Contemporary Comprehensive Review on Arsenic-Induced Male Reproductive Toxicity and Mechanisms of Phytonutrient Intervention
Abstract
1. Introduction
1.1. Exposure to Arsenic in the Environment
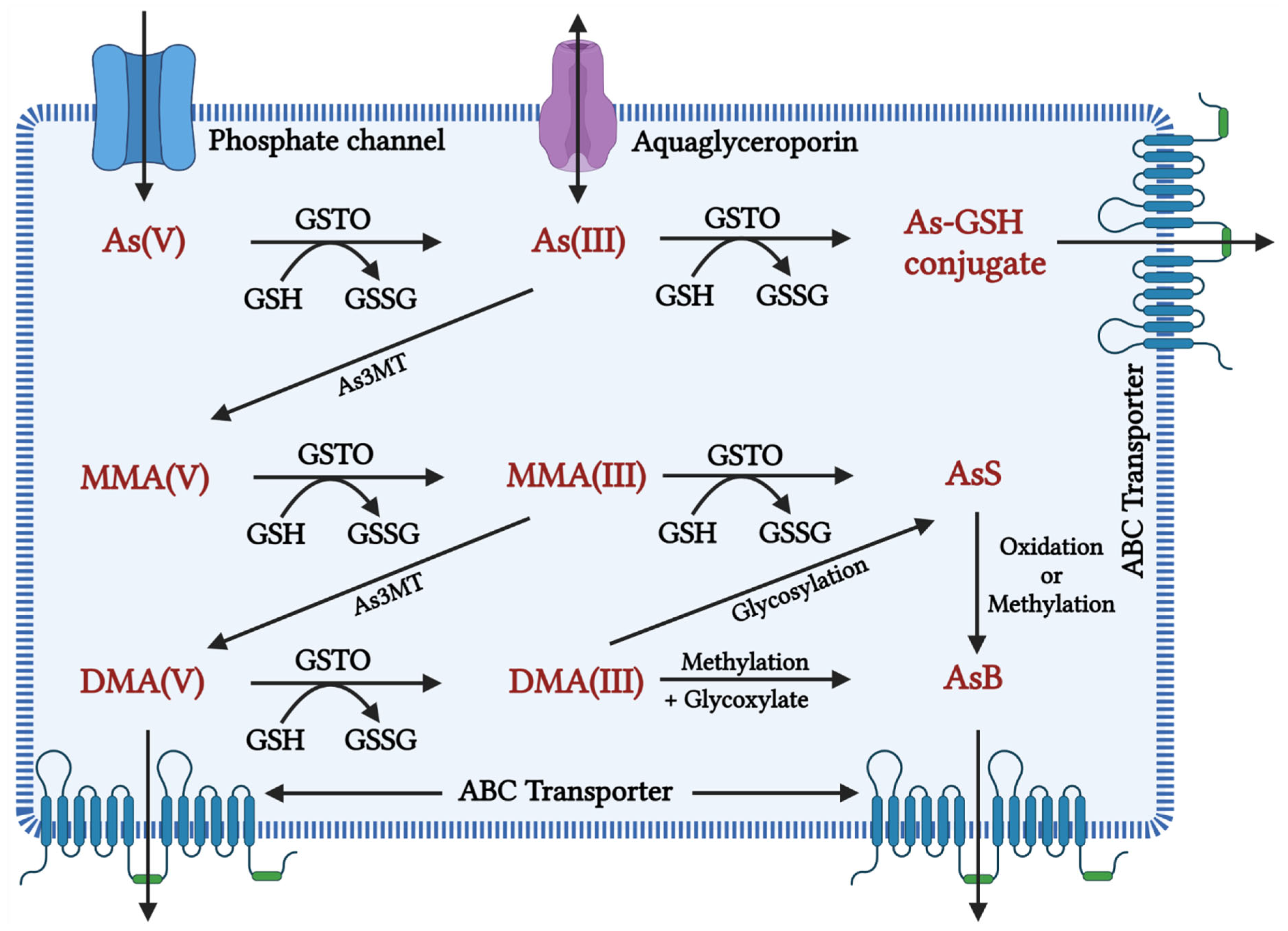
1.2. Metabolism of Arsenic
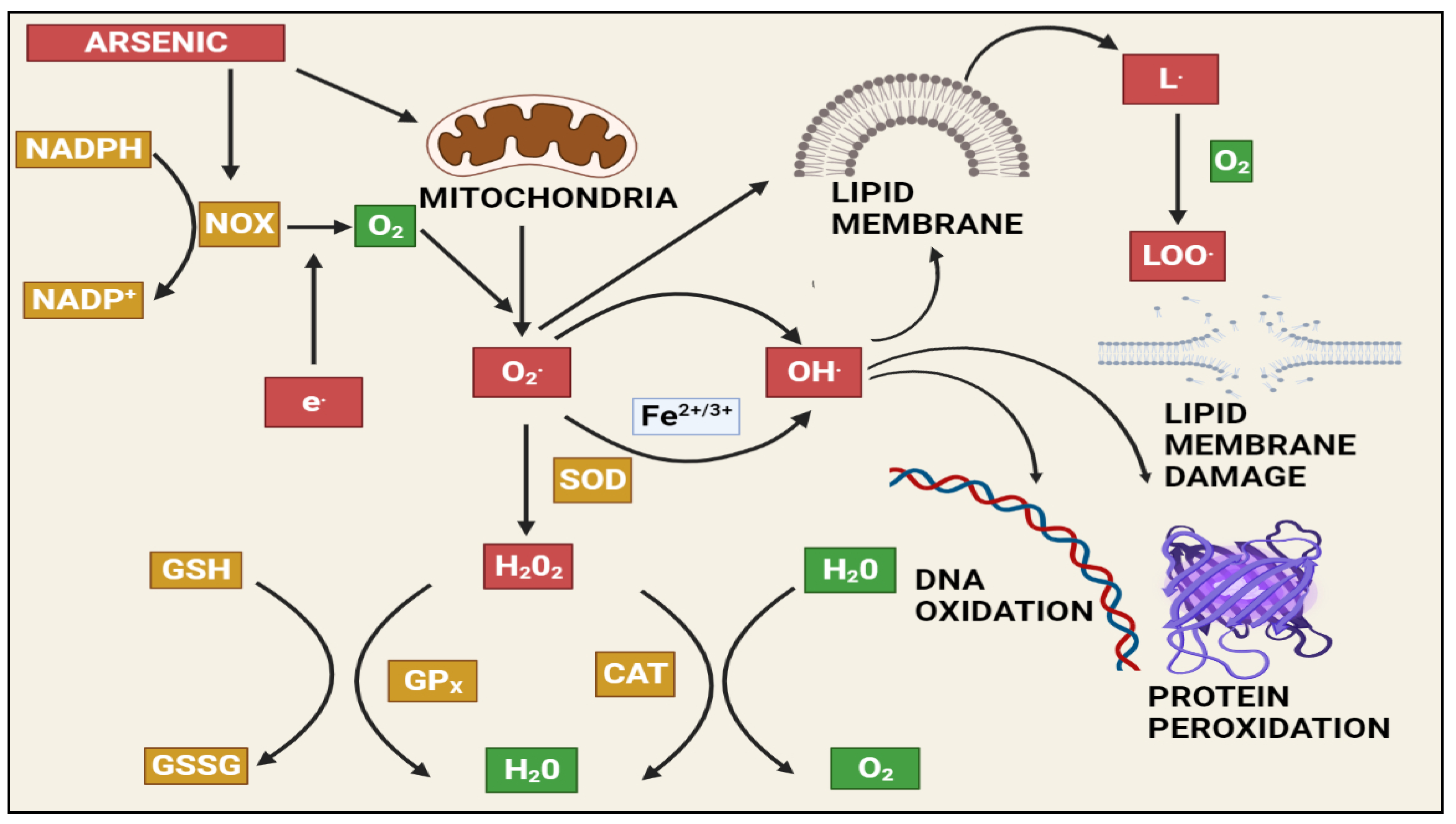
1.3. Arsenic-Induced Oxidative Stress and DNA Damage
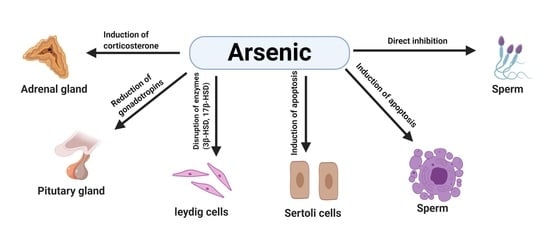
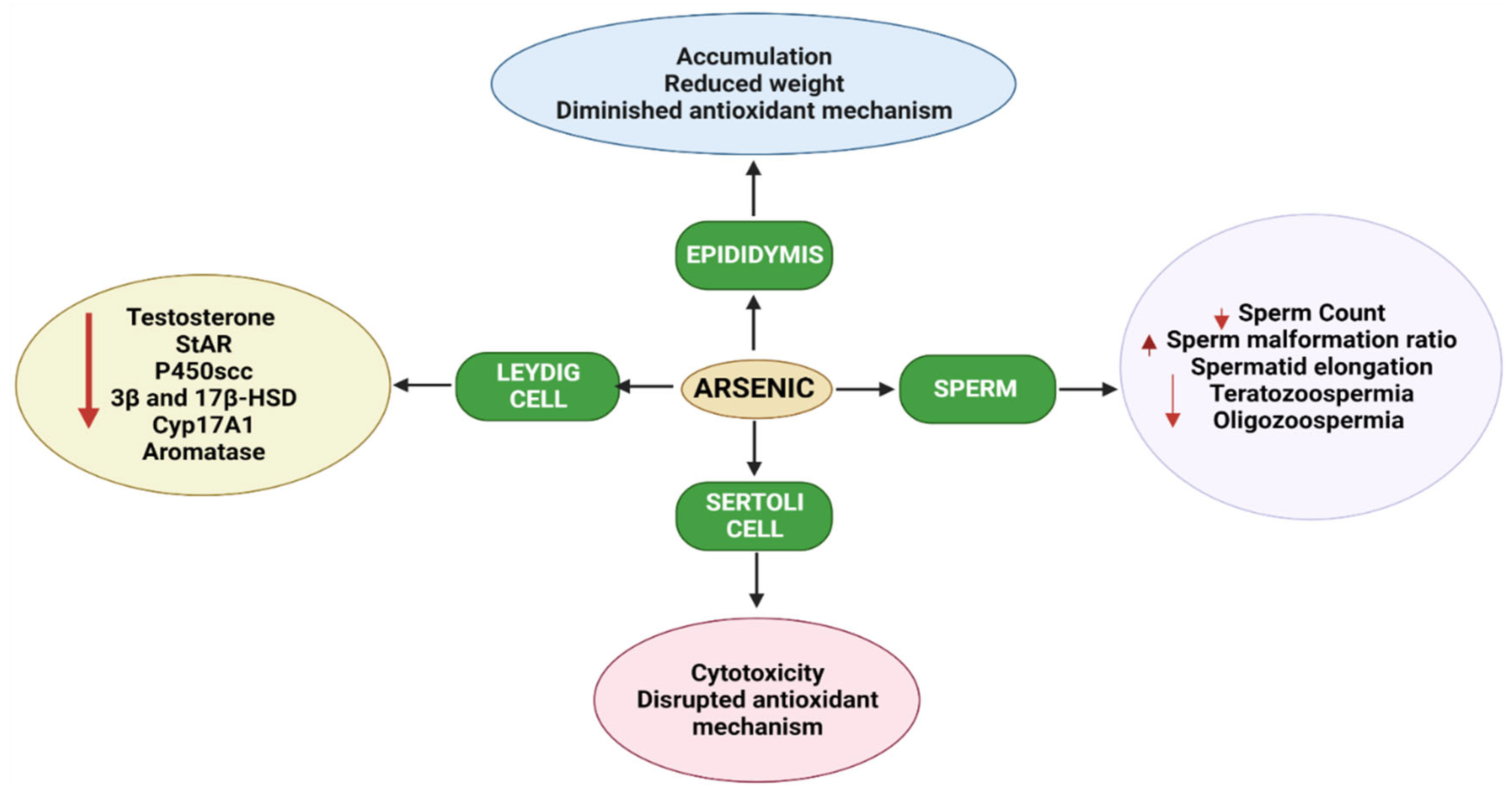
1.4. Effects of Arsenic on the Male Reproductive System in Animals
1.5. Pre-Natal Arsenic Exposure Induced Male Reproductive Toxicity in Animals
| S. No | Test Organism | Arsenic Species | Exposure Regimen | Route of Exposure | Duration of Exposure | Organ/Tissue/Cell Line | Observations | Reference |
|---|---|---|---|---|---|---|---|---|
| RODENTS | ||||||||
| 1 | Mice | As trioxide | 0.3 and 3 mg/kg bw | Subcutaneous | 35 days | Testes | Decreased sperm count, increased seminiferous tubule’s epithelial aberration, and exfoliation of germ cells, altered nucleus/cytoplasm ratio of Leydig cells. | [131] |
| 2 | Mice | As trioxide | 0.2, 2, and 20 mg/kg bw | Drinking water | 180 days | Testes | Reduced sperm motility, altered ultra-structure of acrosome and sperm tail. | [132] |
| 3 | Mice | As trioxide | 0.2, 2, and 20 mg/kg bw | Oral | 180 days | Testes | Reduced spermatid elongation, decreased DDX25 and CRM1 mRNA expression and HMG2 and PGK2 proteins expression. | [133] |
| 4 | Mice | As trioxide | 0.0003, 0.0015, 0.015, and 0.03 mg/kg bw | Oral | 15 days | Sperm | Abnormal head morphology. | [134] |
| 5 | Mice | Sodium arsenate dibasic heptahydrate | 10, 25, 50, 100, and 200 mg/kg bw | Oral | 40 days | Testes and sperm | Decreased sperm kinetics, viability, plasma membrane integrity. Altered SOD, CAT, and GST levels. Reduced sperm count. | [136] |
| 6 | Mice | As trioxide | 0.2, 2, 20 mg/kg bw | Oral | ≈123 days | Testes | Enhanced PI3K, Atg5, Atg12 gene expressions, Increased Beclin1, LC3-I, LC3-II, and p62 protein expressions (F1 generation). | [41] |
| 7 | Mice | As trioxide | 0.2, 2, 20 mg/kg bw | Oral | ≈123 days | Increased number of MDC-labeled autophagic vacuoles, and MDA/GSH ratio in HPG axis of pubertal F1 male in highest dose treated animals. A dose-dependent increase expression of ATG3, ATG5, Beclin genes, protein expression of P62 ATG12, and Becline. Decreased gene expression of PI3K and mTOR gene expression was recorded in the HPG tissues of puberty F1 males. | [37] | |
| 8 | Mice | Sodium arsenite | 0.05 and 1 mg/kg bw | Oral | 1, 2, and 3 days | Testes | Cytotoxicity and disrupted antioxidant mechanisms in the Leydig cells and Sertoli cells. | [144] |
| 9 | Mice | Sodium arsenite | 5 and 50 mg/kg bw | Oral | 180 days | Testes | Reduced LHR, StAR, 3β-HSD, and 17β-HSD expression. Downregulation of StAR, 17β-HSD, and Ddx3y mRNA. | [145] |
| 10 | Mice | As trioxide and antimony | 4 and 15 mg/kg bw | Oral | 60 days | Testes and sperm | Altered sperm count, morphology, survival, testosterone level. Reduced germ cell count, T-AOC, SOD, and MsrB 1 levels. Upregulation of Beclin1, Atg-5, LC3B/LC3A, Caspase-8, Cytc, CC-3, p53, Bax | [146] |
| 11 | Diabetic rats | Sodium arsenite | 10 mg/L | Oral | 40 days | Testes and sperm | Decreased serum testosterone, daily sperm production, motility, and morphology. Impairment of acrosome and plasma membrane integrity. | [135] |
| 12 | Rat | Sodium arsenite | 0.01 and 10 mg/L | Oral | 32 days (PND 21 to PND 53) | Testes | Increased vacuolisation, acidophilic cells, and epithelial degeneration. Increased testicular fluid and inflammatory infiltration. | [125] |
| 13 | Rat | Sodium arsenite | 5 mg/kg bw | Oral | 56 days | Testes | Decreased testicular weights and seminiferous tubule diameter | [16] |
| 14 | Rat | Sodium arsenite | 10 mg/L | Oral | 56 days | Testes | Decreased sperm counts and enhanced sperm head abnormalities. Infertility risk and pre-implantation loss. | [130] |
| 15 | Rat | Sodium arsenite | 1, 5 and 25 mg/L | Oral | 180 days | Testes | Down-regulation Lhr, Star, P450scc, Hsd3b, Cyp17a1, Hsd17b, and Aromatase mRNA expressions. Upregulation H3K9me3 methyltransferase, Suv39h1. Down-regulation of demethylase and Jmjd2a. | [147] |
| 16 | Rat | Sodium arsenite | 10 mg/L | Oral | 30 days (PND 21 to 51) | Testes and epididymis | Overexpression of SOD1, SOD2, CAT, GSTK1, and MT1 in testes and SOD1, CAT, and GSTK1 in epididymis. | [148] |
| 17 | Rat | Sodium arsenite and arsenate | 0.01 and 10 mg/L | Oral | 56 days | Testes | Decreased CAT activity. Increased vacuolisation in seminiferous tubule. | [127] |
| 18 | Rat | Sodium arsenite | 0.01 and 10 mg/L | Oral | 20 days (PND 23 to 53) | Prostate | Tissue damage and delayed maturation of prostate. | [147] |
| 19 | Rat | Sodium fluoride and sodium arsenite | 100 and 50 mg/L | Oral | 113 days | Testes | Reduced FSH, LH, and testosterone levels. Increased Beclin1 and LC3 expression. Decreased p62 expression. | [143] |
| 20 | Rat | Lead and sodium arsenite | 819 and 2.3 mg/L | Oral | 60 days (PND 55–115) | Testes | Decreased reproductive organ weights and daily sperm production. Decreased 3β-HSD and 17β-HSD activities. | [142] |
| 21 | Rat | Sodium arsenite | 1, 5, 25 mg/L drinking water | Oral | 180 days | Reproductive parameters | Compromised sperm counts and motility, serum testosterone. Alteration in proteins related to reproduction such as Vdac3, Prkaca, Hspa41, Spaca1, Ma1b, Gpx4, Safb1, Trim28, Rbp1, Hsd11b1, Mapk3, Gpd2, Ace, Hspa11, Dnaja1, Ybx3, Smcp, Nasp, Cabs1. | [104] |
| 22 | Rat | As trioxide | 1 mg/mL | Oral | 112 days | Testes | Alterations in methylation patterns and reproductive parameters. Morphological aberration in ovaries (F0 and F1) and testicles (F1–F3). Decreased sperm quality (F0–F3, except F2). | [138] |
| 23 | Rat | Sodium arsenite | 10 mg/L | Oral | 21 days (GD 1–21) | Testis and Epididymis | Changes in SOD1, SOD2, CAT, and GSTK1 gene expression. Altered SOD, Catalase, and GSH activities. | [139] |
| 24 | Rat | Sodium arsenite | 0.01 and 10 mg/L | Oral | 56 days | Epididymis | Reduced daily sperm production, number of spermatids | [127] |
| 25 | Rat | Sodium arsenite | 10 mg/L | Oral | 30 days (PND 52 to PND 81) | Epididymis | Lower sperm production, sperm count, motility and quality. | [140] |
| CHICKEN | ||||||||
| 26 | Chicken | As trioxide | 7.5, 15, and 30 mg/kg bw | Oral | 30, 60, and 90 days | Testes | Increased NF-Kβ, TNF-α, i-NOS, COX-2, and PTGEs mRNA over expressions. Increased Hsp70 and HSp90 mRNA expressions. | [149] |
| 27 | Chicken | Copper sulphate and As trioxide | 300 and 30 mg/kg bw | Oral | 28, 56, and 84 days | Testes | Increased mRNA levels of pro-inflammatory cytokines and inflammatory factors. Increased mRNA and protein levels of Hsp60, Hsp70, and Hsp90 | [150] |
| 28 | Chicken | As trioxide | 0.625, 1.25, and 2.5 mg/kg bw | Oral | 30, 60 and 90 days | Testes | Enhanced LC-III, dynein, Beclin-1, ATG-5, and ATG4B expression. | [151] |
| CELL LINE | ||||||||
| 29 | MLTC-1 Line | As trioxide | 3, 6 and 9 µM | NA | 1 day | Leydig cell | Accumulation of autophagosomes. | [152] |
| 30 | MLTC-1 Line | Sodium arsenite | 1, 2, and 4 mg/L | NA | 2 days | Leydig cell | Enhanced mRNA and protein expression levels of 3β-HSD by suppressing H3K9me2/3, whereas genes Star, P450scc, P45-c17, and 17β-HSD were downregulated. | [153] |
| 31 | GC-1 Spermatogonial (SPG) cell line | As trioxide | 10, 20 µM | NA | 1 day | Spermatogonia | Damaged mitochondria upregulated ATG3, p62, LC-3I, and LC-3II mRNA expressions. | [154] |
2. Mechanisms of Arsenic-Induced Male Reproductive Toxicity in Animals
2.1. Arsenic-Induced Oxidative Stress
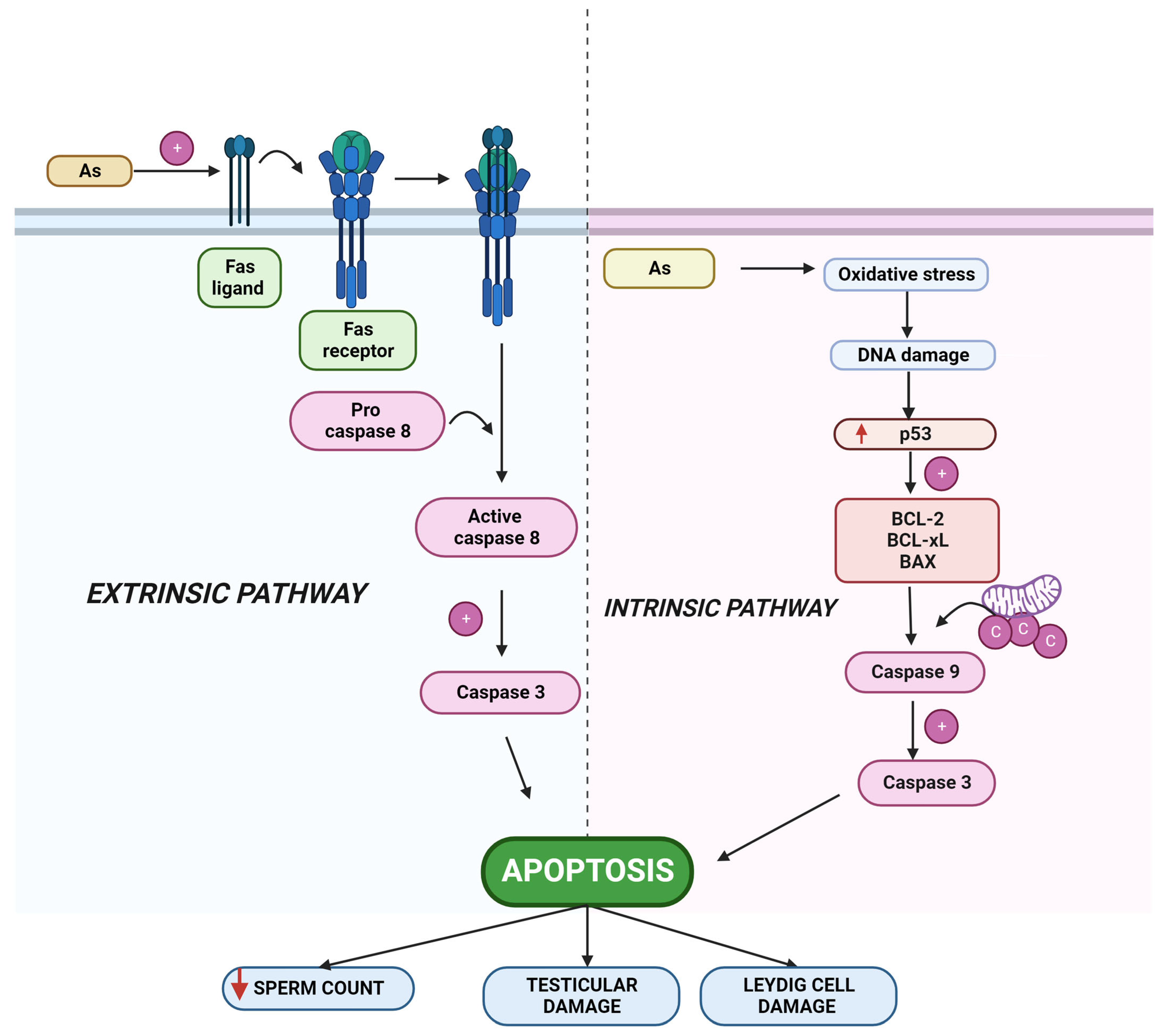
2.2. Arsenic-Induced Apoptosis
2.3. Arsenic-Induced Autophagy
2.4. Arsenic-Induced Inflammation
3. Effects of Prenatal Exposure to Arsenic in Humans
3.1. Effects of Arsenic on the Male Reproductive System in Humans
3.2. Ameliorating Agents for Arsenic Toxicity
3.3. Other Phytonutrients That Promote Male Fertility
4. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SGA | Small-for-gestational age |
| WHO | World Health Organization |
| EPA | Environmental Protection Agency |
| IARC | International Agency for Research on Cancer |
| AS2O3 | Arsenic trioxide |
| CML | Chronic myelogenous leukaemia |
| APL | Acute promyelocytic leukaemia |
| TBARS | Thiobarbituric acid reactive substances |
| HPG axis | The hypothalamic–pituitary–gonadal axis |
| StAR | The steroidogenic acute regulatory |
| 3β-HSD | 3β-Hydroxysteroid dehydrogenase |
| GIT | Gastrointestinal tract |
| AQP | Aquaglyceroporins |
| MFS | Major facilitator superfamily |
| ABC | ATP-binding cassette |
