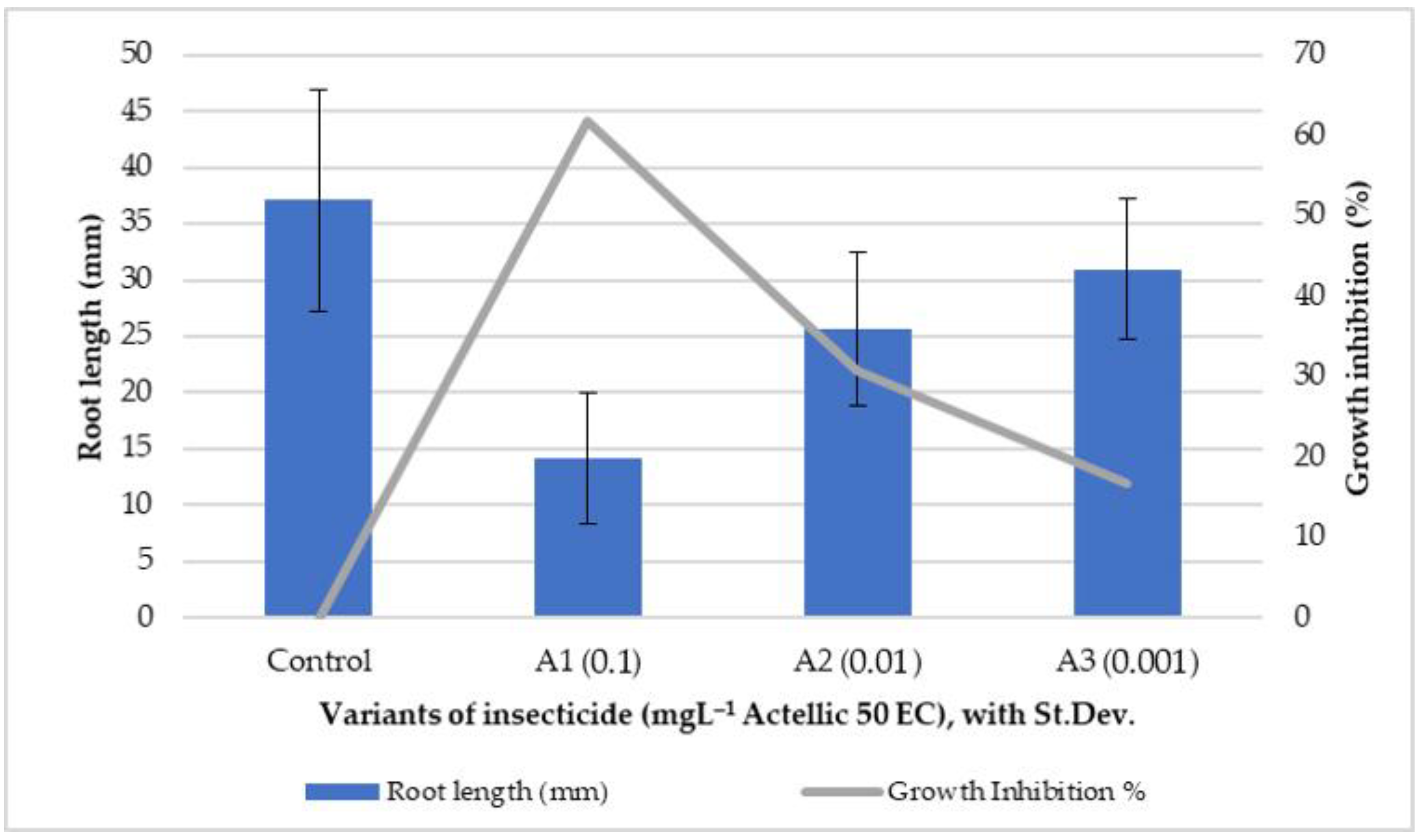

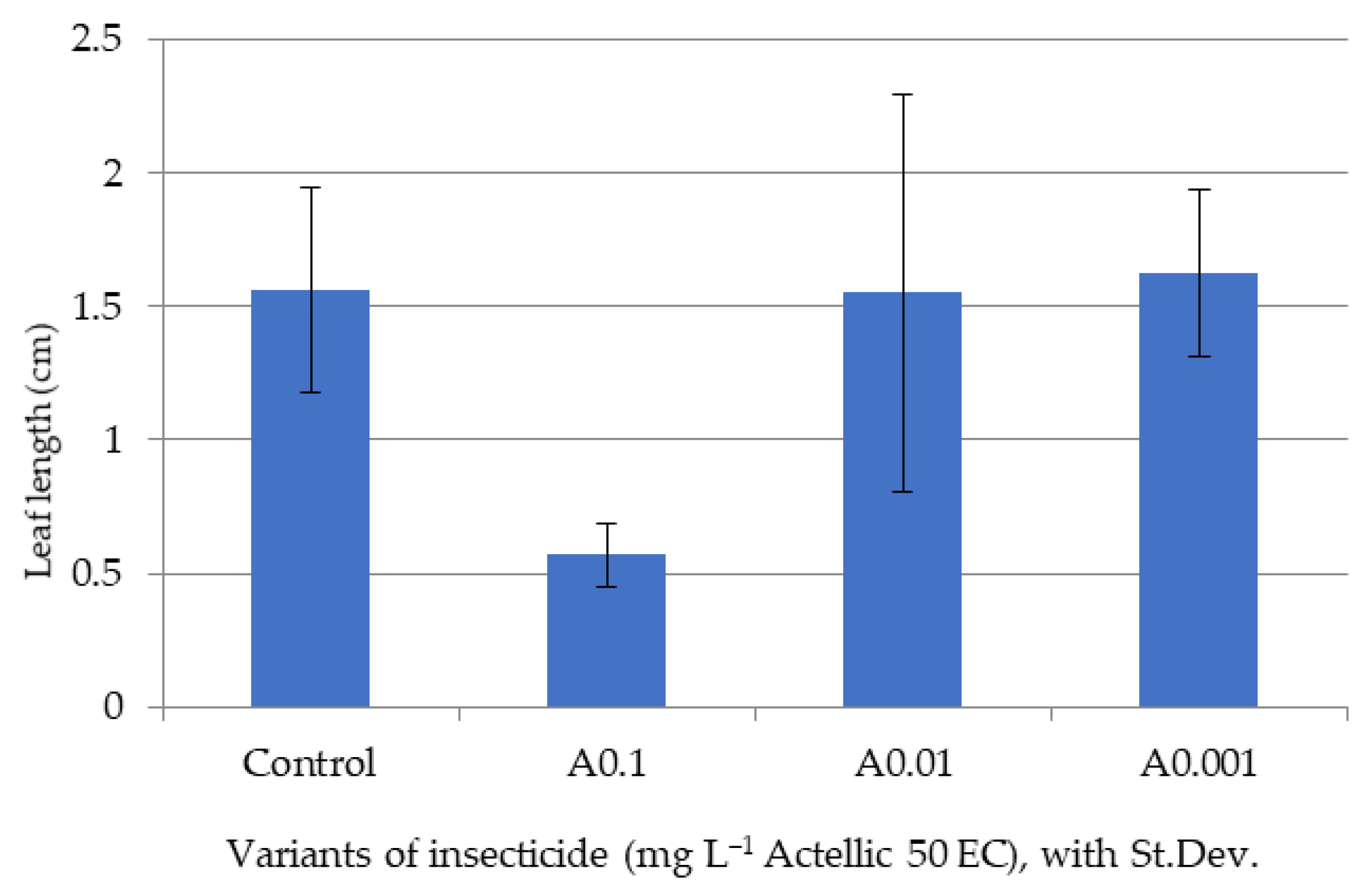
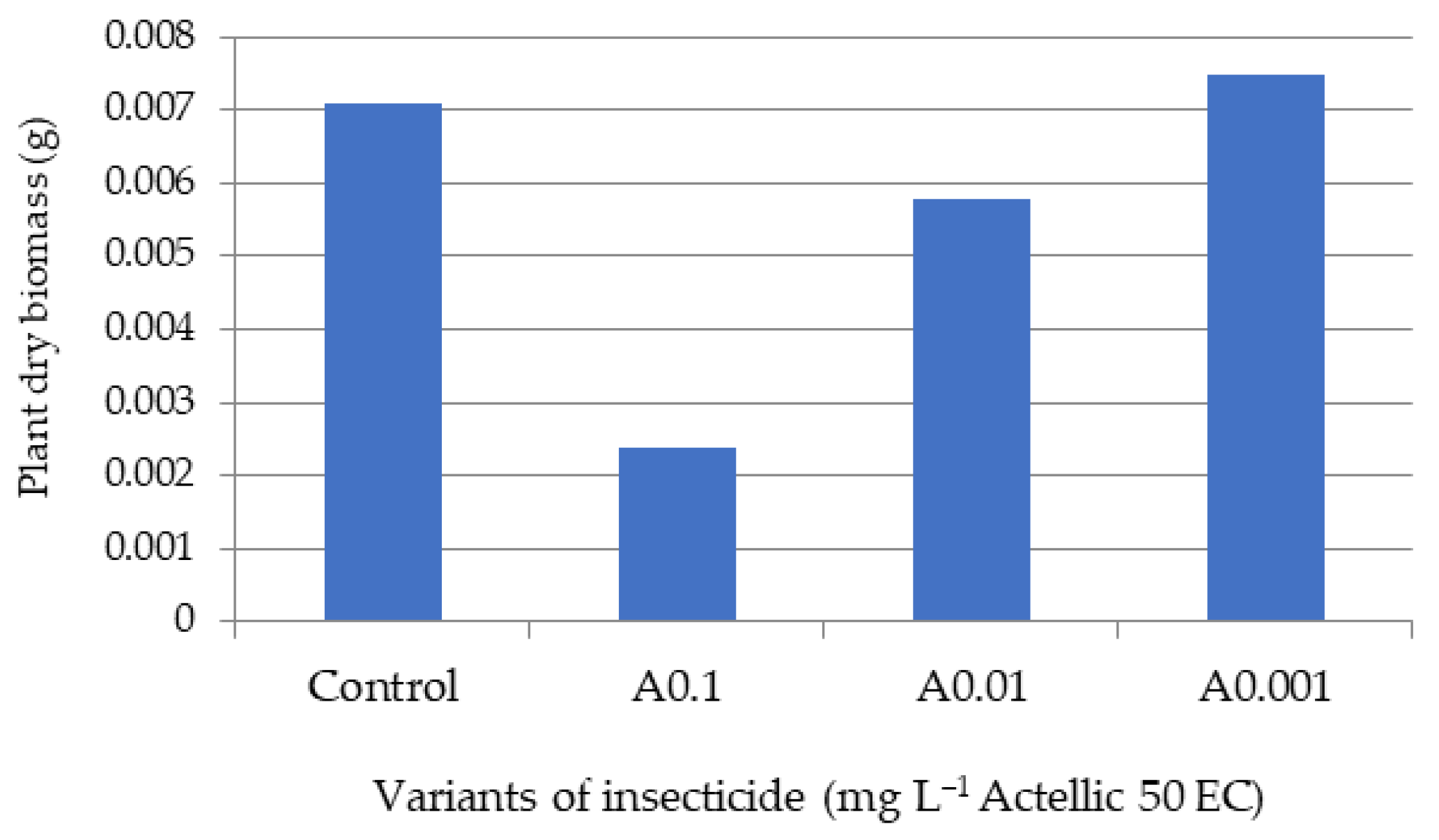
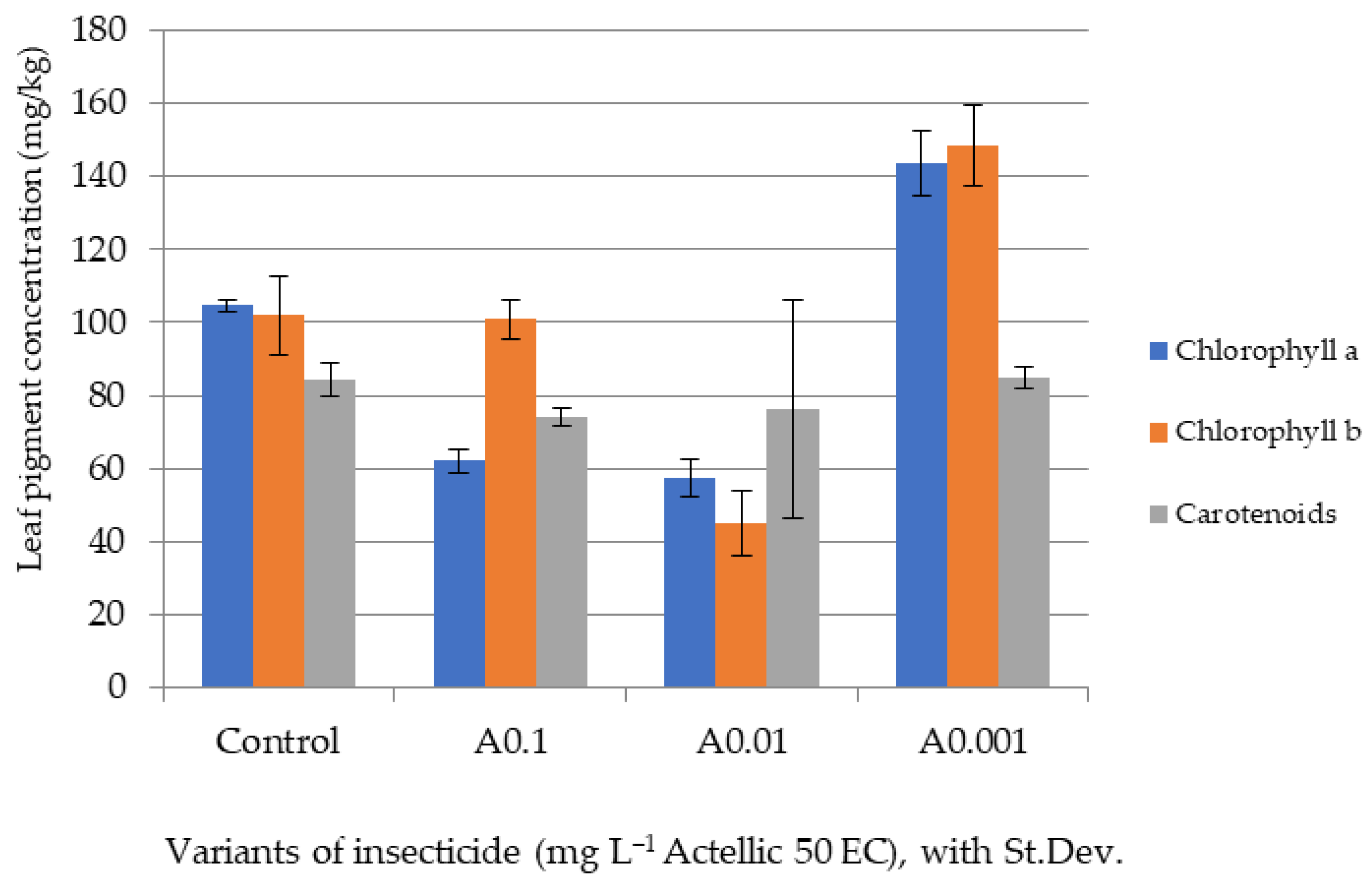


| Triticum aestivum Seeds | Fern Spores | |
|---|---|---|
| Control | Distilled water | Knop solution |
| A1 | 1 mg L−1 of Actellic 50 EC | 1 mg L−1 of Actellic 50 EC |
| A0.1 | 0.1 mg L−1 of Actellic 50 EC | 0.1 mg L−1 of Actellic 50 EC |
| A0.01 | 0.01 mg L−1 of Actellic 50 EC | 0.01 mg L−1 of Actellic 50 EC |
| A0.001 | 0.001 mg L−1 of Actellic 50 EC | 0.001 mg L−1 of Actellic 50 EC |
| Variant | Root | Stem | Fresh Weight | Dry Weight | ||||
|---|---|---|---|---|---|---|---|---|
| 12 h | 24 h | 12 h | 24 h | 12 h | 24 h | 12 h | 24 h | |
| Control | 27.43 ± 3.31 a | 34.27 ± 4.10 a | 31.67 ± 1.87 a | 35.47 ± 2.14 a | 1.02 ± 0.04 ab | 1.20 ± 0.07 a | 0.35 ± 0.02 b | 0.38 ± 0.01 a |