| As3MT | Arsenite methyltransferase |
| MMA | Monomethylarsonic acid |
| DMA | Dimethylarsinic acid |
| As(V) | Inorganic pentavalent arsenic |
| As(III) | Inorganic trivalent arsenic |
| MMA(V) | Methyl arsonate |
| DMA(V) | Dimethyl arsenate |
| GSTO | Glutathione S-transferase omega |
| GSSG | Glutathione disulfide |
| As-GSH | Arsenic glutathione |
| AsS | Arsenic sulphide |
| AsB | Arsenobetaine |
| ROS | Reactive oxygen species |
| ICR | Institute of Cancer Research |
| CC3 | Cleaved caspase 3 |
| CYP17A1 | Cytochrome P450 17A1 |
| DHT | Dihydrotestosterone |
| SD | Sprague Dawley |
| MLTC-1 | Mouse testes Leydig tumor cell lines |
| MDA | Malondialdehyde |
| NAPDH | Nicotinamide adenine dinucleotide phosphate (NADP+) |
| NOX | NAPDH oxidase |
| GPx | Glutathione peroxidase |
| SOD | Supeoxide dismutase |
| CAT | Catalase |
| H2O2 | Hydrogen peroxide |
| LOO | Lipid peroxy radical |
| UMI | Unexplained male infertility |
| VLBW | Very low birth weight |
| PTB | Preterm birth |
| EGCG | Epigallocatechin-3-gallate |
| SMI | Structural membrane integrity |
| FMI | Functional membrane integrity |
| NAC | N-Acetyl Cysteine |
| MSTD | Mean seminiferous tubular diameter |
| MTBS | Mean testicular biopsy scores |
| PCNA | Proliferating cell nuclear antigen |
| DPDS | Diphenyl diselenide |
| BTB | Bllod testicular border |
References
- Rossy, K.M.; Janusz, C.A.; Schwartz, R.A. Cryptic Exposure to Arsenic. Indian J. Dermatol. Venereol. Leprol. 2005, 71, 230–235. [Google Scholar] [PubMed]
- Verma, N.; Rachamalla, M.; Sravan Kumar, P.; Dua, K. Assessment and Impact of Metal Toxicity on Wildlife and Human Health; Woodhead Publishing: Sawston, UK, 2023; pp. 93–110. [Google Scholar] [CrossRef]
- Mandal, B.K.; Suzuki, K.T. Arsenic Round the World: A Review. Talanta 2002, 58, 201–235. [Google Scholar] [CrossRef] [PubMed]
- Antman, K.H. Introduction: The History of Arsenic Trioxide in Cancer Therapy. Oncologist 2001, 6, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Chen, Z.; Lallemand-Breitenbach, V.; de Thé, H. Timeline: How Acute Promyelocytic Leukaemia Revived Arsenic. Nat. Rev. Cancer 2002, 2, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, S.; Frankenberger, W.T. Environmental Biochemistry of Arsenic. Rev. Environ. Contam. Toxicol. 1992, 124, 79–110. [Google Scholar] [CrossRef] [PubMed]
- Sears, M.E.; Kerr, K.J.; Bray, R.I. Arsenic, Cadmium, Lead, and Mercury in Sweat: A Systematic Review. J. Environ. Public Health 2012, 2012, 1–10. [Google Scholar] [CrossRef]
- Leist, M.; Casey, R.; Caridi, D. The management of arsenic wastes: Problems and prospects. J. Hazard. Mater. 2000, 76, 125–138. [Google Scholar] [CrossRef]
- Waxman, S.; Anderson, K.C. History of the Development of Arsenic Derivatives in Cancer Therapy. Oncologist 2001, 6, 3–10. [Google Scholar] [CrossRef]
- Renu, K.; Madhyastha, H.; Madhyastha, R.; Maruyama, M.; Vinayagam, S.; Gopalakrishnan, A.V. Review on molecular and biochemical insights of arsenic-mediated male reproductive toxicity. Life Sci. 2018, 212, 37–58. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Kim, J.-M. Arsenic Toxicity in Male Reproduction and Development. Dev. Reprod. 2015, 19, 167–180. [Google Scholar] [CrossRef]
- Chen, Q.Y.; Costa, M. Arsenic: A Global Environmental Challenge. Annu. Rev. Pharmacol. Toxicol. 2021, 61, 47–63. [Google Scholar] [CrossRef] [PubMed]
- Abdul, K.S.M.; Jayasinghe, S.S.; Chandana, E.P.S.; Jayasumana, C.; De Silva, P.M.C.S. Arsenic and human health effects: A review. Environ. Toxicol. Pharmacol. 2015, 40, 828–846. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.-Y.; Yu, S.-D.; Hong, Y.-S. Environmental Source of Arsenic Exposure. J. Prev. Med. Public Health 2014, 47, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Oremland, R.S.; Stolz, J.F. The Ecology of Arsenic. Science 2003, 300, 939–944. [Google Scholar] [CrossRef]
- Ramos, A.T.D.A.; Diamante, M.A.S.; Lamas, C.D.A.; Dolder, H.; Predes, F.D.S. Morphological and morphometrical changes on adult Wistar rat testis caused by chronic sodium arsenite exposure. Environ. Sci. Pollut. Res. 2017, 24, 27905–27912. [Google Scholar] [CrossRef]
- Xu, W.; Bao, H.; Liu, F.; Liu, L.; Zhu, Y.-G.; She, J.; Dong, S.; Cai, M.; Li, L.; Li, C.; et al. Environmental exposure to arsenic may reduce human semen quality: Associations derived from a Chinese cross-sectional study. Environ. Health 2012, 11, 46. [Google Scholar] [CrossRef]
- Quansah, R.; Armah, F.; Essumang, D.K.; Luginaah, I.; Clarke, E.; Marfoh, K.; Cobbina, S.J.; Nketiah-Amponsah, E.; Namujju, P.B.; Obiri, S.; et al. Association of Arsenic with Adverse Pregnancy Outcomes/Infant Mortality: A Systematic Review and Meta-Analysis. Environ. Health Perspect. 2015, 123, 412–421. [Google Scholar] [CrossRef]
- Podgorski, J.; Berg, M. Global threat of arsenic in groundwater. Science 2020, 368, 845–850. [Google Scholar] [CrossRef]
- Vidosavljevic, M.; Puntaric, D.; Gvozdic, V.; Vidosavljevic, D.; Juric, D.; Begovic, L. Assessment of Arsenic in Hair of the Inhabitants of East Croatia—Relationship to Arsenic Concentrations in Drinking Water. Water 2022, 14, 1558. [Google Scholar] [CrossRef]
- Rahman, M.; Tondel, M.; Ahmad, S.A.; Axelson, O. Diabetes Mellitus Associated with Arsenic Exposure in Bangladesh. Am. J. Epidemiol. 1998, 148, 198–203. [Google Scholar] [CrossRef]
- Kile, M.L.; Christiani, D.C. Environmental Arsenic Exposure and Diabetes. JAMA-J. Am. Med. Assoc. 2008, 300, 845–846. [Google Scholar] [CrossRef] [PubMed]
- Hopenhayn, C.; Ferreccio, C.; Browning, S.R.; Huang, B.; Peralta, C.; Gibb, H.; Hertz-Picciotto, I. Arsenic Exposure from Drinking Water and Birth Weight. Epidemiology 2013, 14, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Bardach, A.E.; Ciapponi, A.; Soto, N.; Chaparro, M.R.; Calderon, M.; Briatore, A.; Cadoppi, N.; Tassara, R.; Litter, M.I. Epidemiology of Chronic Disease Related to Arsenic in Argentina: A Systematic Review. Sci. Total Environ. 2015, 538, 802–816. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.H.; Lopipero, P.A.; Bates, M.N.; Steinmaus, C.M. Arsenic Epidemiology and Drinking Water Standards. Science 2002, 296, 2145–2146. [Google Scholar] [CrossRef]
- Rahman, M.M.; Chowdhury, U.K.; Mukherjee, S.C.; Mondal, B.K.; Paul, K.; Lodh, D.; Biswas, B.K.; Chanda, C.R.; Basu, G.K.; Saha, K.C.; et al. Chronic Arsenic Toxicity in Bangladesh and West Bengal, India—A Review and Commentary. J. Toxicol. Clin. Toxicol. 2001, 39, 683–700. [Google Scholar] [CrossRef]
- Mazumder, D.N.G. Chronic Arsenic Toxicity: Clinical Features, Epidemiology, and Treatment: Experience in West Bengal. J. Environ. Sci. Health Part A Tox Hazard. Subst. Environ. Eng. 2003, 38, 141–163. [Google Scholar] [CrossRef]
- Singh, A.; Ghosh, A.K. Groundwater Arsenic Contamination and Its Implications: A Case Study of Shahpur Block of Bhojpur District, Bihar. Int. J. Mod. Eng. Res. 2014, 4, 8. [Google Scholar]
- Chakraborti, D.; Singh, S.K.; Rahman, M.M.; Dutta, R.N.; Mukherjee, S.C.; Pati, S.; Kar, P.B. Groundwater Arsenic Contamination in the Ganga River Basin: A Future Health Danger. Int. J. Environ. Res. Public Heal. 2018, 15, 180. [Google Scholar] [CrossRef]
- Yang, H.-C.; Rosen, B.P. New mechanisms of bacterial arsenic resistance. Biomed. J. 2016, 39, 5–13. [Google Scholar] [CrossRef]
- Palma-Lara, I.; Martínez-Castillo, M.; Quintana-Pérez, J.; Arellano-Mendoza, M.; Tamay-Cach, F.; Valenzuela-Limón, O.; García-Montalvo, E.; Hernández-Zavala, A. Arsenic exposure: A public health problem leading to several cancers. Regul. Toxicol. Pharmacol. 2020, 110, 104539. [Google Scholar] [CrossRef]
- Wang, W.; Cheng, S.; Zhang, D. Association of inorganic arsenic exposure with liver cancer mortality: A meta-analysis. Environ. Res. 2014, 135, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, A.P.F.; Udoh, K.T.; States, J.C. Arsenic-induced changes in miRNA expression in cancer and other diseases. Toxicol. Appl. Pharmacol. 2020, 409, 115306. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.M.; Chowdhury, A.; Arnold, L.L. Inorganic arsenic: A non-genotoxic carcinogen. J. Environ. Sci. 2016, 49, 28–37. [Google Scholar] [CrossRef]
- Martinez, V.D.; Vucic, E.A.; Becker-Santos, D.D.; Gil, L.; Lam, W.L. Arsenic Exposure and the Induction of Human Cancers. J. Toxicol. 2011, 2011, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Darbandi, M.P.; Taheri, J. Using Sulfur-Containing minerals in medicine: Iranian traditional documents and modern pharmaceutical terminology. Earth Sci. Hist. 2018, 37, 25–33. [Google Scholar] [CrossRef]
- Ommati, M.; Heidari, R.; Manthari, R.K.; Chiranjeevi, S.T.; Niu, R.; Sun, Z.; Sabouri, S.; Zamiri, M.; Zaker, L.; Yuan, J.; et al. Paternal exposure to arsenic resulted in oxidative stress, autophagy, and mitochondrial impairments in the HPG axis of pubertal male offspring. Chemosphere 2019, 236, 124325. [Google Scholar] [CrossRef]
- Leu, L.; Mohassel, L. Arsenic Trioxide as First-Line Treatment for Acute Promyelocytic Leukemia. Am. J. Health-Syst. Pharm. 2009, 66, 1913–1918. [Google Scholar] [CrossRef]
- Ratnaike, R.N. Acute and chronic arsenic toxicity. Postgrad. Med. J. 2003, 79, 391–396. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Baldwin, L.A. Inorganics and Hormesis. Crit. Rev. Toxicol. 2003, 33, 215–304. [Google Scholar] [CrossRef]
- Ommati, M.M.; Manthari, R.K.; Tikka, C.; Niu, R.; Sun, Z.; Sabouri, S.; Zamiri, M.J.; Ahmadi, H.N.; Ghaffari, H.; Heidari, R.; et al. Arsenic-induced autophagic alterations and mitochondrial impairments in HPG-S axis of mature male mice offspring (F1-generation): A persistent toxicity study. Toxicol. Lett. 2020, 326, 83–98. [Google Scholar] [CrossRef]
- Ommati, M.M.; Shi, X.; Li, H.; Zamiri, M.J.; Farshad, O.; Jamshidzadeh, A.; Heidari, R.; Ghaffari, H.; Zaker, L.; Sabouri, S.; et al. The mechanisms of arsenic-induced ovotoxicity, ultrastructural alterations, and autophagic related paths: An enduring developmental study in folliculogenesis of mice. Ecotoxicol. Environ. Saf. 2020, 204, 110973. [Google Scholar] [CrossRef]
- Dastgiri, S.; Mosaferi, M.; Fizi, M.A.H.; Olfati, N.; Zolali, S.; Pouladi, N.; Azarfam, P. Arsenic Exposure, Dermatological Lesions, Hypertension, and Chromosomal Abnormalities among People in a Rural Community of Northwest Iran. J. Health Popul. Nutr. 2010, 28, 14. [Google Scholar] [CrossRef]
- Maloney, M.E. Arsenic in Dermatology. Dermatol. Surg. 1996, 22, 301–304. [Google Scholar] [CrossRef]
- Thakur, M.; Rachamalla, M.; Niyogi, S.; Datusalia, A.K.; Flora, S.J.S. Molecular Mechanism of Arsenic-Induced Neurotoxicity including Neuronal Dysfunctions. Int. J. Mol. Sci. 2021, 22, 10077. [Google Scholar] [CrossRef] [PubMed]
- Duker, A.A.; Carranza, E.J.; Hale, M. Arsenic geochemistry and health. Environ. Int. 2005, 31, 631–641. [Google Scholar] [CrossRef]
- Kannan, G.M.; Tripathi, N.; Dube, S.N.; Gupta, M.; Flora, S. Toxic Effects of Arsenic (III) on Some Hematopoietic and Central Nervous System Variables in Rats and Guinea Pigs. J. Toxicol. Clin. Toxicol. 2001, 39, 675–682. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, Y.; Shao, Y.; Liu, J.; Wang, S.; Xing, M. Oxidative stress-induced skeletal muscle injury involves in NF-κB/p53-activated immunosuppression and apoptosis response in copper (II) or/and arsenite-exposed chicken. Chemosphere 2018, 210, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Bjørklund, G.; Aaseth, J.; Chirumbolo, S.; Urbina, M.A.; Uddin, R. Effects of arsenic toxicity beyond epigenetic modifications. Environ. Geochem. Health 2017, 40, 955–965. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.-S.; Song, K.-H.; Chung, J.-Y. Health Effects of Chronic Arsenic Exposure. J. Prev. Med. Public Health 2014, 47, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, A.; Houshmand, G.; Goudarzi, M.; Sezavar, S.H.; Mehrzadi, S.; Mansouri, E.; Kalantar, M. Ameliorative effect of gallic acid on sodium arsenite-induced spleno-, cardio- and hemato-toxicity in rats. Life Sci. 2018, 217, 91–100. [Google Scholar] [CrossRef]
- Kleiman, N.J.; Quinn, A.M.; Fields, K.G.; Slavkovich, V.; Graziano, J.H. Arsenite accumulation in the mouse eye. J. Toxicol. Environ. Health Part A 2016, 79, 339–341. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Okayasu, R. Arsenic accumulation, elimination, and interaction with copper, zinc and manganese in liver and kidney of rats. Food Chem. Toxicol. 2008, 46, 3646–3650. [Google Scholar] [CrossRef]
- Bae, J.; Jang, Y.; Kim, H.; Mahato, K.; Schaecher, C.; Kim, I.M.; Kim, E.; Ro, S.-H. Arsenite exposure suppresses adipogenesis, mitochondrial biogenesis and thermogenesis via autophagy inhibition in brown adipose tissue. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef]
- Martinez, E.J.; Kolb, B.L.; Bell, A.; Savage, D.D.; Allan, A.M. Moderate perinatal arsenic exposure alters neuroendocrine markers associated with depression and increases depressive-like behaviors in adult mouse offspring. NeuroToxicology 2008, 29, 647–655. [Google Scholar] [CrossRef]
- Pakzad, D.; Akbari, V.; Sepand, M.R.; Aliomrani, M. Risk of neurodegenerative disease due to tau phosphorylation changes and arsenic exposure via drinking water. Toxicol. Res. 2021, 10, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Rattan, S.; Zhou, C.; Chiang, C.; Mahalingam, S.; Brehm, E.; Flaws, J.A. Exposure to endocrine disruptors during adulthood: Consequences for female fertility. J. Endocrinol. 2017, 233, R109–R129. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Holladay, S.D.; Wolf, D.C.; Ahmed, S.A.; Robertson, J.L. Reproductive and Developmental Toxicity of Arsenic in Rodents: A Review. Int. J. Toxicol. 2006, 25, 319–331. [Google Scholar] [CrossRef]
- Biswas, R.; Poddar, S.; Mukherjee, A. Investigation on the Genotoxic Effects of Long-Term Administration of Sodium Arsenite in Bone Marrow and Testicular Cells In Vivo Using the Comet Assay. J. Environ. Pathol. Toxicol. Oncol. 2007, 26, 29–37. [Google Scholar] [CrossRef]
- Jana, K.; Jana, S.; Samanta, P.K. Effects of chronic exposure to sodium arsenite on hypothalamo-pituitary-testicular activities in adult rats: Possible an estrogenic mode of action. Reprod. Biol. Endocrinol. 2006, 4, 9. [Google Scholar] [CrossRef]
- Tikka, C.; Manthari, R.K.; Ommati, M.M.; Niu, R.; Sun, Z.; Zhang, J.; Wang, J. Immune disruption occurs through altered gut microbiome and NOD2 in arsenic induced mice: Correlation with colon cancer markers. Chemosphere 2020, 246, 125791. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wu, R.; Chen, X.; Geng, H.; Hu, Y.; Gao, L.; Fu, J.; Pi, J.; Xu, Y. Developmental arsenic exposure induces dysbiosis of gut microbiota and disruption of plasma metabolites in mice. Toxicol. Appl. Pharmacol. 2022, 450. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Chen, X.; Tian, X.; Qiu, Y.; Wang, J.; Yu, G.; Dong, N.; Feng, J.; Xie, J.; Nalesnik, M.; et al. Co-exposure to inorganic arsenic and fluoride prominently disrupts gut microbiota equilibrium and induces adverse cardiovascular effects in offspring rats. Sci. Total Environ. 2021, 767, 144924. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Sun, P.; Geng, Q.; Fan, H.; Gong, Y.; Hu, Y.; Shan, L.; Sun, Y.; Shen, W.; Zhou, Y. Disrupted spermatogenesis in a metabolic syndrome model: The role of vitamin A metabolism in the gut–testis axis. Gut 2021, 71, 78–87. [Google Scholar] [CrossRef]
- Zhao, Q.; Huang, J.-F.; Cheng, Y.; Dai, M.-Y.; Zhu, W.-F.; Yang, X.-W.; Gonzalez, F.J.; Li, F. Polyamine metabolism links gut microbiota and testicular dysfunction. Microbiome 2021, 9, 1–18. [Google Scholar] [CrossRef]
- Jangra, A.; Verma, M.; Kumar, D.; Chandrika, C.; Rachamalla, M.; Dey, A.; Dua, K.; Jha, S.K.; Ojha, S.; Alexiou, A.; et al. Targeting Endoplasmic Reticulum Stress using Natural Products in Neurological Disorders. Neurosci. Biobehav. Rev. 2022. [Google Scholar] [CrossRef]
- Puppala, E.R.; Jain, S.; Saha, P.; Rachamalla, M.; Np, S.; Yalamarthi, S.S.; Abubakar; Chaudhary, A.; Chamundeswari, D.; Usn, M.; et al. Perillyl alcohol attenuates rheumatoid arthritis via regulating TLR4/NF-κB and Keap1/Nrf2 signaling pathways: A comprehensive study onin-vitro and in-vivo experimental models. Phytomedicine 2022, 97, 153926. [Google Scholar] [CrossRef]
- Zargari, F.; Rahaman, S.; KazemPour, R.; Hajirostamlou, M. Arsenic, Oxidative Stress and Reproductive System. J. Xenobiotics 2022, 12, 214–222. [Google Scholar] [CrossRef]
- Adewoyin, M.; Ibrahim, M.; Roszaman, R.; Isa, M.L.M.; Alewi, N.A.M.; Rafa, A.A.A.; Anuar, M.N.N. Male Infertility: The Effect of Natural Antioxidants and Phytocompounds on Seminal Oxidative Stress. Diseases 2017, 5, 9. [Google Scholar] [CrossRef]
- Khair, A.; Awal, M.A.; Hoque, M.N.; Talukder, A.K.; Das, Z.C.; Rao, D.R.; Shamsuddin, M. Spirulina ameliorates arsenic induced reproductive toxicity in male rats. Anim. Reprod. 2021, 18. [Google Scholar] [CrossRef] [PubMed]
- Kumarathilaka, P.; Seneweera, S.; Ok, Y.S.; Meharg, A.; Bundschuh, J. Arsenic in cooked rice foods: Assessing health risks and mitigation options. Environ. Int. 2019, 127, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Taylor, V.; Goodale, B.; Raab, A.; Schwerdtle, T.; Reimer, K.; Conklin, S.; Karagas, M.R.; Francesconi, K.A. Human exposure to organic arsenic species from seafood. Sci. Total. Environ. 2017, 580, 266–282. [Google Scholar] [CrossRef] [PubMed]