| A1 | 16.53 ± 2.03 b | 10.40 ± 1.51 b | 16.50 ± 1.37 c | 13.70 ± 1.44 c | 0.90 ± 0.05 b | 0.85 ± 0.08 b | 0.35 ± 0.02 ab | 0.39 ± 0.02 a |
| A0.1 | 23.27 ± 1.45 ab | 17.00 ± 1.69 b | 23.83 ± 1.27 b | 25.13 ± 1.30 b | 1.08 ± 0.06 a | 0.98 ± 0.06 ab | 0.40 ± 0.02 a | 0.37 ± 0.01 a |
| A0.01 | 21.87 ± 1.98 ab | 14.07 ± 2.53 b | 24.33 ± 2.00 b | 23.17 ± 2.20 b | 1.00 ± 0.06 ab | 0.96 ± 0.12 ab | 0.37 ± 0.00 ab | 0.38 ± 0.01 a |
| A0.001 | 16.90 ± 2.38 b | 16.00 ± 2.33 b | 23.77 ± 1.74 b | 28.07 ±2.00 b | 0.93 ± 0.02 ab | 1.01 ± 0.07 ab | 0.36 ± 0.02 ab | 0.40 ± 0.02 a |
| F | 3.91 | 12.65 | 10.27 | 18.22 | 2.07 | 2.24 | 2.03 | 0.69 |
| Germination Percentage (%) | ||
|---|---|---|
| Variants | Athyriumfilix-femina | Asplenium scolopendrium |
| Control | 81.33 ± 1.86 a | 75.33 ± 2.33 a |
| A1 | 0.00 | 0.00 |
| A0.1 | 6.67 ± 0.67 d | 1.00 ± 0.58 d |
| A0.01 | 18.00 ± 2.65 c | 13.33 ± 1.86 c |
| A0.001 | 74.00 ± 1.53 b | 60.00 ± 1.73 b |