- Calatayud, M.; Farias, S.S.; de Paredes, G.S.; Olivera, M.; Carreras, N..; Giménez, M.C.; Devesa, V.; Vélez, D. Arsenic exposure of child populations in Northern Argentina. Sci. Total Environ. 2019, 669, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kordas, K.; Queirolo, E.I.; Mañay, N.; Peregalli, F.; Hsiao, P.Y.; Lu, Y.; Vahter, M. Low-level arsenic exposure: Nutritional and dietary predictors in first-grade Uruguayan children. Environ. Res. 2016, 147, 16–23. [Google Scholar] [CrossRef]
- Wei, B.; Yu, J.; Kong, C.; Li, H.; Yang, L.; Guo, Z.; Cui, N.; Xia, Y.; Wu, K. An investigation of the health effects caused by exposure to arsenic from drinking water and coal combustion: Arsenic exposure and metabolism. Environ. Sci. Pollut. Res. 2017, 24, 25947–25954. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, H.; Kay, P.; Slack, R.; Gong, Y.Y.; Carter, A. Human exposure assessment of different arsenic species in household water sources in a high risk arsenic area. Sci. Total. Environ. 2017, 584-585, 631–641. [Google Scholar] [CrossRef]
- Chang, J.Y.; Ahn, S.C.; Lee, J.S.; Kim, J.-Y.; Jung, A.-R.; Park, J.; Choi, J.-W.; Yu, S.D. Exposure assessment for the abandoned metal mine area contaminated by arsenic. Environ. Geochem. Health 2019, 41, 2443–2458. [Google Scholar] [CrossRef]
- Signes-Pastor, A.J.; Vioque, J.; Navarrete-Muñoz, E.M.; Carey, M.; García-Villarino, M.; Fernández-Somoano, A.; Tardón, A.; Santa-Marina, L.; Irizar, A.; Casas, M.; et al. Inorganic arsenic exposure and neuropsychological development of children of 4–5 years of age living in Spain. Environ. Res. 2019, 174, 135–142. [Google Scholar] [CrossRef]
- Samal, A.C.; Kar, S.; Bhattacharya, P.; Santra, S. Human exposure to arsenic through foodstuffs cultivated using arsenic contaminated groundwater in areas of West Bengal, India. J. Environ. Sci. Health Part A Tox Hazard. Subst. Environ. Eng. 2011, 46, 1259–1265. [Google Scholar] [CrossRef]
- Menon, M.; Sarkar, B.; Hufton, J.; Reynolds, C.; Reina, S.V.; Young, S. Do arsenic levels in rice pose a health risk to the UK population? Ecotoxicol. Environ. Saf. 2020, 197, 110601. [Google Scholar] [CrossRef]
- Liao, N.; Seto, E.; Eskenazi, B.; Wang, M.; Li, Y.; Hua, J. A Comprehensive Review of Arsenic Exposure and Risk from Rice and a Risk Assessment among a Cohort of Adolescents in Kunming, China. Int. J. Environ. Res. Public Health 2018, 15, 2191. [Google Scholar] [CrossRef]
- Oguri, T.; Yoshinaga, J. Daily Inorganic Arsenic Intake of the Japanese Estimated by a Probabilistic Approach. Nippon Eiseigaku Zasshi Jpn. J. Hyg. 2014, 69, 177–186. [Google Scholar] [CrossRef][Green Version]
- Rasheed, H.; Kay, P.; Slack, R.; Gong, Y.Y. Arsenic species in wheat, raw and cooked rice: Exposure and associated health implications. Sci. Total. Environ. 2018, 634, 366–373. [Google Scholar] [CrossRef]
- Xue, L.; Zhao, Z.; Zhang, Y.; Liao, J.; Wu, M.; Wang, M.; Sun, J.; Gong, H.; Guo, M.; Li, S.; et al. Dietary exposure to arsenic and human health risks in western Tibet. Sci. Total Environ. 2020, 731, 138840. [Google Scholar] [CrossRef]
- Raeeszadeh, M.; Gravandi, H.; Akbari, A. Determination of some heavy metals levels in the meat of animal species (sheep, beef, turkey, and ostrich) and carcinogenic health risk assessment in Kurdistan province in the west of Iran. Environ. Sci. Pollut. Res. 2022, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Vahter, M. Mechanisms of arsenic biotransformation. Toxicology 2002, 181–182, 211–217. [Google Scholar] [CrossRef]
- National Research Council (US) Subcommittee on Arsenic in Drinking Water. Disposition of Inorganic Arsenic; National Academies Press (US): Washington, DC, USA, 1999. [Google Scholar]
- Lindgren, A.; Vahter, M.; Dencker, L. Autoradiographic Studies on the Distribution of Arsenic in Mice and Hamsters Administered 74As-Arsenite or -Arsenate. Acta Pharmacol. Toxicol. 1982, 51, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, A.; Danielsson, B.R.G.; Dencker, L.; Vahter, M. Embryotoxicity of Arsenite and Arsenate: Distribution in Pregnant Mice and Monkeys and Effects on Embryonic Cells in Vitro. Acta Pharmacol. Toxicol. 1984, 54, 311–320. [Google Scholar] [CrossRef]
- Vahter, M.; Marafante, E.; Lindgren, A.; Dencker, L. Tissue distribution and subcellular binding of arsenic in Marmoset monkeys after injection of 74As-Arsenite. Arch. Toxicol. 1982, 51, 65–77. [Google Scholar] [CrossRef]
- Garbinski, L.D.; Rosen, B.P.; Chen, J. Pathways of arsenic uptake and efflux. Environ. Int. 2019, 126, 585–597. [Google Scholar] [CrossRef]
- Roggenbeck, B.A.; Banerjee, M.; Leslie, E.M. Cellular arsenic transport pathways in mammals. J. Environ. Sci. 2016, 49, 38–58. [Google Scholar] [CrossRef] [PubMed]
- Hughes, M.F. Arsenic toxicity and potential mechanisms of action. Toxicol. Lett. 2002, 133, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Rehman, K.; Naranmandura, H. Arsenic metabolism and thioarsenicals. Metallomics 2012, 4, 881–892. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Ogra, Y. Distributions and chemical forms of arsenic after intravenous administration of dimethylarsinic and monomethylarsonic acids to rats. Toxicol. Appl. Pharmacol. 2004, 198, 336–344. [Google Scholar] [CrossRef]
- Kligerman, A.D.; Doerr, C.L.; Tennant, A.H.; Harrington-Brock, K.; Allen, J.W.; Winkfield, E.; Poorman-Allen, P.; Kundu, B.; Funasaka, K.; Roop, B.C.; et al. Methylated trivalent arsenicals as candidate ultimate genotoxic forms of arsenic: Induction of chromosomal mutations but not gene mutations. Environ. Mol. Mutagen. 2003, 42, 192–205. [Google Scholar] [CrossRef] [PubMed]
- Mass, M.J.; Tennant, A.; Roop, B.C.; Cullen, W.R.; Styblo, M.; Thomas, D.J.; Kligerman, A.D. Methylated Trivalent Arsenic Species Are Genotoxic. Chem. Res. Toxicol. 2001, 14, 355–361. [Google Scholar] [CrossRef]
- Braman, R.S.; Foreback, C.C. Methylated Forms of Arsenic in the Environment. Science 1973, 182, 1247–1249. [Google Scholar] [CrossRef] [PubMed]
- Chouchane, S.; Snow, E.T. In Vitro Effect of Arsenical Compounds on Glutathione-Related Enzymes. Chem. Res. Toxicol. 2001, 14, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, K.; Hayashi, H.; Tachikawa, M.; Kato, K.; Hasegawa, A.; Oku, N.; Okada, S. Metabolic methylation is a possible genotoxicity-enhancing process of inorganic arsenics. Mutat. Res. Toxicol. Environ. Mutagen. 1997, 394, 95–101. [Google Scholar] [CrossRef]
- Ahmad, S.; Kitchin, K.T.; Cullen, W.R. Arsenic Species That Cause Release of Iron from Ferritin and Generation of Activated Oxygen. Arch. Biochem. Biophys. 2000, 382, 195–202. [Google Scholar] [CrossRef]
- Corsini, E.; Asti, L.; Viviani, B.; Marinovich, M.; Galli, C.L. Sodium Arsenate Induces Overproduction of Interleukin-1α in Murine Keratinocytes: Role of Mitochondria. J. Investig. Dermatol. 1999, 113, 760–765. [Google Scholar] [CrossRef]
- Tsai, C.-H.; Yang, M.-H.; Hung, A.C.; Wu, S.-C.; Chiu, W.-C.; Hou, M.-F.; Tyan, Y.-C.; Wang, Y.-M.; Yuan, S.-S.F. Identification of Id1 as a downstream effector for arsenic-promoted angiogenesis via PI3K/Akt, NF-κB and NOS signaling. Toxicol. Res. 2015, 5, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Luo, L.; Alamdar, A.; Zhang, J.; Liu, L.; Tian, M.; Eqani, S.A.M.A.S.; Shen, H. Integrated proteomics and metabolomics analysis of rat testis: Mechanism of arsenic-induced male reproductive toxicity. Sci. Rep. 2016, 6, 32518. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-C.; Yu, H.-S.; Chai, C.-Y. Proteins in the ERK pathway are affected by arsenic-treated cells. Toxicol. Res. 2015, 4, 1545–1554. [Google Scholar] [CrossRef]
- Chen, J.; Fok, K.L.; Chen, H.; Zhang, X.H.; Xu, W.M.; Chan, H.C. Cryptorchidism-induced CFTR down-regulation results in disruption of testicular tight junctions through up-regulation of NF- B/COX-2/PGE2. Hum. Reprod. 2012, 27, 2585–2597. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.-P.; Zheng, X.-M.; Zheng, H.; Liu, X.-J.; Liu, G.-Y.; Wang, X. Downregulation of cold-inducible RNA-binding protein activates mitogen-activated protein kinases and impairs spermatogenic function in mouse testes. Asian J. Androl. 2012, 14, 884–889. [Google Scholar] [CrossRef]
- Danielsson, B.R.G.; Dencker, L.; Lindgren, A.; Tjälve, H. Accumulation of Toxic Metals in Male Reproduction Organs. Arch. Toxicol. Suppl. 1984, 7, 177–180. [Google Scholar] [CrossRef]
- Pant, N.; Kumar, R.; Murthy, R.C.; Srivastava, S.P. Male reproductive effect of arsenic in mice. BioMetals 2001, 14, 113–117. [Google Scholar] [CrossRef]
- Zubair, M.; Ahmad, M.; Qureshi, Z.I. Review on Arsenic-Induced Toxicity in Male Reproductive System and Its Amelioration. Andrologia 2017, 49, e12791. [Google Scholar] [CrossRef]
- Kumar, A.; Raj, V.; Srivastava, A.; Ali, M.; Ghosh, A.K.; Rachamalla, M.; Kumar, D. Autophagy in arsenic exposed population and cancer patients. In Autophagy and Metabolism; Academic Press: Cambridge, MA, USA, 2022; pp. 141–161. [Google Scholar] [CrossRef]
- Roy, S.; Bhattacharya, S. Arsenic-induced histopathology and synthesis of stress proteins in liver and kidney of Channa punctatus. Ecotoxicol. Environ. Saf. 2006, 65, 218–229. [Google Scholar] [CrossRef]
- Ahmed, K.; Mamun, H.A.; Parvin, E.; Akter, M.S.; Khan, M.S. Arsenic induced toxicity and histopathological changes in gill and liver tissue of freshwater fish, tilapia (Oreochromis mossambicus). Exp. Toxicol. Pathol. 2013, 65, 903–909. [Google Scholar] [CrossRef]
- Manthari, R.K.; Tikka, C.; Ommati, M.M.; Niu, R.; Sun, Z.; Wang, J.; Zhang, J.; Wang, J. Arsenic-Induced Autophagy in the Developing Mouse Cerebellum: Involvement of the Blood-Brain Barrier’s Tight-Junction Proteins and the PI3K-Akt-MTOR Signaling Pathway. J. Agric. Food Chem. 2018, 66, 8602–8614. [Google Scholar] [CrossRef] [PubMed]
- Manthari, R.K.; Tikka, C.; Ommati, M.M.; Niu, R.; Sun, Z.; Wang, J.; Zhang, J.; Wang, J. Arsenic Induces Autophagy in De-velopmental Mouse Cerebral Cortex and Hippocampus by Inhibiting PI3K/Akt/MTOR Signaling Pathway: Involvement of Blood–Brain Barrier’s Tight Junction Proteins. Arch. Toxicol. 2018, 92, 3255–3275. [Google Scholar] [CrossRef] [PubMed]
- Ommati, M.M.; Heidari, R.; Zamiri, M.J.; Sabouri, S.; Zaker, L.; Farshad, O.; Jamshidzadeh, A.; Mousapour, S. The Footprints of Oxidative Stress and Mitochondrial Impairment in Arsenic Trioxide-Induced Testosterone Release Suppression in Pubertal and Mature F1-Male Balb/c Mice via the Downregulation of 3β-HSD, 17β-HSD, and CYP11a Expression. Biol. Trace Elem. Res. 2020, 195, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Acevedo-Rodriguez, A.; Kauffman, A.S.; Cherrington, B.D.; Borges, C.S.; Roepke, T.A.; Laconi, M. Emerging Insights into Hypothalamic-Pituitary-Gonadal (HPG) Axis Regulation and Interaction with Stress Signaling. J. Neuroendocrinol. 2018, 30, e12590. [Google Scholar] [CrossRef] [PubMed]
- Chiaverini, N.; de Ley, M. Protective Effect of Metallothionein on Oxidative Stress-Induced DNA Damage. Free Radic. Res. 2010, 44, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Siu, E.; Mruk, D.D.; Porto, C.S.; Cheng, C.Y. Cadmium-induced testicular injury. Toxicol. Appl. Pharmacol. 2009, 238, 240–249. [Google Scholar] [CrossRef]
- Dalton, T.; Fu, K.; Enders, G.C.; Palmiter, R.D.; Andrews, G.K. Analysis of the effects of overexpression of metallothionein-I in transgenic mice on the reproductive toxicology of cadmium. Environ. Health Perspect. 1996, 104, 68–76. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Mruk, D.D. The Blood-Testis Barrier and Its Implications for Male Contraception. Pharmacol. Rev. 2011, 64, 16–64. [Google Scholar] [CrossRef]
- Sanghamitra, S.; Hazra, J.; Upadhyay, S.N.; Singh, R.K.; Amal, R.C. Arsenic induced toxicity on testicular tissue of mice. Indian J. Physiol. Pharmacol. 2008, 52, 84–90. [Google Scholar]
- Wang, L.; Hao, J.; Hu, J.; Pu, J.; Lü, Z.; Zhao, L.; Wang, Q.; Yu, Q.; Wang, Y.; Li, G. Protective Effects of Ginsenosides against Bisphenol A-Induced Cytotoxicity in 15P-1 Sertoli Cells via Extracellular Signal-Regulated Kinase 1/2 Signalling and Antioxidant Mechanisms. Basic Clin. Pharmacol. Toxicol. 2012, 111, 42–49. [Google Scholar] [CrossRef]
- Momeni, H.R.; Oryan, S.; Eskandari, N. Effect of vitamin E on sperm number and testis histopathology of sodium arsenite-treated rats. Reprod. Biol. 2012, 12, 171–181. [Google Scholar] [CrossRef] [PubMed]
- de Medeiros, P.; Samelo, R.R.; Silva, A.P.G.; Santiago, M.; Duarte, F.; Castro, .; Perobelli, J.E. Prepubertal exposure to low doses of sodium arsenite impairs spermatogenesis and epididymal histophysiology in rats. Environ. Toxicol. 2018, 34, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Anwar, N.; Qureshi, I.Z.; Spears, N.; Lopes, F. In vitro administration of sodium arsenite in mouse prepubertal testis induces germ cell loss and apoptosis. Toxicol. Vitro 2020, 67, 104924. [Google Scholar] [CrossRef]
- Souza, A.C.F.; Marchesi, S.C.; Lima, G.D.D.A.; Ferraz, R.P.; Santos, F.C.; da Matta, S.L.P.; Machado-Neves, M. Effects of Sodium Arsenite and Arsenate in Testicular Histomorphometry and Antioxidants Enzymes Activities in Rats. Biol. Trace Element Res. 2015, 171, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Chiou, T.-J.; Chu, S.-T.; Tzeng, W.-F.; Huang, Y.-C.; Liao, C.-J. Arsenic Trioxide Impairs Spermatogenesis via Reducing Gene Expression Levels in Testosterone Synthesis Pathway. Chem. Res. Toxicol. 2008, 21, 1562–1569. [Google Scholar] [CrossRef]
- Wang, Y.-X.; Wang, P.; Feng, W.; Liu, C.; Yang, P.; Chen, Y.-J.; Sun, L.; Sun, Y.; Yue, J.; Gu, L.-J.; et al. Relationships between seminal plasma metals/metalloids and semen quality, sperm apoptosis and DNA integrity. Environ. Pollut. 2017, 224, 224–234. [Google Scholar] [CrossRef]
- Lima, G.D.D.A.; Sertorio, M.N.; Souza, A.C.F.; Menezes, T.P.; Mouro, V.G.S.; Gonçalves, N.M.; de Oliveira, J.M.; Henry, M.; Machado-Neves, M. Fertility in male rats: Disentangling adverse effects of arsenic compounds. Reprod. Toxicol. 2018, 78, 130–140. [Google Scholar] [CrossRef]
- da Silva, R.F.; Borges, C.D.S.; Lamas, C.D.A.; Cagnon, V.H.A.; Kempinas, W.D.G. Arsenic trioxide exposure impairs testicular morphology in adult male mice and consequent fetus viability. J. Toxicol. Environ. Health Part A Curr. Issues 2017, 80, 1166–1179. [Google Scholar] [CrossRef]
- Han, Y.; Liang, C.; Yu, Y.; Manthari, R.K.; Cheng, C.; Tan, Y.; Li, X.; Tian, X.; Fu, W.; Yang, J.; et al. Chronic arsenic exposure lowered sperm motility via impairing ultra-microstructure and key proteins expressions of sperm acrosome and flagellum formation during spermiogenesis in male mice. Sci. Total Environ. 2020, 734, 139233. [Google Scholar] [CrossRef]
- Han, Y.; Liang, C.; Manthari, R.K.; Yu, Y.; Gao, Y.; Liu, Y.; Jiang, S.; Tikka, C.; Wang, J.; Zhang, J. Arsenic influences spermatogenesis by disorganizing the elongation of spermatids in adult male mice. Chemosphere 2019, 238, 124650. [Google Scholar] [CrossRef]
- Kesari, V.P.; Kumar, A.; Khan, P.K. Induction of sperm impairments in mice as a sensitive biomarker of arsenic toxicity. Environ. Monit. Assess. 2014, 186, 3115–3121. [Google Scholar] [CrossRef] [PubMed]
- Souza, A.C.F.; Bastos, D.S.S.; Sertorio, M.N.; Santos, F.C.; Ervilha, L.O.G.; de Oliveira, L.L.; Machado-Neves, M. Combined effects of arsenic exposure and diabetes on male reproductive functions. Andrology 2019, 7, 730–740. [Google Scholar] [CrossRef] [PubMed]
- Guvvala, P.R.; Sellappan, S.; Parameswaraiah, R.J. Impact of arsenic(V) on testicular oxidative stress and sperm functional attributes in Swiss albino mice. Environ. Sci. Pollut. Res. 2016, 23, 18200–18210. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, K.F.; Mellouk, N.; Ungewitter, E.K.; Nicol, B.; Liu, C.; Brown, P.R.; Willson, C.J.; Yao, H.H.-C. In utero exposure to arsenite contributes to metabolic and reproductive dysfunction in male offspring of CD-1 mice. Reprod. Toxicol. 2020, 95, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Nava-Rivera, L.E.; Betancourt-Martínez, N.D.; Lozoya-Martínez, R.; Carranza-Rosales, P.; Guzmán-Delgado, N.E.; Carranza-Torres, I.E.; Delgado-Aguirre, H.; Zambrano-Ortíz, J.O.; Morán-Martínez, J. Transgenerational effects in DNA methylation, genotoxicity and reproductive phenotype by chronic arsenic exposure. Sci. Rep. 2021, 11, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Souza, A.C.F.; Machado-Neves, M.; Bastos, D.S.S.; Santos, F.C.; Ervilha, L.O.G.; Coimbra, J.L.D.P.; Araújo, L.D.S.; de Oliveira, L.L.; Guimarães, S.E.F. Impact of prenatal arsenic exposure on the testes and epididymides of prepubertal rats. Chem. Interact. 2020, 333, 109314. [Google Scholar] [CrossRef]
- Couto-Santos, F.; Viana, A.G.D.A.; Souza, A.C.F.; Dutra, A.A.D.A.; Mendes, T.A.D.O.; Ferreira, A.T.D.S.; Aguilar, J.E.P.; Oliveira, L.L.; Machado-Neves, M. Prepubertal arsenic exposure alters phosphoproteins profile, quality, and fertility of epididymal spermatozoa in sexually mature rats. Toxicology 2021, 460, 152886. [Google Scholar] [CrossRef]
- Souza, A.C.F.; Marchesi, S.C.; Ferraz, R.P.; Lima, G.D.D.A.; de Oliveira, J.A.; Machado-Neves, M. Effects of sodium arsenate and arsenite on male reproductive functions in Wistar rats. J. Toxicol. Environ. Health Part A 2016, 79, 274–286. [Google Scholar] [CrossRef]