| F | 565.29 | 508.69 |
| Variants | Athyrium filix-femina | Asplenium scolopendrium |
|---|---|---|
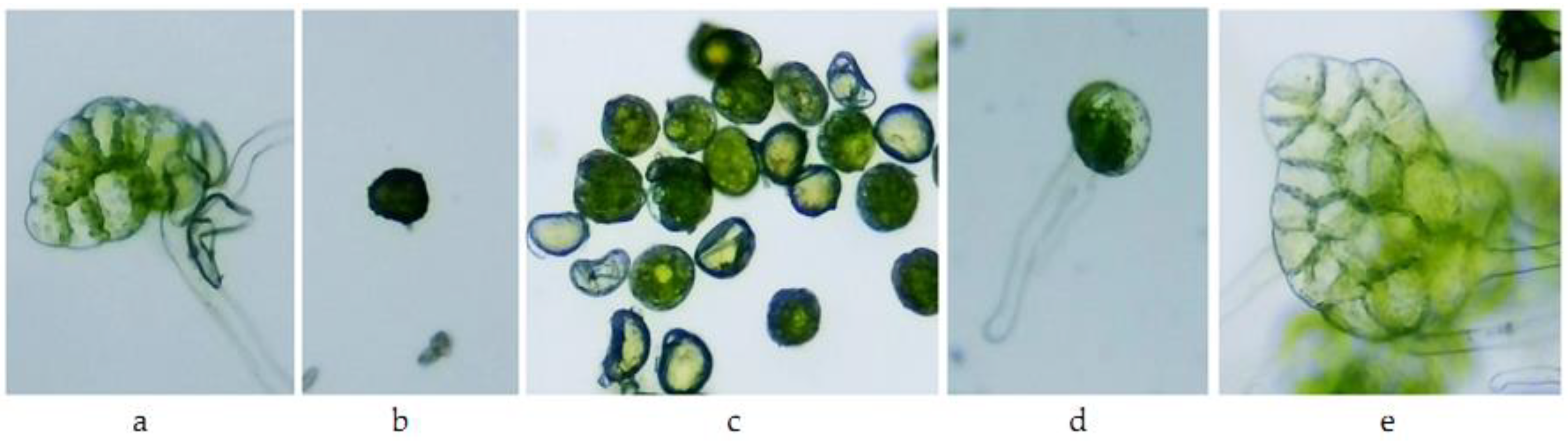
| M | prothallium blade | filament and prothallium blade |
| A1 | ungerminated spores | ungerminated spores |
| A0.1 | ungerminated spores, slightly swollen | ungerminated spores, slightly swollen |
| A0.01 | germinated spores | germinated spores |
| A0.001 | prothallium blade slightly discolored | prothallium blade discolored |
| Lots | 0 h | 24 h | 48 h | 72 h | 96 h | 168 h | 336 h |
|---|---|---|---|---|---|---|---|
| Control | 84.2 ± 2.5 | 82.7 ± 4.22 | 78.86 ± 4.24 | 80.6 ± 2.74 | 83.5 ± 4.56 | 82.4 ± 3.42 | 81.8 ± 6.58 |
| Lot I (Actellic 50EC 0.001 mg L−1) | 81.6 ± 3.48 | 76.7 ± 4.38 * | 74.5 ± 6.48 | 72.78 ± 9.82 * | 74.4 ± 5.26 | 68.3 ± 4.86 * | 64.3 ± 2.48 * |
| Lot II (Actellic 50 EC 0.002 mg L−1 | 88.6 ± 7.22 | 72.6 ± 6.25 * | 65.5 ± 5.48 * | 62.8 ± 6.14 | 65.3 ± 3.92 | 61.3 ± 4.16 * | 56.5 ± 5.48 * |