- Ram, A.K.S.S.; Reddy, K.P.; Girish, B.P.; Supriya, C.; Reddy, P.S. Arsenic aggravated reproductive toxicity in male rats exposed to lead during the perinatal period. Toxicol. Res. 2018, 7, 1191–1204. [Google Scholar] [CrossRef]
- Liu, P.; Li, R.; Tian, X.; Zhao, Y.; Li, M.; Wang, M.; Ying, X.; Yuan, J.; Xie, J.; Yan, X.; et al. Co-exposure to fluoride and arsenic disrupts intestinal flora balance and induces testicular autophagy in offspring rats. Ecotoxicol. Environ. Saf. 2021, 222, 112506. [Google Scholar] [CrossRef]
- Yilmaz, B.O.; Yildizbayrak, N.; Erkan, M. Sodium arsenite-induced detriment of cell function in Leydig and Sertoli cells: The potential relation of oxidative damage and antioxidant defense system. Drug Chem. Toxicol. 2018, 43, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Yi, H.; Huang, L.; An, Q.; Wang, H. Reduced testosterone and Ddx3y expression caused by long-term exposure to arsenic and its effect on spermatogenesis in mice. Environ. Toxicol. Pharmacol. 2018, 63, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhong, G.; Wan, F.; Jiang, X.; Tang, Z.; Hu, T.; Rao, G.; Lan, J.; Hussain, R.; Tang, L.; et al. Evaluation of toxic effects induced by arsenic trioxide or/and antimony on autophagy and apoptosis in testis of adult mice. Environ. Sci. Pollut. Res. 2021, 28, 54647–54660. [Google Scholar] [CrossRef]
- Alamdar, A.; Tian, M.; Huang, Q.; Du, X.; Zhang, J.; Liu, L.; Shah, S.T.A.; Shen, H. Enhanced histone H3K9 tri-methylation suppresses steroidogenesis in rat testis chronically exposed to arsenic. Ecotoxicol. Environ. Saf. 2019, 170, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Couto-Santos, F.; Souza, A.C.F.; Bastos, D.S.S.; Ervilha, L.O.G.; Dias, F.C.R.; Araújo, L.D.S.; Guimarães, S.E.F.; de Oliveira, L.L.; Machado-Neves, M. Prepubertal exposure to arsenic alters male reproductive parameters in pubertal and adult rats. Toxicol. Appl. Pharmacol. 2020, 409, 115304. [Google Scholar] [CrossRef]
- Sun, X.; Li, S.; He, Y.; Zhao, H.; Wang, Y.; Zeng, X.; Xing, M. Arsenic-induced testicular toxicity in Gallus gallus: Expressions of inflammatory cytokines and heat shock proteins. Poult. Sci. 2017, 96, 3399–3406. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Zhao, H.; Wang, Y.; Liu, J.; Li, J.; Chai, H.; Xing, M. Arsenic and/or copper caused inflammatory response via activation of inducible nitric oxide synthase pathway and triggered heat shock protein responses in testis tissues of chicken. Environ. Sci. Pollut. Res. 2017, 25, 7719–7729. [Google Scholar] [CrossRef]
- Shao, Y.; Zhao, H.; Wang, Y.; Liu, J.; Li, J.; Luo, L.; Xing, M. The apoptosis in arsenic-induced oxidative stress is associated with autophagy in the testis tissues of chicken. Poult. Sci. 2018, 97, 3248–3257. [Google Scholar] [CrossRef]
- Liang, C.; Feng, Z.; Manthari, R.K.; Wang, C.; Han, Y.; Fu, W.; Wang, J.; Zhang, J. Arsenic induces dysfunctional autophagy via dual regulation of mTOR pathway and Beclin1-Vps34/PI3K complex in MLTC-1 cells. J. Hazard. Mater. 2020, 391, 122227. [Google Scholar] [CrossRef]
- Alamdar, A.; Xi, G.; Huang, Q.; Tian, M.; Eqani, S.A.M.A.S.; Shen, H. Arsenic activates the expression of 3β-HSD in mouse Leydig cells through repression of histone H3K9 methylation. Toxicol. Appl. Pharmacol. 2017, 326, 7–14. [Google Scholar] [CrossRef]
- Chen, H.; Liu, G.; Qiao, N.; Kang, Z.; Hu, L.; Liao, J.; Yang, F.; Pang, C.; Liu, B.; Zeng, Q.; et al. Toxic effects of arsenic trioxide on spermatogonia are associated with oxidative stress, mitochondrial dysfunction, autophagy and metabolomic alterations. Ecotoxicol. Environ. Saf. 2019, 190, 110063. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Jenisova, Z.; Feszterova, M.; Baros, S.; Liska, J.; Hudecova, D.; Rhodes, C.J.; Valko, M. Arsenic: Toxicity, Oxidative Stress and Human Disease. J. Appl. Toxicol. 2011, 31, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Tariba Lovaković, B. Cadmium, Arsenic, and Lead: Elements Affecting Male Reproductive Health. Curr. Opin. Toxicol. 2020, 19, 7–14. [Google Scholar] [CrossRef]
- Anwar, N.; Qureshi, I.Z. In vitro application of sodium arsenite to mice testicular and epididymal organ cultures induces oxidative, biochemical, hormonal, and genotoxic stress. Toxicol. Ind. Health 2019, 35, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Ji, P.-Y.; Li, Z.-Y.; Wang, H.; Dong, J.-T.; Li, X.-J.; Yi, H.-L. Arsenic and sulfur dioxide co-exposure induce renal injury via activation of the NF-κB and caspase signaling pathway. Chemosphere 2019, 224, 280–288. [Google Scholar] [CrossRef]
- Zhong, G.; Wan, F.; Wu, S.; Jiang, X.; Tang, Z.; Zhang, X.; Huang, R.; Hu, L. Arsenic or/and antimony induced mitophagy and apoptosis associated with metabolic abnormalities and oxidative stress in the liver of mice. Sci. Total Environ. 2021, 777, 146082. [Google Scholar] [CrossRef]
- Qi, Y.; Li, H.; Zhang, M.; Zhang, T.; Frank, J.; Chen, G. Autophagy in Arsenic Carcinogenesis. Exp. Toxicol. Pathol. 2014, 66, 163–168. [Google Scholar] [CrossRef]
- Zeinvand-Lorestani, M.; Kalantari, H.; Khodayar, M.J.; Teimoori, A.; Saki, N.; Ahangarpour, A.; Rahim, F.; Alboghobeish, S. Autophagy upregulation as a possible mechanism of arsenic induced diabetes. Sci. Rep. 2018, 8, 11960. [Google Scholar] [CrossRef]
- Tang, Q.; Bai, L.; Zou, Z.; Meng, P.; Xia, Y.; Cheng, S.; Mu, S.; Zhou, J.; Wang, X.; Qin, X.; et al. Ferroptosis is newly characterized form of neuronal cell death in response to arsenite exposure. NeuroToxicology 2018, 67, 27–36. [Google Scholar] [CrossRef]
- Meng, P.; Zhang, S.; Jiang, X.; Cheng, S.; Zhang, J.; Cao, X.; Qin, X.; Zou, Z.; Chen, C. Arsenite induces testicular oxidative stress in vivo and in vitro leading to ferroptosis. Ecotoxicol. Environ. Saf. 2020, 194, 110360. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.; Zhang, X.; Zhu, P.; Hao, J.-H.; Tao, F.-B.; Xu, D.-X. Maternal serum arsenic level during pregnancy is positively associated with adverse pregnant outcomes in a Chinese population. Toxicol. Appl. Pharmacol. 2018, 356, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Y.; Chen, S.W.; Zhao, F.; Zhang, H.M.; Zhang, W.L.; Qu, Y.L.; Liu, Y.C.; Gu, H.; Cai, J.Y.; Cao, Z.J.; et al. Association of arsenic with unexplained recurrent spontaneous abortion: A case-control study. Zhonghua Yu Fang Yi Xue Za Zhi 2019, 53, 470–474. [Google Scholar] [PubMed]
- Liang, C.; Han, Y.; Ma, L.; Wu, X.; Huang, K.; Yan, S.; Li, Z.; Xia, X.; Pan, W.; Sheng, J.; et al. Low levels of arsenic exposure during pregnancy and maternal and neonatal thyroid hormone parameters: The determinants for these associations. Environ. Int. 2020, 145, 106114. [Google Scholar] [CrossRef] [PubMed]
- Zubair, M.; Ahmad, M.; Saleemi, M.K.; Gul, S.T.; Ahmad, N.; Umar, S. Protective effects of vitamin E on sodium arsenite-induced toxicity, testicular measurements and histopathological studies of testes in Teddy goat bucks. Andrologia 2016, 49, e12699. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Cui, Y.; Wu, H.; Niu, Q.; Lu, X.; Wang, L.; Huang, F. Association of maternal arsenic exposure with birth size: A systematic review and meta-analysis. Environ. Toxicol. Pharmacol. 2019, 69, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Almberg, K.S.; Turyk, M.E.; Jones, R.M.; Rankin, K.; Freels, S.; Graber, J.M.; Stayner, L.T. Arsenic in drinking water and adverse birth outcomes in Ohio. Environ. Res. 2017, 157, 52–59. [Google Scholar] [CrossRef]
- Yin, G.; Xia, L.; Hou, Y.; Li, Y.; Cao, D.; Liu, Y.; Chen, J.; Liu, J.; Zhang, L.; Yang, Q.; et al. Transgenerational male reproductive effect of prenatal arsenic exposure: Abnormal spermatogenesis with Igf2/H19 epigenetic alteration in CD1 mouse. Int. J. Environ. Health Res. 2021, 32, 1248–1260. [Google Scholar] [CrossRef]
- Tian, M.; Wang, Y.-X.; Wang, X.; Wang, H.; Liu, L.; Zhang, J.; Nan, B.; Shen, H.; Huang, Q. Environmental doses of arsenic exposure are associated with increased reproductive-age male urinary hormone excretion and in vitro Leydig cell steroidogenesis. J. Hazard. Mater. 2020, 408, 124904. [Google Scholar] [CrossRef]
- Shen, H.; Xu, W.; Zhang, J.; Chen, M.; Martin, F.L.; Xia, Y.; Liu, L.; Dong, S.; Zhu, Y.-G. Urinary Metabolic Biomarkers Link Oxidative Stress Indicators Associated with General Arsenic Exposure to Male Infertility In a Han Chinese Population. Environ. Sci. Technol. 2013, 47, 8843–8851. [Google Scholar] [CrossRef]
- He, Y.; Zou, L.; Luo, W.; Yi, Z.; Yang, P.; Yu, S.; Liu, N.; Ji, J.; Guo, Y.; Liu, P.; et al. Heavy metal exposure, oxidative stress and semen quality: Exploring associations and mediation effects in reproductive-aged men. Chemosphere 2019, 244, 125498. [Google Scholar] [CrossRef]
- Sharma, G.; Kumar, M. Antioxidant and modulatory role of Chlorophytum borivilianum against arsenic induced testicular impairment. J. Environ. Sci. 2012, 24, 2159–2165. [Google Scholar] [CrossRef] [PubMed]
- Prathima, P.; Pavani, R.; Sukeerthi, S.; Sainath, S.B. α-Lipoic acid inhibits testicular and epididymal oxidative damage and improves fertility efficacy in arsenic-intoxicated rats. J. Biochem. Mol. Toxicol. 2017, 32, e22016. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Mukhopadhyay, P.K. Casein- and pea-enriched high-protein diet can take care of the reprotoxic effects of arsenic in male rats. Andrologia 2020, 52, e13560. [Google Scholar] [CrossRef] [PubMed]
- Samadder, A.; Das, J.; Das, S.; Khuda-Bukhsh, A.R. Dihydroxy-isosteviol-methyl-ester, an active biological component of Pulsatilla nigricans, reduces arsenic induced cellular dysfunction in testis of male mice. Environ. Toxicol. Pharmacol. 2012, 34, 743–752. [Google Scholar] [CrossRef]
- Mehrzadi, S.; Bahrami, N.; Mehrabani, M.; Motevalian, M.; Mansouri, E.; Goudarzi, M. Ellagic acid: A promising protective remedy against testicular toxicity induced by arsenic. Biomed. Pharmacother. 2018, 103, 1464–1472. [Google Scholar] [CrossRef]
- Guvvala, P.R.; Ravindra, J.P.; Selvaraju, S.; Arangasamy, A.; Venkata, K.M. Ellagic and ferulic acids protect arsenic-induced male reproductive toxicity via regulating Nfe2l2, Ppargc1a and StAR expressions in testis. Toxicology 2018, 413, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Li, S.G.; Xu, S.Z.; Niu, Q.; Ding, Y.S.; Pang, L.J.; Ma, R.L.; Jing, M.X.; Wang, K.; Ma, X.M.; Feng, G.L.; et al. Lutein alleviates arsenic-induced reproductive toxicity in male mice via Nrf2 signaling. Hum. Exp. Toxicol. 2015, 35, 491–500. [Google Scholar] [CrossRef]
- Li, S.G.; Ding, Y.S.; Niu, Q.; Xu, S.Z.; Pang, L.J.; Ma, R.L.; Jing, M.X.; Feng, G.L.; Liu, J.M.; Guo, S.X. Grape Seed Proanthocyanidin Extract Alleviates Arsenic-induced Oxidative Reproductive Toxicity in Male Mice. Biomed. Environ. Sci. 2015, 28, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Ince, S.; Avdatek, F.; Demirel, H.H.; Arslan-Acaroz, D.; Goksel, E.; Kucukkurt, I. Ameliorative effect of polydatin on oxidative stress-mediated testicular damage by chronic arsenic exposure in rats. Andrologia 2015, 48, 518–524. [Google Scholar] [CrossRef]
- Guvvala, P.R.; Ravindra, J.P.; Rajani, C.V.; Sivaram, M.; Selvaraju, S. Protective role of epigallocatechin-3-gallate on arsenic induced testicular toxicity in Swiss albino mice. Biomed. Pharmacother. 2017, 96, 685–694. [Google Scholar] [CrossRef]
- Reddy, P.S.; Rani, G.P.; Sainath, S.; Meena, R.; Supriya, C. Protective effects of N-acetylcysteine against arsenic-induced oxidative stress and reprotoxicity in male mice. J. Trace Elements Med. Biol. 2011, 25, 247–253. [Google Scholar] [CrossRef] [PubMed]
- El-Khadragy, M.F.; Al-Megrin, W.A.; Alomar, S.; Alkhuriji, A.F.; Metwally, D.M.; Mahgoub, S.; Amin, H.K.; Habotta, O.A.; Moneim, A.E.A.; Albeltagy, R.S. Chlorogenic acid abates male reproductive dysfunction in arsenic-exposed mice via attenuation of testicular oxido-inflammatory stress and apoptotic responses. Chem. Interact. 2020, 333, 109333. [Google Scholar] [CrossRef] [PubMed]
- Ola-Davies, O.; Ajani, O.S. Semen characteristics and sperm morphology of Pistia stratiotes Linn. (Araceae) protected male albino rats (Wistar strain) exposed to sodium arsenite. J. Complement. Integr. Med. 2016, 13, 289–294. [Google Scholar] [CrossRef]
- Adedara, I.A.; Adebowale, A.A.; Atanda, O.E.; Fabunmi, A.T.; Ayenitaju, A.C.; Rocha, J.B.; Farombi, E.O. Selenium abates reproductive dysfunction via attenuation of biometal accumulation, oxido-inflammatory stress and caspase-3 activation in male rats exposed to arsenic. Environ. Pollut. 2019, 254, 113079. [Google Scholar] [CrossRef]
- da Silva, R.F.; Borges, C.D.S.; e Silva, P.V.; Missassi, G.; Kiguti, L.R.A.; Pupo, A.S.; Junior, F.B.; Anselmo-Franci, J.A.; Kempinas, W.D.G. The Coadministration of N-Acetylcysteine Ameliorates the Effects of Arsenic Trioxide on the Male Mouse Genital System. Oxidat. Med. Cell. Longev. 2015, 2016, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Uygur, R.; Aktas, C.; Caglar, V.; Uygur, E.; Erdogan, H.; Ozen, O.A. Protective effects of melatonin against arsenic-induced apoptosis and oxidative stress in rat testes. Toxicol. Ind. Health 2013, 32, 848–859. [Google Scholar] [CrossRef]
- Fouad, A.A.; Al-Sultan, A.I.; Yacoubi, M.T. Coenzyme Q10 counteracts testicular injury induced by sodium arsenite in rats. Eur. J. Pharmacol. 2011, 655, 91–98. [Google Scholar] [CrossRef]
- Baltaci, B.B.; Uygur, R.; Caglar, V.; Aktas, C.; Aydin, M.; Ozen, O.A. Protective effects of quercetin against arsenic-induced testicular damage in rats. Andrologia 2016, 48, 1202–1213. [Google Scholar] [CrossRef]
- Jahan, S.; Rehman, S.; Ullah, H.; Munawar, A.; Ain, Q.U.; Iqbal, T. Ameliorative effect of quercetin against arsenic-induced sperm DNA damage and daily sperm production in adult male rats. Drug Chem. Toxicol. 2015, 39, 290–296. [Google Scholar] [CrossRef]
- Jahan, S.; Iftikhar, N.; Ullah, H.; Rukh, G.; Hussain, I. Alleviative effect of quercetin on rat testis against arsenic: A histological and biochemical study. Syst. Biol. Reprod. Med. 2014, 61, 89–95. [Google Scholar] [CrossRef]
- Durg, S.; Shivaram, S.B.; Bavage, S. Withania somnifera (Indian ginseng) in male infertility: An evidence-based systematic review and meta-analysis. Phytomedicine 2018, 50, 247–256. [Google Scholar] [CrossRef]
- Altoé, L.S.; Reis, I.B.; Gomes, M.; Dolder, H.; Pirovani, J.M. Could vitamin C and zinc chloride protect the germ cells against sodium arsenite? Hum. Exp. Toxicol. 2016, 36, 1049–1058. [Google Scholar] [CrossRef]
- Ajibade, T.O.; Olayemi, F.O. Polyphenol-rich fraction ofAlchornea cordifolialeaf ameliorates arsenite-induced infertility in male rats. Andrologia 2020, 52, e13754. [Google Scholar] [CrossRef] [PubMed]
- Ogunlade, B.; Adelakun, S.A.; Ukwenya, V.; Elemoso, T. Potentiating response of D- Ribose-L-Cysteine on Sodium arsenate- induced hormonal imbalance, spermatogenesis impairments and histomorphometric alterations in adult male Wistar rat. JBRA Assist. Reprod. 2021, 25, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Sampayo-Reyes, A.; Taméz-Guerra, R.S.; de León, M.B.; Vargas-Villarreal, J.; Lozano-Garza, H.G.; Rodríguez-Padilla, C.; Cortés, C.; Marcos, R.; Hernández, A. Tocopherol and selenite modulate the transplacental effects induced by sodium arsenite in hamsters. Reprod. Toxicol. 2017, 74, 204–211. [Google Scholar] [CrossRef]
- Mwaeni, V.K.; Nyariki, J.N.; Jillani, N.; Omwenga, G.; Ngugi, M.; Isaac, A.O. Coenzyme Q10 Protected against Arsenite and Enhanced the Capacity of 2,3-Dimercaptosuccinic Acid to Ameliorate Arsenite-Induced Toxicity in Mice. BMC Pharmacol. Toxicol. 2021, 22, 1–19. [Google Scholar] [CrossRef]
- Zubair, M.; Ahmad, M.; Jamil, H.; Deeba, F. Toxic effects of arsenic on semen and hormonal profile and their amelioration with vitamin E in Teddy goat bucks. Andrologia 2016, 48, 1220–1228. [Google Scholar] [CrossRef] [PubMed]
- Raeeszadeh, M.; Karimfar, B.; Amiri, A.A.; Akbari, A. Protective Effect of Nano-Vitamin C on Infertility due to Oxidative Stress Induced by Lead and Arsenic in Male Rats. J. Chem. 2021, 2021, 1–12. [Google Scholar] [CrossRef]
- Raeeszadeh, M.; Karimi, P.; Khademi, N.; Mortazavi, P. The Effect of Broccoli Extract in Arsenic-Induced Experimental Poisoning on the Hematological, Biochemical, and Electrophoretic Parameters of the Liver and Kidney of Rats. Evid.-Based Complement. Altern. Med. 2022, 2022, 1–9. [Google Scholar] [CrossRef]

| S. No | Countries | Population (Subjects) | Sample Size | Sample | Detected Levels of Arsenic | Reference |
|---|---|---|---|---|---|---|
| 1 | Argentina | Children (3–15 years) | 101 | Hair, Urine | 110–1311 μg/kg | [73] |
| 2 | Montevideo, Uruguay | Children (5–8 years) | 328 | Drinking water | 9.9 μg/L | [74] |
| 0.45 μg/L | ||||||
| 3 | Shaanxi province, China | Adults | 96 | Drinking water | 4.52 µg/L | [75] |
| Indoor air | 0.03 mg/m3 | |||||
| Soil | 14930 mg/kg | |||||
| 4 | Inner Mongolia | Adults | 96 | Drinking water | 144.71 µg/L | |
| Soil | 10190 mg/kg | |||||
| 5 | Villages of Pakistan | Children (≤16 years) | 223 | Ground water | 15. 63 µg/kg/day (arsenate) 0.09 µg/kg/day (arsenite) | [76] |
| Adult | 15.07 µg/kg/day (arsenate) 0.26 µg/kg/day (arsenite) | |||||