| Lots | 0 h | 24 h | 48 h | 72 h | 96 h | 168 h | 336 h |
|---|---|---|---|---|---|---|---|
| Control | 142 ± 11.36 | 146 ± 6.82 | 144 ± 5.56 | 148 ± 4.86 | 146 ± 8.56 | 148 ± 5.74 | 138 ± 9.52 |
| Lot I (Actellic 50EC 0.001 mg L−1) | 148 ± 7.62 | 128.7 ± 6.38 * | 122.1 ± 7.58* | 113.78 ± 9.82 * | 94.4 ± 7.56 * | 98.3 ± 7.83 | 111.3 ± 8.65 * |
| Lot II (Actellic 50EC 0.002 mg L−1) | 138.4 ± 11.14 | 107.8 ± 8.65 * | 95.5 ± 7.56 * | 82.4 ± 6.87 * | 74.3 ± 3.94 * | 78.3 ± 4.47 | 88.5 ± 9.84 * |
| Lots | Red Blood Cells/mL Blood | White Blood Cells/mL Blood | Glucose (mg/100 mL Blood) |
| Control lot | 958,625 ± 11,310 | 51,250 ± 5648 | 64.54 ± 9.28 |
| Lot I (Actellic 50 EC 0.001 mg L−1) | 1,106,500 ± 28,550 * | 56,680 ± 6545 * | 74.63 ± 6.68 * |
| Lot II (Actellic 50 EC 0.002 mg L−1) | 1,118,600 ± 35,560 * | 62,480 ± 4850 * | 83.03 ± 7.42 * |