| 6 | Indae metal mine area | Residents (mean age of 66.8 years) | 50 | Urine | Arsenite (1.45 µg/L), arsenate (0.74 µg/L), MMA (2.43 µg/L), DMA (27.63 µg/L), and arsenobetaine (88.62 µg/L) | [77] |
| 7 | Spain | Children (4–5 years) | 400 | Urine | 2.74–7.54 µg/L | [78] |
| S. No | Country | Sample (s) | Arsenic Levels | Reference |
|---|---|---|---|---|
| 1 | Kolkata, India | Rice, grain, and vegetable | 76 µg/kg and 41.4 µg/kg | [79] |
| 2 | UK | Rice | 130 μg/kg | [80] |
| 3 | Kunming, China | Rice | 3520 µg/kg | [81] |
| 4 | Japan | Rice, hijiki | 19 µg/kg and 59 µg/kg | [82] |
| 5 | Pakistan | Raw rice | 92.5 ± 41.88 μg/kg | [83] |
| Cooked rice | 79.21 ± 76.42 μg/kg | |||
| Wheat | 116.38 ± 51.38 μg/kg | |||
| 6 | West Bengal, India | Boro and Aman rice | 194 μg/kg and 156 μg/kg | [79] |
| Arum and radish | 780 and 674 μg/kg | |||
| Urine | 154–276 µg/L | |||
| 7 | Ngari area, Western Tibet | Barley | 180 ± 210 µg/kg | [84] |
| Vegetable | 400 ± 570 µg/kg, | |||
| Meat | 210 ± 160 µg/kg | |||
| Dairy products | 180 ± 80 µg/kg | |||
| Daily intake | 3600 µg/kg | |||
| 8 | Kurdistan province, Iran | Sheep meat | 230 ± 140 µg/kg | [85] |
| Beef meat | 200 ± 90 µg/kg | |||
| Turkey meat | 880 ± 210 µg/kg | |||
| Ostrich meat | 850 ± 130 µg/kg |
| S. No | Experimental Model | Phytonutrient | Treatment | Route of Administration | Duration | Organ/Tissue | Observations | Reference |
|---|---|---|---|---|---|---|---|---|
| 1 | Mice | C. borivilianum | Sodium arsenite 4 mg/kg bw, C. borivilianum 100, 200, 400, and 800 mg/kg bw | Oral | 30 days | Testes | Decreased acid phosphatase, alkaline phosphatase, and cholesterol levels. | [174] |
| 2 | Mice | Ellagic acid and Ferulic acid | Sodium arsenate dibasic heptahydrate–200 ppm and Ellagic acid 50mg/kg and Ferulic acid 50 mg/kg | Oral | 40 days | Testes and sperm | Restored sperm morphology characteristics, anti-oxidant levels, enhanced expression of Nfe2l2 and StAR, reduced Ppargc1a. | [179] |
| 3 | Mice | Lutein | As trioxide–5 mg/kg bw and Lutein 40 mg/kg bw | Oral | 35 days | Testes and sperm | Increased sperm count. Enhanced expression of Nrf-2 and downstream genes (HO-1, GST and NQO1). | [180] |
| 4 | Mice | Grape seed proanthocyanidin extract | As trioxide–4 mg/kg bw, grape seed proanthocyanidin extract 100 and 200 mg/kg bw | Oral | 35 days | Testes | Reduction in MDA, 8-OHdG, increased T-AOC and activities of GSH and SOD. Elevated expression of genes related to Nrf-2 signalling pathway. | [181] |
| 5 | Mice | Epigallocatechin (EGCG) | Sodium arsenite heptahydrate–200 ppm, Epigallocatechin 3–20 mg/kg bw | Intraperitoneal | 40 days | Testes | Restored sperm morphology, SMI, FMI, serum testosterone, antioxidant system. | [183] |
| 6 | Mice | N-acetyl cysteine (NAC) | Arsenic trioxide–0.3 and 3 mg/kg bw | Subcutaneous | 35 days | Sperm and Seminal vesicle | Restored seminal vesicle weight, sperm motility, daily sperm production. | [188] |
| NAC–40mM | Oral | |||||||
| 7 | Mice | N-acetyl cysteine (NAC) | Sodium arsenite–4 ppm, NAC–75 mg/kg bw | Intraperitoneal | 40 days | Testes | Restored weight of testes, epididymis, seminal vesicles and ventral prostate and increase in sperm parameters, 3βHSD, 17βHSD, and SOD catalase activities. | [184] |
| 8 | Mice | Chlorogenic acid (CGA) | Sodium arsenite–5 mg/kg and CGA–100 and 200 mg/kg bw | Oral | 28 days | Testes | Antioxidant, anti-inflammatory, anti-apoptotic, and activates Nrf-2 pathway. | [185] |
| 9 | Rat | α-lipoic acid | Sodium arsenite–25 mg/L and α-lipoic acid–70 mg/kg bw | Intraperitoneal | 56 days | Testes | Restoration of testicular architecture, testicular sperm production, 3β and 17β-HSDs. | [175] |
| 10 | Rat | FHPD | As trioxide–3 mg/kg bw 7% pea and 15% casein were added. | Oral | 30 days | Testes and sperm | Restored the number of total motile spermatozoa, maintains testosterone levels, restored anti-oxidant levels. | [176] |
| 11 | Rat | Ellagic acid | Sodium arsenite 10 mg/kg bw Ellagic acid 10 and 30mg/kg bw | Oral | 14 days | Testes | Restored serum testosterone, testicular anti-oxidant level and structural changes. | [178] |
| 12 | Rat | Polydatin | As trioxide–100 mg/L, Polydatin–50,100 and 200 mg/kg bw | Oral | 60 days | Sperm | Enhanced sperm membrane integrity, sperm morphology, enhanced epididymal sperm motility. | [182] |
| 13 | Rat | Melatonin | Sodium arsenite–5 mg/kg bw and Melatonin–25 mg/kg bw | Oral | 30 days | Testes and sperm | Improved body weight, testicular weight, reduced the TUNEL positive germ cells enhanced PCNA index. | [189] |
| 14 | Rat | Co-enzyme Q10 | Sodium arsenite–10 mg/kg bw Co-enzyme Q10–10 mg/kg | Intraperitoneal | 5 days | Testes | Restored serum testosterone, restored anti-oxidant, TNF-α, NO, restored testis architecture and active spermatogenesis, reduction in i-NOS, NF-kβ, Fas ligand and caspase-3 in testis. | [190] |
| 15 | Rat | Quercetin | Sodium arsenite–10 mg/kg bw Quercetin–50 mg/kg bw | Oral | 15 days | Testes | Restored testicular architecture, reduced TUNEL positive cells. | [191] |
| 16 | Rat | Quercetin | Sodium arsenite–50 ppm Quercetin–50 mg/kg bw | Oral | 49 days | Epididymis | Recovered daily sperm production, sperm count, and reversed sperm DNA damage. | [192] |
| 17 | Rat | Quercetin | Sodium arsenite–50 ppm Quercetin–50 mg/kg bw | Oral | 49 days | Testes | Restored GSH, CAT, SOD, POD, TBARS, and testosterone. | [193] |
| 18 | Rat | Withania somnifera | Sodium arsenite–8 mg/kg bw Withania somnifera–100 mg/kg bw | Oral | 30 days | Testes and sperm | Restored sperm morphology characteristics, serum testosterone, decreased LPO, restored spermatogenesis, and testicular architecture. | [194] |
| 19 | Rat | Zinc chloride and Vitamin C | Sodium arsenite–5 mg/kg bw Zinc chloride–20 mg/kg bw Vitamin C–100 mg/kg bw | Oral | 60 days | Testes and epididymis | Restored sperm morphology, count, and seminiferous tubule diameter. | [195] |
| 20 | Rat | Pistia stratiotes | Pistia stratiotes–100 mg/kg Sodium arsenite–2.5 mg/kg | Oral | 14 days | Sperm | Restored sperm motility, viability, count, and semen volume. | [186] |
| 21 | Rat | Alchornea cordifolia | Sodium arsenite–7 mg/kg bw Alchornea cordifolia–100 µg/kg bw | Oral | 30 days | Testes | Enhanced testosterone, FSH, spermatozoa count, and motility. Expression of Androgen receptor binding protein and anti-apoptotic B-cell lymphoma-2. | [196] |
| 22 | Rat | D-Ribose-L-Cysteine | Sodium arsenate–8 mg/kg bw D-Ribose-L-Cysteine–10 and 30 mg/kg bw | Oral | 28 days | Testes | Restored sperm count, motility and viability, LH, FSH, and testosterone and CAT, SOD, GSH. | [197] |
| 23 | Rat | Selenium and Diphenyl diselenide (DPDS) | Sodium arsenite–60 µg/L Selenium–0.25 mg/kg bw and DPDS–2.5 mg/kg bw | Oral | 45 days | Testes and epididymis | Suppressed inflammation, myeloperoxidase activity, NO, TNF-α, and IL-1. | [187] |
| 24 | Teddy goat buck | Vitamin E | Sodium arsenite–5 mg/kg bw Vitamin E–200 mg/kg bw | Oral | 84 days | Testes | Enhanced spermatogenesis, restored germinal epithelium, and enhanced testosterone, FSH, LH, and ameliorated histopathological lesions. Serum LH, FSH, and testosterone were restored and improved semen quality. | [167] |
| 25 | Hamster | α- tocopherol succinate (α-TOS) and sodium selenite (SS) | Sodium arsenite 100 ppm α-TOS–6 mg/kg bw and SS–0.025mg/kg bw | Oral | 22 days | Placenta and fetus | Decreased teratogenic effects. SS increased methylation process and α-TOS enhanced antioxidant activity. | [198] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rachamalla, M.; Chinthada, J.; Kushwaha, S.; Putnala, S.K.; Sahu, C.; Jena, G.; Niyogi, S. Contemporary Comprehensive Review on Arsenic-Induced Male Reproductive Toxicity and Mechanisms of Phytonutrient Intervention. Toxics 2022, 10, 744. https://doi.org/10.3390/toxics10120744
Rachamalla M, Chinthada J, Kushwaha S, Putnala SK, Sahu C, Jena G, Niyogi S. Contemporary Comprehensive Review on Arsenic-Induced Male Reproductive Toxicity and Mechanisms of Phytonutrient Intervention. Toxics. 2022; 10(12):744. https://doi.org/10.3390/toxics10120744
Chicago/Turabian StyleRachamalla, Mahesh, Joshi Chinthada, Sapana Kushwaha, Sravan Kumar Putnala, Chittaranjan Sahu, Gopabandhu Jena, and Som Niyogi. 2022. "Contemporary Comprehensive Review on Arsenic-Induced Male Reproductive Toxicity and Mechanisms of Phytonutrient Intervention" Toxics 10, no. 12: 744. https://doi.org/10.3390/toxics10120744
APA StyleRachamalla, M., Chinthada, J., Kushwaha, S., Putnala, S. K., Sahu, C., Jena, G., & Niyogi, S. (2022). Contemporary Comprehensive Review on Arsenic-Induced Male Reproductive Toxicity and Mechanisms of Phytonutrient Intervention. Toxics, 10(12), 744. https://doi.org/10.3390/toxics10120744