| Actellic 50 EC Concentration (mg/L water) | Percentage of Hatching (Days) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| Control | 0 | 0 | 6.66 | 33.33 | 59.94 | 86.58 | 86.58 | 86.58 | 86.58 |
| Lot I.1 (0.1) | 0 | 0 | 0 | 6.66 * | 26.64 * | 33.33 * | 56.61 * | 63.27 * | 66.6 * |
| Lot I.2 (0.01) | 0 | 0 | 0 | 29.95 * | 43.27 * | 59.92 * | 63.25 * | 73.25 * | 73.25 * |
| Lot I.3 (0.001) | 0 | 0 | 3.33 | 36.63 | 59.94 | 73.25 | 73.25 | 82.58 | 82.58 |
| Variable | Experimental lots | ||||||
|---|---|---|---|---|---|---|---|
| Start Point | 24 h | 48 h | 72 h | 96 h | 120 h | ||
| Percentage of survival | Control II 0.1 mg L−1Actellic 50 EC 0.01 mg L−1Actellic 50 EC 0.001 mg L−1Actellic 50 EC | 100 100 100 100 | 100 70 * 80 * 100 | 100 70 * 80 * 100 | 100 60 * 80 * 100 | 100 60 * 70 * 100 | 100 50 * 70 * 100 |
| Variable (Mean ± SD) | Control II | Lot II.1 | Lot II.2 | Lot II.3 |
|---|---|---|---|---|
| Volume of the tadpoles (mL) | 0.604 ± 0.020 a | 0.475 ± 0.029 d | 0.501 ± 0.015 c | 0.566 ± 0.018 b |
| Body length of the tadpoles (cm) | 3.74 ± 0.126 a | 3.1 ± 0.105 c | 3.4 ± 0.176 b | 3.66 ± 0.117 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paunescu, A.; Ponepal, C.M.; Tofan, L.; Brinzea, G.; Tantu, M.M.; Mihaescu, C.F.; Draghiceanu, O.A.; Popoviciu, D.R.; Fagaras, M.M.; Vasile, D.; et al. Ecotoxicological Risk Assessment of Actellic 50 EC Insecticide on Non-Target Organisms in Parallel with the Application of Standardized Tests. Toxics 2022, 10, 745. https://doi.org/10.3390/toxics10120745
Paunescu A, Ponepal CM, Tofan L, Brinzea G, Tantu MM, Mihaescu CF, Draghiceanu OA, Popoviciu DR, Fagaras MM, Vasile D, et al. Ecotoxicological Risk Assessment of Actellic 50 EC Insecticide on Non-Target Organisms in Parallel with the Application of Standardized Tests. Toxics. 2022; 10(12):745. https://doi.org/10.3390/toxics10120745
Chicago/Turabian StylePaunescu, Alina, Cristina Maria Ponepal, Lucica Tofan, Gheorghita Brinzea, Monica Marilena Tantu, Cristina Florina Mihaescu, Oana Alexandra Draghiceanu, Dan Razvan Popoviciu, Marius Mirodon Fagaras, Daniela Vasile, and et al. 2022. "Ecotoxicological Risk Assessment of Actellic 50 EC Insecticide on Non-Target Organisms in Parallel with the Application of Standardized Tests" Toxics 10, no. 12: 745. https://doi.org/10.3390/toxics10120745
APA StylePaunescu, A., Ponepal, C. M., Tofan, L., Brinzea, G., Tantu, M. M., Mihaescu, C. F., Draghiceanu, O. A., Popoviciu, D. R., Fagaras, M. M., Vasile, D., & Soare, L. C. (2022). Ecotoxicological Risk Assessment of Actellic 50 EC Insecticide on Non-Target Organisms in Parallel with the Application of Standardized Tests. Toxics, 10(12), 745. https://doi.org/10.3390/toxics10120745







